Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Percutaneous image-guided needle biopsy (PNB) is the cornerstone for diagnosis and treatment of many diseases involving the liver. Minimally invasive, PNB is an effective outpatient procedure with a low complication rate. The success of PNB requires proper patient selection, optimal procedural technique, and optimal postprocedure management.
Interventional radiology (IR, which also stands for interventional radiologists) plays a central role in patient management because of the need for image-guided tissue sampling procedures. Several oncology trials have demonstrated that molecular and biomarker targeted therapies have a higher chance of clinical success, and such studies require tissue for analysis. PNB may also prevent unnecessary surgery for lesions with benign pathology. Finally, tissue obtained at PNB can allow for histologic grading that has both prognostic and treatment implications.
The role of PNB has increased beyond simply obtaining a diagnosis, and tissue obtained from PNB can now predict disease susceptibility to treatment and provide prognostic information. In addition, tissue-based biomarkers can be used to prescribe a tumor-specific treatment regimen because they can predict response to targeted therapies and/or immunotherapy. As a result, an increased volume of tissue per biopsy and repeated sampling from the same target are often required.
Because the results of PNB are so impactful for optimal care of patients with a liver mass or at risk for cirrhosis, obtaining adequate specimens is critically important. This also holds true for clinical trials in which inadequate specimens have been shown to be a cause of ineligibility for oncology patients. For example, in the National Cancer Institute–Molecular Analysis for Therapy Choice (NCI-MATCH) trial, almost 15% of biopsy samples were inadequate for molecular analysis; this proved to be a major impediment in the treatment of patients for whom a trial was the last resort. Therefore, with PNB, IR can help improve patient outcomes through the study of biomarkers and optimization of specimen acquisition techniques. This chapter will explore the equipment and modalities used to perform PNB, the preprocedural and postprocedural patient care, sample adequacy concepts, and quality metrics, as well as future perspectives in the era of personalized medicine.
PNB involves advancing a needle under imaging guidance into a target to obtain tissue or cells for diagnosis. PNB can be performed using aspiration techniques with a thin hollow needle (18–25 gauge) for cytologic evaluation or by using a cutting tool (core needle biopsy [CNB]), which uses a larger needle (9–20 gauge) that has a capturing mechanism to allow for the extraction of a piece of tissue. Of the two, only CNB allows for gross histologic evaluation ( Fig. 23.1 ). Both techniques can provide material for immunohistochemistry and molecular characterization.
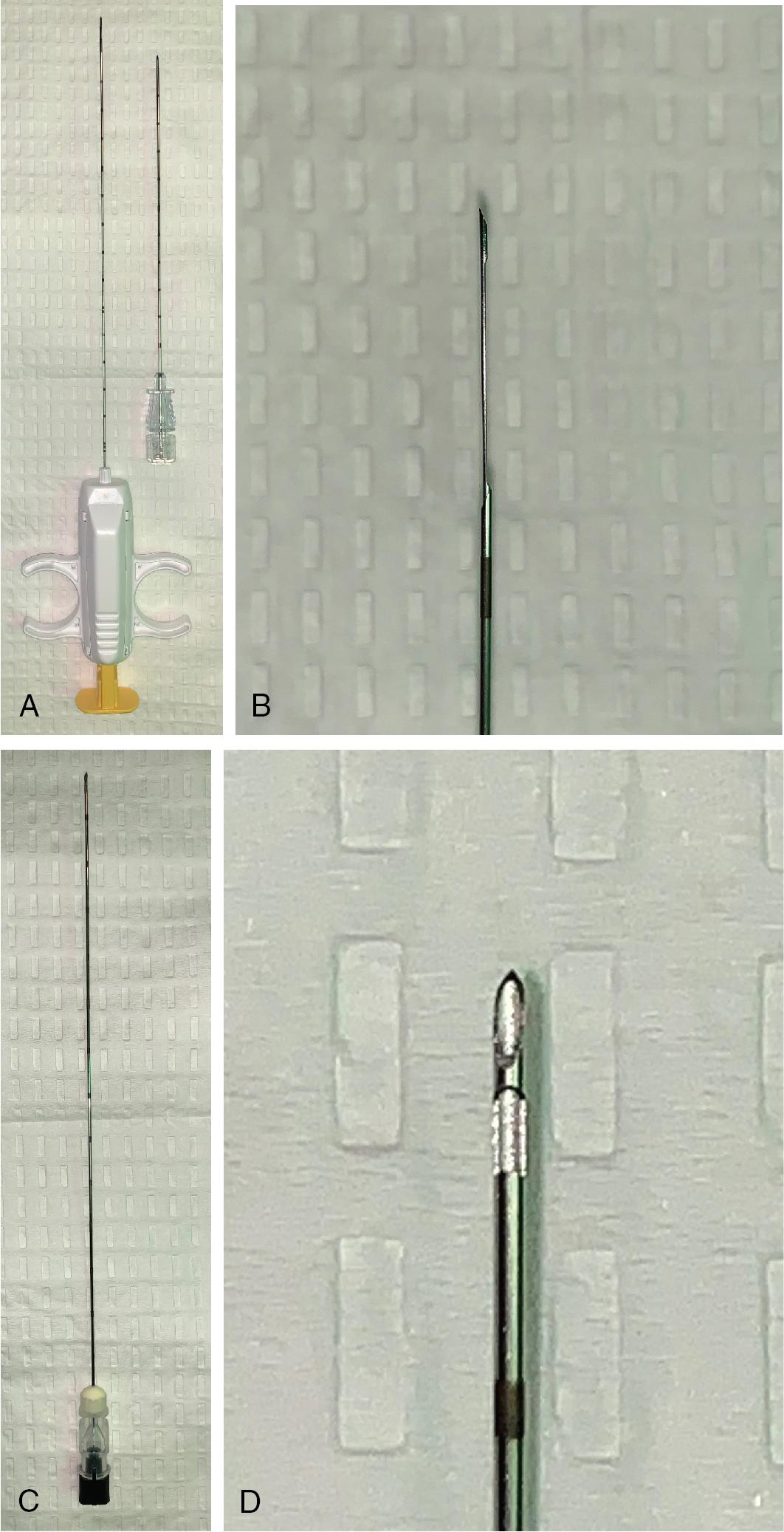
Aspiration techniques include fine needle aspiration (FNA), fine needle capillary sampling (FNCS), and large needle aspiration (LNA). These techniques are generally considered safer and potentially less traumatic compared with CNB. During FNA, samples are acquired by using suction from a syringe until tissue/cells are collected. FNCS consists of placing a fine needle into the target, followed by rotating the tip within the target without aspiration until the sample ascends the needle by capillary action. An LNA is similar to an FNA but performed with a larger bore needle, is often useful to aspirate fluid for cytology, and may be preferred for thicker fluids/secretions. Ideally, an on-site cytopathologist or cytotechnologist can provide an immediate evaluation of the sample quality; this has been shown to increase the sensitivity of the biopsy, shorten the procedure time, and minimize the number of passes required to obtain a diagnostic specimen. ,
A CNB is most commonly performed with 16- to 21-gauge needles that have an automated cutting mechanism and a variable throw or length of tissue sampled (5–30 mm). The specific needles are selected based on target size and presence of vascular structures or other organs in the path of or beyond the margins of the targeted lesion. The needle length is chosen after determining the depth of the target, based on the preprocedural imaging. Although FNA can provide excellent diagnostic information for metastatic disease, infection, and lymphoma, CNB samples are more likely to render definitive diagnosis for most primary liver tumors. To confirm adequacy of the specimen, a touch preparation can be prepared on a glass slide for immediate evaluation by a cytopathologist or cytotechnologist. Before placement in formalin or saline, the core of tissue is placed on a glass slide and gently moved over the slide to allow some cells to collect on the surface. Care should be taken to avoid excessive vigorous touch preparations because this has been shown to deplete the cellularity and DNA content of the specimen.
Core samples are commonly placed in formalin. Occasionally, specimens may be sent “fresh” to pathology in saline or on saline-soaked gauze for special studies. Because cells placed in saline eventually undergo cell lysis related to osmotic shifts of saline into the cell, a specimen in saline needs to be fixed or frozen within a few hours to avoid deterioration of the tissue sample. Tissue also may be snap frozen for future studies. The preferred method for processing tissue may vary from institution to institution, and the preference of the pathologists reviewing the material should be ascertained before initiating a biopsy. For core biopsies obtained to evaluate organ parenchyma, end-cut rather than side-notch needles may yield more diagnostic samples in terms of number of portal triads. In the setting of organ dysfunction or failure, no touch preparation is required; specimens are sent in formalin or saline, depending on the indication and the preference of the pathologist.
Many authors advocate performing both FNA and CNB to maximize the diagnostic yield of every biopsy. CNB is particularly useful in most solid primary liver lesions, such as well-differentiated hepatocellular carcinoma (HCC) or nodular hyperplasia in the setting of cirrhosis and in confirmation of benign diagnoses, including hemangioma, adenoma, or focal nodular hyperplasia (FNH; see Chapter 87 ). ,
For more information, see Chapter 13, Chapter 14, Chapter 15, Chapter 16, Chapter 17 .
Preprocedural target evaluation is crucial to adequately select the best imaging modality for a specific target lesion. Multiple imaging modalities are available. For a summary of advantages and disadvantages of each imaging modality, please refer to Table 23.1 .
| MODALITY | REAL TIME | COMMON TARGETS | LIMITATIONS | ADVANTAGES |
|---|---|---|---|---|
| Ultrasound | Yes |
|
|
|
| CT | Nearly (CT fluoroscopy) |
|
|
|
| Fluoroscopy | Yes |
|
|
|
| X-Ray | No | Stereotactic – breast | Ionizing radiation | Visualize and sample calcifications |
| MR | Nearly (MR Fluoroscopy) |
|
|
|
| PET-CT | No, although often used in combination with CT |
|
|
|
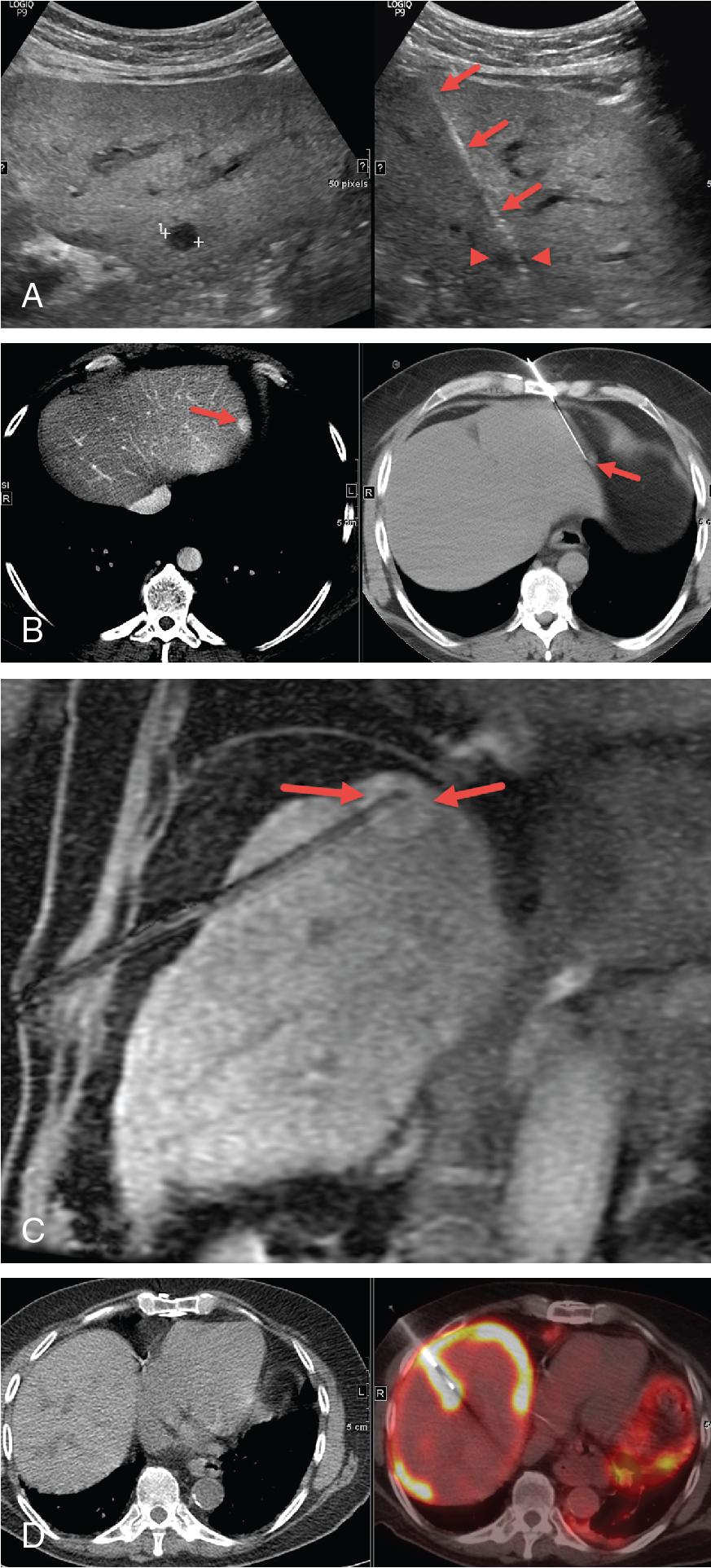
For many lesions that can be seen with ultrasonography (US), ultrasound is ideal for PNB. Because it is located just under the diaphragm, the liver is susceptible to respiratory motion. Smaller lesions and lesions in proximity to critical structures benefit the most from US because of the ability to visualize the needle in real-time as it is advanced from the skin into the target. Another advantage of US is the ability to image in virtually any plane, which allows the operator to plan trajectories that might be impossible using computed tomography (CT) or magnetic resonance (MR) guidance. The fact that US does not use ionizing radiation is also relevant, especially for children and pregnant patients. US has been shown to result in a shorter procedure time and a lower cost when compared with CT-guided interventions. The ability to successfully and safely place a biopsy needle in a lesion is unfortunately operator-dependent and has a relatively steep learning curve. Further, US imaging is limited or impossible in air-filled/gas-filled structures, such as the lung or bowel, and the sound waves cannot penetrate bone.
CT is a common modality for guiding PNBs because it provides superb anatomic detail. IRs are very familiar with cross-sectional imaging, making it the modality of choice for deep intra-abdominal structures or for those that cannot be adequately imaged with US (i.e., lung, pancreas, adrenal glands, retroperitoneal lymph nodes, bone). Many manufacturers offer CT fluoroscopy as an option on diagnostic scanners. This produces CT images in near real time. CT fluoroscopy can expose the operator to ionizing radiation, but some physicians prefer it because of the near immediacy between needle manipulation and image availability.
Cone-beam CT (CBCT), also sometimes referred to as C-arm CT , uses a flat-panel x-ray detector that rotates around the patient; the x-rays are divergent, forming a cone. Images can be reconstructed in multiple planes, and three-dimensional reconstructions can be performed. The soft tissue resolution is not nearly as good as with conventional CT, but the resolution for lung and bone is adequate for biopsy guidance. Further, available biopsy path–planning and needle-navigation software may assist the operator with needle placement.
MR imaging (MRI)–guided biopsy has been made possible by the advent of open-bore MRI systems that provide access to patients during imaging and the availability of nonferrous biopsy needles and monitoring equipment. The superior contrast resolution of MRI allows for the targeting of lesions that are difficult to visualize with US and noncontrast CT, and the ability of MRI to image in any plane enhances the safe targeting of lesions where access in the axial plane would be more dangerous or more difficult. However, there is a limited selection of MRI-safe biopsy needles and there is considerable artifact on the MRI images; this makes biopsy of small lesions challenging.
Fluoroscopy is useful in the abdomen for guiding bile duct biopsies. Benign and malignant biliary strictures often have similar cholangiographic appearances and rarely can be distinguished based on imaging alone , (see Chapter 16 and 20 ). Lesions originating within the duct may be sampled by either an endoluminal or a direct percutaneous approach. Percutaneous transhepatic biliary drainage allows direct access to the biliary tract for endoluminal biopsy (see Chapter 31 ). Biopsy forceps or brush-biopsy catheters can be used through the existing tract to obtain tissue samples of suspicious areas. The sensitivity of forceps biopsy is in the range of 40% to 80%, higher than that of brush biopsy, which is in the range of 30% to 60%. Specificity for each approaches 98%. , , Combining forceps and brush biopsy of the bile duct may provide superior results to either alone. Studies have noted the sensitivity of brushing alone of 49%, forceps alone of 69%, and combined of 80%; with specificity for malignancy of 100%. However, a new percutaneous forceps biopsy technique cites sensitivity of 93.3%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 70%, with overall accuracy of 94.2%. Alternatively, after the biliary tree is opacified, a direct PNB of a bile duct lesion may be targeted with fluoroscopy, using a transhepatic approach. , After contrast injection into the indwelling biliary drainage catheter to delineate the targeted bile duct abnormality, a fine needle is advanced through the abdomen to the target and a specimen is obtained. Confirmation of accurate needle position is made by obtaining oblique fluoroscopic images and by real-time fluoroscopy, when the needle is seen to move the duct or the indwelling catheter or both. Fluoroscopy can also be used to guide nontargeted transvenous biopsies of the liver.
Occasionally, a lesion is only well demonstrated by 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging (see Chapter 18 ). In these cases, it is possible to use the combination of PET and CT to guide accurate needle placement. Certain lesions may also have varying FDG avidity; PET guidance allows the most hypermetabolic portion of a lesion to be targeted. Operators should be mindful of the patient as a source of radiation; the major source of radiation to the operator during PET-guided interventions was found to be the time spent in close proximity to the patient.
New technology that requires further development into clinical practice allows for the fusion of multiple modalities. For example, CT and MRI scans can be overlaid with real-time US images to achieve the clarity of the CT or MR and the real-time visualization capabilities of US. PET images can also be fused. Additionally, robotic guidance systems have entered the market in an effort to optimize speed and accuracy for needle placement ( Fig. 23.2 ).
With the exception of nontarget liver biopsy, all patients undergoing PNB should have preprocedural imaging. Careful evaluation of the images by an IR is mandatory for the procedure to be successful because adequate imaging will determine the proper imaging modality for guidance, patient positioning, and preferred access/sampling technique. Main objectives for PNB may include diagnosis of the etiology of diffuse parenchymal diseases, microbiology diagnosis in infectious diseases, histologic diagnosis of a focal lesion, histologic classification of a malignancy, pathologic staging of a malignancy, or molecular diagnostic testing.
Absolute contraindications for a PNB are rare and are basically limited to lack of safe access. Relative contraindications are related to conditions that increase the risk for complications and include coagulopathy (depending on target location), inability to sedate or provide general anesthesia, and significant comorbidities. Table 23.2 lists the general approach to common medication classes that should be considered before any biopsy procedure.
| MEDICATION CLASS | LOW PROCEDURAL RISK | HIGH PROCEDURAL RISK | MEDICATION RESUME |
|---|---|---|---|
| Glycoprotein IIb/IIIa inhibitor | Withhold 24 hours before procedure | 24 hours | |
| Direct thrombin inhibitors | Continue | Withhold 2–4 hours before procedure and check activated partial thromboplastin time (aPTT) | 4–6 hours after procedure |
| Direct factor Xa inhibitors | Continue | eGFR ≥30 mL/min: withhold 4 doses eGFR <30 mL/min: withhold 6 doses Emergent: Use reversal agents as appropriate (i.e., andexanet alfa). |
24 hours |
| Nonsteroidal antiinflammatory drugs (NSAIDs) | Continue | Hold for five days if possible, minimum three days | 24 hours after procedure |
| Parenteral direct P2Y 12 inhibitors | Defer until patient is off medication. If emergent, withhold 1 hour before procedure, discuss with cardiology | Defer until patient is off medication. If emergent, withhold one hour before procedure, discuss with cardiology | 4–6 hours after procedure |
| Intermediate-acting NSAID | Continue | Hold morning of procedure | 24 hours after procedure |
| Reversible phosphodiesterase II inhibitor | Continue | Continue | 4–6 hours after procedure |
| Thienopyridines (P2Y 12 platelet inhibitors) | Continue | Withhold for five days before procedure | 4–6 hours after procedure |
| Low molecular weight heparin | Continue |
|
12 hours after procedure |
| Heparin | Continue | Stop 4–6 hours before procedure. | 6–8 hours after procedure |
| Phosphodiesterase (PDE) inhibitor | Continue | Hold morning of procedure | Resume day after procedure |
| Reversible adenosine diphosphate (ADP) receptor antagonist | Continue | Withhold five days before procedure | Day after procedure |
| Vitamin K antagonist |
|
|
|
The indication for liver biopsy can be broadly grouped into two categories: (1) Random (nontarget) liver biopsy for diagnosis of hepatocellular disease, and (2) targeted liver biopsy for tissue diagnosis of a liver mass. With refinements in abdominal imaging, benign diseases are often confidently diagnosed on imaging alone, obviating the need for a biopsy; this is particularly helpful in cases of suspected adenomas and hemangiomas because these highly vascular lesions have a higher risk for procedural complications.
Certain locations in the liver can be more technically difficult in terms of visualizing and accessing the lesion, particularly with masses that are closer to the dome and/or more anterior. In these instances, patient cooperation with deep inspiration and/or decubitus or semirecumbent positioning can be helpful, although the need for deep inspiration in particular limits the ability to sedate the patient for the procedure, because sedated patients are typically not able to cooperate with breathing instructions. When multiple liver lesions with similar diagnostic imaging characteristics are present, the choice of which lesion to biopsy is made based on lesion visibility and location. A needle trajectory that passes through normal parenchyma before accessing the lesion is preferred to minimize the risk for hemorrhage and tract seeding.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here