Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Lupus erythematosus is a complex disorder associated with numerous clinical signs and symptoms and a wide range of laboratory abnormalities. It shows a spectrum of varying prognoses, ranging from a benign, solely cutaneous variant (localized discoid) through to a potentially fatal systemic illness. The range of subtypes is shown in Table 17.1 .
|
Although the precise etiology is unknown, it is thought that interplay of genetic factors, autoantibodies, immune complexes, hormones, and other factors is responsible for the development of the illness. There is evidence suggesting that the incidence of the systemic variant is increasing, but due to earlier diagnosis and more effective therapy, the mortality rate has significantly diminished and the 10-year overall survival rate in adults now exceeds 90%. The presence of renal and/or neurological involvement, however, remains a poor prognostic indicator.
Pediatric systemic lupus erythematosus (SLE) is an aggressive illness with considerable mortality, largely due to the incidence of renal disease. Even with corticosteroid and immunosuppressive therapy the death rate is as high as 15%.
Discoid lupus erythematosus (DLE), the commonest form, is subdivided into localized and generalized variants. This is of prognostic importance because only about 1% of patients with localized DLE develop systemic disease, but approximately 5% of those with the generalized form (in particular those with persistent anemia, leukopenia, thrombocytopenia, false-positive Wassermann reaction, and high titer antinuclear factor) develop full-blown SLE. Discoid lesions develop in up to 20% of patients with systemic lupus. Periungual telangiectasia, sclerodactyly, and the presence of Raynaud phenomenon may also signify potential disease progression. Patients with DLE should not be made unduly worried about the risk of developing systemic involvement, which is not high.
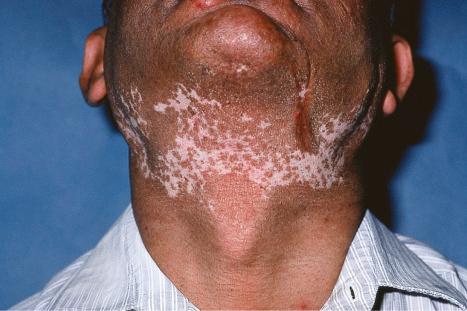
DLE is persistent and affects twice as many females as males. Although any age group may be involved, it is most common in the third, fourth, and fifth decades, with a peak incidence in the late thirties. Presentation in childhood is rare. Lesions typically arise on sun-exposed sites and patients frequently experience photosensitivity; there may be spring and summer exacerbation. In the localized form, the head and neck are usually affected, but in the generalized variant, lesions may also be present on the dorsal aspect of the arms, hands, and fingers and on the ‘V’ of the neck. Nonexposed sites, including the trunk, upper limbs, and the palms and soles, are also commonly involved.
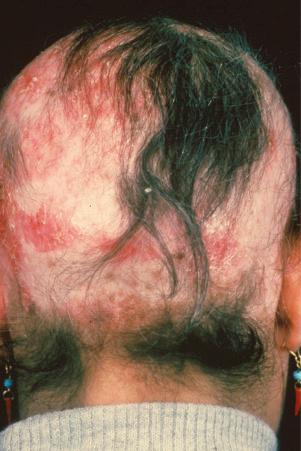
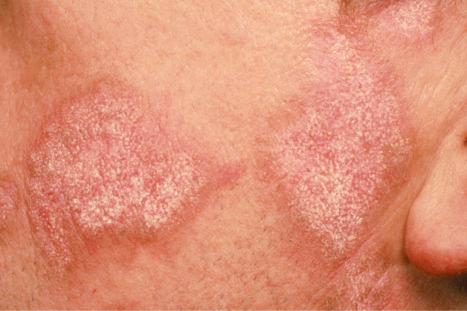
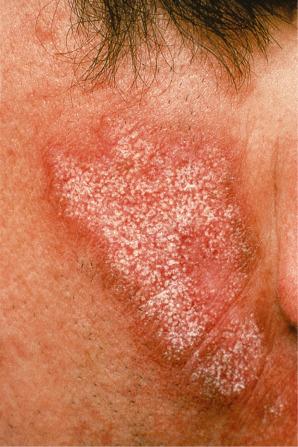
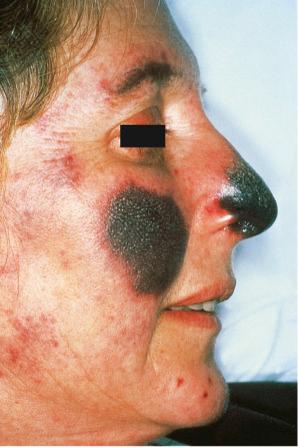
Facial plaques occur most often on the cheeks ( Fig. 17.1 ). Other sites affected include the bridge of the nose, the ears, the neck, and the scalp ( Figs 17.2–17.6 ). The associated scarring of the scalp is followed by permanent alopecia ( Figs 17.7 and 17.8 ). Scalp involvement, which is more common in those patients affected by the disease when they were young, is chronic, and correlates with longstanding severe illness. Ocular involvement mainly encompasses the eyelids, followed by orbit and cornea with blepharitis being the most common symptom.
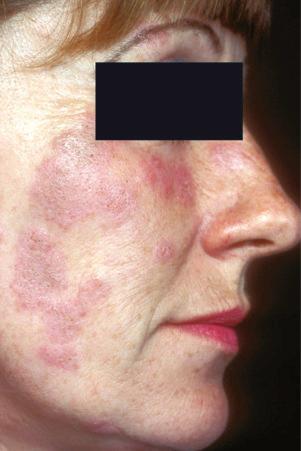
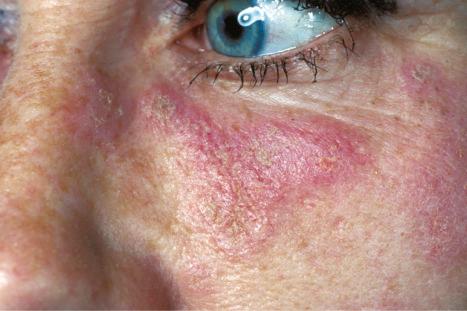
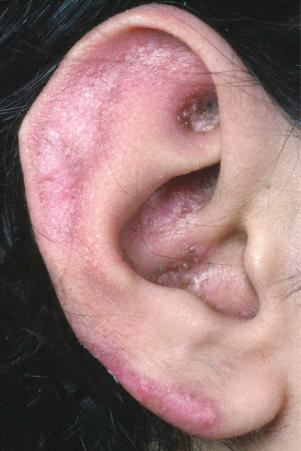
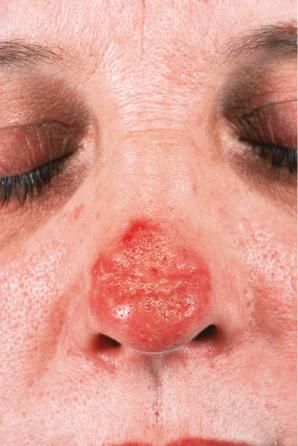
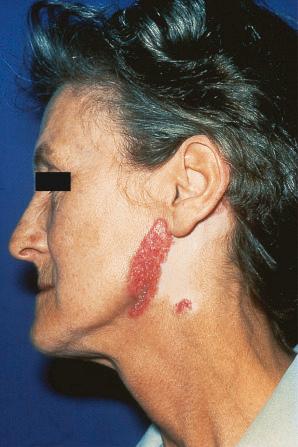
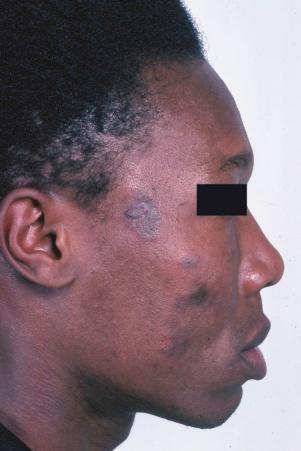
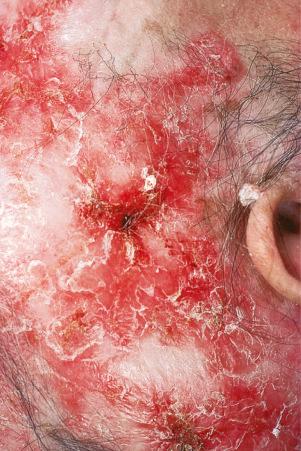
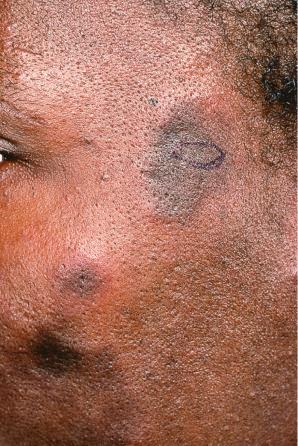
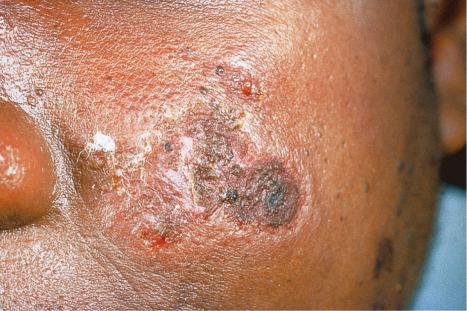
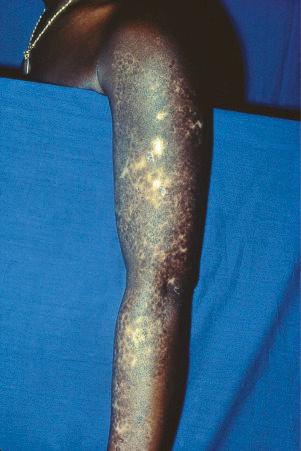
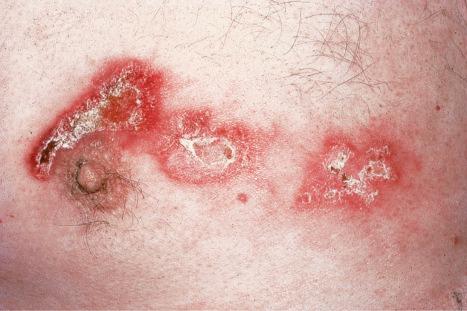
Early lesions appear edematous and erythematous. An established plaque of DLE, which may measure up to 10 cm across, is usually covered with an adherent scale accompanied by epidermal atrophy, follicular dilatation, and plugging ( Figs 17.9 and 17.10 ). If the scale is removed the horny plugs are often seen attached to its undersurface (‘carpet tacks’ sign). Telangiectasia is a common finding and lesions heal with scarring, which is often marked. In dark- or black-skinned individuals the plaque may be hypo- or hyperpigmented and this may be particularly disfiguring ( Figs 17.11–17.14 ).
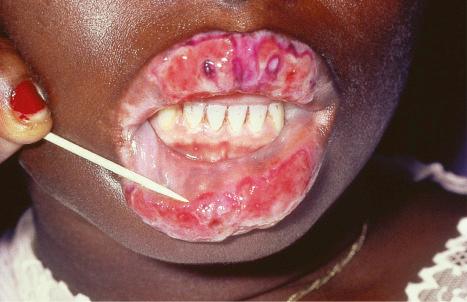
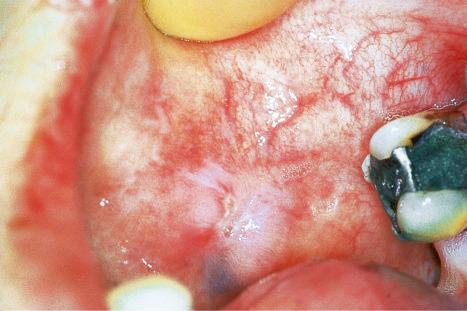
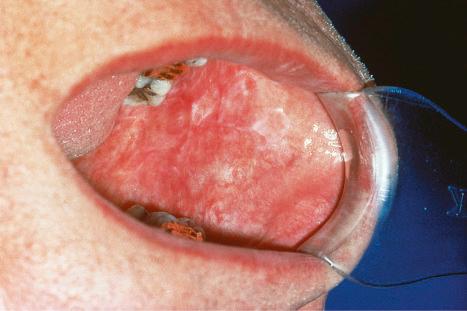
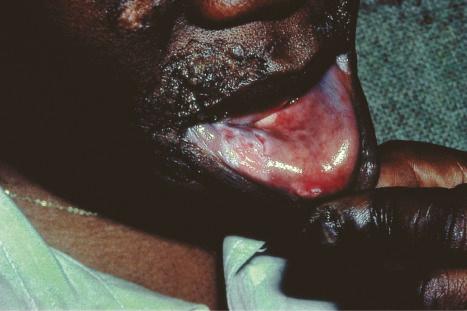
Oral involvement occurs in 20% to 25% of patients with DLE, with the vermilion border of the lower lip, alveolar processes, labial and buccal mucosae being particularly affected ( Figs 17.15–17.17 ). Chronic lesions are typically erythematous and atrophic with a scalloped white keratotic border and adjacent telangiectasia. Erosions and ulcers are additional features. Lesions are sometimes indistinguishable from atrophic lichen planus. Involvement of the tongue manifests as erythema, fissuring, and atrophy of the papillae. Chronic lupus cheilitis is associated with cicatricial scarring and an increased risk of squamous carcinoma. Genital skin and perianal mucosal involvement has occasionally been documented.
DLE has been described in association with osteopoikilosis, α 1 -antitrypsin deficiency, polyarteritis nodosa, Addison disease, chronic granulomatous disease, dystrophic calcinosis cutis, allergic contact dermatitis, acquired partial lipoatrophy, as a reaction to tattoo, and in a patient with scleroderma, deep linear morphea, and chronic hepatitis C virus infection. Exceptionally, localized DLE has been reported in the area previously affected by herpes zoster and at the site of previous traumatic injury.
Systemic symptoms are usually absent in patients with localized DLE. In the generalized form, in which cutaneous plaques may be quite widespread, a small percentage may have Raynaud phenomenon and arthralgia ( Figs 17.23–17.25 ). Laboratory abnormalities are more common in the generalized than in the localized variant. Anemia is not seen in localized DLE, but is sometimes a feature of the generalized variant. Leukopenia, a raised erythrocyte sedimentation rate (ESR), and hypergammaglobulinemia can occur in both. Antinuclear factor (diffuse pattern), a positive Wassermann reaction, and rheumatoid factor may also be features. Anti-double-stranded DNA (dsDNA) antibodies are detected in a minority of patients with disseminated DLE, and these patients frequently transform to the systemic disease; occasionally, antibodies to single-stranded DNA (ssDNA) are present. Urinalysis and renal function tests are normal.
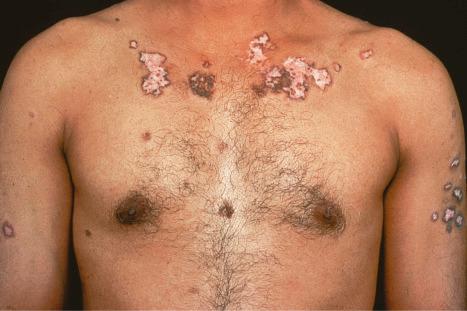
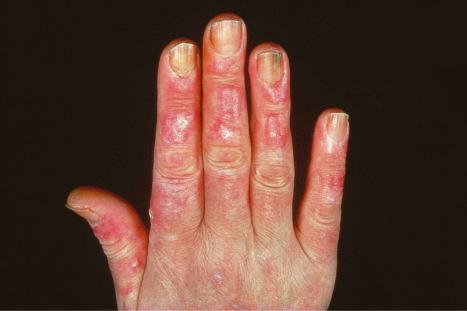
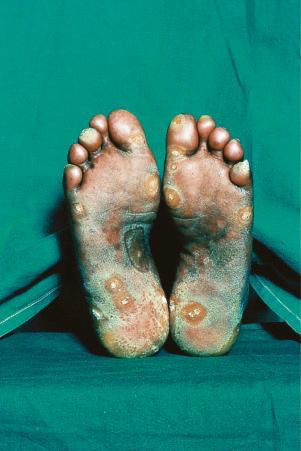
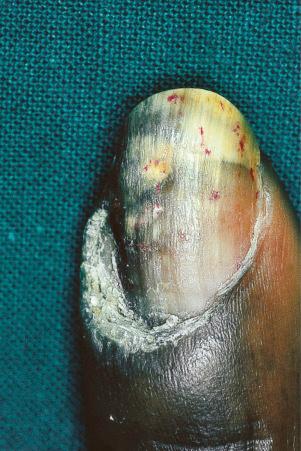
Verrucous (hypertrophic) DLE presents as warty, hyperkeratotic papules and plaques with a predilection for the face, scalp, mucous membranes of the lips, and upper limbs ( Figs 17.18 and 17.19 ). It affects approximately 2% of patients with chronic discoid disease. Although verrucous (hypertrophic) hyperkeratotic skin changes have been observed in patients with SLE, such cases are exceptional. Sometimes the palms and soles are affected and occasionally the nails ( Figs 17.20 and 17.21 ). Rare manifestations include nodular keratoacanthoma-like, squamous cell carcinoma-like, and hypertrophic lichen planus-like lesions on the arms and hands ( Fig. 17.22 ). A variant with associated necrosis of the subcutaneous tissue is known as lupus erythematosus hypertrophicus et profundus.
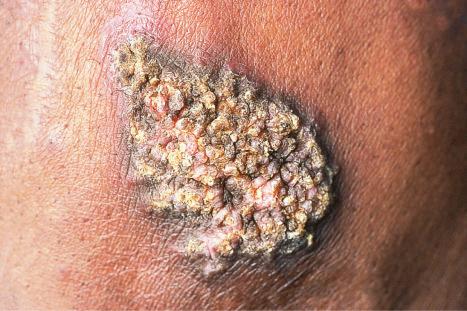
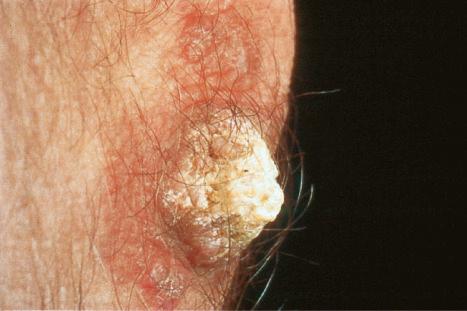
Cutaneous squamous cell carcinoma and less often basal cell carcinoma may arise in patients with chronic lesions including the hypertrophic variant. Squamous cell carcinoma occurs predominantly in males, particularly affects the scalp, and is sometimes associated with early metastases. A case of atypical fibroxanthoma has recently been reported in a scar of DLE.
Lupus erythematosus tumidus is a distinctive subset of cutaneous lupus clinically presenting mainly in patients with DLE and rarely in patients with SLE. It is characterized by erythematous papules, plaques or even nodules with an urticarial appearance arising mainly on sun-exposed skin of the face, neck, and trunk. Scarring does not occur. This variant of lupus is discussed in more detail in Chapter 8 .
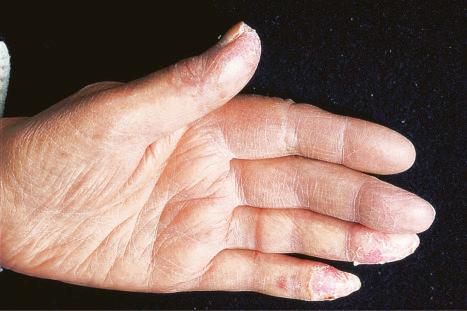
Chilblain lupus erythematosus (CHLE), which accounts for approximately 11% of DLE cases, develops during the winter months or following exposure to cold, wet, and damp conditions. Although CHLE appears most frequently in sporadic patients, familial occurrence has recently been reported. Patients with familial CHLE display mutations in the TREX1 gene located on chromosome 3p, encoding a DNA-specific 3′-5′ exonuclease 1. In addition, a mutation in SAMHD1 (SAM domain and HD domain-containing protein 1), a deoxynucleotide-degrading phosphohydrolase, has been reported in a single family. Autosomal dominant inheritance has been demonstrated in these patients. While familial CHLE generally presents in early childhood, sporadic CHLE usually affects middle-aged females. Patients develop itchy, painful, papuloerythematous or blue-purple plaques and nodules on the fingers, heels, and soles of the feet; the hands, calves, knees, knuckles, elbows, nose, and ears are less often affected ( Fig. 17.26 ). Hyperkeratotic fissured lesions and ulcers are sometimes also present. CHLE may present with depigmentation mimicking vitiligo. Although patients usually develop chilblains many years after the typical discoid rash, lesions may develop simultaneously and sometimes the chilblains are the sole manifestation. Some patients with sporadic CHLE have an associated cryofibrinogenemia or cold agglutinin. About 20% of patients with sporadic CHLE develop SLE, particularly those who develop discoid and perniotic lesions simultaneously and those with DLE-erythema multiforme-like syndrome in addition to perniosis. None with the familial CHLE have progressed to SLE.
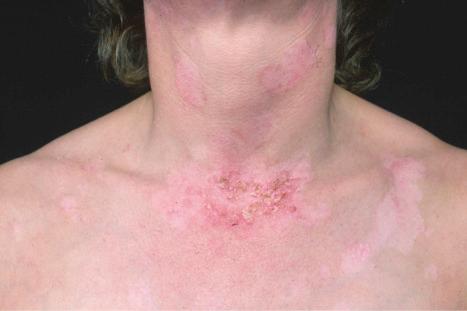
The lupus erythematosus-erythema multiforme-like syndrome (Rowell syndrome) is rare. Patients, mostly middle-aged women, develop recurrent episodes of annular lesions on the limbs and, to a lesser extent, on the face, neck, chest, and mouth, in addition to the features of any variant of lupus erythematosus. Involvement of palms and soles is exceptional. Early lesions are erythematous papules, which become annular and may vesiculate at the edge. The condition usually heals without scarring, but in severe disease bullae may develop, become necrotic, and ulcerate. Patients with this variant also have erythrocyanosis, chilblains, and Raynaud phenomenon. It has been suggested that Rowell syndrome and toxic epidermal necrolysis-like skin lesions with SLE represent different ends of the same illness spectrum, with most of the latter examples being induced by drugs. The serum of patients with lupus erythematosus-erythema multiforme-like syndrome contains speckled antinuclear factor (the most consistent feature, present in 88% of the patients), rheumatoid factor, and anti-Ro/La antibody. In rare cases, anti-Ro/La and rheumatoid factor are negative. Association with antiphospholipid syndrome has exceptionally been documented.
Lupus erythematosus profundus (panniculitis) is another uncommon variant that may develop in association with either DLE or SLE. This is discussed in detail in Chapter 10 .
Occasionally, a DLE-like dermatosis develops in female carriers of X-linked chronic granulomatous disease. Mothers of affected children may occasionally show similar lesions, presenting with bluish-red, infiltrated, scaly papules on the face and hands, sometimes associated with photosensitivity. Additional features include recurrent aphthous-like ulcerative stomatitis and perniosis.
X-linked chronic granulomatous disease is inherited as a recessive trait, and patients (usually boys) have severe and recurrent infections due to defective neutrophil bactericidal activity. Heterozygous female carriers display a partial defect, indicated by diminished nitroblue tetrazolium reductions, but are usually free from infections. An autosomal recessive variant in which discoid lupus-like lesions may occur has also been documented. DLE lesions have been reported in both carriers and chronic granulomatous disease patients; however, development of SLE in chronic granulomatous disease patients is extremely rare. Subacute cutaneous lupus erythematosus-like lesions and lupus tumidus may also occur. DLE has also been described in non-X-linked hyper-IgM syndrome.
Subacute cutaneous lupus erythematosus (SCLE) accounts for 5% to 10% of patients with lupus erythematosus. Approximately 50% have SLE as defined by the revised American Rheumatology Association diagnostic criteria (see below). SCLE predominates in Caucasians and is uncommon in blacks, Koreans, and Chinese. Females are affected more often than males (2.3 : 1), and the mean age at presentation is about 40 years. This variant of lupus occurs very rarely in children. Nail dystrophy affecting the majority of nails has been reported in a child with SCLE.
The eruption, which is often widely distributed, consists of symmetrical, nonscarring, and nonindurated erythematosquamous lesions ( Fig. 17.27 ). Unique presentation along the lines of Blaschko have also been reported. Absence of induration has been regarded as a useful clinical discrimination feature between SCLE and DLE. In addition, the absence of follicular plugging, adherent scale, and dermal atrophy helps to distinguish the subacute lesion from the discoid variant. Pruritus is very rare. Lesions may become papulosquamous (psoriasiform) or annular, the latter coalescing to produce polycyclic and gyrate configurations ( Fig. 17.28 ). In some patients both patterns are seen. Crusting and vesiculation are sometimes evident on the active border of the annular lesions. Pityriasiform and erythema multiforme-like lesions have also been described, as has occasional chronic leukoderma. Exceptionally, the disease may present as generalized poikiloderma or erythroderma. In addition, 15% to 30% of patients develop features of typical DLE, usually located on the scalp or face. The malar erythema of SLE is also sometimes evident (15%). Subtle hypopigmentation, telangiectasia, nonscarring alopecia, livedo reticularis, Raynaud phenomenon, and mucous membrane ulcers are additional features. Dystrophic calcification is exceptional. Cutaneous leukocytoclastic vasculitis is seen in a minority of patients and appears to be self-limited and not associated with a worsened prognosis.
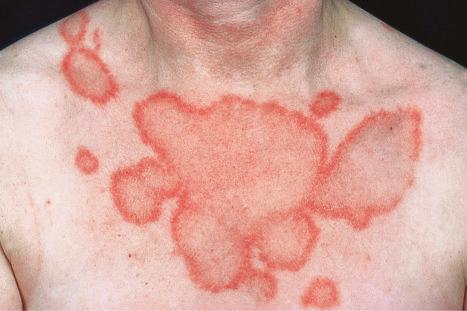
Photosensitivity is of major importance and lesions are therefore typically seen on the face, neck, upper part of back and chest, shoulders, extensor aspect of the arms, backs of hands, and fingers ( Figs 17.29 and 17.30 ).
Patients with SCLE have an increased incidence of human leukocyte antigen (HLA)-DR3 (75%), HLA-B8, and HLA-A1, and there is a significant association with inherited homozygous C2 and C4 deficiency. HLA-DR2 is also present at a higher frequency, particularly in those with papulosquamous rather than annular skin lesions. Antinuclear antibodies are found in approximately 50% of patients, while anti-Ro (SS-A) antibodies are present in approximately 65%, particularly those with annular polycyclic lesions. Anti-La (SS-B) antibodies are also often evident. Children with SCLE usually display anti-Ro (SS-A) antibodies in the majority of cases, while anti-La (SS-B) antibodies are rarely noted. In addition, ANA are detected in over 70% of pediatric SCLE patients.
The course of SCLE tends to be relatively benign, but systemic manifestations are quite common and may be severe. Severe extracutaneous disease appears to be more common in men with papulosquamous SCLE. The type of cutaneous lesion, however, has not always been proven to correlate with the severity of the extracutaneous manifestations. Renal disease has been reported to be uncommon but a recent study found its frequency to be as similar and equally severe as in SLE. Rarely, patients develop other more serious manifestations of SLE.
Patients with SCLE have an increased incidence of both rheumatoid arthritis and Sjögren syndrome. Those with SCLE and Sjögren syndrome have high titers of anti-Ro antibodies, a high incidence of cutaneous vasculitis, and an increased risk of severe neuropsychiatric and pulmonary involvement. SCLE has been documented in association with porphyria cutanea tarda, hepatitis B virus infection, radiotherapy, radioiodine treatment, squamous cell carcinoma of the head and neck region, and breast, hepatocellular, and lung carcinoma. An exceptional association with inclusion body myositis and interstitial myositis with mitochondrial changes has also been documented. A relationship with lichen planus and Kikuchi-Fujimoto disease is exceptional. Clinically and histologically identical cases have been documented following therapy with hydrochlorothiazide, griseofulvin, antihistamines, terbinafine, calcium channel blockers, nifedipine, angiotensin-converting enzyme (ACE) inhibitors, proton pump inhibitors, interferon, phenytoin, bupropion, ticlopidine, capecitabine, lansoprazole, leflunomide, fluorouracil, leuprorelin, efalizumab, acebutolol, tamoxifen, docetaxel, statin, citalopram, golimumab, rituximab, gemcitabine, adalimumab, bevacizumab, paclitaxel, ranibizumab, terbinafine, pazopanib, norfloxacin, minocycline, pemetrexed plus carboplatin chemotherapy, imiquimod, mitotane, and anastrozole.
Patients with SLE, but also patients with hydralazine-induced lupus and neonatal lupus, occasionally develop a rare cutaneous eruption morphologically mimicking Sweet syndrome. Several alternative designations have been used in the literature for such a condition, for example neutrophilic dermatosis or neutrophilic urticarial dermatosis owing to its clinical resemblance to urticaria. The frequency of neutrophilic dermatosis in SLE patients has been estimated to be below 5%. Importantly, however, neutrophilic dermatosis can be an initial manifestation of SLE in as much as one-third of the patients. Nevertheless, this reaction pattern is not specific for SLE and can also be observed in diverse diseases, for example rheumatoid arthritis, Sjögren syndrome, cryopyrin-associated periodic syndromes, Schnitzler syndrome, and adult-onset Still disease.
Patients present with erythematous, pink or violaceous non-itchy plaques or slightly elevated papules. Mucosal involvement is absent. The sites of predilection include the extremities, followed by trunk, face, and head and neck area. Palmoplantar involvement has also been reported. All age groups can be affected, from the neonatal period to elderly patients, but the eruption most commonly develops in the fourth decade of life. There is a striking female predominance for patients with SLE and neonatal lupus, while hydralazine-induced lupus typically develops in males. Systemic symptoms, including fever, malaise, and arthritis, are present in a subset of the patients.
In addition to the variable cutaneous manifestations, lesions may be found in virtually any organ or system in the body in SLE. The guidelines of the American Rheumatology Association are valuable in establishing the diagnosis: any patient who has experienced four or more of the criteria, either serially or concurrently, is considered to have SLE ( Table 17.2 ). Nevertheless, in 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE were proposed and represent evolution and refinement of the previous ARA guidelines. The SLICC criteria increase the total number of criteria from 11 to 17 ( Table 17.3 ). To be diagnosed with SLE, the following criteria need to be met: (1) fulfillment of at least four criteria (of these at least one clinical and one immunological criterion), or (2) lupus nephritis as the sole criterion in the presence of ANA or anti-dsDNA antibodies.
| Criterion | Definition |
|---|---|
| Malar rash | Fixed erythema, flat or raised, over malar eminences tending to spare nasolabial folds |
| Discoid rash | Erythematous, raised patches with adherent keratotic scaling and follicular plugging; atrophic scarring may occur in old lesions |
| Photosensitivity | Skin rash as result of unusual reaction to sunlight (observed by physician or recounted by patient) |
| Oral ulcers | Oral or nasopharyngeal ulceration, usually painless, observed by physician |
| Arthritis | Nonerosive arthritis involving two or more peripheral joints, characterized by tenderness, swelling, or effusion |
| Serositis | Pleurisy (convincing history of pleuritic pain or rub heard by physician or evidence of pleural effusion). Pericarditis (confirmed by ECG or rub or evidence of pericardial effusion) |
| Renal disorder | Persistent proteinuria (> 0.5 g/day or > 3 if quantification is not performed). Cellular casts (may be RBC, hemoglobin, granular, tubular, or mixed) |
| Neurological disorder | Seizures (in absence of offending drugs or known metabolic derangements, e.g., uremia ketoacidosis or electrolyte imbalance). Psychosis (in absence of offending drugs or known metabolic derangements, e.g., uremia ketoacidosis or electrolyte imbalance) |
| Hematological disorder | Hemolytic anemia with reticulocytosis. Leukopenia (< 4000/mm 3 on two or more occasions). Lymphopenia (< 1500/mm 3 on two or more occasions). Thrombocytopenia (< 1500/mm 3 in absence of offending drug therapy) |
| Immunological disorder | Positive LE cell preparation. Anti-DNA (antibody to native DNA in abnormal titer). Anti-SM (presence of antibody to SM nuclear antigen). False positive serological test result for syphilis known to be positive for at least 6 months and confirmed by Treponema pallidum immobilization or fluorescent treponemal antibody absorption test |
| Antinuclear antibody | Abnormal ANA titer by immunofluorescence or equivalent assay at any time and in absence of drugs known to be associated with drug-induced lupus syndrome |
| Clinical criteria |
|
| Immunological criteria |
|
* Criteria are cumulative and need not be present concurrently
SLE is characterized by a marked female predominance (9 : 1) and usually presents in the third, fourth, and fifth decades. There is a high incidence among Afro-Caribbeans, with a maximum prevalence of 1/150 females in Jamaica. In the United States the incidence is approximately 1/100.
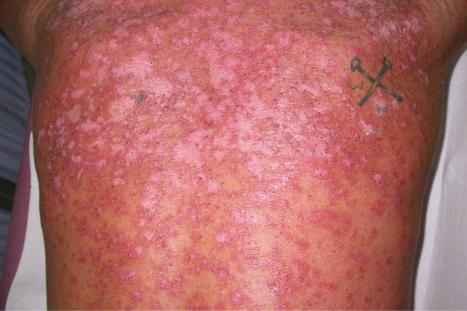
Cutaneous involvement occurs in 75% to 88% of patients. Lesions are highly polymorphic and may mimic many other dermatoses ( Table 17.4 ). The ‘butterfly rash’ is typical and is a slightly scaly, sometimes edematous, erythema that is particularly distributed on the bridge of the nose and on the cheeks ( Fig. 17.31 ). Photosensitivity is common, affecting more than 50% of patients, and erythematous or violaceous maculopapular eruptions may develop, particularly in white patients, at other light-exposed areas, such as the ‘V’ of the neck, and the forearms. Sensitivity is toward both ultraviolet (UV) A and B light. Approximately 15% of patients, particularly Afro-Caribbeans, have lesions similar to those of DLE, apparently associated with a less severe disease and a lower frequency of renal involvement. SCLE-like features are present in 10% to 15% of patients. It has been suggested that patients with SLE and SCLE lesions have a more favorable prognosis.
|
|
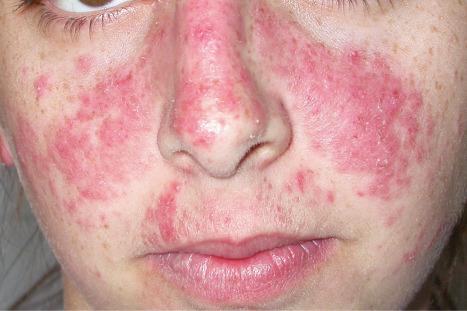
Alopecia is important, occurring in about 20% of patients, and may be scarring or nonscarring. The nonscarring lesions, which are more common, often constitute a fairly diffuse hair loss occurring as a non-specific response to stress (telogen effluvium). Fractured frontal hairs are characteristic.
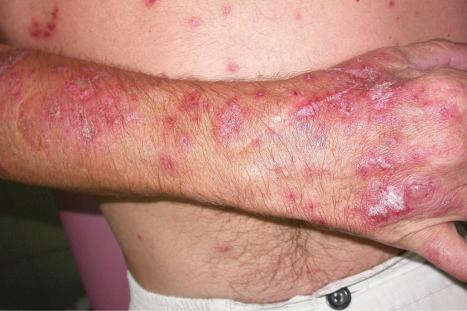
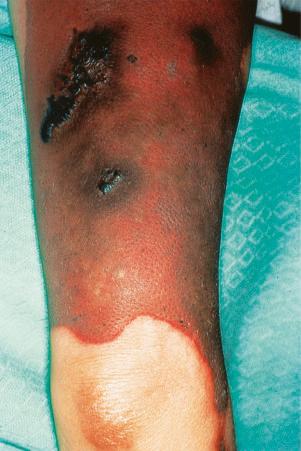
Raynaud phenomenon occurs in 10% to 40% of patients. Purpura and ecchymoses are common and may be partly due to corticosteroid therapy, but thrombocytopenia and immune complex-mediated vasculitis also play a role in the pathogenesis. Vasculitis, which occurs in up to 30% of patients, may also result in infarcts, ulcers, digital nodules, scars, and gangrene ( Figs 17.32–17.34 ). Livedo reticularis that particularly affects the arms and thighs and periarticular sites may be a presenting feature in up to 10% of patients, and the changes of atrophie blanche have been documented occasionally (livedoid vasculitis) ( Fig. 17.35 ). Livedo reticularis is often a feature of the anticardiolipin syndrome and presenting lesions identical to those seen in Degos disease (malignant atrophic papulosis) have been described occasionally. Vasculitic features correlate with renal and central nervous system involvement. Urticaria is frequently found.
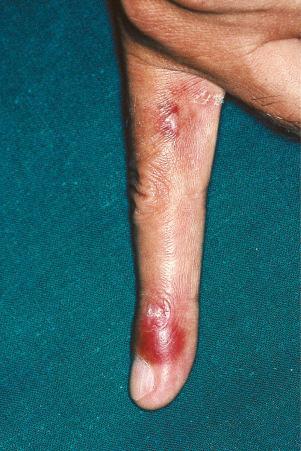
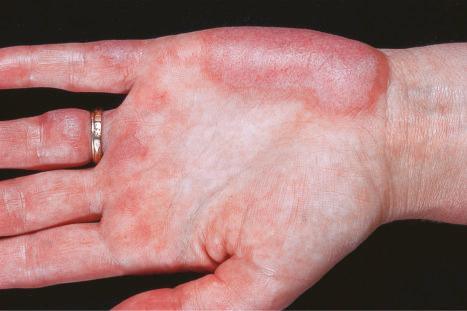
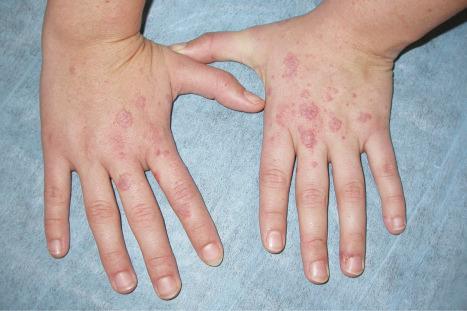
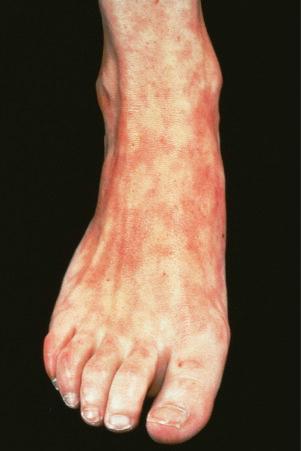
Erythromelalgia, a burning sensation accompanied by erythema following exposure to heat, has also been noted. Localized and diffuse hyperpigmentation and urticarial lesions, including urticarial vasculitis, are less commonly found. Digital manifestations, in addition to infarction and ulceration, include periungual and knuckle erythema, nail fold telangiectases, cuticular lesions, and splinter hemorrhages. Telangiectases may also be seen on the fingertips and palms. Some patients develop red lunulae. The associated erythema multiforme-like and perniotic lesions are described above. Involvement of the mucous membranes occurs in about 10% of patients: painless ulceration is most common, but other features include erythema, petechiae, erosions, and hemorrhage. The central part of the hard palate, lips, and buccal mucosa are particularly affected.
Additional lesions that are occasionally seen include persistent eyelid edema and erythema, rheumatoid-like nodules, dermal mucinosis, bullous variants, thrombotic thrombocytopenic purpura, mixed cryoglobulinemia, and soft tissue calcifications. Vesicles and bullae in SLE may complicate extreme basal cell hydropic degeneration, represent coexpression of an autoimmune bullous dermatosis, or signify a specific dermatitis herpetiformis-like eruption (bullous dermatosis of SLE). There is a recognized association with porphyria cutanea tarda.
Approximately 5% to 10% of patients with SLE are antinuclear antibody negative. This seems to correlate with a specific subtype. Patients are usually HLA-DR3 positive and have Sjögren syndrome and an increased incidence of pulmonary involvement, psychiatric manifestations, cutaneous vasculitis, and hypergammaglobulinemic purpura.
Non-specific symptoms are common and include chronic tiredness, weight loss, fever, malaise, and weakness. Arthralgia and myalgia occur in 90% of patients. The myalgia may be disabling, but objective muscle weakness is rare. Similarly, although arthralgia may be marked, there is usually little clinical evidence of joint damage. Effusions are common. Approximately 25% of patients have frank arthritis, either a migratory polyarthritis or a chronic progressive polyarthritis with deformity. Patients sometimes develop avascular bone necrosis due either to the primary disease or to steroid therapy. Very rarely, involvement of the tendons is associated with the development of contractures.
Lesions of the cardiovascular system manifest as cardiomegaly, pericarditis, pericardial effusion, and/or endocarditis (Libman-Sacks valvulitis). In SLE, the mitral valve is predominantly affected, but patients with the lupus anticoagulant syndrome appear to have a particular risk of developing aortic valve disease. Conduction defects and congestive cardiac failure are additional features.
Respiratory involvement may present as pleurisy, with or without effusion, bacterial pneumonia or, very rarely, lupus pneumonitis. Pulmonary hypertension has been estimated to be present in 0.5% to 14% of SLE patients, especially those associated with antiphospholipid antibodies.
Involvement of the central nervous system is an important cause of morbidity and mortality. It may affect up to 40% of patients. Encephalitis, meningitis, vasculitis, and coagulation defects give rise to a wide range of clinical manifestations, including convulsions, hemiplegias, chorea, and psychoses. Convulsions and coma are an indication of severe involvement and portend a grave outcome. Peripheral neuritis affects up to 12% of patients; ocular lesions, including conjunctivitis, fundal hemorrhages, and cotton wool exudates, occur in 25%.
Renal manifestations develop in approximately 45% of patients, and progressive renal involvement is an important cause of morbidity and mortality (nephrotic syndrome and lupus glomerulonephritis). Evidence of active renal disease includes the presence in the urine of more than five red blood cells/high-power field. Proteinuria of greater than 1 g/24 hours, oval fat bodies, granular, hyaline, and red blood cell casts may also be detected.
Generalized lymphadenopathy is present in about 50% of the patients, hepatomegaly in 20%, and splenomegaly in 10%.
Gastrointestinal manifestations are uncommon; the most important is esophageal involvement leading to loss of peristalsis and dilatation reminiscent of that seen in scleroderma.
Laboratory investigation commonly reveals anemia, leukopenia, lymphopenia, thrombocytopenia, and a raised ESR. False-positive reactions to reagin and treponemal tests for syphilis are common, 10% to 20% of patients are positive for the Coombs test and rheumatoid factor, and 10% to 50% have circulating anticoagulants.
While lupus erythematosus (LE) cell preparation used to be the basic screening test for SLE, it has now been superseded by testing for antinuclear factor. This and other autoantibodies will be discussed further under pathogenesis. Serum complement levels are often low in patients with active disease (CH 50 and C3); estimations of C3 levels are of particular value in following disease activity.
SLE is characterized by relapses, with remissions of variable duration sometimes lasting a decade or more. There is still, however, significant mortality. Causes of death include nephritis, infections, and central nervous system involvement.
An association between SLE and inherited complement deficiency, involving the early components of the classic pathway including C1r, C1s, C1q, C4, and C2, has been described. Deficiency of C2 is inherited in an autosomal recessive fashion; up to 60% of homozygotes develop lupus erythematosus characterized by an erythematous, papulosquamous, SCLE-like photosensitive dermatosis, a low incidence of renal involvement, and arthralgia. Additional features may include urticarial vasculitis, malar erythema, and nail fold abnormalities. Discoid lesions are sometimes evident, and recently a patient with C2 and C4 deficiency and LE panniculitis has been documented. Patients are, however, often (but not invariably) negative for antinuclear factor and manifest a negative lupus band test on unaffected skin. There is an association with HLA-DR2, and over 60% of patients possess anti-Ro antibodies.
Numerous drugs have been implicated in the induction of SLE including:
isoniazid, hydralazine, procainamide, rifampicin, quinidine, penicillamine, terbinafine, carbamazepine, phenytoin, sertraline, valpromide, amiodarone, atenolol, sulfonamides, methimazole, COL-3, hydrochlorothiazide, minocycline, spironolactone, captopril, methyldopa, gold salts, penicillin, streptomycin, phenylbutazone, reserpine, griseofulvin, clonidine, oral contraceptives, captopril, interleukin (IL)-2, hydroxyurea, clobazam, clozapine, ciprofloxacin, cefuroxime, nafcillin, celiprolol, hydralazine, para-amino salicylic acid, yohimbine, infliximab, adalimumab, pegylated interferon alpha-2B, conjugated estrogens, antitumor necrosis factor alpha (TNF-α), and etanercept.
Transient skin and serological manifestations of SLE have been documented in two children with trichophyton mentagrophytes infection. The disease has also been linked to hepatitis B vaccination and with exposure to insecticides.
Sjögren syndrome coexists in approximately 10% to 20% of patients with SLE. SLE has also been described in association with scleroderma, morphea, rheumatoid arthritis, eosinophilic fasciitis, dermatomyositis, lichen sclerosus, pemphigus (including pemphigus vulgaris, foliaceous, and paraneoplastic pemphigus), hypergammaglobulinemia of Waldenström, dermatitis herpetiformis, ulcerative colitis, alopecia areata, autoimmune thyroiditis, myasthenia gravis, acanthosis nigricans, Sweet syndrome, porphyria, gout, sarcoidosis, psoriasis, lichen planus, and cutaneous T-cell lymphoma. It is likely that many of these associations are chance associations except for those diseases with an autoimmune basis.
Neonatal lupus erythematosus is very rare, occurring in approximately 1 in 20 000 live births and most commonly presents in female infants. It is associated with maternal anti-Ro (SS-A) antibodies (95%) and/or anti-La (SS-B) antibodies. Anti-U1-ribonucleoprotein (RNP) antibodies may also be present. Anticardiolipin antibodies have been described in a single case. Transplacental transfer of anti-Ro and anti-La antibodies results in an SCLE-like eruption consisting of erythematous annular scaly plaques that particularly affect the periorbital region (‘owl-eye’ or ‘eye-mask’) and scalp, and to a lesser extent, the trunk and extremities ( Figs 17.36 and 17.37 ). The skin changes can already be present at birth. Nevertheless, they typically develop in infants at the age of 4 to 6 weeks, are usually self-limiting, and in the majority of cases resolve by 15 to 17 weeks without any sequelae. These cutaneous lesions often appear after exposure to ultraviolet light. Photosensitivity in neonatal lupus erythematosus patients appears to show racial differences, being more frequent in Caucasians and Americans than in Japanese infants. Crusted lesions and cutis marmorata telangiectasia congenita are additional features in some cases. Exceptionally, discoid lesions, panniculitis (lupus profundus), multiple morphea, erosions, targetoid lesions, and alopecia may also occur. Atrophy and scarring are very rare but residual hypopigmentation and telangiectasia are present in about 25% of patients. Babies in subsequent pregnancies may present manifestations of the disease and the overall recurrence rate for neonatal lupus-associated cardiac disease is 17%.
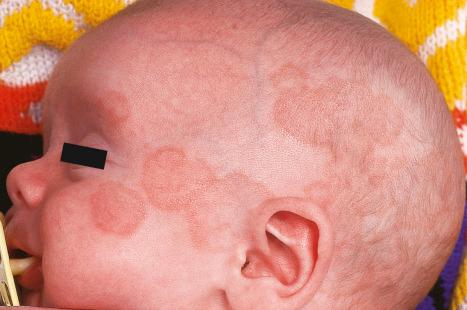
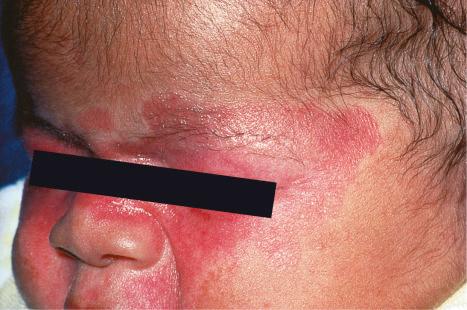
Neonatal lupus erythematosus is often associated with cardiac abnormalities. Complete heart block represents the most serious complication developing either in utero or after birth. Circulating autoantibodies to annexin A6 were detected in a single patient with neonatal lupus erythematosus-related dilated cardiomyopathy. The mortality associated with cardiac involvement is around 19%. Hematological complications have been estimated to develop in 10% to 35% of patients with neonatal lupus; the most common abnormalities are mild anemia and mild thrombocytopenia. Multiple mucocutaneous and visceral hemangiomas have been reported in a single patient. Severe hematological and liver involvement without evidence of cutaneous or cardiac involvement has exceptionally been reported. Liver involvement in neonatal lupus is rare and usually presents as a cholestatic hepatitis. Other rare associations include sigmoidal telangiectasia with rectal bleeding, painful plantar atrophy, central nervous system vasculitis, stroke, intraventricular hemorrhage, and extensive aortic aneurysm.
The levels of maternal autoantibodies in the infant decrease quickly during the first weeks of life and generally become undetectable by the age of 1 year.
The disease also occurs in identical and nonidentical twins. A single case has been described in association with Turner syndrome. Neonates who survive until infancy have a fairly good prognosis, but there is an overall mortality of about 10% due to heart disease. Mothers of affected infants are commonly asymptomatic initially but they may have systemic lupus, Sjögren syndrome, rheumatoid arthritis, leukocytoclastic vasculitis or an overlap syndrome.
A number of mothers who present with no symptoms often subsequently develop evidence of connective tissue disease, particularly systemic lupus (in about 20% of cases) and Sjögren syndrome.
Despite enormous research efforts, the precise etiology and pathogenesis are unknown, but the condition is certainly multifactorial. Lupus erythematosus is characterized by B-cell hyperactivity in association with defective suppressor T-cell function. Patients develop a wide range of autoantibodies, many of which result in immune complexes with resultant systemic manifestations, including vasculitis and glomerulonephritis. In addition to immunological factors, familial, genetic, and hormonal influences play a part. It is believed that in lupus erythematosus there is a genetic predisposition; sex-associated and environmental factors are necessary to promote the development of the disease.
Autoantibodies are important in the diagnosis of lupus erythematosus and have a significant role in its pathogenesis, either by direct cytotoxic effects (such as the lymphopenia induced by antilymphocyte antibodies) or by immune complex deposition. Until the 1960s the presence of LE cells in a patient's blood was regarded as pathognomonic for lupus erythematosus. This was changed, however, by the discovery of antinuclear factor (antinuclear antibody) with direct immunofluorescent techniques. Antinuclear antibody is present in the serum of 90% to 95% of patients with SLE, in 30% of patients with localized DLE, and in 50% of patients with generalized DLE. It should also be noted that 10% of the normal population have antinuclear antibody in the serum, albeit at low concentration. Antinuclear antibody has at least four subtypes:
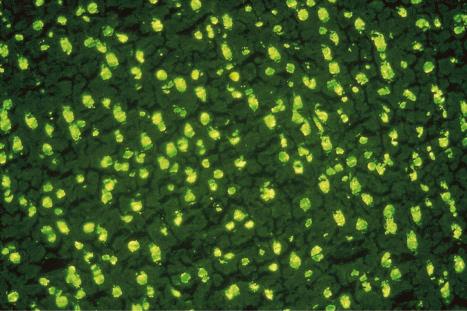
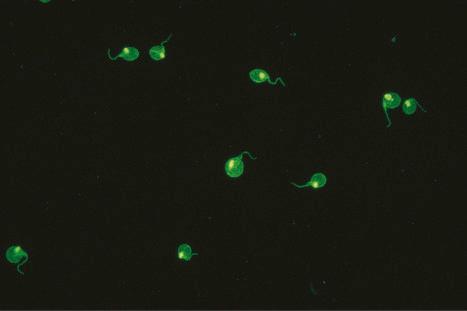
A homogeneous or diffuse pattern is most commonly seen ( Figs 17.38 and 17.39 ).
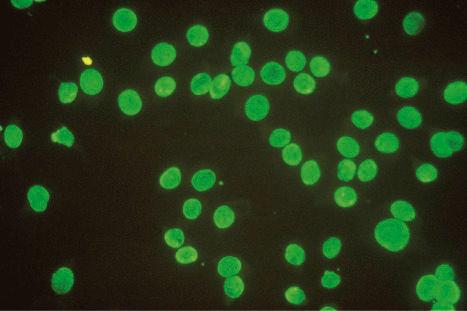
Speckled fluorescence representing an antibody to saline-soluble nuclear protein is present in patients with the lupus erythematosus-erythema multiforme syndrome ( Fig. 17.40 ).
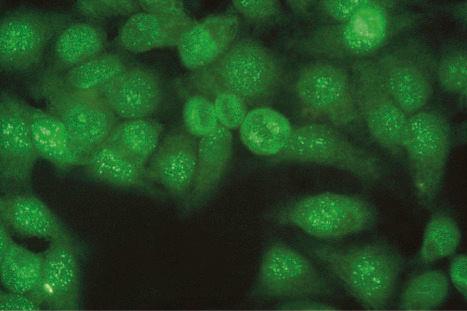
The nucleolar pattern (representing antinucleolar RNA antibody) is occasionally evident in lupus erythematosus, although it is more common in systemic sclerosis.
The peripheral (outline) staining pattern reflects high titer anti-DNA antibody and is a marker of active systemic disease.
Patients with lupus erythematosus develop antibodies to a variety of nuclear and cytoplasmic antigens; these autoantibodies, both singly and in combination, have varying associations, which may allow a prediction of (to some extent, at least) the course of the disease in individual patients ( Table 17.5 ). Antibodies to native (double-stranded) DNA (nDNA) are pathognomonic for idiopathic (classic) SLE ( Fig. 17.41 ). They are rarely seen in the drug-induced variant and are only very occasionally present in patients with DLE. The presence of anti-nDNA antibody in association with hypocomplementemia is indicative of active disease and is often accompanied by severe renal involvement.
| Antigen | Antibody | Significance | Comment |
|---|---|---|---|
| Nuclear constituents | Anti-native DNA Antihistone Anti-single stranded DNA |
Highly specific for SLE associated with hypocomplementemia and glomerulonephritis 30% of SLE patients 90% of SLE patients, associated with glomerulonephritis |
Found in NZB/NZW model Found in high titer in drug-induced lupus-like disease May be detected in ANF-negative SLE patients |
| Small nuclear ribonuclear protein | Sm nRNP La (SS-B) |
Highly specific for SLE 15% of SLE patients May occur in SLE but also present in MCTD and PSS Found in 10% of SLE |
May be associated with low incidence of renal disease |
| Small cytoplasmic ribonuclear protein | Ro (SS-A) | Found in 25% of SLE patients Found in SCLE Found in 50% of complement-deficient LE-like syndromes Found in all neonatal lupus |
May be detected in ANF-negative SLE |
Antihistone antibodies may be found in approximately 30% of patients with idiopathic SLE, but are particularly associated with the drug-induced variant. The latter is caused by a wide variety of drugs, including hydralazine, procainamide hydrochloride, phenytoin, and isoniazid. Symptoms develop in up to 20% of patients taking procainamide and antinuclear antibodies are present in 50%. Procainamide-induced SLE-like syndrome is characterized by the presence of leukocyte-specific antinuclear antibody. Clinical features include malaise, pneumonitis with pleural effusion, arthralgia, arthritis, and serositis; renal, central nervous system, and cutaneous lesions are less common and are usually mild. Although antinuclear antibodies are often present, anti-DNA antibodies are not formed in most cases of drug-induced lupus. Drug-induced lupus usually, but not invariably, undergoes remission on withdrawal of the drug.
Antibodies to ssDNA occur in 90% of patients with classic SLE and in approximately 20% of patients with disseminated DLE, and may also be found in other connective tissue diseases. Their presence in disseminated DLE correlates with an increased risk of developing SLE. Patients with SLE who are negative for antinuclear antibody may have anti-ssDNA antibodies in their serum. Antibodies to the soluble nuclear antigen Sm are highly specific for SLE. They may also be found in a group of patients with good prognosis who are positive for antinuclear factor, but anti-nDNA-negative, and who have mild nonprogressive glomerulonephritis, hypocomplementemia, mild central nervous system involvement, and conspicuous cutaneous eruptions.
Antibodies to ribonucleoprotein are detected in 23% of patients with SLE, but are much more commonly associated with mixed connective tissue disease (MCTD): they may also be found in patients with progressive systemic sclerosis. Anti-La (SS-B) antibodies are detected in the serum of 10% of patients with SLE. Anti-La antibodies, which are often present in Sjögren syndrome, are almost invariably accompanied by anti-Ro (SS-A) antibodies. The latter are particularly associated with SCLE, complement-deficient lupus erythematosus, circulating IgG or IgM anticoagulant, and neonatal lupus erythematosus. About 15% to 20% of patients with SLE have detectable antineutrophil cytoplasmic antibodies (ANCA). A large multicenter study of 645 childhood SLE patients from Brazil demonstrated anti-Ro (SS-A) and/or anti-La (SS-B) antibodies to be associated with mild cutaneous and musculoskeletal involvement.
The circulating anticoagulant (antiphospholipid or lupus anticoagulant) is associated with paradoxical thrombosis, spontaneous abortion, premature labor, intrauterine death, labile hypertension, cutaneous necrosis, gangrene, ecchymoses, purpura, leg ulcers, atrophie blanche, livedo reticularis, and false-positive syphilis serology – the lupus anticoagulant syndrome ( Table 17.6 ). The lupus anticoagulant syndrome is more accurately known as the antiphospholipid syndrome because not all patients have associated SLE. It is due to the presence of circulating antiphospholipid antibodies which inhibit coagulation in vitro, but more importantly are associated with a greatly increased risk of thrombotic phenomena affecting both arteries and veins ( Figs 17.42 and 17.43 ). About 50% of patients with SLE present with antiphospholipid antibodies but only half of these will develop manifestations of the disease. Presentation in the skin may also be with papules and nodules or lesions resembling pyoderma gangrenosum. Interestingly, patients with antiphospholipid antibodies and SLE appear to be more at risk of developing anetoderma. The incidence, however, is not high. Interestingly, patients with clinically significant antiphospholipid antibodies were found to have reduced prevalence of acute cutaneous lupus in comparison to patients without significant antiphospholipid antibodies. Rarely, patients develop reactive angioendotheliomatosis. Systemic involvement includes deep venous thrombosis often complicated by pulmonary embolism, arterial occlusion with resultant strokes, transient ischemic attacks, multi-infarct dementia, myocardial infarction, and gangrene.
| Group | Criteria |
|---|---|
|
Thrombosis |
Recurrent fetal loss |
|
|
Positive anticardiolipin antibody assay; isotype IgG or IgM |
| Positive lupus anticoagulant test | |
|
Hemolytic anemia/positive direct Coombs test Migraine |
| Endocardial/valvular lesions | |
| Transient visual loss | |
| Chorea | |
|
|
|
|
|
|
|
|
|
|
|
* Laboratory tests should be positive on two occasions 2 months apart.
The association of cutaneous thrombotic lesions, livedo racemosa, hypertension, and cerebrovascular disease is known as Sneddon syndrome. This presents mainly in young females and is exceptional in children. Antiphospholipid antibodies are present in around 41% of patients.
How antiphospholipid antibody induces thrombosis is unknown. Theories of inhibition of protein C (a natural vitamin K-dependent anticoagulant) function and a suppressive effect on endothelial cell prostacyclin have not yet been substantiated. The antiphospholipid syndrome has also been documented in other autoimmune diseases including rheumatoid arthritis, hemolytic anemia, thrombocytopenic purpura, ulcerative colitis, factor V Leiden mutation, and mesothelioma. It may also complicate treatment with a number of drugs, including phenothiazines and procainamide, develop during viral illnesses, including acquired immunodeficiency syndrome (AIDS), and can present with an underlying lymphoma. The antiphospholipid syndrome is further discussed elsewhere.
Anti-Ro antibodies have been shown to bind to the epidermis in neonatal and subacute lupus erythematosus, and to the conducting system and myocardium in neonatal lupus erythematosus. They also bind to keratinocytes in vitro and have been shown to react with the epidermis when infused into human-skin-grafted mice. This – combined with the disappearance of the cutaneous manifestations as maternal IgG is removed from the circulation – suggests that in neonatal lupus erythematosus, anti-Ro antibodies are of major pathogenetic significance. Ro antigens are present in the nuclei and cytoplasm of keratinocytes and it has been demonstrated that UVB is capable of translocating these antigens to the surface of cultured keratinocytes. Anti-Ro antibodies in the sera bind to the antigens in keratinocytes and appear to be important in inducing antibody-dependent keratinocyte damage.
Anti-C1q antibodies are present in roughly 30% of patients with SLE. A combination of anti-C1q antibodies, anti-dsDNA antibodies, and low complement has been proven to be the most reliable serological marker of lupus nephritis.
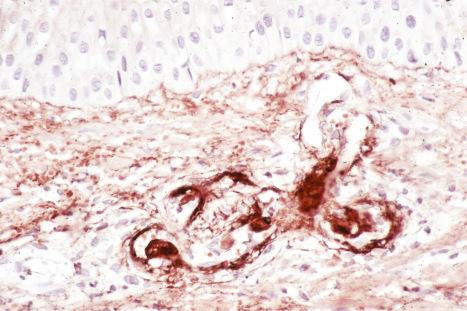
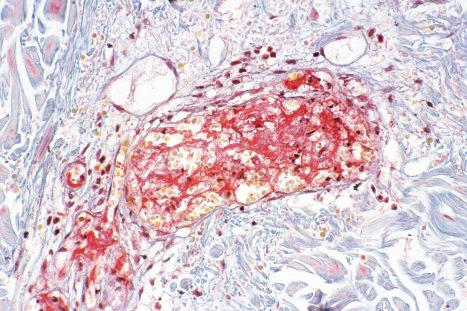
Immune complexes have been shown to be important in the pathogenesis of both vasculitis and glomerulonephritis in SLE; their presence may be inferred by the detection of immunoglobulin and complement in blood vessel walls in skin biopsies ( Fig. 17.44 ). High concentrations of anti-nDNA, anti-ssDNA, and anti-Ro (SS-A) antibodies have been detected in the renal cortex of patients with lupus glomerulonephritis. The finding of large numbers of T lymphocytes and macrophages with minimal or no B cells in the dermal infiltrate of skin lesions suggests that cell-mediated immunity may be particularly important in the pathogenesis of lesions at this site. Both delayed hypersensitivity and antibody-dependent cellular cytotoxicity mechanisms have been proposed.
There is a striking female predominance in SLE, suggesting that female sex hormones are of pathogenetic significance. Interestingly, in the experimental model of SLE in rabbits, females have a much more serious form of glomerulonephritis than do their male counterparts and this difference can be negated by castration or the administration of male sex hormones.
There is, without question, a genetic predisposition to the development of SLE. There are many examples of familial incidence, and immunoglobulin abnormalities have often been documented in asymptomatic relatives; indeed, as many as seven members in a single family have been recorded. Lupus erythematosus has also been reported in identical twins. HLA typing has revealed an increased incidence of HLA-A1, HLA-B8, HLA-B15, HLA-DR2, and HLA-DR3 in SLE. It has been suggested that expression of the disease may be inherited as an autosomal dominant trait. HLA-DR4 is associated with hydralazine-induced lupus erythematosus. The frequencies of HLA-DRw6 and HLA-B8 are increased in DLE. It has recently been demonstrated that patients with polymorphic light eruption and HLA-DRB1*0301 have a higher risk of developing either subacute or discoid lupus erythematosus. The familial clustering of polymorphic light eruption in relatives of persons with lupus erythematosus suggests that these diseases may share a similar pathogenesis.
SCLE has been demonstrated to be associated with the TNF-308A allele. Association between SCLE and single nucleotide polymorphism in the TNF-alpha (TNF-α) gene promoter and gene that encodes C1qA chain of C1q has been demonstrated. Transcription of TNF-α appears to be photoregulated in subacute lupus. TNF-alpha, produced by keratinocytes, has been localized within refractory lesions of SCLE, suggesting its potential role in the pathogenesis of SCLE. Abnormal regulation of DNA methylation in CD4+ T lymphocytes has recently been implicated in the pathogenesis of SCLE. Triggering mechanisms in lupus erythematosus include UV light (both naturally occurring and artificial), drugs, and, possibly, viruses. Sunlight commonly worsens the cutaneous manifestations and may exacerbate systemic disease. Antibodies to UV DNA have been identified in patients with SLE. These may be important in the pathogenesis of the photosensitivity-induced lesions. Lesions are typically worse in spring and summer, and cutaneous lesions can be induced artificially by UV irradiation. Dysregulation of apoptosis has been suggested as an important mechanism in the pathogenesis of lupus erythematosus. A reduction of bcl-2 expression in epidermal basal cells is associated with overexpression of FAS antigen and this appears to correlate with the extent of apoptosis in the epidermis.
Despite early enthusiasm for a viral etiology for lupus erythematosus, extensive research has not provided convincing evidence. For many years paramyxovirus-like inclusions within the cytoplasm of endothelial cells were thought to represent evidence of a viral cause. They are now known to represent a non-specific membranous byproduct (tubuloreticular body) of organelle degeneration. An association with parvovirus (erythrovirus) B19 has been suggested. Two experimental virus-induced animal models – Aleutian mink disease and New Zealand black–white hybrid mouse disease – have been described. Recent investigations have shown that the latter is associated with a primary stem cell defect. Bone marrow grafts can therefore transfer the disease to previously irradiated normal recipients and induce tolerance defects. Bone marrow cultures produce B cells with an increased capacity for antibody synthesis. Murine lupus shows a striking similarity to its human counterpart. Interestingly, native (double-stranded) DNA antibodies were detected more often among smokers with SLE than in nonsmokers with SLE. Indeed, a large study encompassing 405 patients with lupus erythematosus confirmed high association between smoking, discoid lupus, and lupus tumidus.
The lupus band test is particularly important in the diagnosis of lupus erythematosus. It is also, to a lesser extent, of some value in assessing prognosis. By either immunofluorescence or immunoperoxidase techniques, the presence of immunoglobulin and complement is sought at the dermal–epidermal junction ( Fig. 17.45 ). IgM is most commonly identified, although IgG, IgA, and C3 are also frequently present. IgG deposits appear to be the most specific for lupus erythematosus. Other factors that may be identified include properdin, C1q, and C4. Deposition of the membrane attack complex (MAC) has also been shown to be a relatively sensitive and specific marker of cutaneous lupus erythematosus. Although deposits are usually homogeneous, granular and thready (reticular) patterns are also recognized. Homogeneous bands are typical of chronic lesions, granular bands are seen in uninvolved skin, and thready deposits are usually a feature of early lesions. Ultrastructurally, the immunoreactants are present in the sub-basal lamina connective tissue and intimately associated with reduplicated lamina densa ( Fig. 17.46 ). The immunoglobulin deposition does not necessarily correlate with the clinical or histologic presence of cutaneous lesions and is therefore unlikely to be of pathogenetic significance.
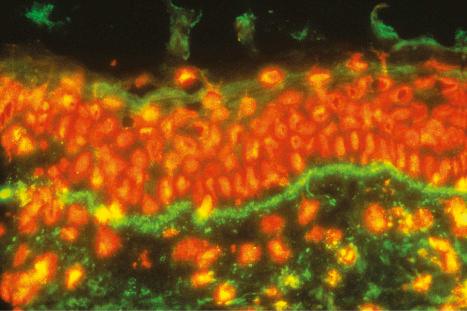
The lupus band test is positive in involved skin in approximately 50% to 94% of patients with SLE, 60% to 80% of those with the discoid variant, 60% of patients with SCLE, and 50% of infants with neonatal lupus erythematosus. Lesional mucosa also shows immunoreactant deposition and this can be of particular value in patients where histologic distinction from lichen planus proves impossible. It may also be positive in uninvolved skin in up to 67% of patients with systemic disease. The prevalence of a positive test in uninvolved skin depends upon the site, with the highest incidence in skin from the shoulder (70%). Sun-exposed skin, such as the dorsal aspect of the forearms, is more frequently positive (60–70%) than non-sun-exposed skin (50–60%). However, the observation that 20% of specimens of sun-exposed normal skin from healthy young adults show a positive lupus band test indicates that non-sun-exposed skin is the substrate of choice.
Positivity also depends upon the duration of the disease (lesions less than 3 months old are often negative) and the effect of previous steroid therapy. Much less often, positive immunofluorescence is seen in the uninvolved skin of patients with DLE. The results must, however, be taken in the context of the clinical information. A positive IgM lupus band test may be seen in unrelated conditions, such as solar keratosis, polymorphous light eruption, rosacea, lymphocytic infiltrate, and dermatomyositis, and as a consequence of UV radiation. These latter conditions are usually associated with C3 deposition or a single immunoglobulin class, particularly IgM, in contrast to the multiple immunoglobulin subclasses found in lupus erythematosus. In nonlupus conditions the lupus band is usually fainter and patchy. With such a significant false-positive rate, it is stressed that the results of a lupus band test must be interpreted with considerable caution and always in the context of the clinical information and histologic features.
A characteristic particulate dust-like deposition of immunoglobulin (predominantly IgG) affecting the basal cells of the epidermis has been described in patients with SCLE. Sometimes suprabasal epidermis, adnexal epithelium, and the dermal cellular infiltrate show similar fluorescence. This pattern of deposition correlates with the presence of Ro antibodies. It is also occasionally evident in SLE. In some cases speckled nuclear staining of keratinocytes for IgG is seen in connective tissue diseases. In the setting of SLE, this finding appears to be associated with a lower incidence of renal disease.
The histopathological features of the various subsets of lupus erythematosus (with the exceptions of lupus erythematosus profundus and bullous dermatosis of SLE) show considerable overlap and their distinction by microscopic techniques is often difficult.
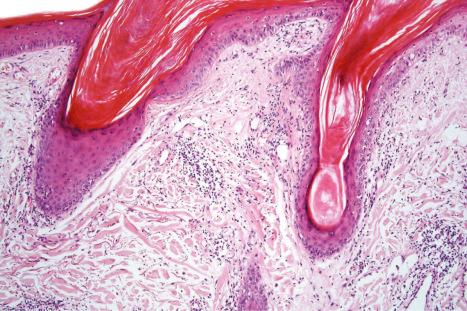
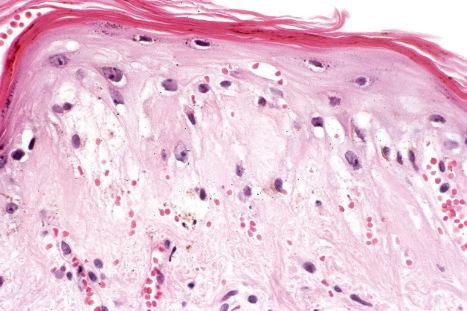
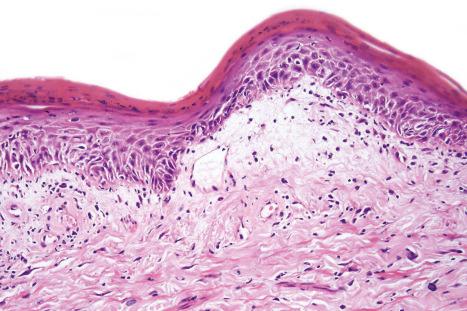
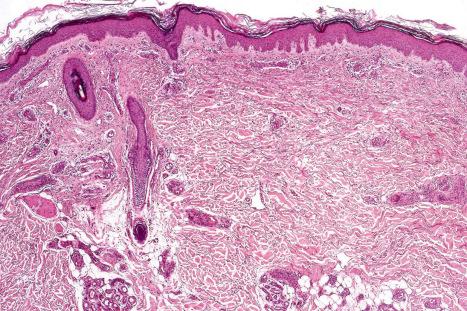
DLE displays variable features, depending upon the stage of the disease. The active lesion is characterized by hyperkeratosis and follicular dilatation with keratin plugging; occasionally, focal parakeratosis is present ( Figs 17.47–17.49 ). The epidermis is usually atrophic and rather flattened, although sometimes there is acanthosis ( Fig. 17.50 ). The most significant features seen at the dermal–epidermal junction are:
liquefactive degeneration of the basal layer of the epidermis ( Fig. 17.51 ),
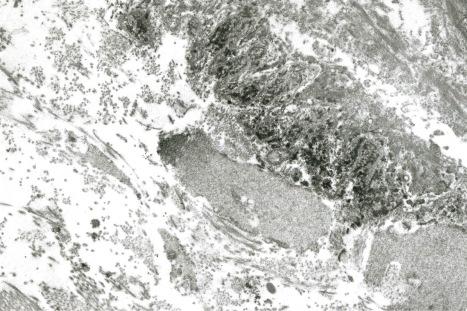
basement membrane thickening ( Fig. 17.52 ), which may be accentuated by use of the periodic acid-Schiff (PAS) reaction.
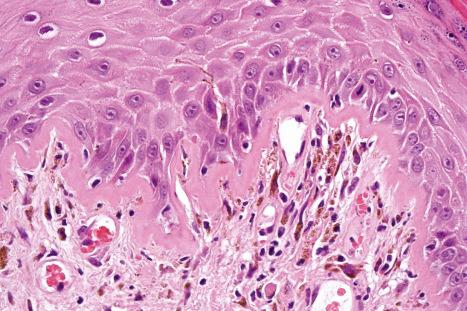
These two features may additionally affect follicular epithelium, and basement membrane thickening of the dermal blood vessels is also sometimes evident ( Fig. 17.53 ). Liquefactive degeneration of the basal layer of the epidermis is often accompanied by pigmentary incontinence ( Fig. 17.54 ). In some instances the epidermal changes are accompanied by cytoid body formation, but this is not usually as conspicuous as in lichen planus ( Fig. 17.55 ). Amyloid formation may occasionally be seen.
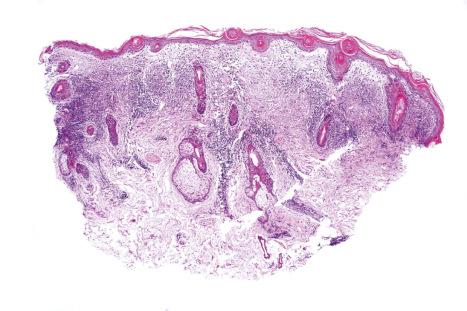
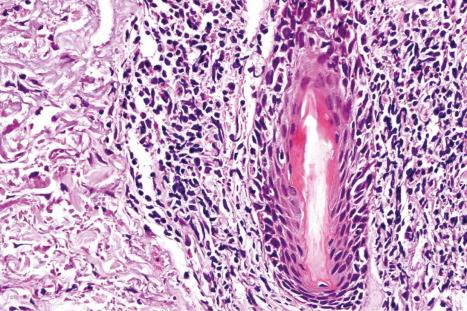
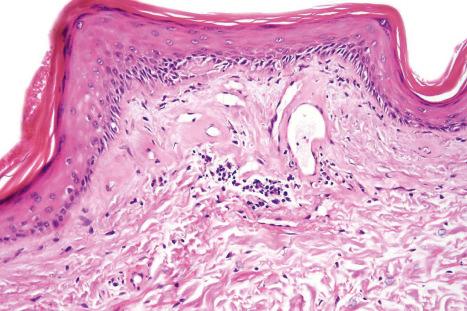
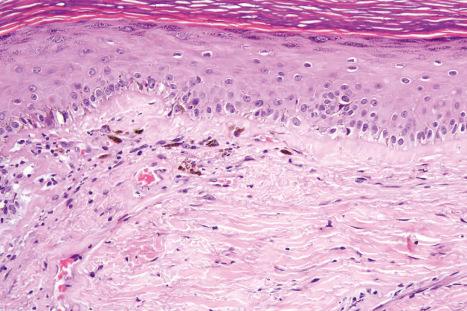
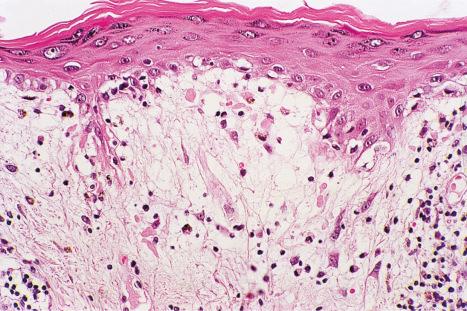
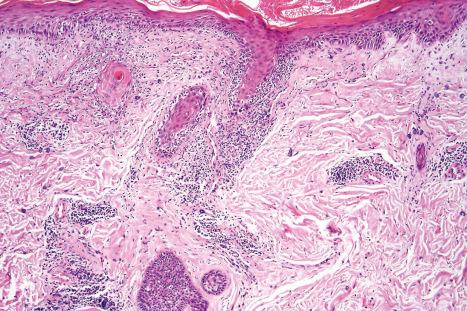
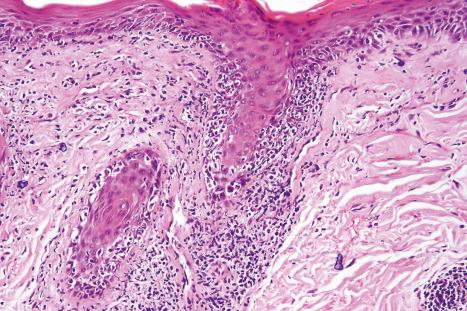
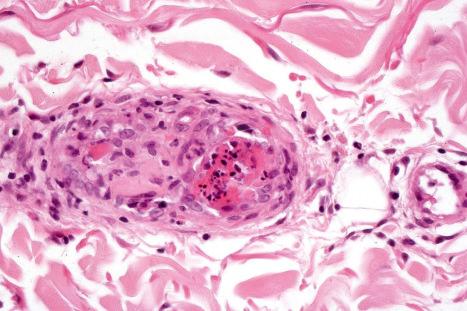
The papillary dermis is edematous, and telangiectatic vessels are often present ( Fig. 17.56 ). Focal extravasations of red blood cells are sometimes seen ( Fig. 17.57 ). Characteristic of DLE is a perivascular and periappendageal chronic inflammatory cell infiltrate of lymphocytes and variable numbers of histiocytes ( Figs 17.58 and 17.59 ). The most common cells are the T lymphocytes, both helper and suppressor cells. Neutrophil polymorphs and plasma cells are not usually evident. However, the presence of neutrophils and leukocytoclasia has been described in the lupus-like lesions of a patient with a lupus-like dermatosis with X-linked chronic granulomatous disease. CD4+ cells predominate in DLE skin lesions. The majority of these T lymphocytes are Ia+, indicating an activated state. Occasional CD4+ cells are present in the epidermis closely related to foci of basal keratinocyte damage. Epidermal Langerhans cells are usually reduced in number. B lymphocytes are generally present in only small numbers. The infiltrate is typically sparse in the papillary dermis, but focal dense aggregates are characteristically found in the reticular dermis and may sometimes extend into the subcutaneous fat.
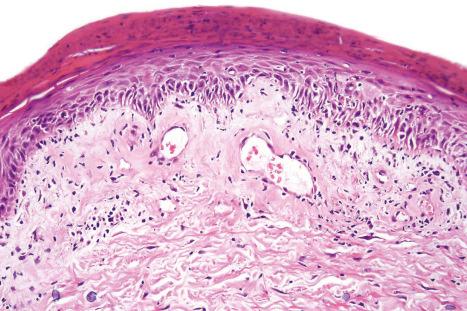
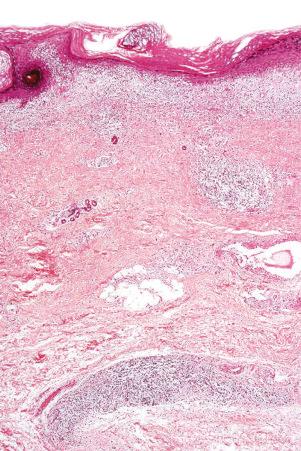
An increase in glycosaminoglycans (acid mucopolysaccharides) in the dermis is a common feature of the acute lesion ( Fig. 17.60 ); in tumid lupus this is very marked ( Figs 17.61 and 17.62 ). Most uncommonly, however, dermal deposition of mucin in discoid lupus can be extensive, presenting clinically as papulonodular mucinosis. Very rarely, dermal calcification has been documented ( Fig. 17.63 ). Exceptionally ‘fibrinoid’ change of the dermal collagen is present ( Fig. 17.64 ).
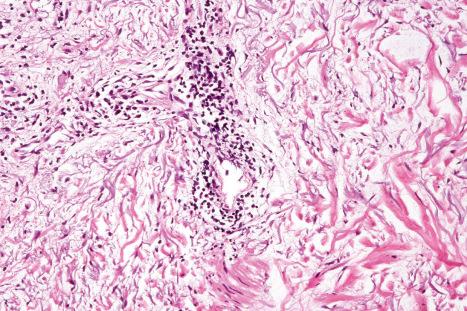
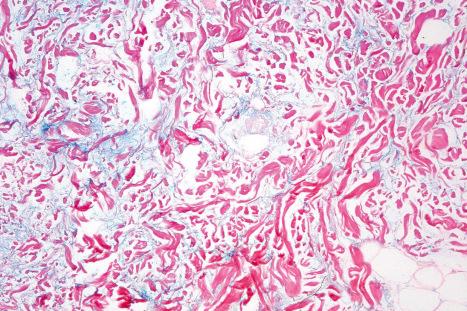
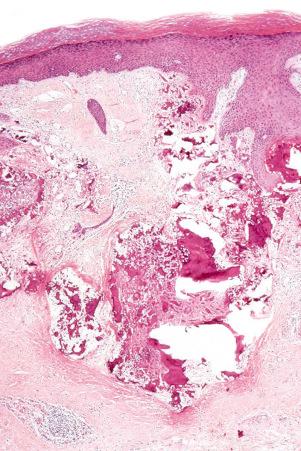
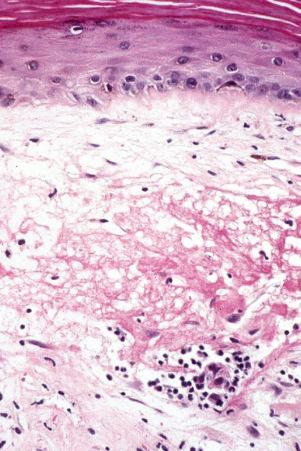
The presence of plasmacytoid dendritic cells representing more than 10% of the dermal inflammatory cells infiltrate, their arrangement in clusters, or their presence at the dermal–epidermal junction has been demonstrated to have significant diagnostic value in discoid and verrucous (hypertrophic) lupus erythematosus.
In the healing lesion of DLE there is hyperkeratosis and the epidermis may be atrophic or, more commonly, slightly thickened. The basement membrane region is characteristically markedly thickened. The dermis is fibrosed, sometimes to a degree that resembles lichen sclerosus ( Fig. 17.65 ). In hair-bearing sites, particularly the scalp, there may be very marked follicular plugging with associated chronic inflammation, and in advanced disease there is often complete loss of the pilosebaceous structures with replacement by collagenous fibrous strands ( Fig. 17.66 ). Sebaceous gland atrophy or loss occurs early in scalp involvement, and the chronic inflammatory changes are centered at the level of the mid follicle. It has been suggested that this may represent a focus of follicular stem cells and therefore chronic inflammation at this site could readily result in permanent hair loss. Secondary amyloid deposition, derived from degenerating epidermal keratinocytes, can occasionally be detected in the papillary dermis.
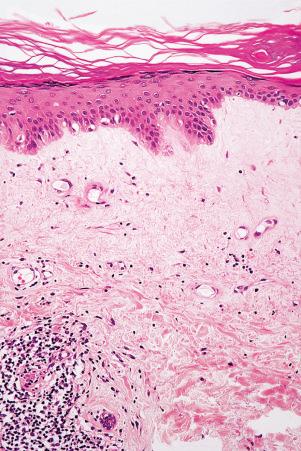
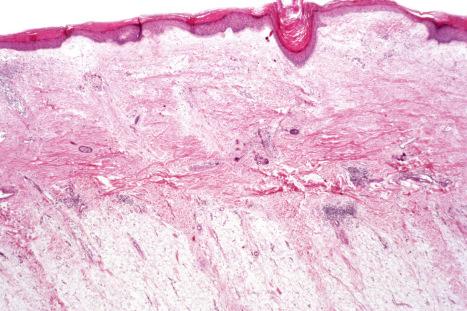
Rarely, DLE may present as a dense superficial and deep perivascular and periappendageal infiltrate in the absence of significant epidermal changes: distinction from lymphocytic infiltrate of Jessner and polymorphous light eruption is, therefore, histologically impossible.
In hypertrophic lesions there is marked hyperkeratosis, hypergranulosis, and irregular acanthosis with papillomatosis in addition to the features of basal cell damage. Cytoid bodies are often conspicuous in the lower epidermis. Amyloid deposition has been reported as a frequent finding. The features are commonly histologically indistinguishable from hypertrophic lichen planus. The presence of intraepidermal elastic fibers, frequently associated with transepidermal elimination of the elastotic material, has been reported in hypertrophic lupus erythematosus. This is not a feature of lichen planus. Resolving or evolving keratoacanthoma may sometimes be mimicked.
Biopsy from patients with lupus pernio reveals a cuffed perivascular lymphocytic infiltrate with edema, red cell extravasation, and variable vascular fibrinoid change ( Figs 17.67–17.70 ). Some biopsies show frank lymphocytic vasculitis. The inflammatory infiltrate often extends into the deep dermis and subcutaneous adipose tissue. Interface epidermal changes, ranging from focal vacuolar changes to a lichenoid tissue reaction, are often present. Chronic inflammation around sweat glands is sometimes seen.
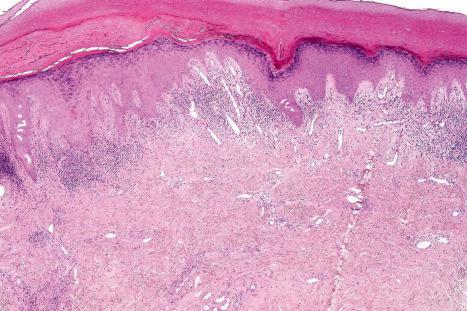
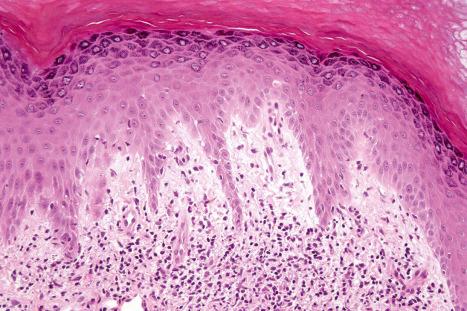
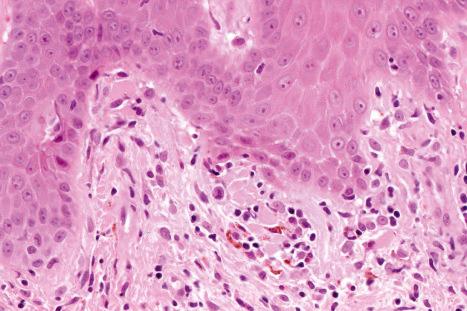
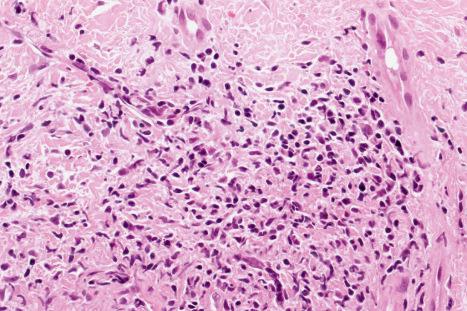
Mucous membrane lesions are frequently difficult to distinguish from lichen planus. They are characterized by hyperkeratosis, often accompanied by parakeratosis. The epithelium may be atrophic or acanthotic. Hydropic degeneration of basal keratinocytes, sometimes associated with cytoid body formation, accompanies a dense lymphohistiocytic infiltrate in which plasma cells are often numerous. The presence of a deep perivascular chronic inflammatory cell infiltrate favors the diagnosis of DLE. Direct immunofluorescence is, however, frequently necessary to establish the diagnosis.
The histologic features of lupus erythematosus profundus are considered in Chapter 10 .
Although the histologic features of SCLE show considerable overlap with those of DLE, there are diagnostic pointers. In SCLE, the hyperkeratosis tends to be mild, atrophy is more marked, and the epidermal ridge pattern is often effaced ( Fig. 17.71 ). Parakeratosis may sometimes be a feature. Basement membrane thickening is minimal or absent, and hair follicles are often unaffected or show only slight keratin plugging. In DLE, the inflammatory cell infiltrate is denser, occupies the papillary and reticular dermis, and often shows perifollicular accentuation. In SCLE, lymphocytic exocytosis may be conspicuous and satellite cell necrosis is not uncommon ( Fig. 17.72 ). Liquefactive degeneration of the basal layer of the epidermis is usually present although often it is only mild ( Figs 17.73 and 17.74 ). Sometimes, however, it is sufficiently marked that subepidermal vesiculation results. Homogenization of the papillary dermal collagen is occasionally seen. Colloid body formation and pigmentary incontinence are often inconspicuous, but sometimes they are a major feature. The inflammatory cell infiltrate is typically mild, superficially located, and perivascular in distribution. In some examples, however, it presents as a lichenoid band. Variable amounts of eosinophils can also be seen in the dermal inflammatory cell infiltrate. This is an entirely non-specific finding not related to the possible drug-induced SCLE.
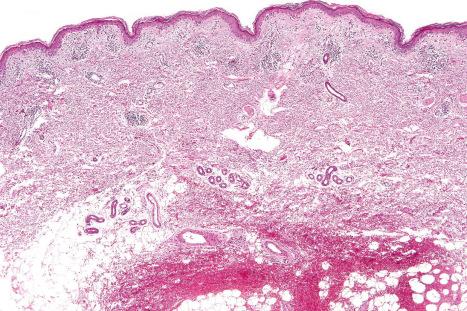
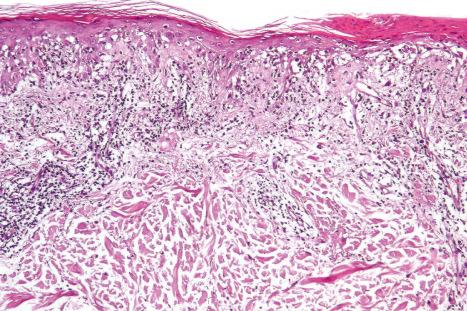
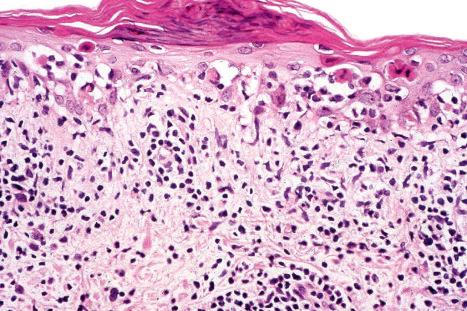
The histopathological features in cutaneous lesions of patients without SCLE and antibodies to SS-A (Ro) are very similar to those seen in lesions of patients with SCLE. These patients often have different clinical diseases including Sjögren syndrome and rheumatoid arthritis.
The histologic features of neutrophilic dermatosis of the SLE are variable. The intensity of neutrophilic infiltrate typically varies from paucicelullar neutrophilic infiltrates in the papillary dermis to moderately or highly cellular neutrophilic infiltrates in perivascular and interstitial distribution frequently admixed with nuclear debris spanning the entire dermis, with possible extension into the subcutaneous fatty tissue. Neutrophilic inflammatory cell infiltrate is generally accompanied by leukocytoclasia, while vasculitis is absent as a rule. In addition to dermal neutrophilic infiltrates, a recent paper found the presence of neutrophils within the epithelium of the epidermis, hair follicles, sebaceous glands, and sweat glands to be highly suggestive of neutrophilic urticarial dermatosis. Additional histologic features, which are helpful indicators of possible association with lupus erythematosus, and are present to a variable extent and frequency, include interface dermatitis, vacuolar degeneration of basal keratinocytes, thickening of the basement membrane, follicular plugging, and dermal mucin deposition. Direct immunofluorescence testing usually reveals a positive lupus band test.
Histologic differential diagnosis mainly depends upon the intensity of the neutrophilic infiltrate. Examples with paucicellular neutrophilic infiltrates in the papillary dermis should mainly be distinguished from bullous SLE, dermatitis herpetiformis, and linear IgA disease – immunofluorescence analysis is crucial for this distinction. Differential diagnosis of more prominent neutrophilic infiltrates includes Still disease, Sweet syndrome, Behçet disease, and infections.
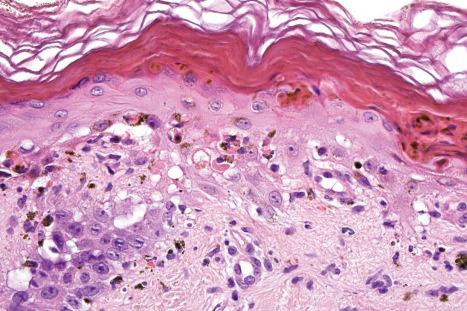
The histologic features of SLE are variable. The changes in early lesions, which may be very mild and subtle, often comprise only slight epidermal basal cell liquefactive degeneration, papillary dermal edema, and a mild chronic inflammatory cell infiltrate, and are seen, for example, in malar erythema ( Fig. 17.75 ). On other occasions the appearances are similar to those of SCLE.
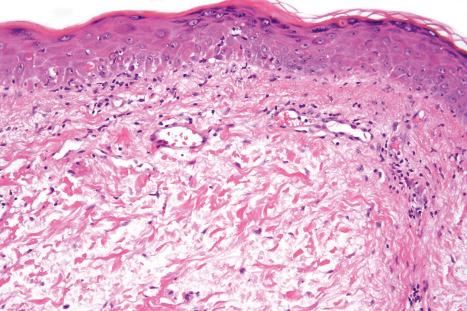
The histology of SLE is often indistinguishable from that of the discoid variant ( Fig. 17.76 ). Fibrinoid degeneration of the dermal collagen is, however, more common in SLE than in DLE. Dermal mucin deposition is often seen between collagen bundles. In most cases, this feature is subtle, but mucin deposition may be very prominent. A thick basal membrane is more commonly seen in lesions of DLE. As with DLE, the commonest cell type is the T lymphocyte, but which subset predominates is uncertain. Both CD4+ and CD4+ subset preponderance have been documented. It may be that differences in the age of the lesion, effects of treatment, and antibody specificity can account for this apparent anomaly. CD4+ lymphocytes are present in large numbers in the epidermis at sites of basal keratinocyte hydropic degeneration.
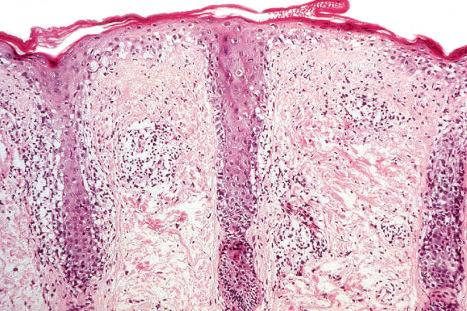
The histopathological features of the cutaneous lesions of the antiphospholipid syndrome comprise both venous and arterial thrombosis in the absence of any evidence of vasculitis ( Figs 17.77–17.79 ). In early lesions, endothelial cell damage may be marked and erythrocyte extravasation is common. Older lesions are characterized by marked vascular proliferation, commonly in a lobular distribution accompanied by hemosiderosis. Hobnail reactive endothelial cells and eosinophilic hyaline globules are sometimes evident. A lymphocytic infiltrate accompanied by variable numbers of plasma cells is often seen. In some patients, the clinical and histologic features of Degos disease and anetoderma have been documented.
Renal lesions may be detected in 70% to 80% of patients. The histologic features are subdivided into six classes according to the International Society of Nephrology/Renal Pathology Society 2003 classification of lupus nephritis, representing an evolution of the previous WHO classification:
In class I lesions (minimal mesangial lupus nephritis) no abnormalities can be detected by light microscopy. Mesangial deposition of immune complexes can be identified by immunofluorescence, electron microscopy, or both.
Class II lesions (mesangial proliferative lupus nephritis) are characterized by any degree of mesangial hypercellularity (defined as three or more mesangial cells per mesangial area in a 3-micron thick section) associated with mesangial immune deposits. Rare small immune deposits involving peripheral capillary walls can occasionally be detected by immunofluorescence or electron microscopy. Features not acceptable are subendothelial deposits on light microscopy as well as any segmental or global glomerular scars resulting from previous glomerular endocapillary proliferation, necrosis, or crescents. Immunofluorescence reveals mesangial deposition of IgG and complement and mesangial electron-dense deposits may be detected by electron microscopy. Mesangial lupus glomerulonephritis is the mildest form of glomerular lesion and is present in about 10% of patients with renal involvement.
Class III lesions (focal lupus nephritis) are distinguished by active or inactive focal, segmental, or global endo- or extracapillary glomerulonephritis involving < 50% of all glomeruli, associated with focal subendothelial immune deposits, with or without mesangial proliferation. Focal segmental proliferative glomerulonephritis is found in about 30% of patients. In this variant, only scattered glomeruli are affected (focal) and usually only a portion of the tuft is involved (segmental). Lesions may be proliferative and necrotizing. Involved glomeruli usually show mesangial and endothelial proliferation, polymorph infiltration, fibrin deposition, and karyorrhexis; occasionally, hematoxylin bodies are evident. Hematoxylin bodies are regarded as the histologic hallmark of SLE. They are round-to-oval structures, which stain purple/red–pink/blue with hematoxylin and eosin. They stain positively with the Feulgen reaction and are von Kossa negative. Hematoxylin bodies are thought to be the in vivo counterpart of the LE cell phenomenon. Although frequently sought they are often very difficult to find. Electron microscopy typically reveals mesangial and subendothelial electron-dense (immune complex) deposits. Clinically, patients present with hematuria and proteinuria, although a proportion may develop chronic renal failure.
Class IV lesions (diffuse lupus nephritis) are characterized by active or inactive focal, segmental or global endo- or extracapillary glomerulonephritis involving ≥ 50% of all glomeruli, associated with diffuse subendothelial immune deposits, with or without mesangial proliferation. They can be further subclassified as diffuse segmental (IV-S) and diffuse global (IV-G) lupus nephritis. Diffuse lupus glomerulonephritis occurs in about 50% of patients with SLE who have the nephritic/nephrotic syndrome or hypertension. Almost all glomeruli are affected and, in addition to mesangial and endothelial proliferation, the epithelial cells may participate, with the formation of crescents. Careful examination of the periphery of the glomerular tuft often reveals regularly thickened capillaries – ‘wire-loop’ lesions, thought to be specific for SLE.
Class V (lupus membranous glomerulonephritis) lesions show global or segmental subepithelial granular immune deposits with or without mesangial alterations. Scattered subendothelial immune deposits can also be present. Class V lesions can be associated with both class III and class IV lesions. Lupus membranous glomerulonephritis is found in about 10% of patients with SLE and presents as proteinuria or the nephrotic syndrome. Histologically, there is uniform thickening of the glomerular capillaries without any significant inflammatory cell infiltration. Advanced glomerulosclerosis can be present. Ultrastructurally, the electron-dense deposits are found subepithelially.
Class VI lesions (advanced sclerosis lupus nephritis) are characterized by global sclerosis without residual activity in ≥ 90% of glomeruli, related to lupus nephritis. They can represent progression of class III, IV or V lesions.
In addition to glomerular lesions, patients with renal involvement may manifest acute necrotizing vasculitis.
The heart is involved in about 50% of patients with SLE. Acute lesions of fibrinoid degeneration and hematoxylin bodies may be seen in the pericardium, but it is more common to find obliteration of the pericardial sac by fibrous, sometimes gelatinous, adhesions. Fibrinoid necrosis of the myocardial collagen is usually found in the connective tissue septa and occasionally also affects the associated arteries. Libman-Sacks endocarditis is the best-known cardiac manifestation of SLE and is believed to occur in 30% to 60% of the patients who come to autopsy. Small granular vegetations have been found on the surfaces of all valves, although the mitral and tricuspid are most often affected. The vegetations may spread to involve the chordae tendinae and adjacent endocardium. Histologically, they consist of fibrin overlying degenerate collagen, with an associated chronic inflammatory cell infiltrate. Infective endocarditis is an occasional, but important, complication. Increased quantities of glycosaminoglycans are usually evident and sometimes hematoxylin bodies are present.
The histologic lesions of pulmonary involvement include interstitial pneumonitis, fibrosing alveolitis, and infarction. In a large autopsy study of patients with SLE, pleuropulmonary involvement has been detected in 97.8% of patients, and included pleuritis (77.8%), bacterial infections (57.8%), primary and secondary alveolar hemorrhage (25.6%), distal airways alterations (21.1%), opportunistic infections (14.4%), and thromboembolism (7.8%). Direct immunofluorescent examination of lung biopsies may reveal the presence of both immunoglobulin and complement.
Lymph nodes often show reactive hyperplasia; occasionally there are striking pathological features, which may be confused with a lymphomatous infiltrate. These changes consist of a necrotizing lymphadenitis with prominent hematoxylin bodies. Surviving follicles show reactive hyperplasia and conspicuous plasma cells and immunoblasts. The thymus may show prominent germinal center formation, with an increase in the size and number of Hassall corpuscles. Perisplenitis (‘sugar icing’) of the splenic capsule is common and a characteristic histologic finding is concentric fibrosis (‘onion skinning’) of the adventitia of the central (penicillary) arteries of the Malpighian corpuscles.
Joint manifestations include fibrinoid degeneration within the synovium, rheumatoid features, and arteritis. A variety of lesions may be seen in the liver, including fatty change, focal hepatocyte necroses, and evidence of vasculitis. Central nervous system manifestations have an ischemic pathogenesis, most probably on an immune complex-mediated vasculitic basis. There is some evidence to incriminate an antineuronal autoantibody. The cardiac pathology of neonatal lupus erythematosus comprises fibrosis and calcification of the atrioventricular node and, to a lesser extent, the sinoatrial node. Focal lymphocytic myocarditis and endocardial fibroelastosis may also be evident.
The histologic features and immunofluorescence pattern of the cutaneous lesions in neonatal disease are similar to those seen in SCLE. The most frequently documented findings include hydropic degeneration of basal keratinocytes and adnexal epithelium, dermal edema as well as a superficial and occasionally deep perivascular and periadnexal lymphohistiocytic chronic inflammatory cell infiltrate. Eosinophils may be prominent in urticaria-like SCLE lesions. In addition, a subset of patients with neonatal lupus display histologic features of neutrophilic dermatosis (Sweet syndrome-like), including histiocytic variant ( Ch15 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here