Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
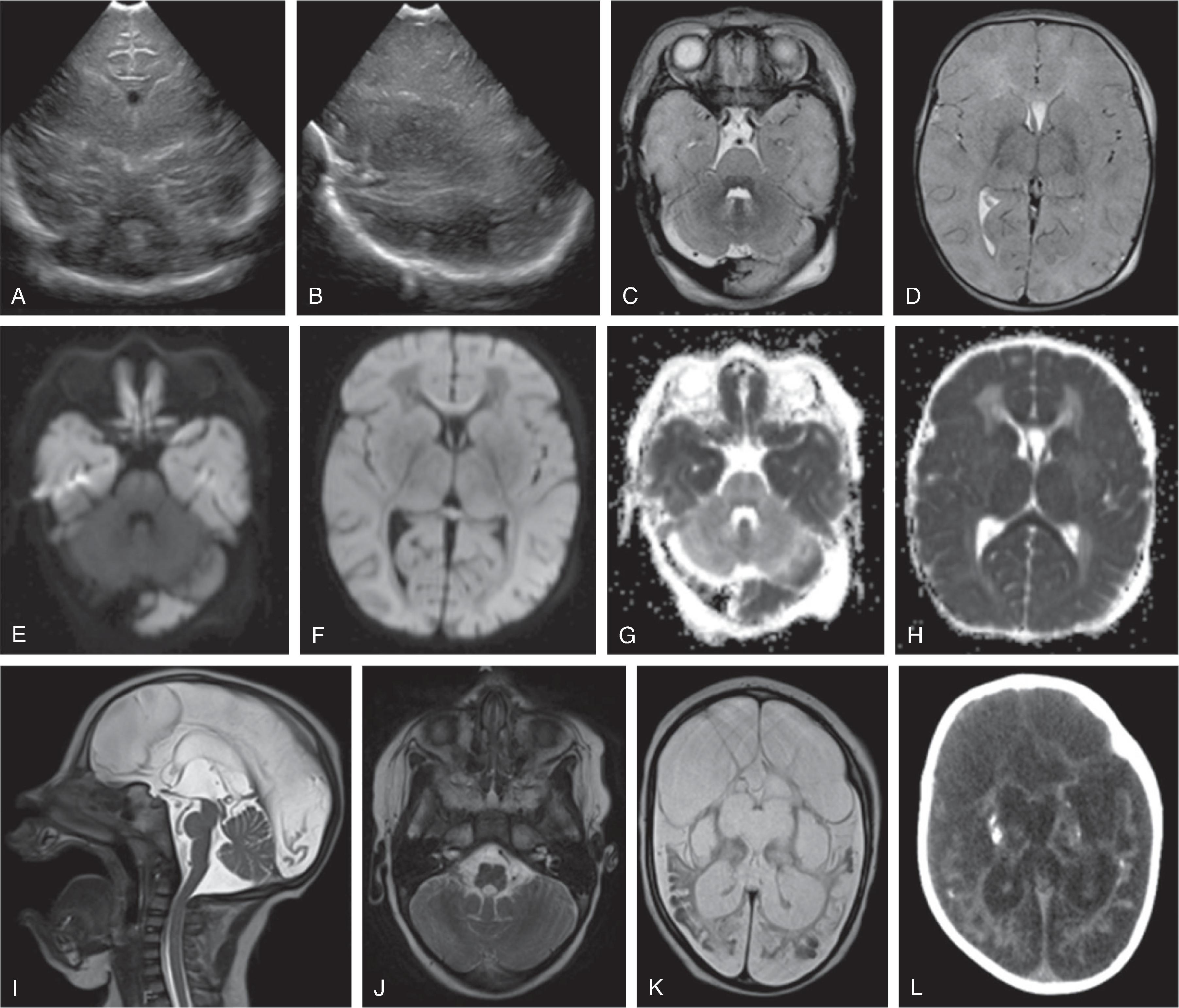
Background
Hypoxic ischemic injury (HII) typically refers to a combination of a hypoxic and hypoperfusion injury to the fetal or neonatal brain.
Clinical manifestations of HII include low Apgar scores (0–3 at 5 minutes and ≤5 at 10 minutes), umbilical cord pH < 7.1, poor cry, weak suck, seizures, diminished movement, absent neonatal reflexes, and hypotonia.
Depending on the maturity of the brain, duration and/or degree of hypoxic-ischemic injury, and coexisting fetal/neonatal or maternal risk factors (e.g., congenital heart disease, neonatal sepsis, chorioamnionitis, placental abruption, surfactant deficiency disease), various patterns of injury may be observed.Imaging
In a full-term neonate, mild HII affects the deep white matter (typically periatrial white matter), moderate HII affects the watershed cerebral cortex and subcortical white matter, and severe HII affects the basal ganglia, thalami, and sensorimotor cortex. The combinations of severity and duration of the hypoxia and hypoperfusion has also been reported to follow the following patterns:•Mild/moderate, and gradual: cerebral white matter injury•Moderate, prolonged and/or intermittent: severe cerebral hemisphere +/- deep gray nuclei•Severe, and brief: deep gray nuclei +/- brainstem•Severe, and prolonged: cerebral hemirspheres, deep gray matter nuclei, and brainstem
In a premature infant, mild to moderate HII affects the periventricular white matter leading to periventricular leukomalacia and germinal matrix hemorrhage, and severe HII affects the thalami.
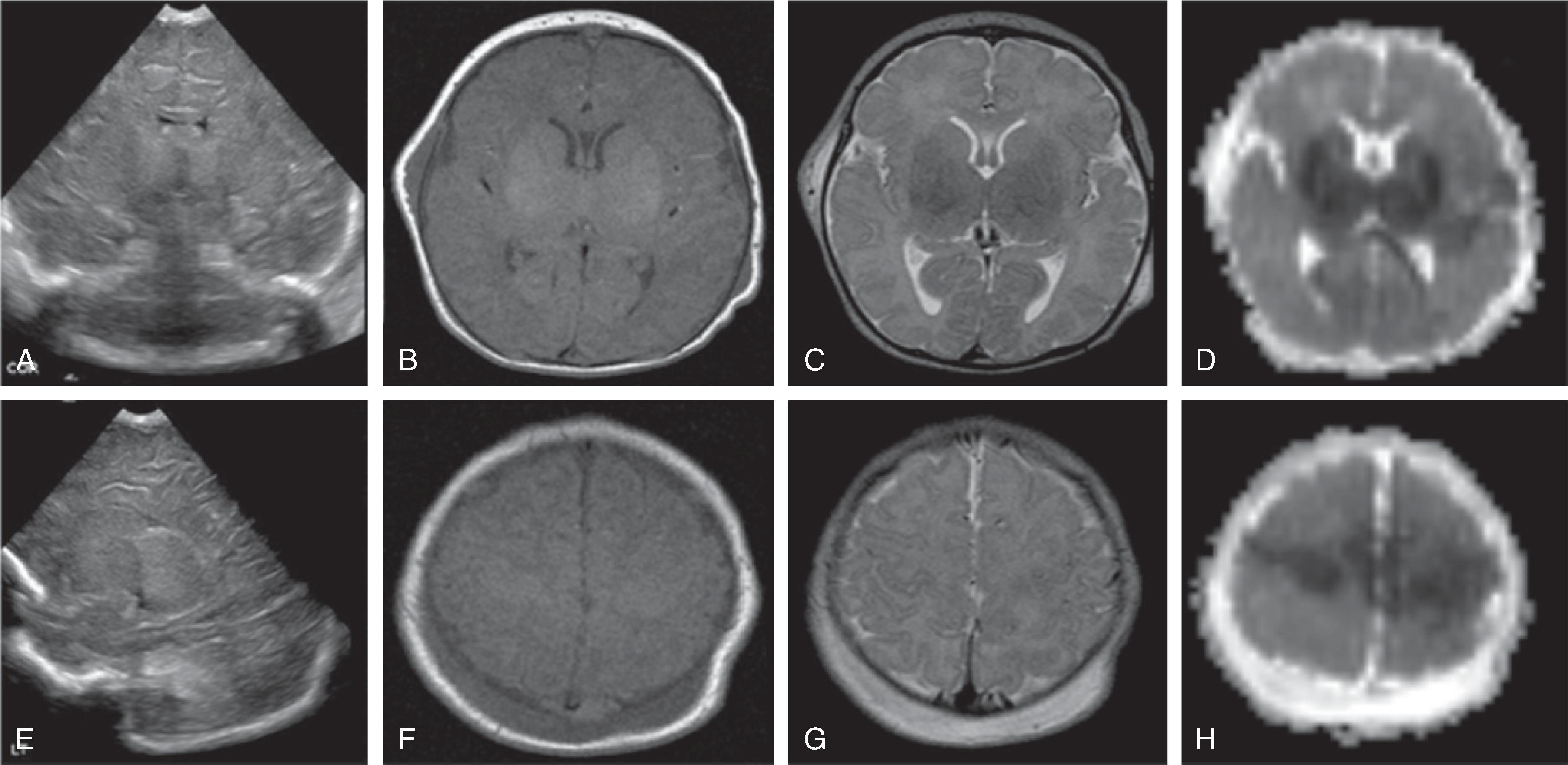
Transfontanellar ultrasound imaging may show hyperechogenicity of the hemispheric white matter relative to the cortical gray matter with resultant increased corticomedullary differentiation, slit-like ventricles, and lowered resistive index (RI) values sampled within a branch of the circle of Willis 24 to 48 hours after injury. Micro- or macrocystic leukoencephalopathy may be seen starting 8 to 10 days after the initial injury.
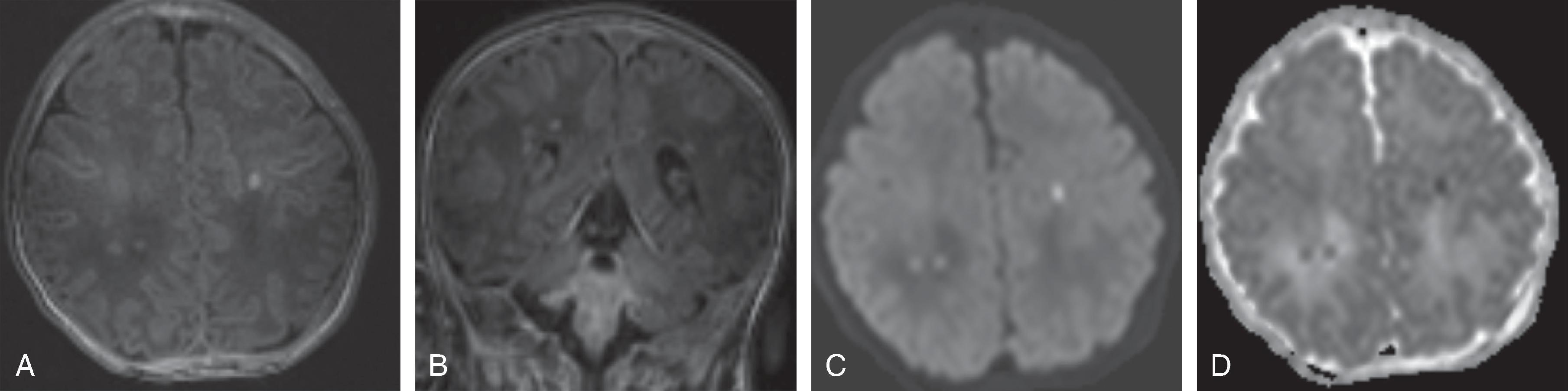
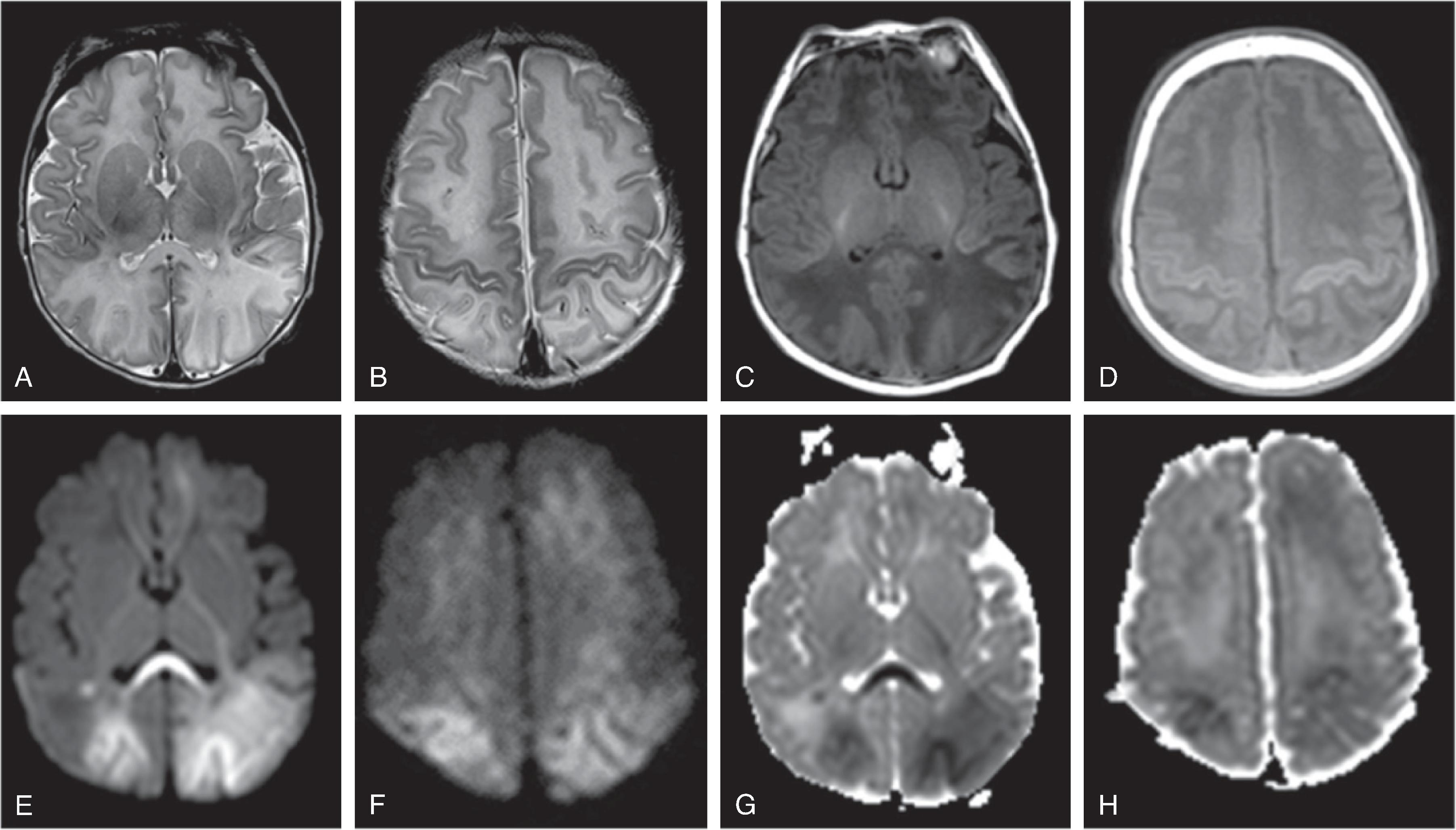
MRI typically shows the distribution of injury in better detail. Diffusion-weighted imaging (DWI) shows reduced diffusion (low ADC) within the first 8 to 10 days after injury followed by pseudonormalization of diffusion (ADC isointensity) followed by progressive increased diffusion (increased ADC) after a brief period of pseudonormalization. DWI is most sensitive for the extent of injury at 3 to 5 days from the HII. Therapeutic hypothermia may delay the temporal evolution of the diffusion characteristics (pseudonormalization occurring approximately at day 10 with cooling versus day 6–8 without cooling).
Conventional T1W and T2W MRI may underestimate injury within the first 24 to 48 hours of injury and appear normal in this time frame. Afterwards depending on the type of insult, the hemispheric white matter may be T2 hyperintense, T1 hypointense. Focal T1-hyperintensity may be seen within the cortical or central gray matter, in particular affecting the ventrolateral thalami and posterior putamina as well as perirolandic cortex.
Relative T2-hyperintensity and T1-hypointensity of the posterior limb of the internal capsule (PLIC-signal) are considered to be linked to a poor prognosis.
Acute 1 H MRS typically reveals increased levels of lactate, while subacute and chronic 1H MRS may show reduced N-Acetyl aspartate (NAA), increased choline (Cho), and decreased Creatine (Cr). A lactate/NAA ratio of >0.3 in the acute phase was associated with poor outcome.
ASL perfusion has shown increased cerebral blood flow in the deep gray matter at 4 to 7 days after HII and associated with adverse clinical outcome.
In the chronic phase, various combinations of a micro- or macrocystic hemispheric leukoencephalopathy may be seen accompanied by hemispheric white matter volume loss, size-reduced central gray matter, cortical gray matter thinning, ex vacuo enlargement of the cerebrospinal fluid (CSF) spaces, microcephaly, and global hypomyelination.
Differential diagnosis includes inborn errors of metabolism, infection/sepsis, and congenital heart disease.
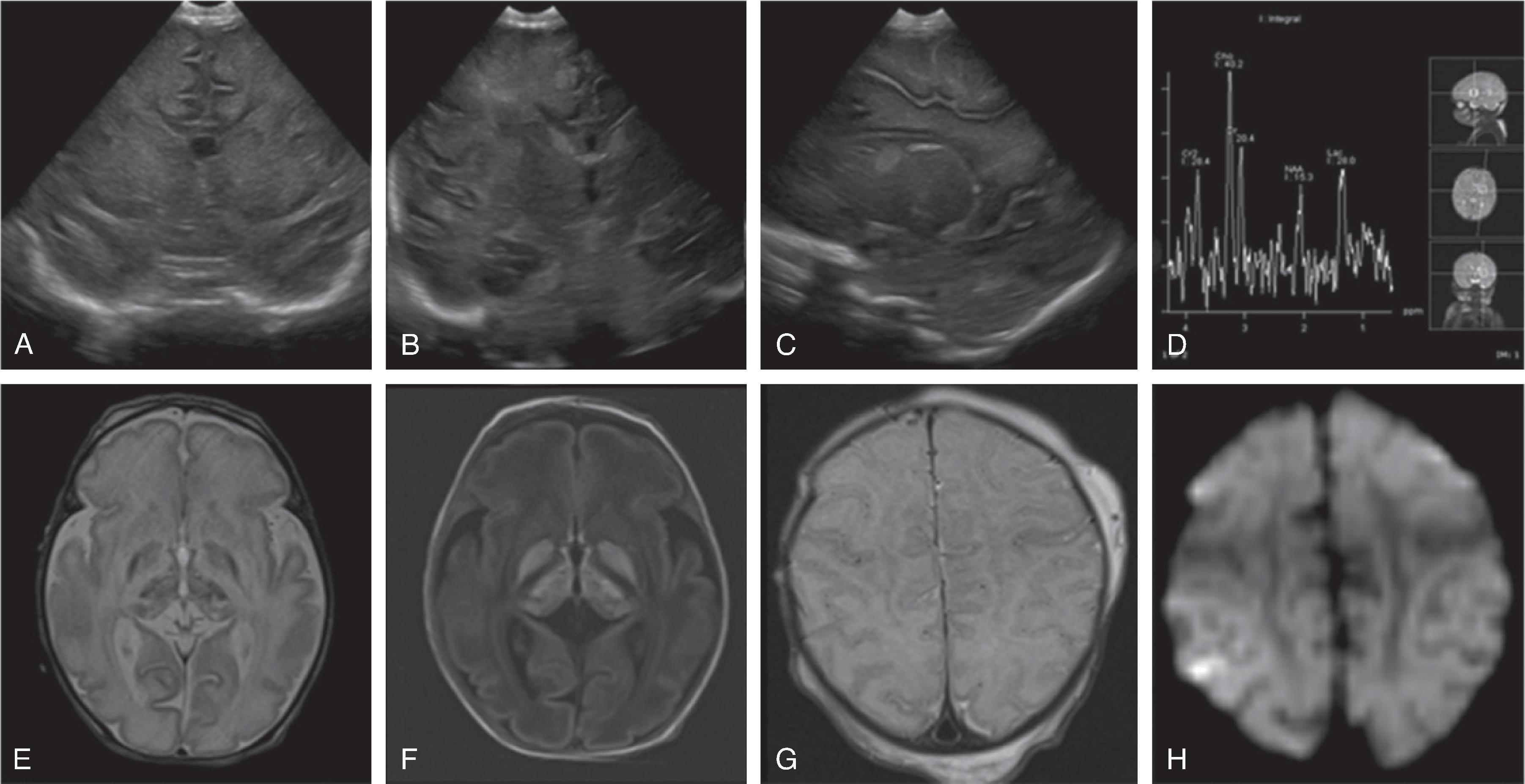
Background
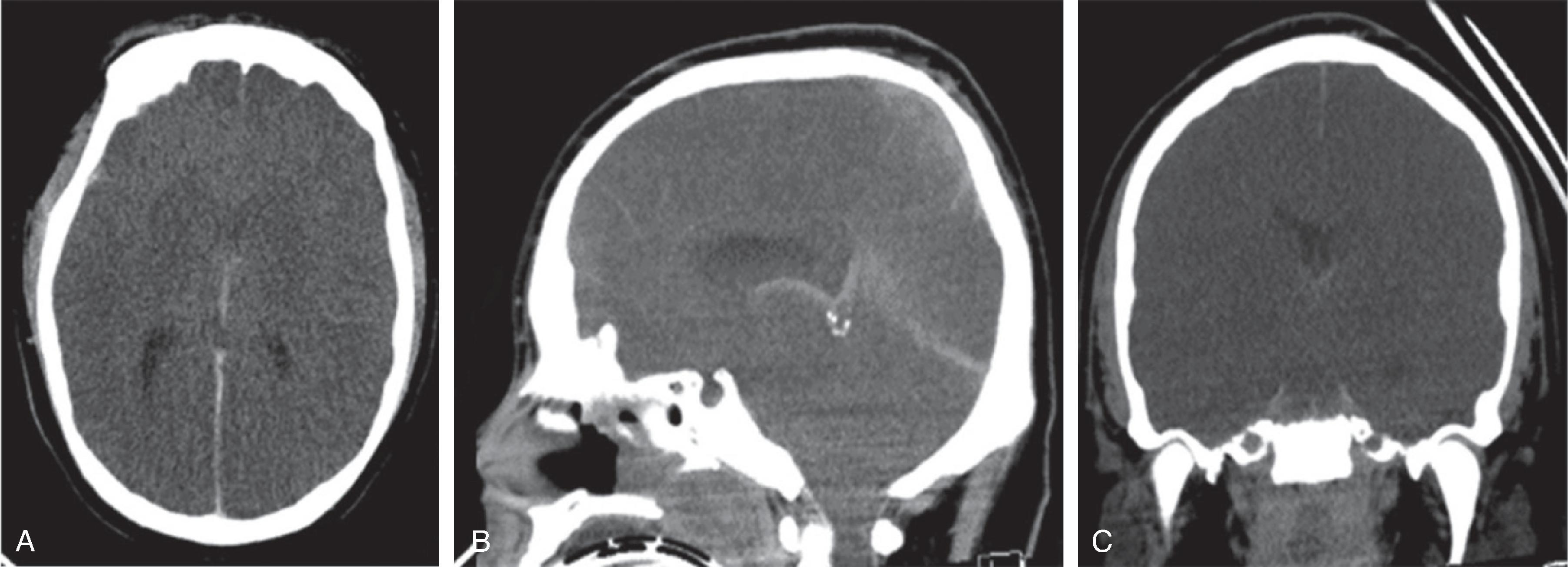
Beyond the perinatal time period, global hypoxic or anoxic injury may result from a variety of reasons, including a cardiopulmonary arrest, prolonged seizures, strangulation, child abuse, near drowning, severe asthma attack, and smoke or carbon monoxide inhalation.
Hypoxia refers to a critically reduced or impaired delivery of oxygen to the central nervous system; anoxia is an extreme form of hypoxia with virtually no oxygen delivered to the brain at all.
Depending on the duration and degree of hypoxia as well as possible coexisting pathologies, different patterns and distributions of injuries may be seen affecting white and gray matter.Imaging
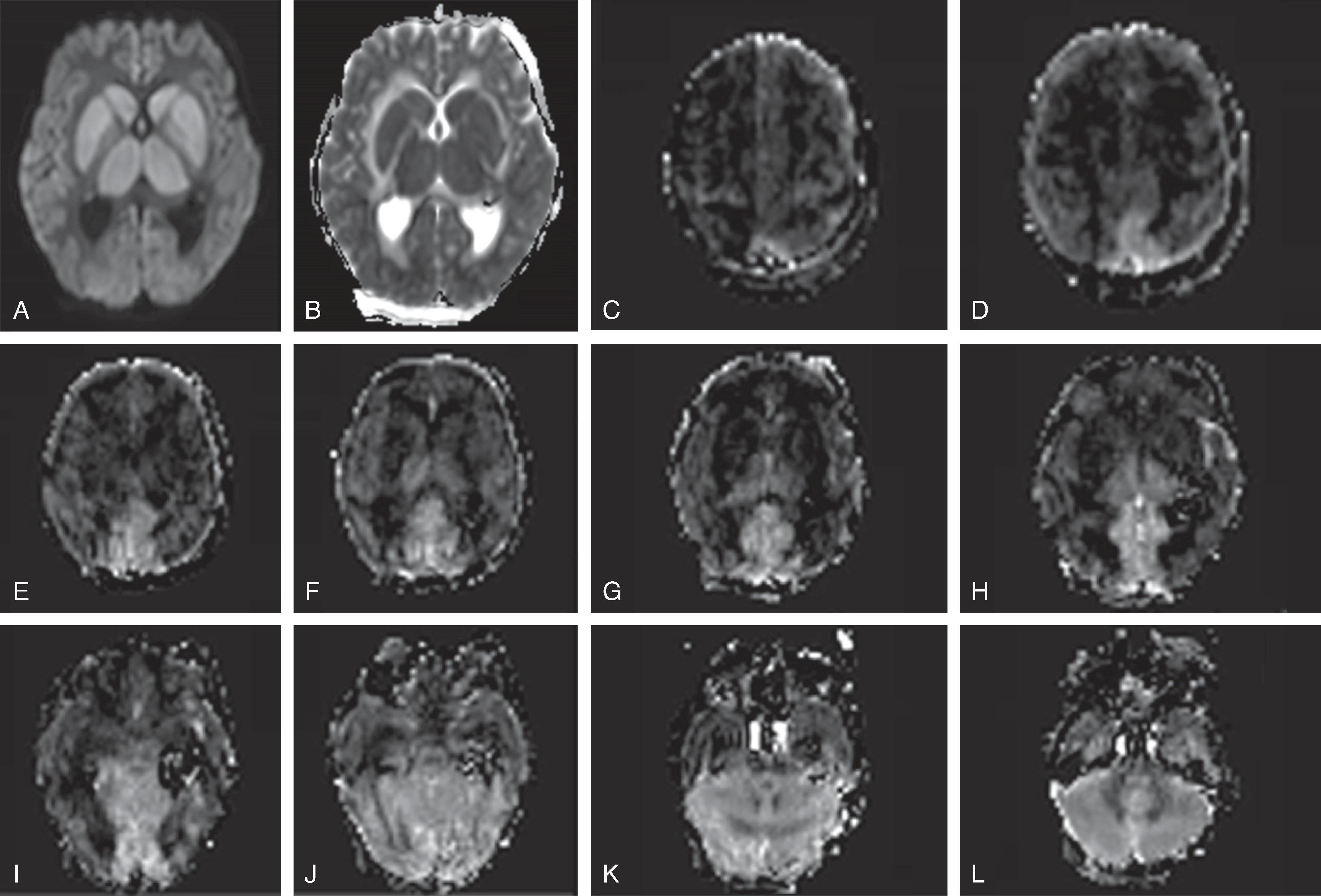
CT may be negative within the first 12 to 24 hours after hypoxic injury; MRI and in particular diffusion-weighted imaging may show hypoxic injury within 3 to 6 hours.
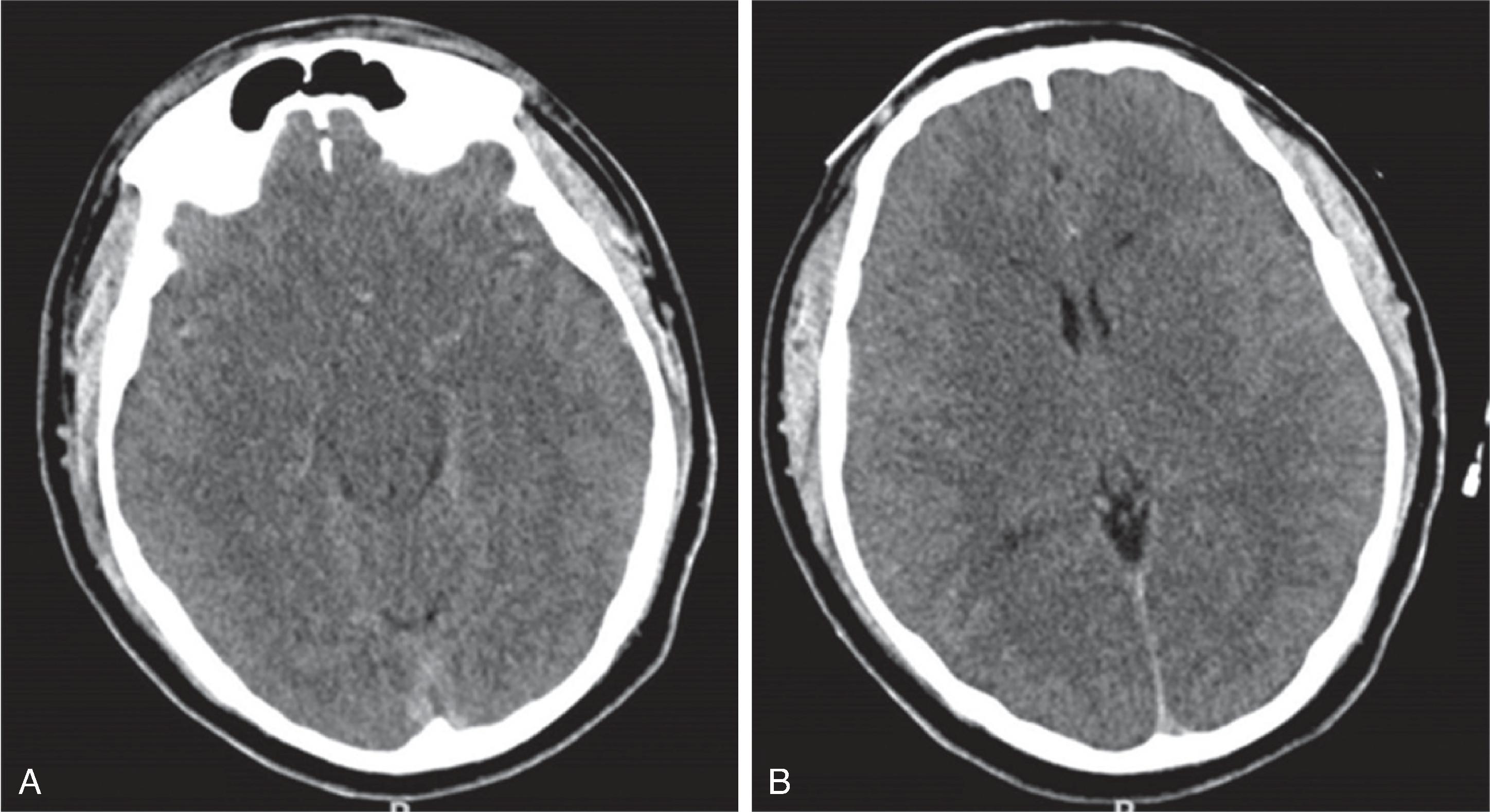
Earliest signs of injury on CT include a global brain swelling with loss of the normal gray-white matter differentiation, effaced cerebral sulci, and small or compressed ventricles. On follow-up, progressive white and gray matter hypodensity will develop with reappearance of the sulci and ventricles. In the chronic phase, progressive volume loss, possibly with cystic transformation of the injured brain tissue, ensues.
Due to protective rearrangement of the brain perfusion and differing vulnerability of the brain tissue, the brainstem and cerebellum may be partially spared, resulting in the so-called “white cerebellum” sign, also known as reversal sign or dense cerebellum sign on CT due to the contrast of the injured edema low density supratentorial brain with respect to the normal density if the brainstem and cerebellum. Slow flow within the arterial circle of Willis and venous circle of Trolard may result in relative hyperdensity of the vessels compared to the hypodense brain tissue.
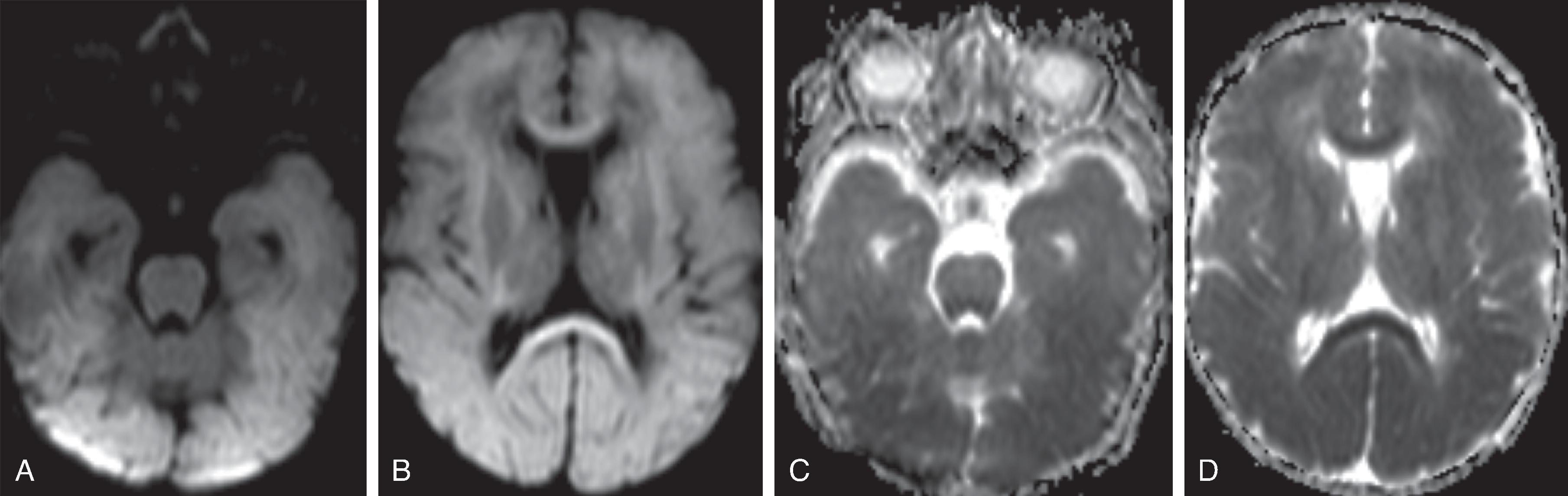
Acute and subacute MRI and DWI typically reveal diffuse T2-hyperintense brain swelling, DWI-hyperintense/ADC-hypointense cytotoxic edema of the injured tissue, hypoperfusion on perfusion-weighted imaging, engorged hypointense intraparenchymal veins on susceptibility-weighted imaging and increased lactate and decreased N-acetyl aspartate on 1 H MRS of the injured tissue. On follow-up, cystic encephalomalacia with volume loss is present.
Tonsillar and uncal herniation may result from the global supratentorial brain swelling with resultant effacement of the basal cisterns.
With carbon monoxide poisoning there is injury to the central gray matter, in particular the globus pallidi.
Background
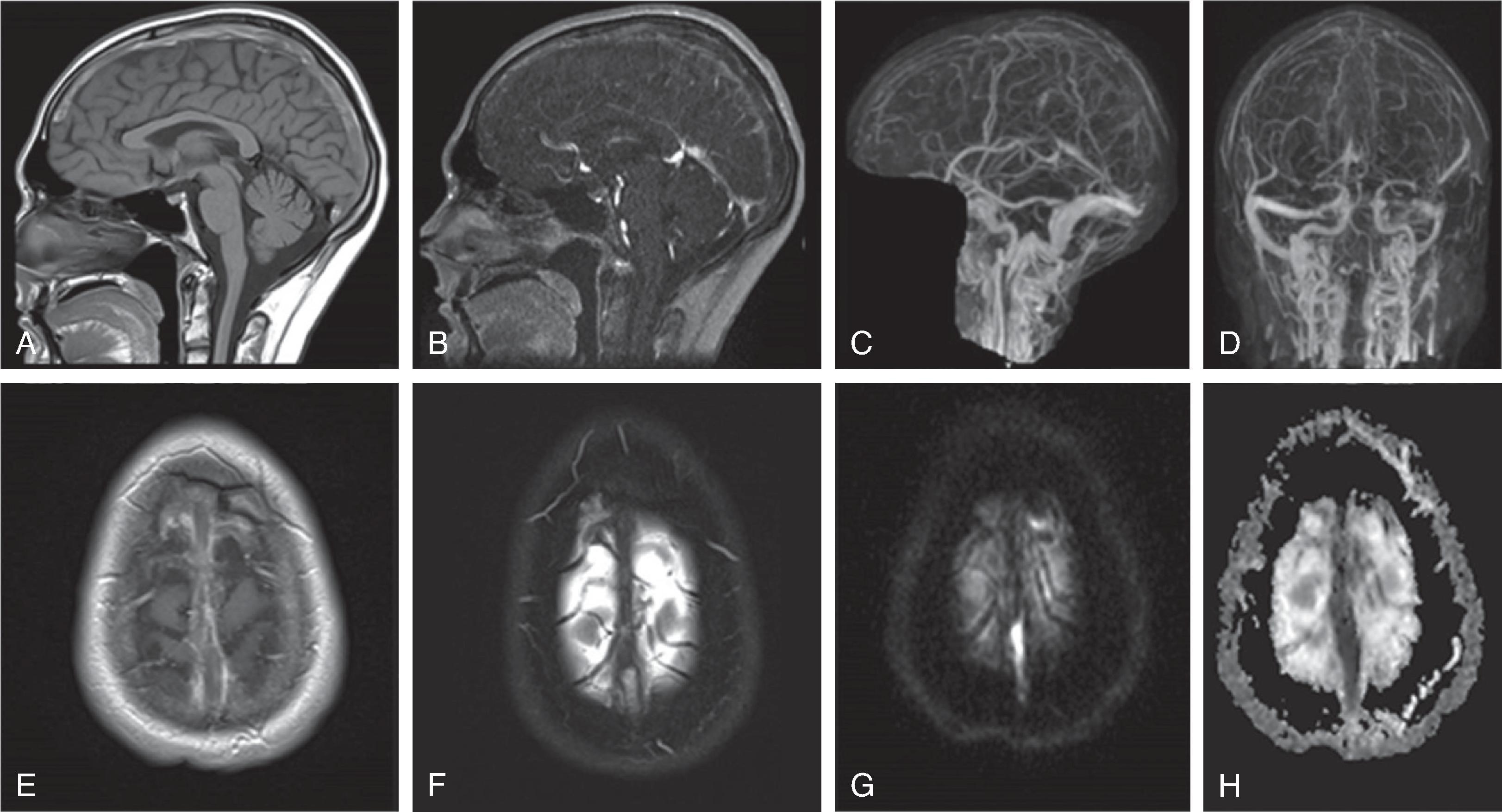
Acute ischemic stroke in children is considered a rare event more common in newborns than older children, occuring in 1/3500 live births compared to 1-2/100,000 during childhood; however, with increasing public and clinical awareness and the wider availability of high-end, sensitive and specific imaging, acute pediatric strokes are increasingly recognized.
Arterial strokes may be encountered in any age group, including the immediate postnatal and occasionally even fetal period.
Children may present with a range of symptoms, including minor or major focal neurologic deficits, irritability, and seizures.
Causes of pediatric arterial ischemic stroke by category include vasculopathy (ex. sickle cell disease, moyamoya, radiotherapy-related vasculopathy, collagen vascular disorders, dissection, focal cerebral arteriopathy, primary and secondary vasculitis, and others), coagulation disorders, congenital and acquired cardiac disease, infection, and genetic disorders.
Acute stroke in the neonatal period may be of thromboembolic nature, including classic thrombi as complication of a chorioamnionitis, placental infarctions or rarely from amniotic fluid thrombi. In addition, congenital vascular anomalies, including a vein of Galen aneurysmal malformation or a dural arteriovenous malformation or fistula, also increase the incidence of acute strokes, possibly aggravated by steal phenomena.
Treatment for acute ischemic stroke in children depends on the etiology. Acute thrombotic vascular occlusion can be treated with tissue plasminogen activator if onset is less than 4.5 hours. Prognosis depends on location of the stroke and age at time of injury. Younger patients have less clinical deficits compared to older children with similar infarct size and location. The mortality rate after childhood stroke is ~2-5%.Imaging
Focal neonatal strokes appear hyperechogenic on transfontanellar ultrasound on acute imaging with developing hypoechogenicity on progressive evolution and eventual anechoic cystic transformation in the chronic phase.
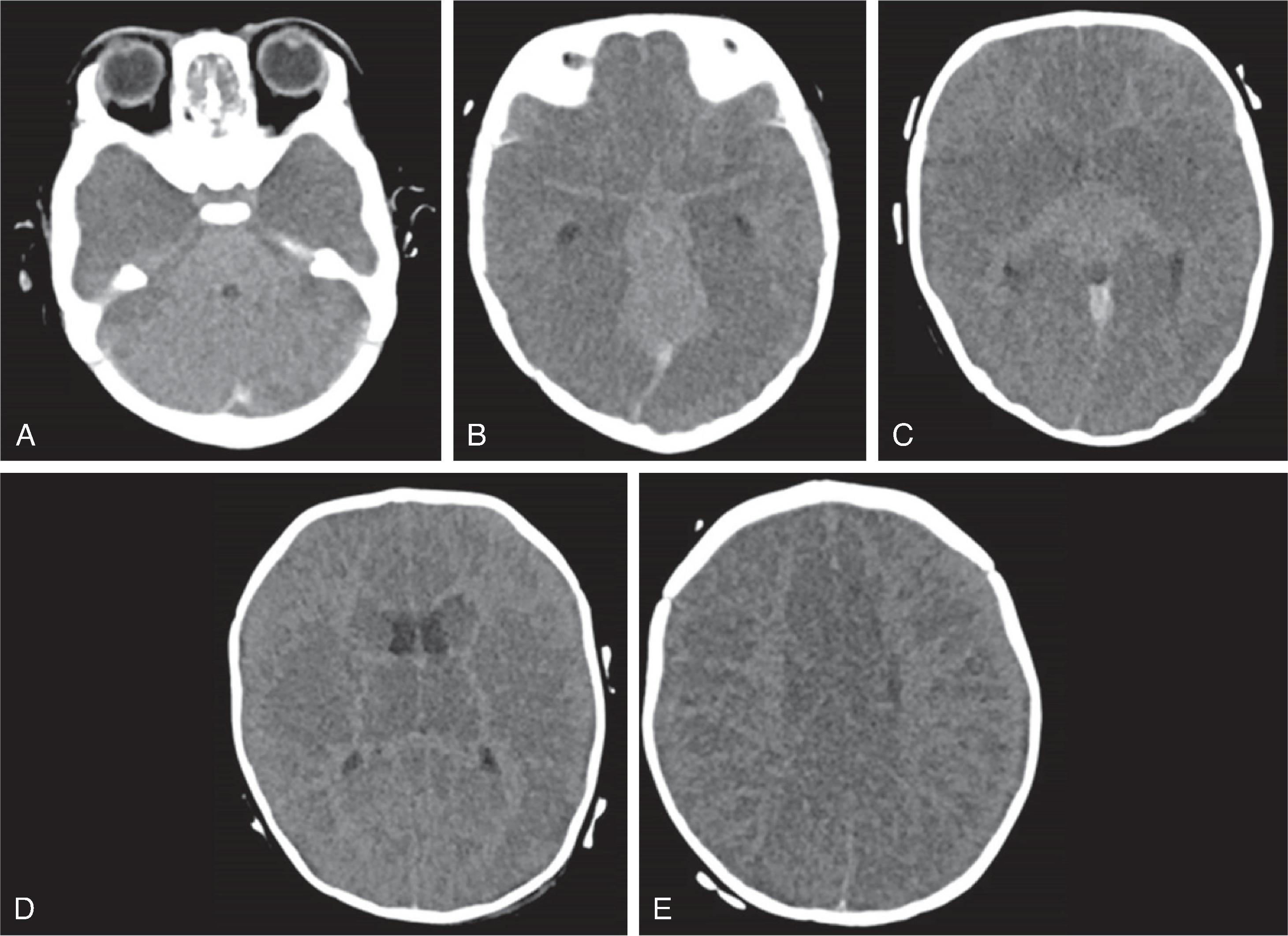
CT may be negative within the first 12 to 24 hours, but is useful for excluding acute hemorrhage. In the acute phase, signs of acute ischemic stroke on CT can include hyperdense thrombus, loss of cortical gray-white differentiation including loss of the insular ribbon, hypodense deep gray nuclei, gyral swelling, and wedge shaped parenchymal hypodensity. In the subacute phase ~1-3 weeks after injury, the hypodensity persists but there is decreased swelling. In the chronic phase, there is parenchymal hypodensity and volume loss.
On MRI, diffusion restriction (DWI hyperintense-ADC hypointense) pattern can be seen within 3-6 hours and persists from ~10-14 days. After 10-14 days the ADC signal will become isointense which is known as pseudonormalization and a few days later the ADC signal will become hyperintense. T2/FLAIR is often normal in the first 6-12 hours of an acute ischemic stroke, after which there is development of T2/FLAIR hyperintensity. Initially, there is no contrast enhancement of the infarct, but at ~3 days there is blood brain barrier breakdown related infarct enhancement which can then persist for 6-12 weeks. Vascular imaging with CTA, or MRA can identify vascular occlusion, stenosis or dissection which can help indicate etiologies such as embolic cause, vasculopathy, or dissection.
On follow-up imaging the ischemic area will be resorbed and evolve into a cerebrospinal fluid–filled tissue defect. Depending on the location, Wallerian degeneration or distant volume loss may be seen along the course of affected white matter tracts, most commonly the corticospinal tract seen as small size of the cerebral peduncle, ventral pons and medulla.
If multiple small ischemic lesions are noted, particular etiologies to consider include an embolic source, infection and coagulopathy.
Hemorrhagic transformation of an ischemic stroke is rarely seen in the pediatric patient population.
Background
Initially described as an arterial stenotic disease that did not correlate with moyamoya, sickle cell arteriopathy, dissection, vasculitis, post varicella arterioapthy or other known pathologies.
FCA is monophasic with a stereotypical history of early progression within days to weeks, and nonprogression at 6 months with improvement or resolution in a subset of patients.
More recent literature by a group of authors seeks to classify this poorly understood entity into three groups based on arterial stenosis/ irregularity to include banding/occlusion (not attributable to thromboembolism) by definition involving the intracranial ICA and its proximal branches with M1 segment most common. This includes:
FCA-inflammation type: includes post varicella arteriopathy, and transient cerebral arteriopathy. FCA may predominately be a function of inflammation that is infectious or postinfectious.
FCA-dissection type: sequelae of trauma.
FCA-undetermined.Imaging
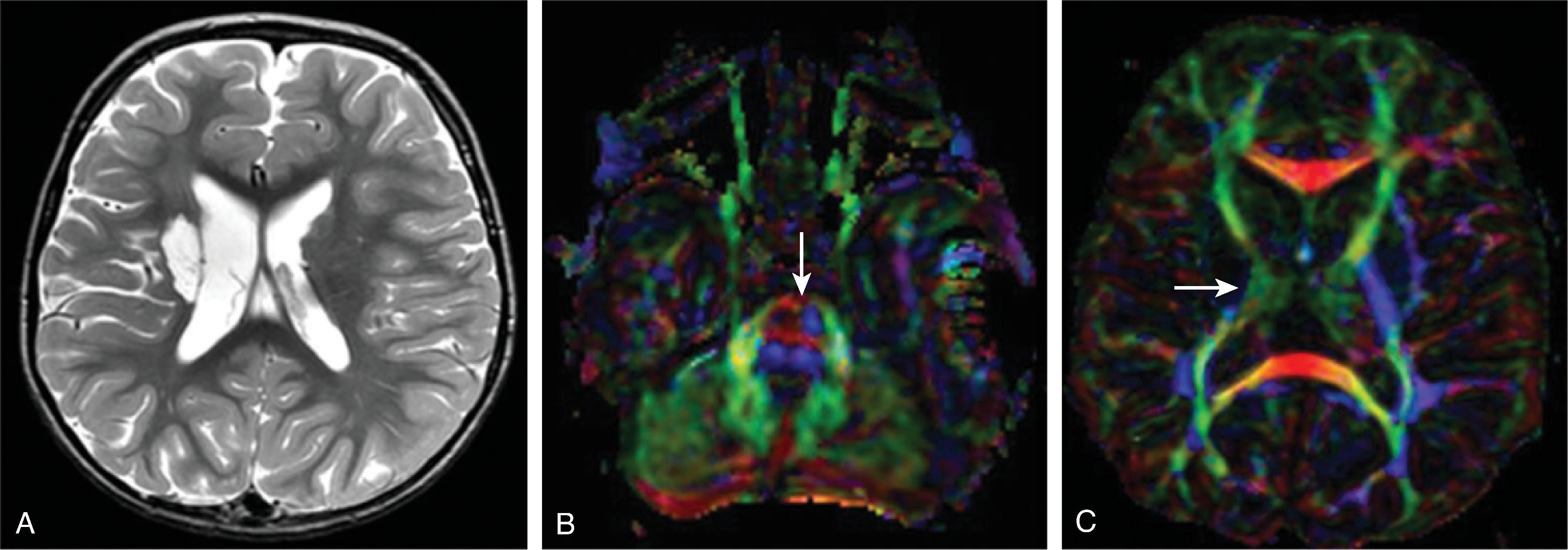
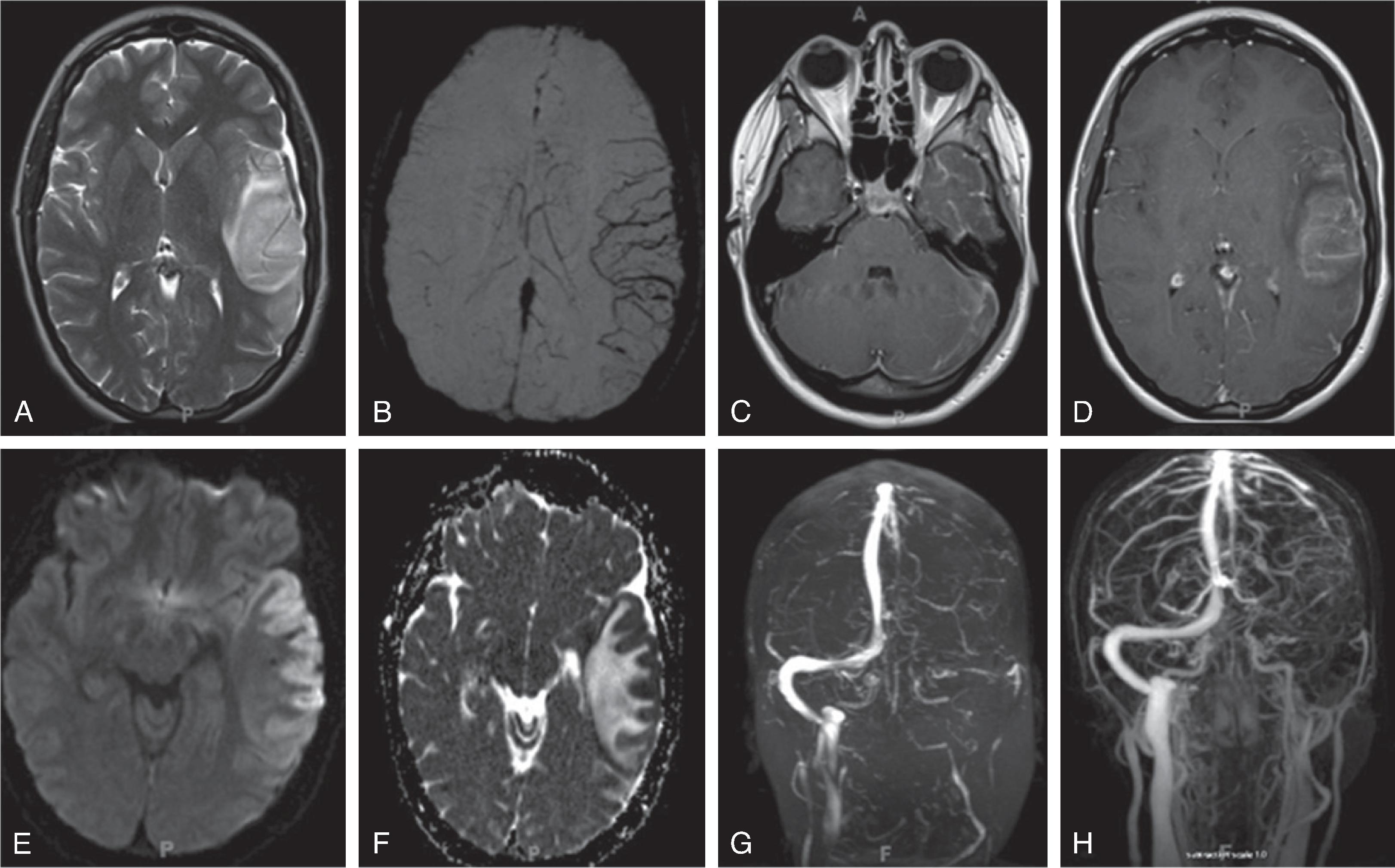
Acute unilateral infarct at presentation predominately involves the lentiform nucleus, caudate nucleus + distal MCA territory is classical. Recurrent strokes may occur in up to 30%. Note that multi-territorial infarcts in a cardiac patient strongly suggest cardioembolic stroke rather than FCA.
Most patients present with >50% stenosis in the involved segments; vessel occlusion may occur. Vessel wall enhancement may be present.
Differentiation from primary and secondary CNS vasculitis may be difficult.
Background
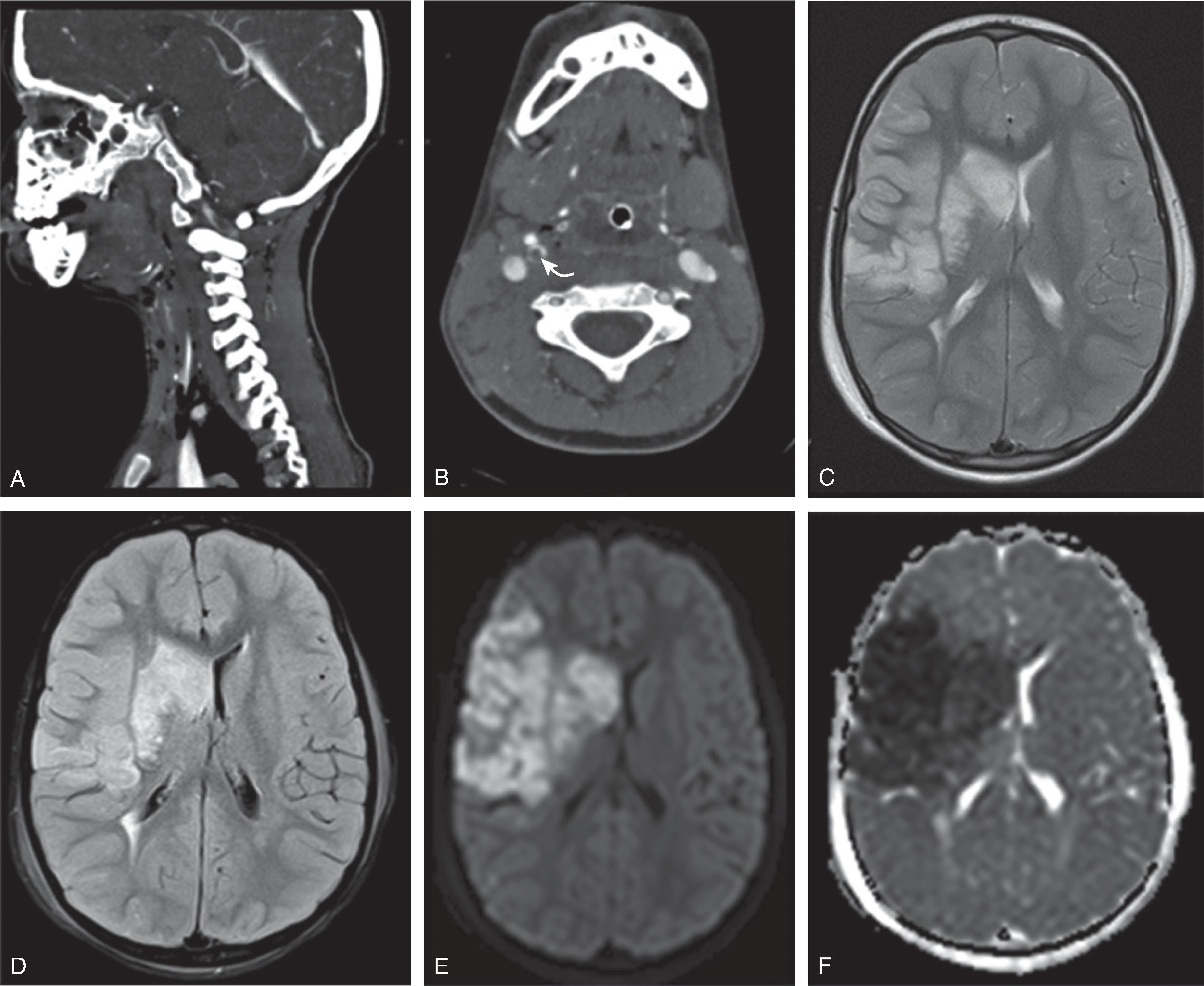
Vascular dissections are rare in children but may occur after minor trauma to the neck vasculature or may be spontaneous in children with preexistent connective tissue disorders including, Ehlers-Danlos or Loeys-Dietz syndrome.
Traumatic vascular dissections are more frequently encountered if the pediatric neck is exposed to a sudden acceleration-deceleration or rotatory angulation force. The immature protective reflexes and musculoskeletal stability of the neck relative to the head make young children especially vulnerable for these sudden acceleration-deceleration events.
Traumatic dissections are often seen at the skull base but may extend into the cranial vault up to the level of the distal internal carotid artery.
The internal carotid artery is more frequently affected than the vertebral artery.Imaging
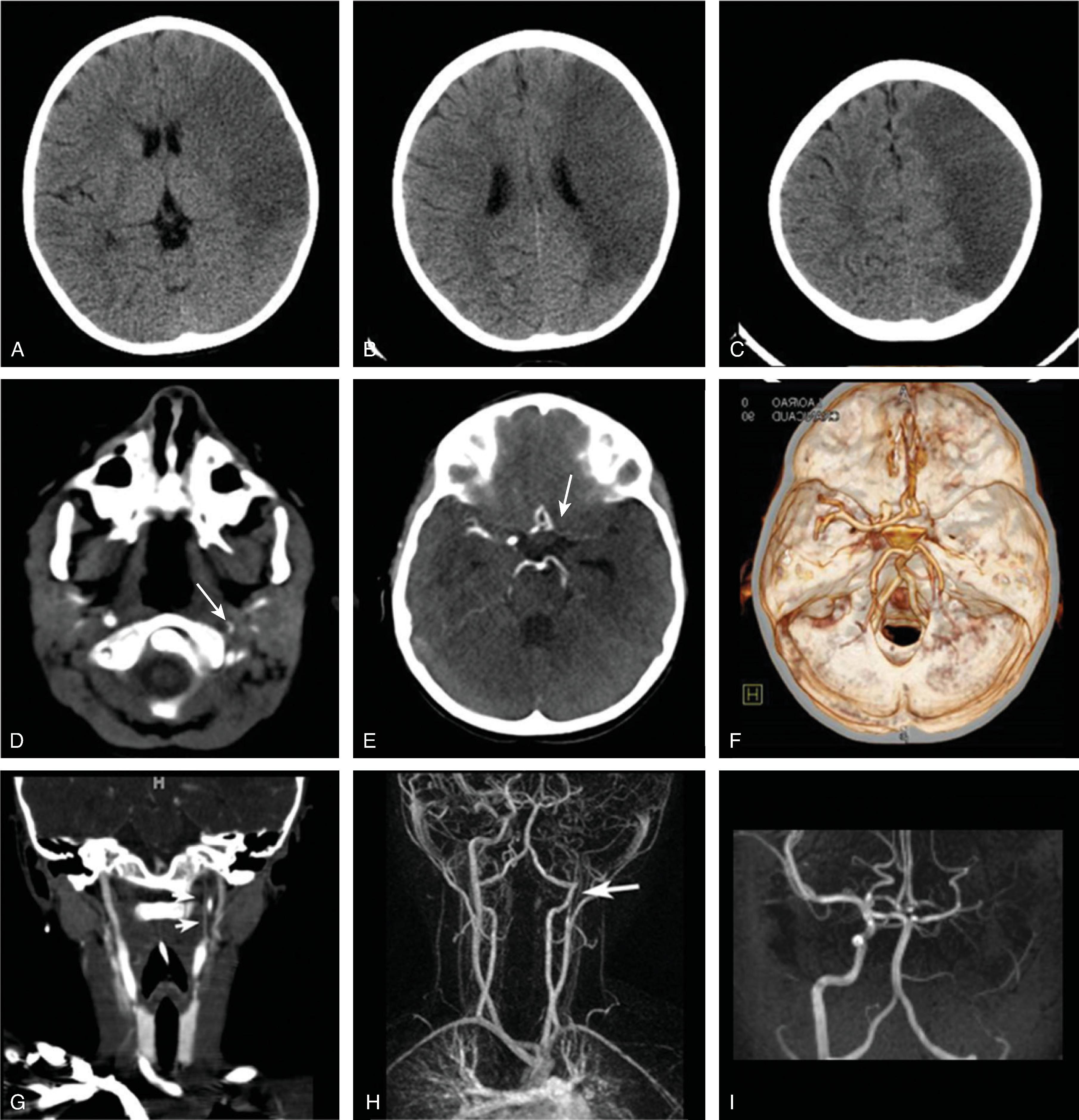
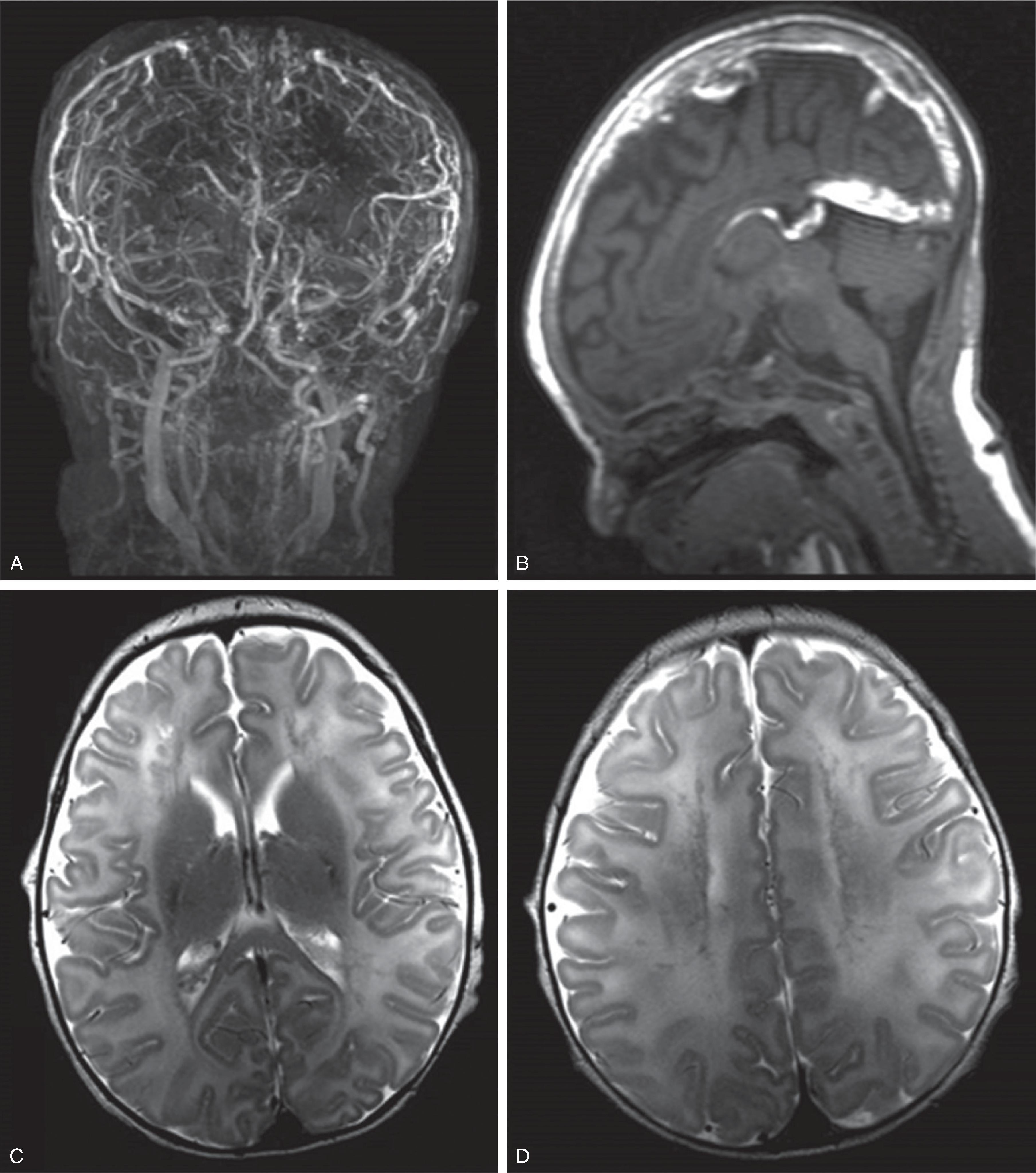
CTA, and MRA studies are highly diagnostic and show a classic tapering of the injured vessel with a narrowed/compressed true lumen. A T1-hyperintense thrombus within the false, subintimal lumen is best seen on fat-saturated T1-weighted images acquired perpendicular to the longitudinal axis of the affected artery.
Ultrasound imaging and duplex/doppler sonography may be helpful to evaluate the neck vasculature; however, care should be given to not injure the vessel by the ultrasound probe or possibly dislodge a thrombus.
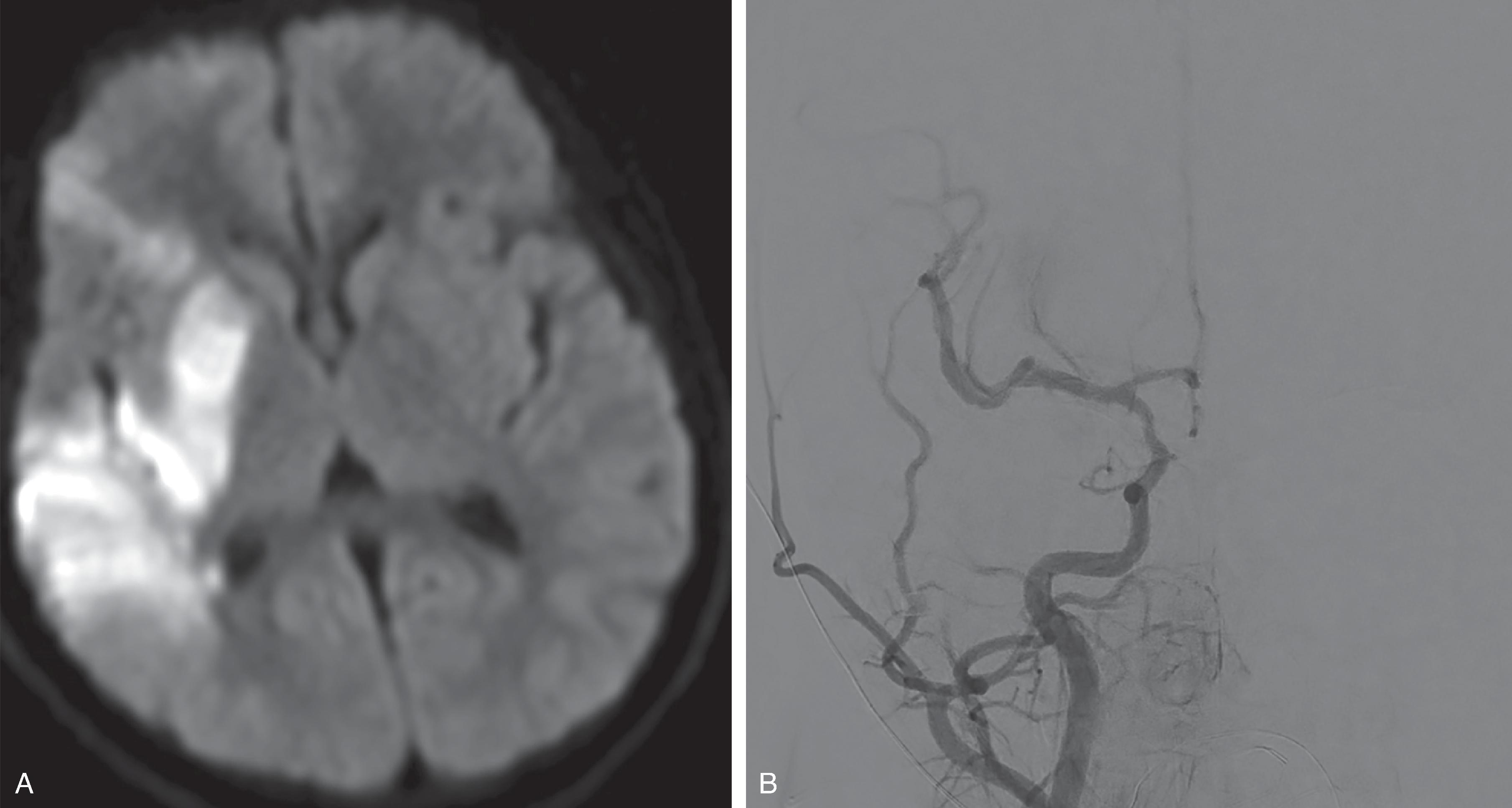
Focal strokes typically result from propagation of the thrombus. In addition, a “showering” of peripheral emboli may result in multiple small central or peripheral ischemic lesions.
The stroke-related parenchymal imaging findings are similar to the previously described acute stroke imaging findings.
Background
Twin-twin transfusion syndrome (TTTS) is a rare prenatal complication of a monochorionic-diamniotic twin pregnancy in which anomalous connections between placental blood vessels allow for a hemodynamic imbalance between both twins.
TTTS occurs in 10%–15% of monochorionic twin pregnancies.
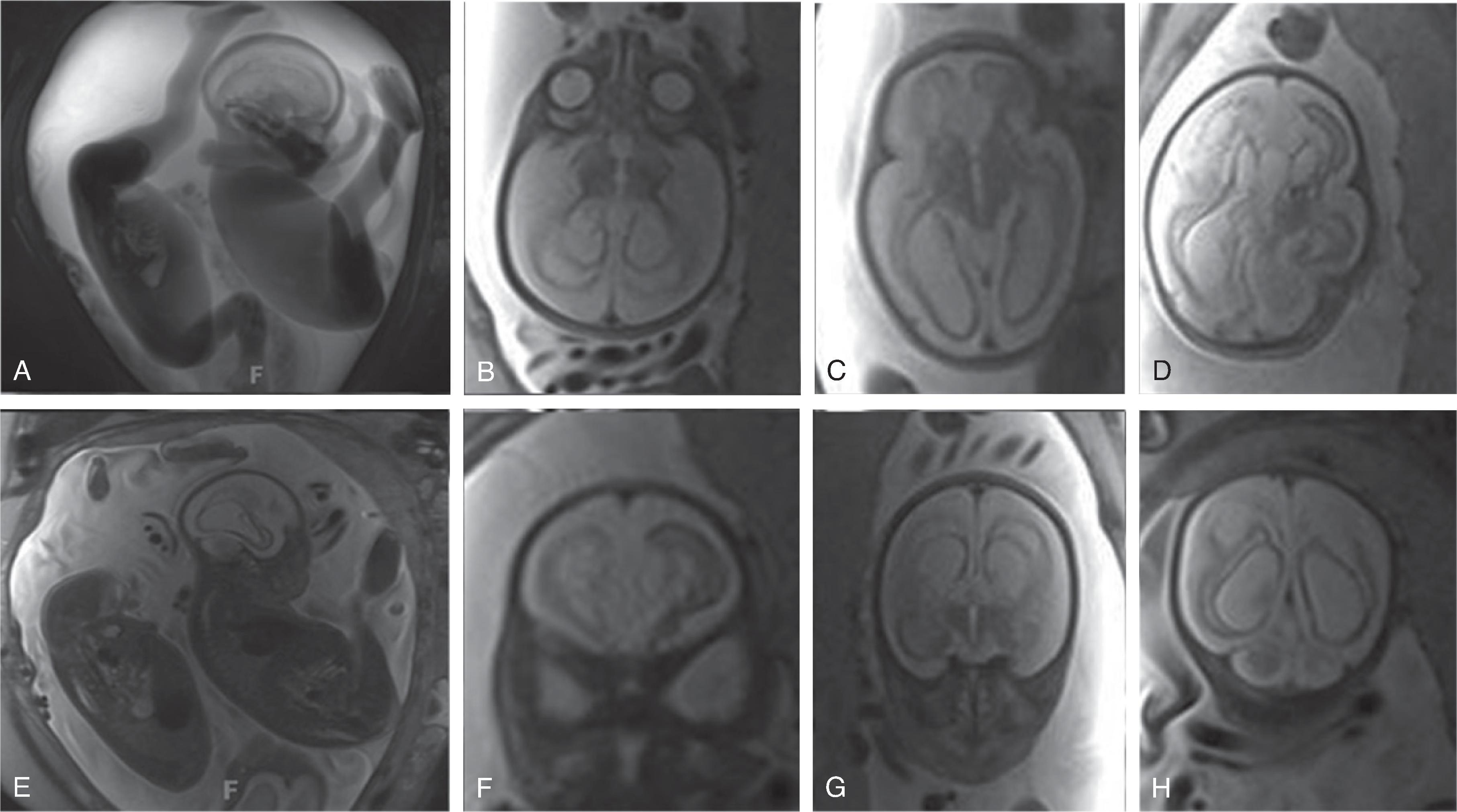
In TTTS the imbalance results in the shunting of blood from one fetus to the other. The donor fetus typically has a decreased blood volume with resultant low urine output, small urinary bladder, and small amount of amniotic fluid (oligohydramnios), while the recipient fetus has to deal with an increased blood volume, with resultant increased urine output, large urinary bladder, large amount of amniotic fluid (polyhydramnios), and possibly heart failure with hydrops.
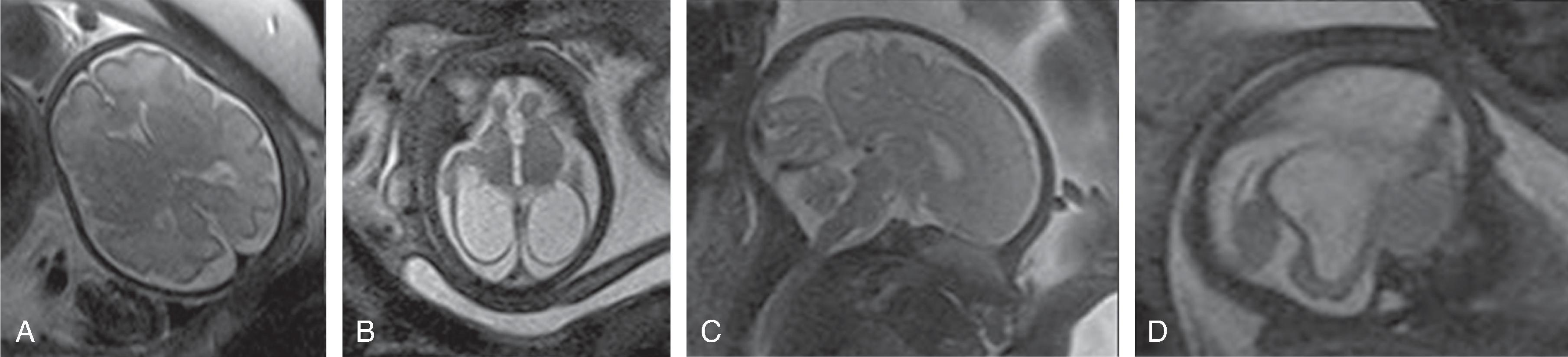
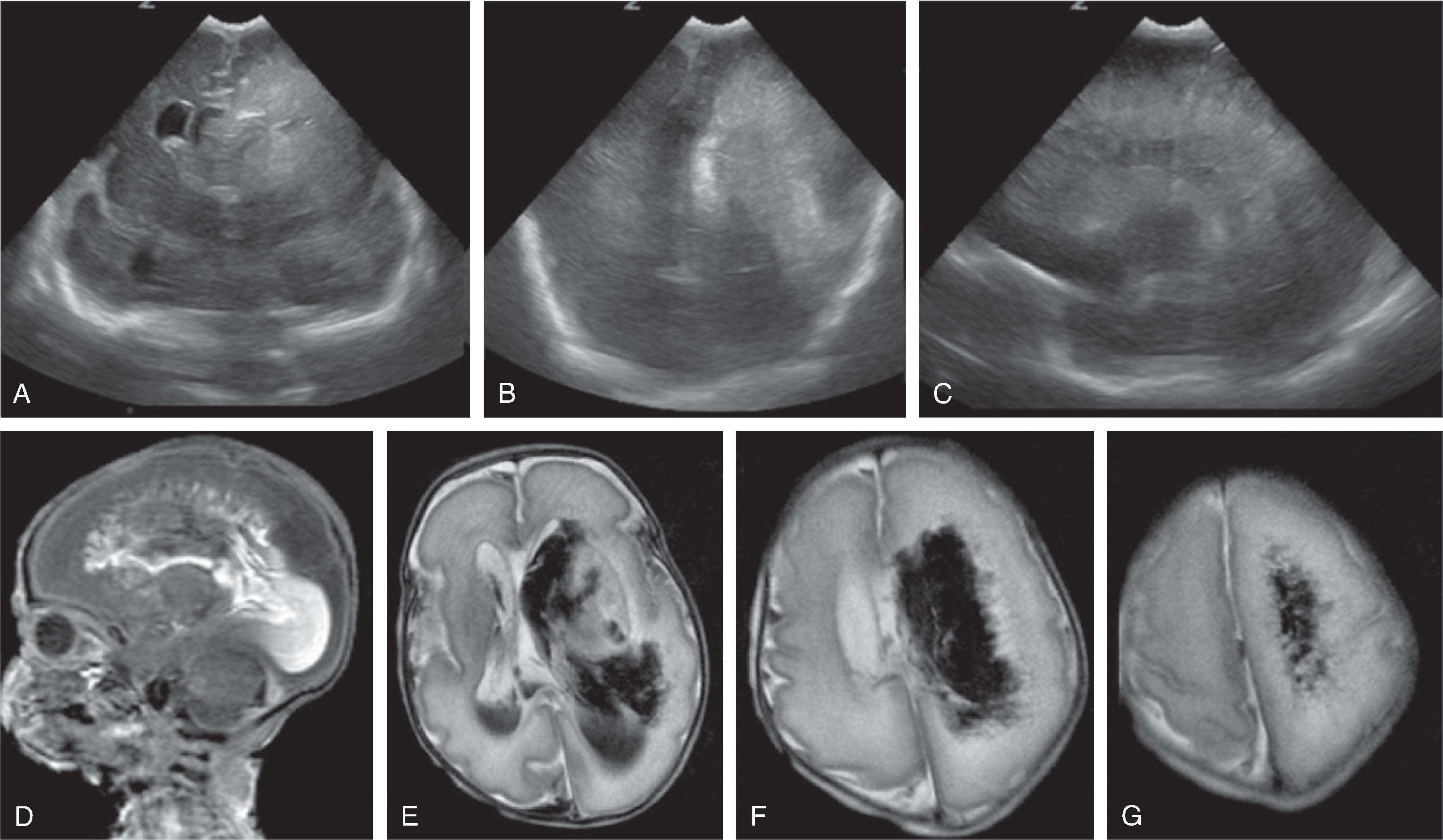
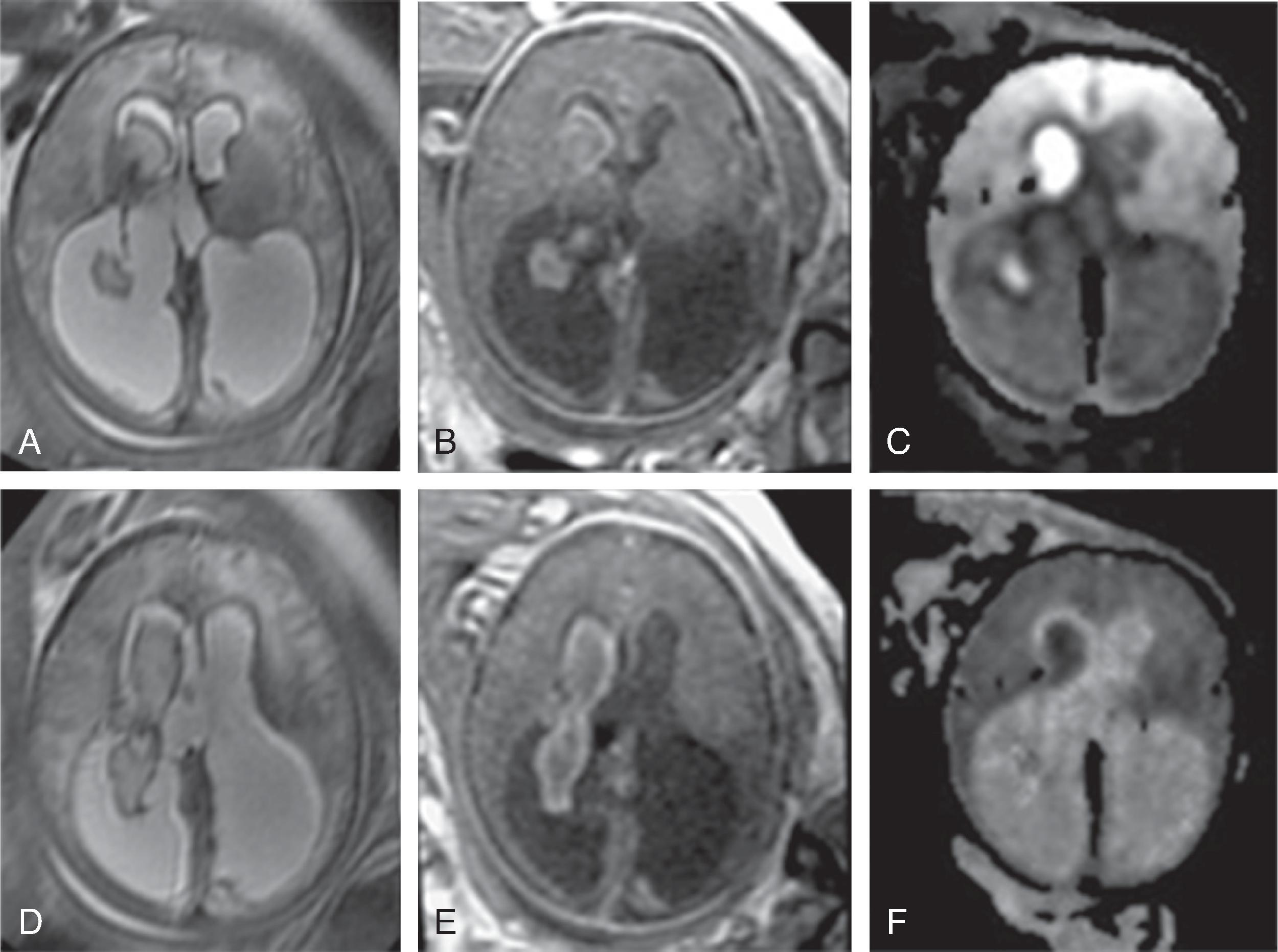
Neurologic complications include focal or global ischemic injury to the brain, cerebral atrophy, ventriculomegaly, and intracerebral hemorrhages. The exact etiology of the cerebral injury remains unclear and is likely multifactorial in origin. Hypoxic-ischemic injury due to cerebral hypoperfusion may be observed in the donor fetus, while hyperviscosity and polycythemia may be causative of cerebral injury in the recipient twin. Arterial strokes typically occur in the recipient.Imaging
Fetal MRI may show watershed ischemia, focal strokes, progressive global hemispheric white matter volume loss, ventriculomegaly, possible microcephaly, and overall reduced size of the fetus for gestational age. Intracerebral hemorrhages are less frequent.
If one fetus dies, thromboembolism over the anomalous placental connections may progressively injure the surviving twin with possible devastating outcome.
Treatment options include fetoscopic laser coagulation of the anomalous placental connections, allowing for more balanced hemodynamics. Postintervention intracerebral hemorrhages have been reported in the recipient, possibly due to hemorrhagic embolic strokes originating from the placenta.
Background
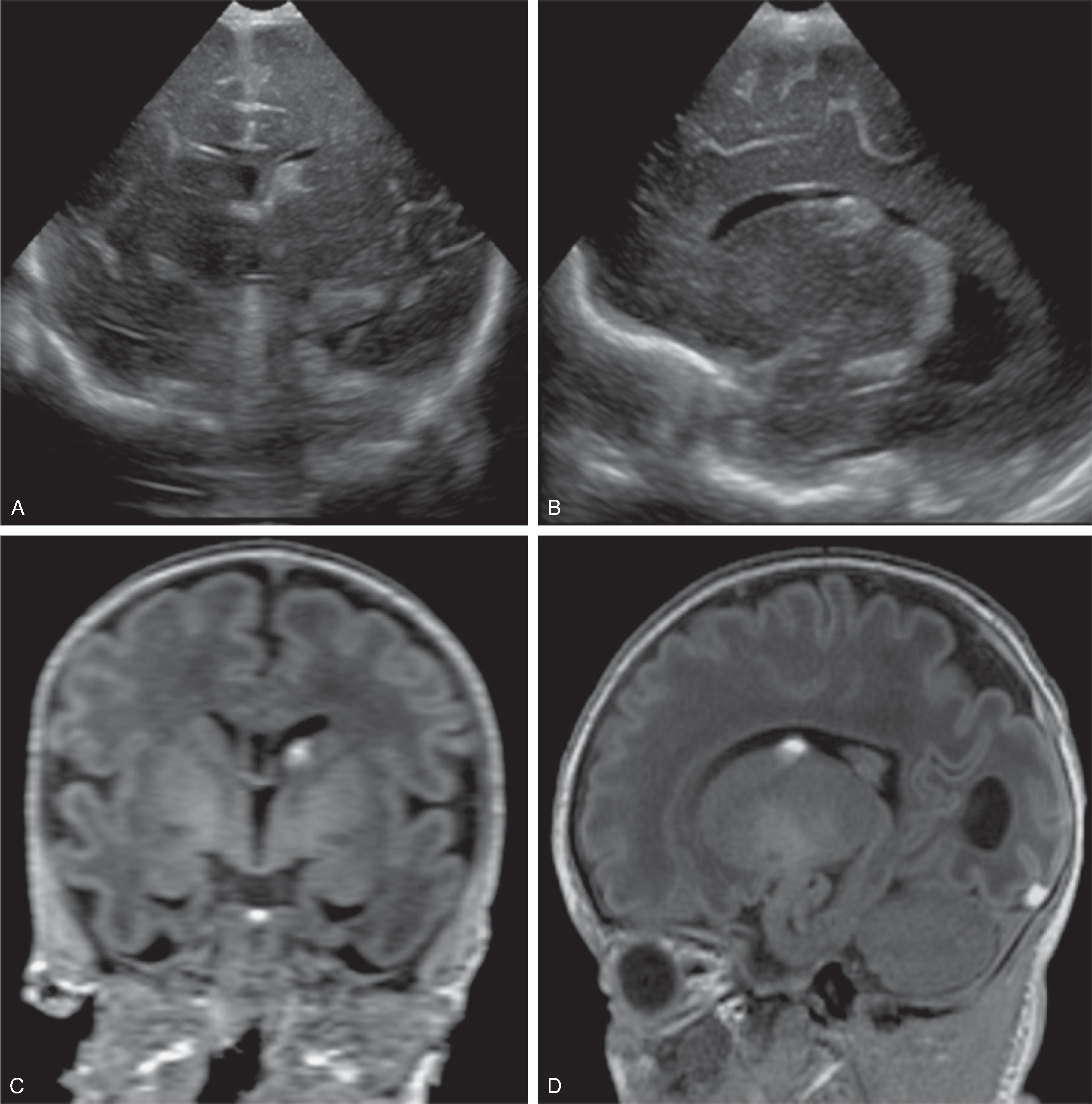
The germinal matrix is a transient, highly metabolically active, highly perfused cell layer along the ventricles from which the precursors of the cortical neurons and hemispheric glial cells originate and subsequently radially migrate peripherally in a programmed pattern.
The germinal matrix is most active during early gestation and will progressively involute with only a small residual component at the caudothalamic groove by 32–34 weeks of gestation.
The high vascularity makes the germinal matrix vulnerable for hemorrhages, especially if concomitant risk factors like congenital heart disease, sepsis, low birth weight, prolonged labor, or coagulation disorders are present.
The hemorrhage can be confined to the germinal matrix or depending on the size rupture into the ventricular system. In addition, complicating periventricular venous infarction with or without hemorrhagic conversion may occur.
Intraventricular blood products may result in obstructive supratentorial hydrocephalus due to adhesions, in particular at the level of the sylvian aqueduct.
Based upon the progressive involution of the germinal matrix, these hemorrhages typically occur in premature children and more frequently in the area of the caudothalamic groove.Imaging
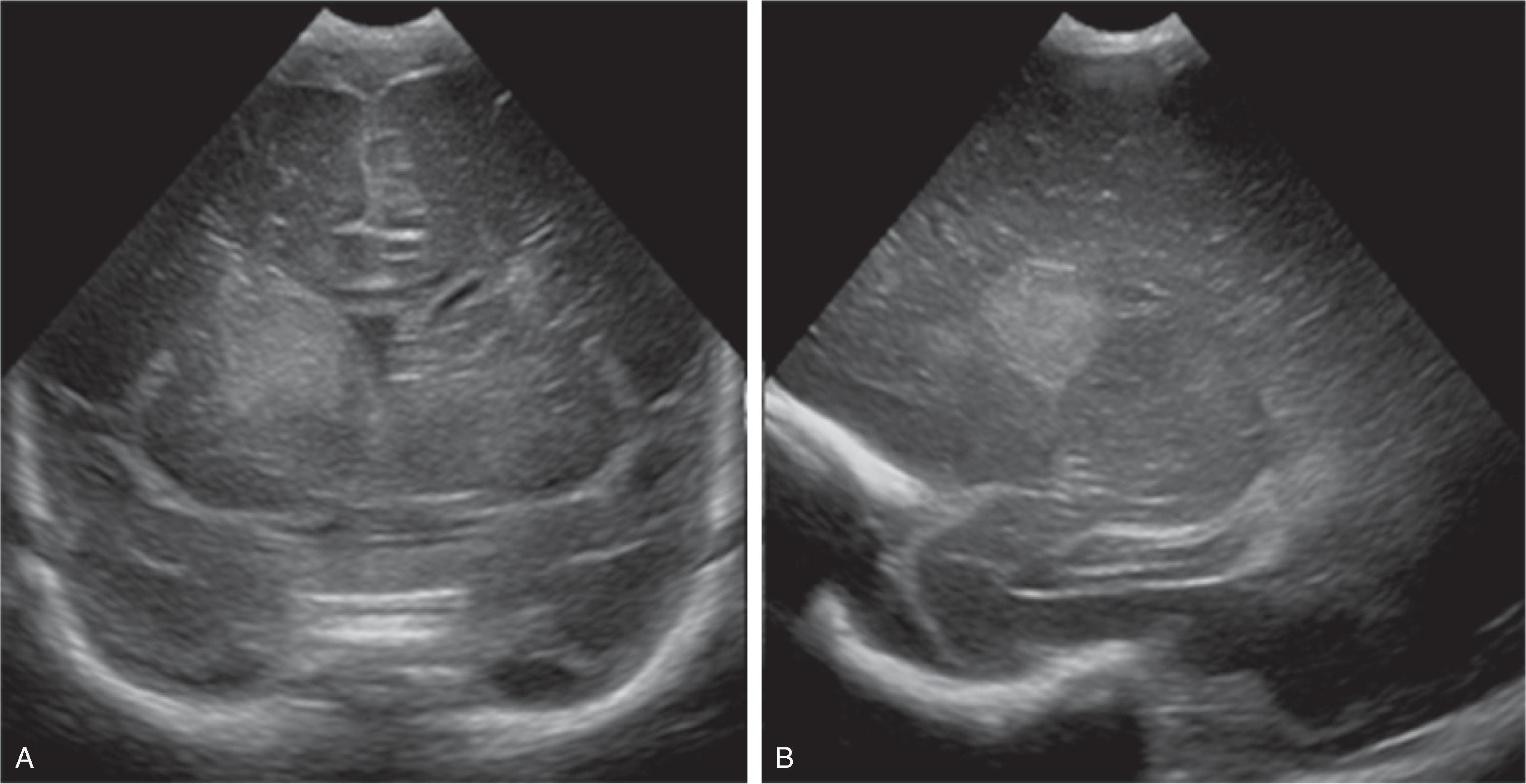
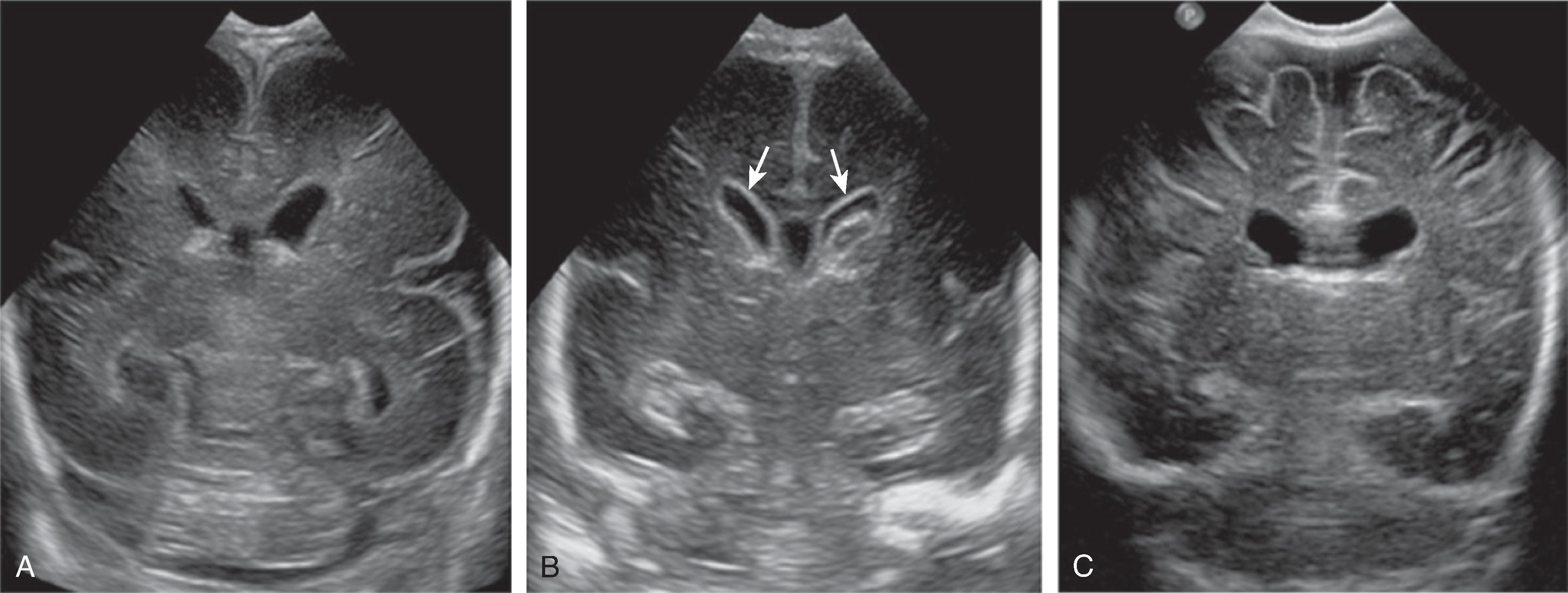
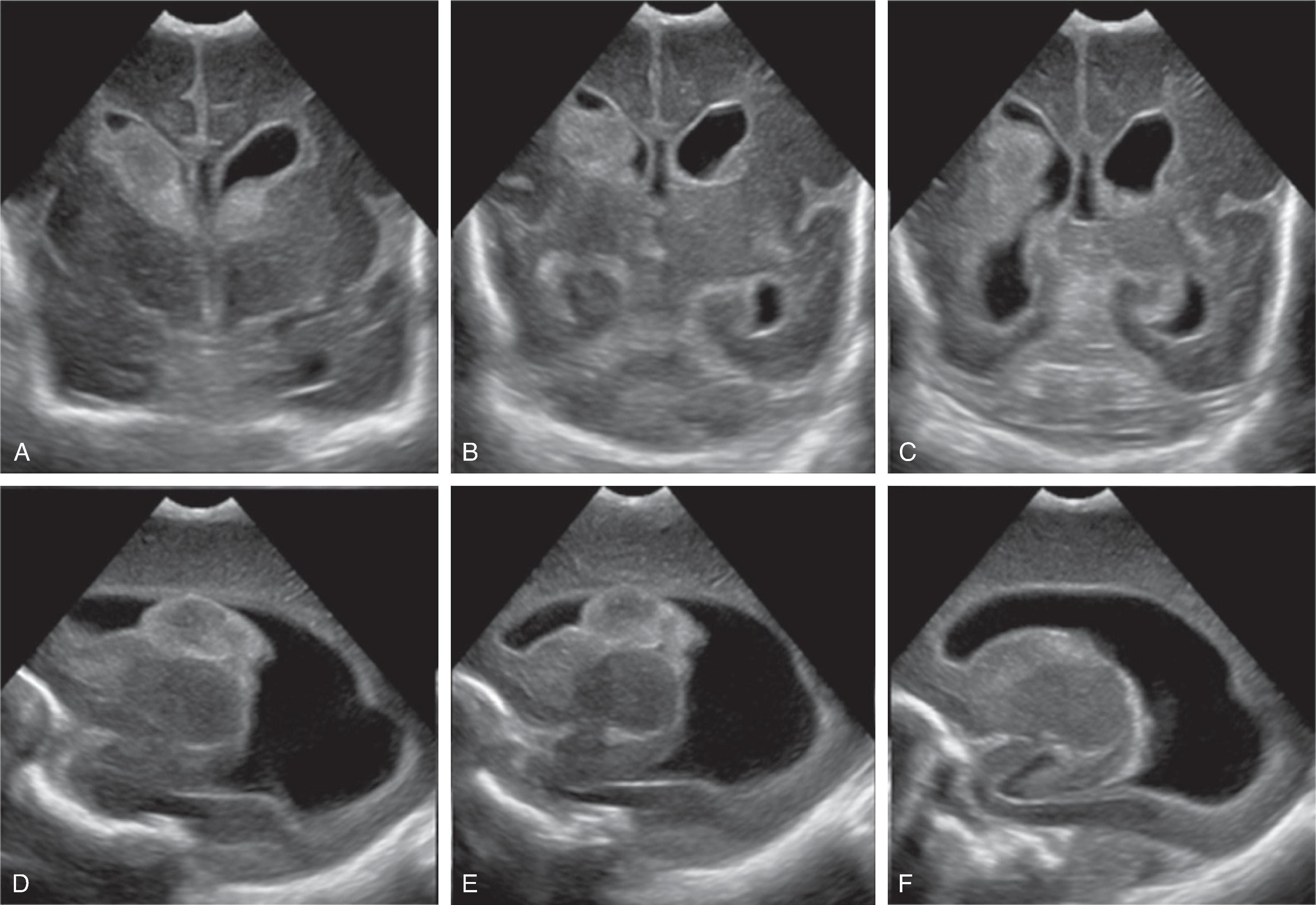
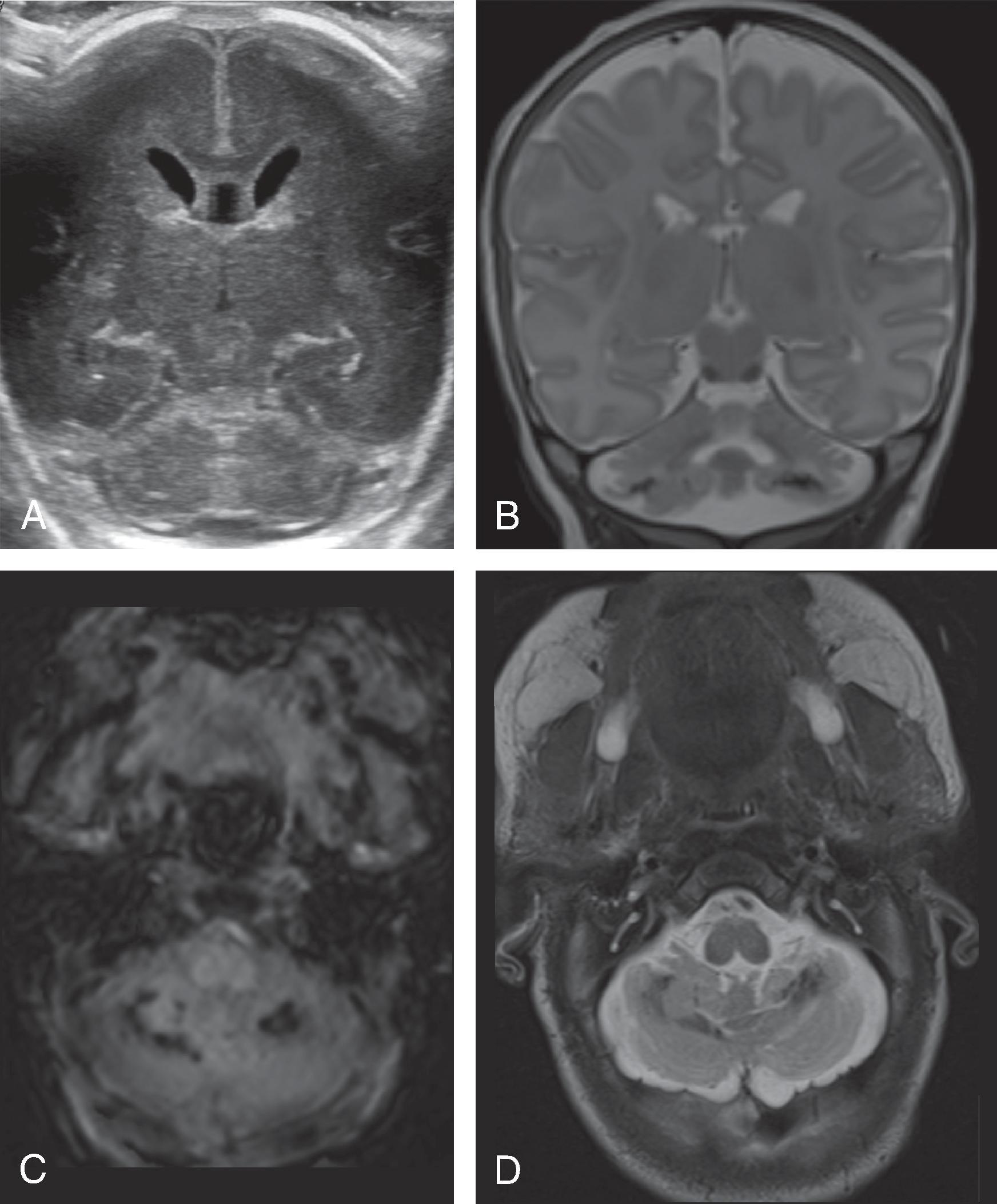
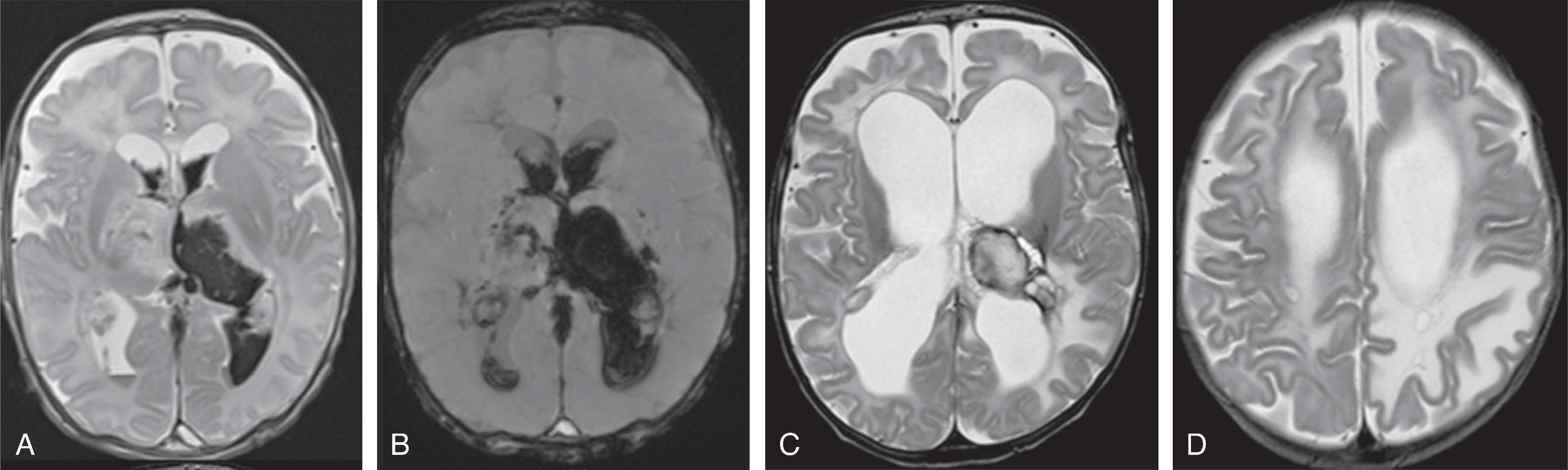
Germinal matrix hemorrhages can be classified as grades 1–3, and periventricular (hemorrhagic) venous infarction (formerly known as grade 4).
Grade 1: Hemorrhage confined to the germinal matrix
Grade 2: Hemorrhage in the germinal matrix with extension into normal-size ventricles
Grade 3: Hemorrhage in the germinal matrix with extension into enlarged ventricles
Periventricular (hemorrhagic) venous infarction is characterized by a focal hemorrhage in the germinal matrix along the course of the veins that drain the periventricular white matter. The impaired venous drainage results in venous stasis, which may evolve into a venous ischemia possibly complicated by an additional hemorrhagic conversion. On follow-up, the ischemic lesion may evolve to a porencephalic cyst incorporated into the adjacent lateral ventricle.
Differential diagnosis includes hemorrhage within the choroid plexus, which may also extend into the ventricular system.
Posterior fossa germinal matrix hemorrhage is less common than supratentorial germinal matrix hemorrhages.
Transfontanellar ultrasound is the imaging method of choice. Acute hemorrhages appear hyperechogenic and will progressively involute on follow-up examination. The center may become hypoechogenic. A hyperechogenic lining of the ventricles indicates intraventricular blood products, resulting in a chemical inflammation. A periventricular hemorrhagic venous ischemia appears in the acute phase as a fan-shaped hyperechogenicity extending from the germinal matrix into the adjacent white matter. The immediate subcortical white matter is typically spared as well as the overlying cortical ribbon because this part of the brain drains into an unaffected, functional superficial venous system.
If hydrocephalus develops, hyperechogenic blood products may be noted in the region of the sylvian aqueduct just superior to a normal-size fourth ventricle.
CT and MRI studies are rarely indicated in the acute phase; follow-up MRI may allow for better depiction of the infarcted periventricular white matter and remaining intraventricular blood products (SWI-hypointense hemosiderin staining).
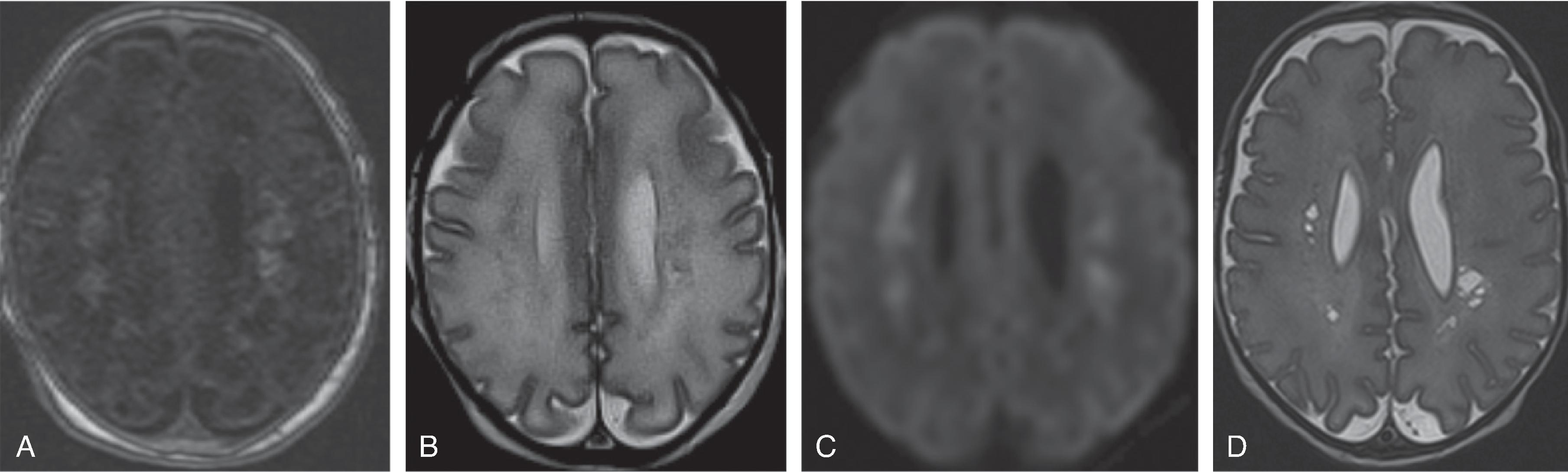
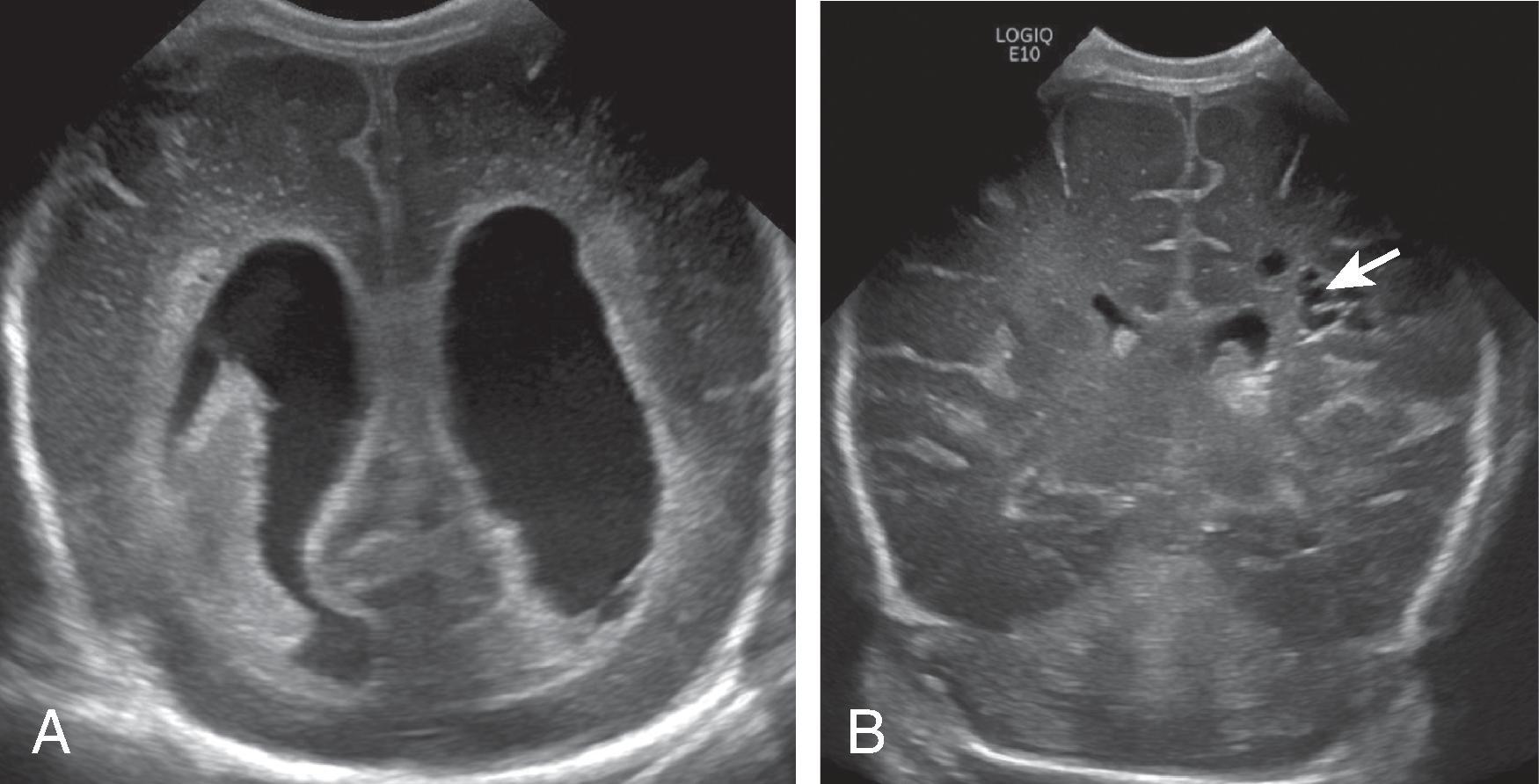
Background
Periventricular leukomalacia (PVL) is periventricular white matter injury in premature neonates that can be caused by hypoxic-ischemic injury, infections, metabolic disorders, and congenital heart disease. The periventricular white matter location has been presumed to be due to the watershed arterial region in premature infants; however, other factors may be important, including location of immature oligodendrocytes, which are susceptible to ischemic injury.
The severity of white matter injury correlates with clinical motor and cognitive outcomes.
The periatrial white matter is the most common location of PVL detected on imaging.Imaging
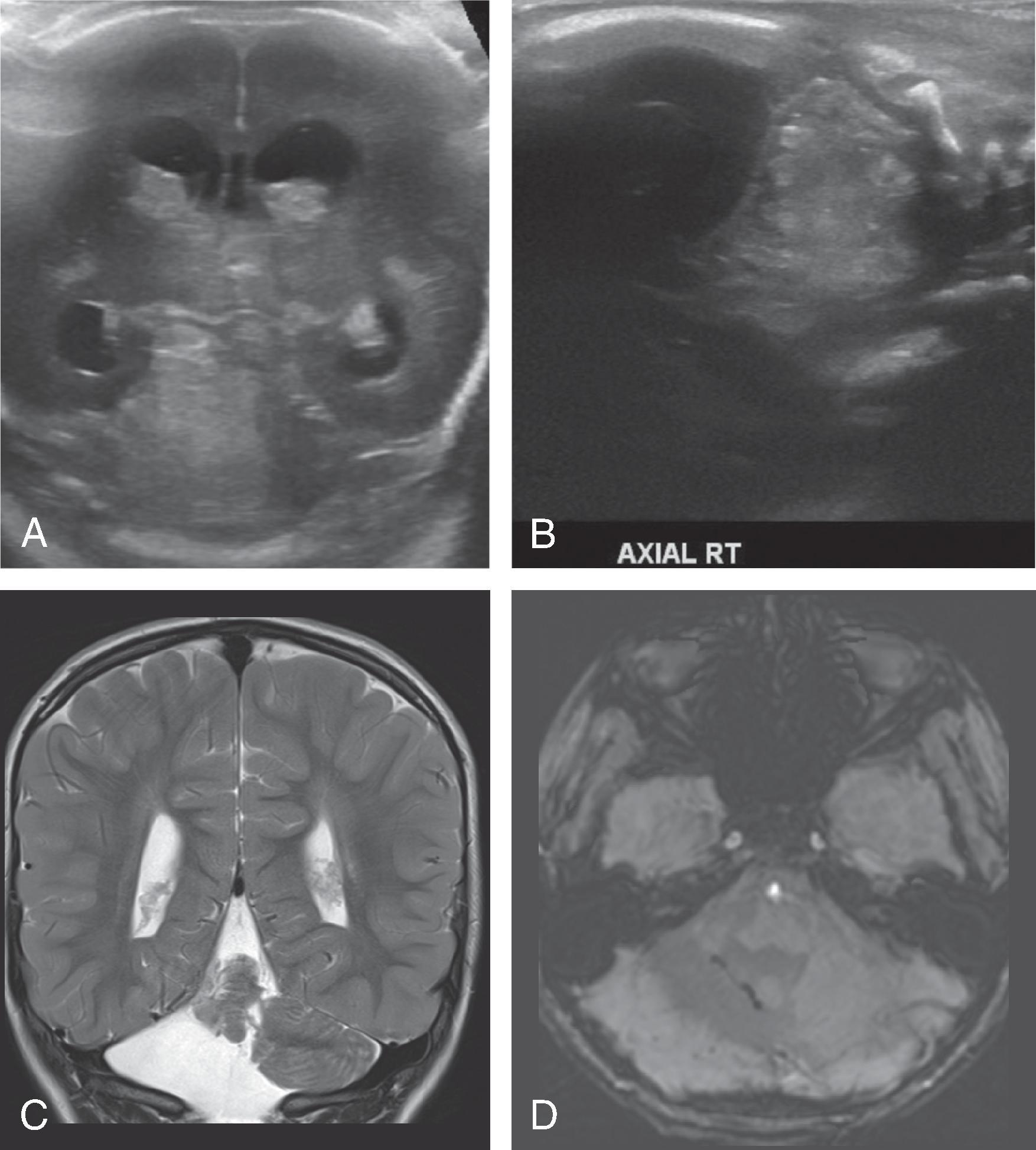
On ultrasound, PVL is suspected when the periventricular echogenicity is similar or greater than the choroid plexus. Cystic PVL appears as anechoic rounded areas adjacent to the ventricles.
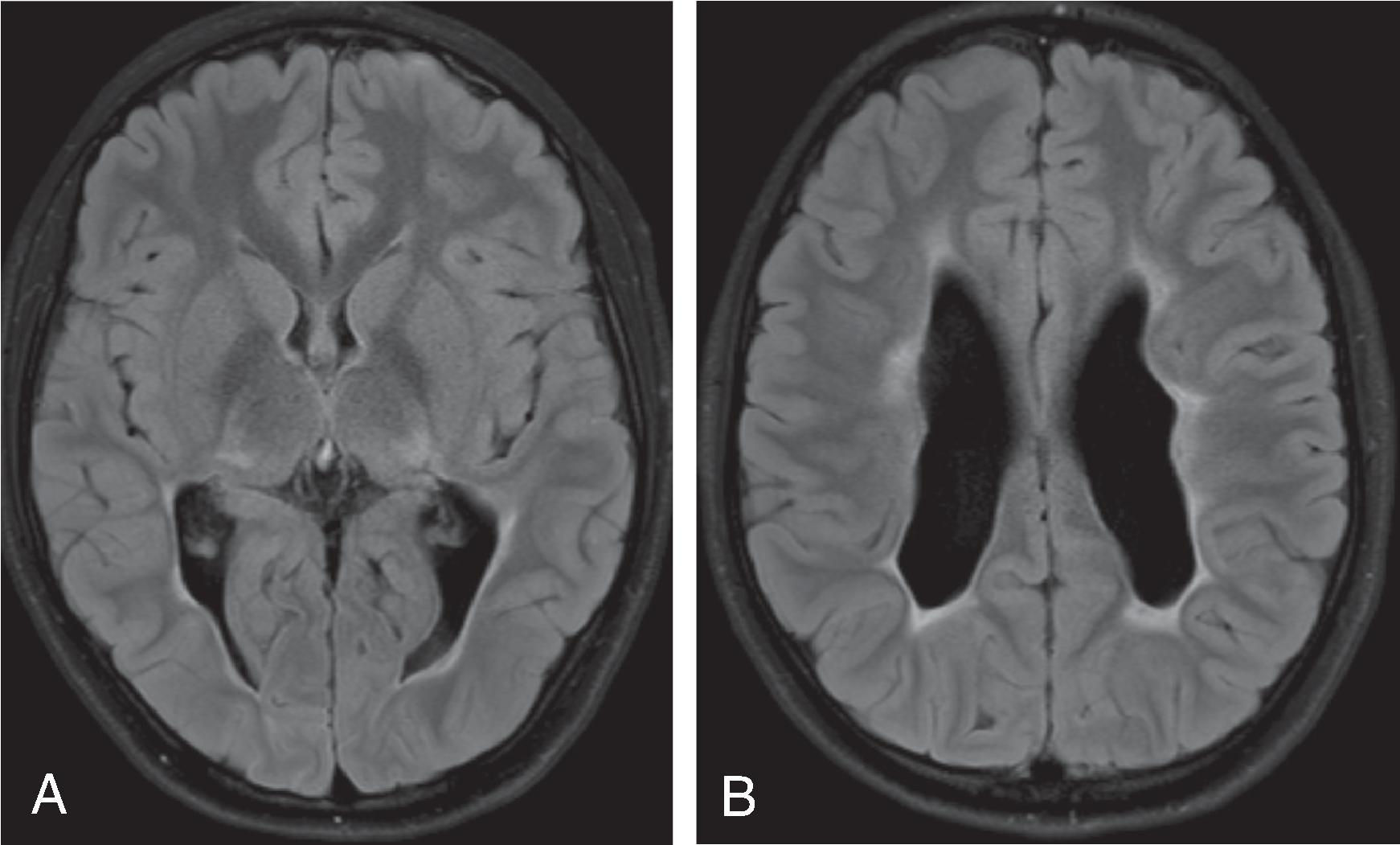
MRI is more sensitive and specific for the diagnosis of PVL than ultrasound. Acute and subacute noncystic PVL typically demonstrates focal areas of T1W hyperintense, T2W hypointense signal in the periatrial white matter. Cystic PVL demonstrates focal T2 hyperintensity. Chronic stages of PVL appear as focal or confluent periventricular T2/FLAIR hyperintensities, and depending on the degree of white matter volume loss, are associated with thinning of the corpus callous, ex vacuo dilatation of the lateral ventricles, angulated ventricular margins, thalamic volume loss, and posterior limb internal capsule T2/FLAIR hyperintensity.
Background
Extracorporeal membrane oxygenation (ECMO) is progressively being used in critically sick pediatric patients including neonates.
The indications for ECMO include life-threatening cardiopulmonary failure, congenital heart disease, congenital diaphragmatic hernia, meconium aspiration syndrome, pulmonary hypertension, respiratory distress syndrome, systemic organ failure, and sepsis.
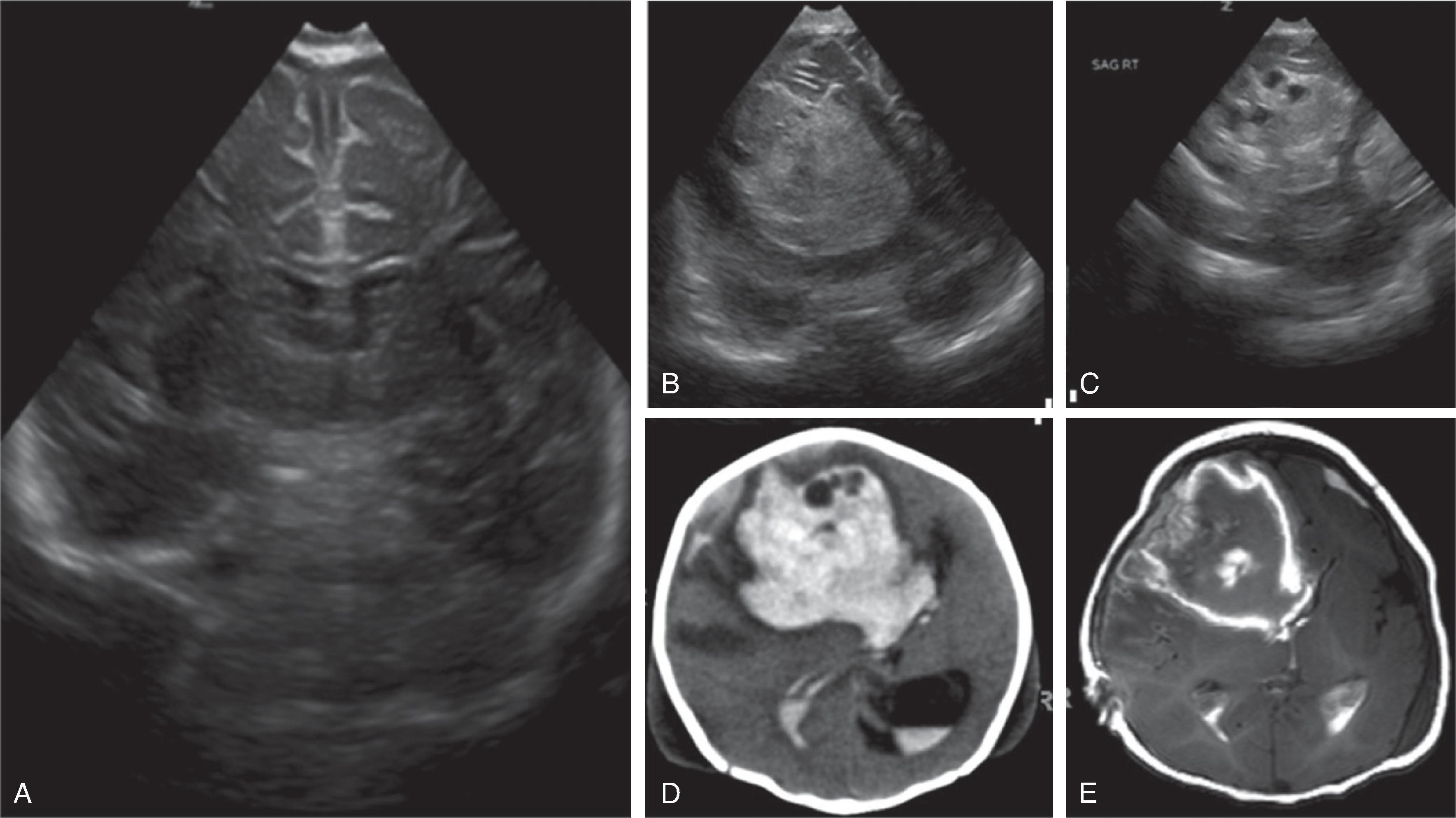
Common neurologic complications include intracerebral hemorrhage and acute stroke.Imaging
Daily transfontanellar head ultrasound (HUS) examination may be used in neonates to identify hemorrhage and stroke early.
On HUS an acute focal hemorrhage typically appears as a mass-like hyperechogenic area. The size of the bleeding can be substantial and result in compression and displacement of adjacent brain structures, including ventricles. A midline shift and possible downward herniation may occur. Most hemorrhages occur in the supratentorial brain.
Studies have suggested that the resistive index values measured within branches of the anterior circle of Willis may show an increased variability in the 24 to 48 hours prior to a hemorrhage.
Focal strokes follow the imaging characteristics of classic strokes; in the acute phase a mild to moderate hyperechogenicity is seen within arterial territories. Mild mass effect may be noted. The cortical ribbon, cerebral white matter, or central gray matter may be affected.
CT and MRI may identify the extent of the hemorrhage and/or stroke in better detail but are typically less available or contraindicated because of the ECMO system.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here