Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypertrophic cardiomyopathy is a disease process that varies broadly in its clinical presentation and has been associated with many different genetic mutations.
Obstructive disease is present in a subset of patients, where the severity of obstruction varies depending on loading conditions and adrenergic state in the individual patient.
Imaging with echocardiography and magnetic resonance imaging are primarily used to make the diagnosis.
Medical management is indicated in symptomatic patients as an initial intervention. Patients at high risk for sudden cardiac death should be considered for an implantable cardioverter-defibrillator.
If an experienced surgical team is available, myectomy is considered to be the treatment of choice in good surgical candidates when symptoms persist despite optimal medical therapy.
Alcohol septal ablation provides an excellent treatment option when surgical myectomy is not thought to be optimal for an individual patient. Careful attention to anatomic details and appropriate selection of patients are requisites for procedural success.
We thank Dr. Gus Theodos, Dr. Shikhar Agarwal, and Dr. Matthew “Casey” Becker for their contributions to earlier versions of this chapter.
By virtue of the broad variability in its phenotypic expression, hypertrophic cardiomyopathy (HCM) is a unique cardiovascular condition with a potential for the development of clinical symptoms during any phase of life. The genetic foundation of HCM has been directly related to abnormalities of the genes encoding the cardiac sarcomere unit and may result in a complex disease phenotype that encompasses a spectrum of clinical and pathologic presentations. In the past, the nomenclature regarding HCM was often misleading. Idiopathic hypertrophic subaortic stenosis or hypertrophic obstructive cardiomyopathy (HOCM) typically described only a subset of patients with this disorder. With improved understanding of the clinical heterogeneity of this process, hypertrophic cardiomyopathy appears to be a more appropriate descriptive term.
The rapid demystification of the genetic underpinnings of HCM has greatly expanded understanding of this entity. HCM is inherited in an autosomal dominant fashion, with more than 12 genes identified as being involved in the phenotypic manifestation. Two of those genes ( MYH7 and MYBPC3 ) account for approximately half of the known cases of familial HCM. Traditionally, the diagnosis of HCM has been primarily clinical, involving the use of echocardiography to evaluate for certain characteristic features such as asymmetric septal hypertrophy or systolic anterior motion of the mitral valve (SAM) with left ventricular outflow tract (LVOT) obstruction. Although there have been dramatic advances in understanding of the genetic predisposition for this disease state, the utility of genetic study for the absolute diagnosis remains preliminary. However, the future holds promise that genetics will become a more reliable tool for establishing and confirming this diagnosis. The use of genotyping in risk stratification is also evolving.
Given the heterogeneity of the disease process even within the same family, its clinical course and long-term outcomes differ significantly. Therefore management strategies span the range from close outpatient follow-up to surgical remodeling of the myocardium. HCM appears to be an evolving process in some patients, and the phenotype changes with age. This presents a challenging dilemma in terms of grasping the clinical course of this disorder. Consequently, therapeutic strategies need to be individualized for each patient.
The prevalence of this genetic disorder is estimated at 0.16% to 0.29% (≈1:625 to 1:344 individuals) in the general adult population, making it one of the more common cardiac genetic disorders known. No distinct ethnic or geographic pattern of distribution has been identified. Estimating prevalence of the disease based on imaging evidence of left ventricular hypertrophy (LVH) alone may be misleading, as phenotypic expression of the underlying genetic mutations may not occur until later in life. The clinical heterogeneity of this disorder plays into the difficulty in establishing a diagnosis. Often, the presentation lacks the classic features on echocardiography, and coexisting diagnoses that can cause myocardial hypertrophy, such as arterial hypertension and aortic stenosis, may also be present. HCM is a disease process that is known to evolve with age, and the development of LVH has been observed to occur more frequently with advancing age. This can make diagnosis of HCM challenging and suggests that repeat evaluation at periodic intervals may be required to establish a diagnosis. Although it is not routinely accounted for in general practice, it is not uncommon to see patients with HCM in tertiary referral centers.
The heterogeneity of HCM lies not only in its varied presentations but also in its natural history in the patient population. Taking all patients with HCM into account, the disease is actually relatively benign; nearly two-thirds of patients with HCM have normal lifespans and minimal or no morbidity. However, the spectrum of disease also includes a subset of patients with severe, life-limiting symptoms including heart failure, arrhythmias, syncope, chest pain, and sudden death.
Attempts to understand the links between genotype, phenotype, and natural history have yielded only limited clinical associations. Selection bias played a significant role in the initial attempts to characterize patient outcomes. Earlier studies from tertiary referral centers implied ominously high annual mortality rates of 3% to 6%; however, this work was limited by a significant referral bias. More recent data from regional and community-based centers suggested an annual mortality rate of approximately 1.5%, and mortality has been reduced to 0.5% per year with introduction of implantable cardiac defibrillators (ICDs) to the HCM population. However, in selected populations, the annual mortality rate may be as high as 5% to 6%, particularly in those symptomatic patients who are eventually referred to larger centers.
The clinical course of the HCM population is often difficult to predict and poses a challenge to clinicians. Some genotype-phenotype correlations are being revealed, though the clinical usefulness of these correlations is not yet clear. For example, there is evidence that clinical phenotypes caused by the two most common HCM genes ( MYH7 and MYBPC3 ) cause more cardiomyocyte hypertrophy and less risk of systolic dysfunction than those caused by genes affecting thin myofilament proteins (e.g., TNNT2 ).
The most feared and least predictable clinical manifestation is sudden cardiac death (SCD), particularly in the younger population. More commonly, patients develop symptoms such as angina, syncope, or exertional dyspnea. These symptoms can become progressively worse over time, and such patients can progress toward end-stage heart failure with failure of the left ventricle (LV). HCM patients may also develop atrial fibrillation (AF), putting them at risk for embolic strokes. Many HCM patients remain asymptomatic and have a comparably normal life expectancy. However, at some point even they are at risk for the development of SCD or AF. The challenge for clinicians is to closely monitor those who eventually develop symptoms and to offer timely therapy when it is indicated.
Although the spectrum of clinical presentation in HCM is large, most patients are actually asymptomatic and are diagnosed as the result of a murmur on examination, an abnormal electrocardiogram (ECG), or unexplained LVH discovered by echocardiography. The complex pathophysiologic interplay among LVOT obstruction, diastolic dysfunction, myocardial ischemia, arrhythmias, and mitral regurgitation typically results in the presenting complaints of exertional dyspnea, chest discomfort, syncope or near syncope, and SCD. Symptomatic patients who will have an adverse clinical course typically follow one of several pathways: (1) those at high risk for SCD; (2) progressive symptoms of exertional dyspnea and chest pain associated with presyncope or syncope in the setting of preserved LV function; (3) development of progressive congestive heart failure due to severe LV remodeling, which results in systolic dysfunction; and (4) consequences of supraventricular or ventricular arrhythmias such as AF or ventricular tachycardia (VT).
SCD is the most common source of mortality attributable to HCM, despite occurring in only a small minority of identified HCM patients (∼5%). In addition, SCD is the single leading cause of cardiovascular death among young people as well as the most common cause of mortality in competitive athletes. It is most commonly observed in asymptomatic children and young adults, and it appears that there is no advanced age at which the risk of SCD becomes negligible. Whereas SCD is the most feared and dramatic complication of HCM, those at high risk for SCD actually constitute only a small fraction of the disease spectrum, and much effort has been devoted to the premorbid identification of this subset of patients. Currently identified risk factors for SCD include prior cardiac arrest or sustained VT, family history of SCD, unexplained syncope or near-syncope, LV thickness greater than 30 mm, hypotensive response during exercise stress testing, and nonsustained VT on Holter monitoring ( Table 58.1 ). In addition, an LVOT gradient greater than 30 mm Hg has been associated with an increased risk of SCD, progression to heart failure, and morbidity related to arrhythmia, including stroke. However, an incremental increase in the subaortic gradient above 30 mm Hg has not been demonstrated to impart any additional risk. It is uncommon for HCM patients to suffer SCD without at least one of the aforementioned risk factors (<3%), though risk of SCD does not differ with respect to number of risk factors present. There does appear to be a subset of HCM patients without the aforementioned conventional risk factors who are at risk for SCD, underscoring the importance of identifying additional markers of risk. In this regard, it has been shown that extensive late gadolinium enhancement (LGE) by cardiac magnetic resonance imaging (CMRI) is associated with increased risk of SCD in HCM, including in patients without traditional risk factors. LV apical aneurysm, elevated LVOT gradient, and end-stage heart failure with systolic dysfunction and ventricular remodeling may represent other SCD risk factors. It has been suggested that the etiology of SCD in this population is related to the development of complex ventricular tachyarrhythmia, often during mild to moderate physical exertion and with a circadian predilection for the early morning hours.
|
Chest pain, both typical and atypical in character, is a common feature in HCM and has been reported in up to 80% of patients in this population. In many cases, angiography reveals normal coronary arteries. Despite this finding, numerous studies incorporating nuclear single-photon emission computed tomography (SPECT), positron emission tomography (PET), and magnetic resonance imaging (MRI) technologies have demonstrated significant reversible and nonreversible myocardial perfusion defects in this subset of patients, including autopsy findings of myocardial infarction in up to 15% of such patients.
Collectively, these data have led to a mounting body of evidence suggesting that microvascular dysfunction may have a pivotal role in the development of myocardial ischemia and infarction in this group. The etiology of microvascular dysfunction is probably multifactorial and due in part to arteriolar medial hypertrophy, which results in reduced luminal diameter, impaired coronary vasodilatory response, and a supply-demand mismatch due to an abnormally thickened ventricle. In addition, early work has suggested that evidence of microvascular dysfunction, as demonstrated by PET, is an independent predictor of increased mortality and may portend a worse prognosis years before the development of clinical deterioration.
Syncope in patients with HCM is not an uncommon phenomenon and has a diverse array of possible etiologies, making the exact determination of mechanism challenging. Whereas it is regarded as an ominous prognostic sign and a known risk factor for SCD in the younger population, syncope in the adult population has not been independently associated with premature death, and recurrent episodes are rarely reported in patients who have suffered SCD. Arrhythmic sources of syncope may be supraventricular (e.g., AF or flutter) or ventricular (e.g., VT or fibrillation). Hemodynamic mechanisms of syncope all result in a sudden and severe reduction in cardiac output that may involve ischemia, outflow tract obstruction, or severe diastolic dysfunction. Additionally, it has been suggested that activation of LV baroreceptors due to elevated intracavitary pressures may induce reflex hypotension and a consequent syncopal episode in a selected subgroup of patients.
Heart failure—as manifested by a symptom complex of exertional dyspnea, orthopnea, and progressive fatigue—is most commonly encountered in adult patients with HCM, but it has been described in the juvenile population as well. Usually, in the setting of preserved systolic function, symptoms are most commonly the consequence of diastolic dysfunction due to an abnormally thickened and noncompliant ventricle. The combined influence of other variables such as ischemia, AF, and mitral regurgitation may also play a significant role in the development of hemodynamic decompensation in this population. A smaller number of patients with HCM and heart failure may have significantly reduced LV systolic function and chamber enlargement. It is important to recognize this subset of patients, given the potential alteration in therapeutic strategy.
AF complicates the course of approximately 20% of patients with HCM and is associated with an increased risk of heart failure–related death. The risk seems to be substantially greater in the subset of patients with outflow tract obstruction or an earlier onset of arrhythmia (<50 years of age). Advanced age, left atrial enlargement, and congestive symptoms are independently linked with the development of AF. Although it is strongly associated with an increased risk of fatal and nonfatal stroke, AF does not appear to be a risk factor for the development of SCD, and approximately one-third of patients have no long-term sequelae from this arrhythmia.
Severe functional deterioration due to dyspnea, chest pain, palpitations, or pulmonary edema may complicate the course of the chronically affected. This is most likely caused by the loss of atrial contraction, reduction in diastolic filling time, and exacerbation of underlying ischemia.
The nature of the clinical presentation may also be affected by a particular patient’s age or gender. In contrast to their younger counterparts, older adult patients with HCM often develop marked symptomatology at an advanced age (>55 years), have lesser degrees of LVH usually confined to the septum, and have a dynamic subaortic gradient due to restricted excursion of the often anteriorly displaced mitral leaflets and posteriorly directed septal motion. Whereas HCM seems to have a male predominance, female patients often are diagnosed at a later age, are more symptomatic, and are at a greater risk of death due to heart failure or stroke.
Given its safety and ubiquity, two-dimensional echocardiography is the most common method for establishing the clinical diagnosis of HCM via identification of a thickened, nondilated LV in the absence of comorbidities known to cause such a degree of LVH (i.e., hypertension, aortic stenosis, or physiologic hypertrophy of athletes). Although LVH was classically thought to involve primarily the ventricular septum, its morphologic expression is extremely heterogeneous, and virtually any pattern of thickening may be observed. In addition, there are significant differences in the pattern of hypertrophy between young and elderly patients. Elderly patients are often found to have an elliptical ventricular cavity with hypertrophy predominantly of the basal septum. In contrast, younger patients (<55 years) often have a crescent-shaped ventricular cavity associated with diffuse hypertrophy of the interventricular septum.
Whereas a maximal wall thickness greater than 15 mm is the traditional echocardiographic benchmark for HCM, the degree of hypertrophy exhibits considerable variability (with a mean thickness of approximately 22 mm). It is important to realize, however, that lack of characteristic LVH (>15 mm) on echocardiographic examination does not exclude the presence of an HCM gene mutation. Therefore serial echocardiographic assessment may be necessary for adequate identification of suspected carriers, especially in the younger population, in whom the development of LVH may be delayed until after puberty.
LVOT obstruction is observed in approximately two-thirds of patients with HCM and is usually dynamic in nature. Subaortic obstruction is due to SAM of the anterior mitral leaflet, which results in mitral-septal contact during midsystole. Obstruction may not be present under resting conditions but can be provoked by pharmacologic (i.e., amyl nitrite) or physiologic (i.e., Valsalva) maneuvers. Significant mitral regurgitation frequently accompanies SAM owing to distortion of the valvular apparatus and malcoaptation of the anterior and posterior leaflets ( Figs. 58.1 and 58.2 ).
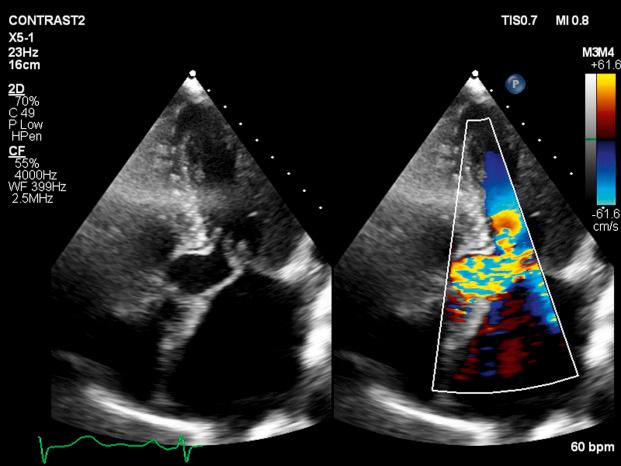
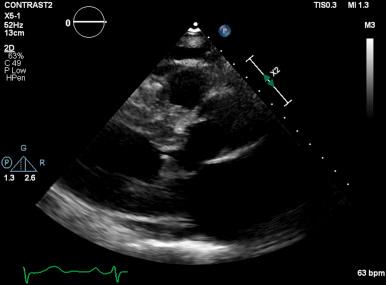
Primary abnormalities of the mitral valve and associated structures are also commonly observed in HCM, including leaflet elongation, papillary muscle hypertrophy, and abnormal origins or insertions of papillary muscles. Mitral regurgitation is also observed in up to 30% of patients who do not demonstrate obstructive physiology; it is primarily caused by leaflet prolapse, chordal rupture, or trauma resulting in calcification or fibrosis. Less commonly, a midcavitary gradient is formed because of the anomalous insertion of the anterolateral papillary muscle directly onto the anterior mitral leaflet or an exaggerated proliferation of midventricular papillary musculature coming into apposition with the ventricular septum.
Whereas the threshold for therapeutic intervention has traditionally been a gradient of greater than 50 mm Hg, it has been demonstrated that the presence of a resting LVOT gradient of 30 mm Hg or greater is an independent predictor of death from heart failure or stroke, progression of heart failure symptoms, and reduced functional capacity as well as SCD. It is important not to misinterpret the Doppler spectral display of mitral regurgitation as an LVOT gradient, given its frequent presence in the setting of obstruction and its close spatial orientation to the LVOT. In the setting of SAM, mitral regurgitation is usually posteriorly directed into the left atrium and is often difficult to distinguish from LVOT flow. It is most useful to sweep anterior to posterior with continuous Doppler imaging to distinguish these two flows.
Given the magnitude of LVH associated with HCM, it is not surprising that more than 80% of patients have evidence of diastolic dysfunction by echocardiogram. This is manifested by reduced maximal flow velocity in early diastole, an increase in isovolumic relaxation time, and an increased atrial contribution to ventricular filling. These findings are similar in patients with and without an LVOT gradient or cardiac symptoms, suggesting that diastolic dysfunction may be an earlier clinical manifestation in the spectrum of this disease process. Several studies have suggested that the presence of significant diastolic dysfunction by transthoracic or tissue Doppler echocardiography implies an increased risk of cardiac arrest, VT, or progression to significant cardiac symptoms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here