Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The serum sodium concentration ([Na + ]) is the ratio of sodium to water in the extracellular fluid (ECF) compartment. It is determined by the relationship among total body sodium, potassium, and water, the last of which typically is the main determinant. Dysnatremias, or derangements in serum [Na + ], include both hyponatremia and hypernatremia. This chapter focuses on the etiology and management of hypernatremia, a hyperosmolar state defined by serum [Na + ] exceeding 145 mEq/L.
Hypernatremia itself is not a disorder but rather a laboratory abnormality caused by an underlying pathologic process. The diagnostic approach to hypernatremia must consider the determinants of the serum [Na + ]. Although excesses in total body sodium and, very rarely, excesses in total body potassium can lead to hypernatremia, the majority of cases result from deficits in total body water (TBW).
To simplify the management of hypernatremia, this chapter considers the balance of TBW and sodium separately. This important concept will be illustrated by dividing the water and sodium components of the ECF compartment into separate theoretical beakers ( Fig. 8.1 ). The goal is to determine appropriate, timely therapies for a disorder that is associated with a high degree of morbidity and mortality.
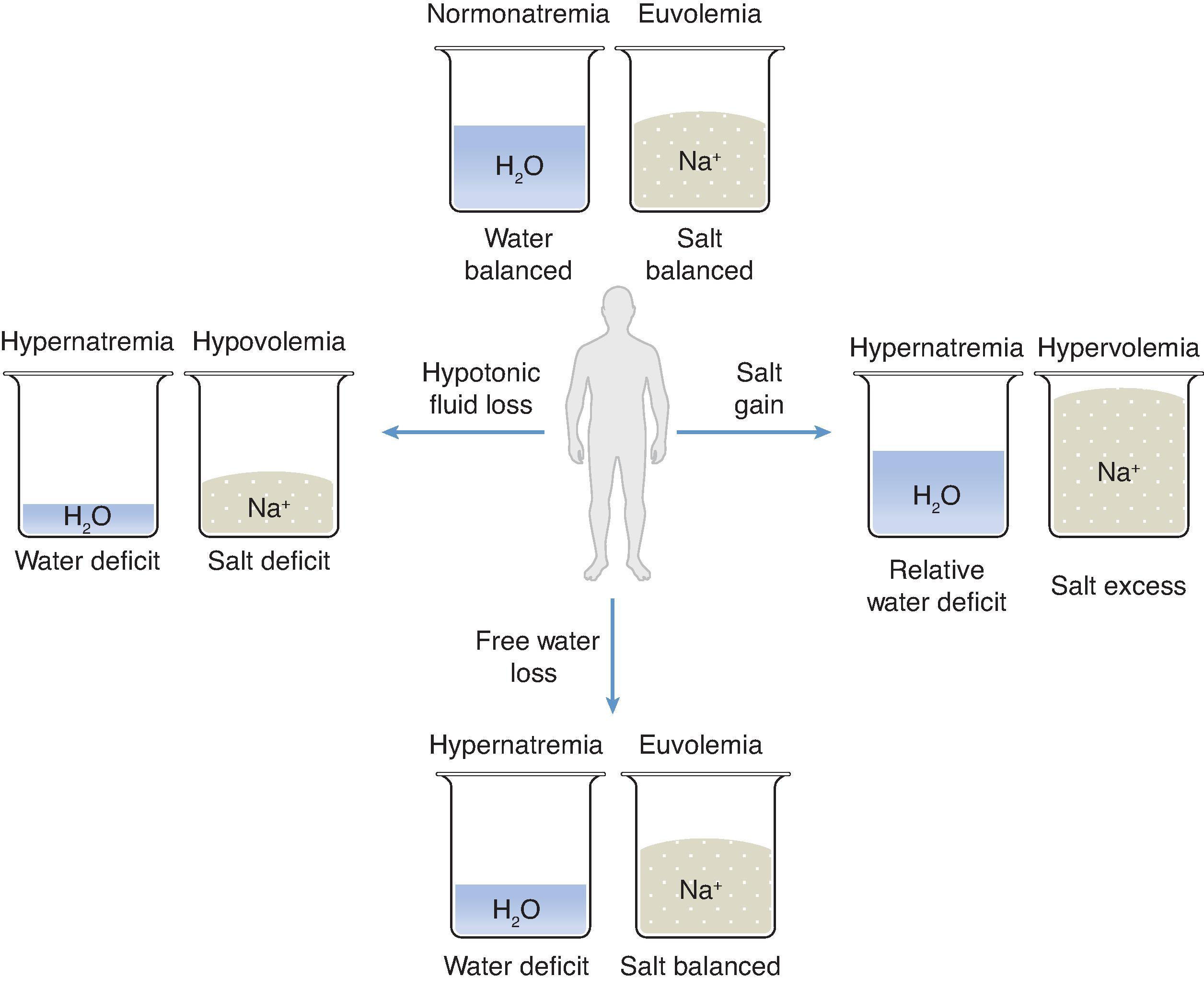
Normal serum [Na + ] is 135 to 145 mEq/L. This range is generally maintained despite large individual variations in salt and water intake. Hypernatremia is always a water problem and sometimes also a salt problem. There is no predictable relationship between serum [Na + ] (a measure of osmolality and tonicity) and total body salt or volume status. More specifically, although hypernatremia confirms the presence of a relative water deficit, the isolated laboratory finding of a serum [Na + ] greater than 145 mEq/L does not reveal anything about a person’s volume status. As a result, hypernatremia may occur in the setting of hypovolemia, euvolemia, or hypervolemia, as indicated in Fig. 8.1 .
Comparing the terms “dehydration” and “volume depletion” illustrates the distinction between water and sodium balance. Although dehydration is commonly used to describe a person’s volume status, this use is inaccurate. Dehydration does not equal volume depletion. Rather, dehydration is a description of water balance, whereas volume depletion refers to a person’s sodium balance. Although these two clinical scenarios may coexist, they should not be confused. It is important that the two terms are not used interchangeably and that a patient’s water and sodium balance be considered independently when addressing dysnatremias clinically.
Hypernatremia always reflects a hyperosmolar state, whereas the reverse is not always true. For example, hyperosmolality may also be a consequence of severe hyperglycemia or elevated blood urea nitrogen, as is seen in kidney failure. Furthermore, hyperosmolality does not necessarily mean hypertonicity. For example, uremia is a hyperosmolar but not a hypertonic state. Urea is an ineffective osmole because it can freely cross cell membranes, unlike sodium; therefore, urea contributes to osmolality but not to tonicity. In contrast to urea, sodium, which is unable to cross cell membranes freely, is an effective osmole and is the primary cation that affects plasma osmolality (P osm ). Hypernatremia is a hypertonic state in which water will flow from the intracellular to the extracellular space, resulting in cellular dehydration and shrinkage.
Hypernatremia is all about water. A more accurate term for hypernatremia might be “hypoaquaremia” because it literally means a state in which there is too little water in the intravascular space and, therefore, too little water in the intracellular space. To begin any discussion of hypernatremia (or hyponatremia), it is important to understand that dysnatremias are actually disorders of water homeostasis.
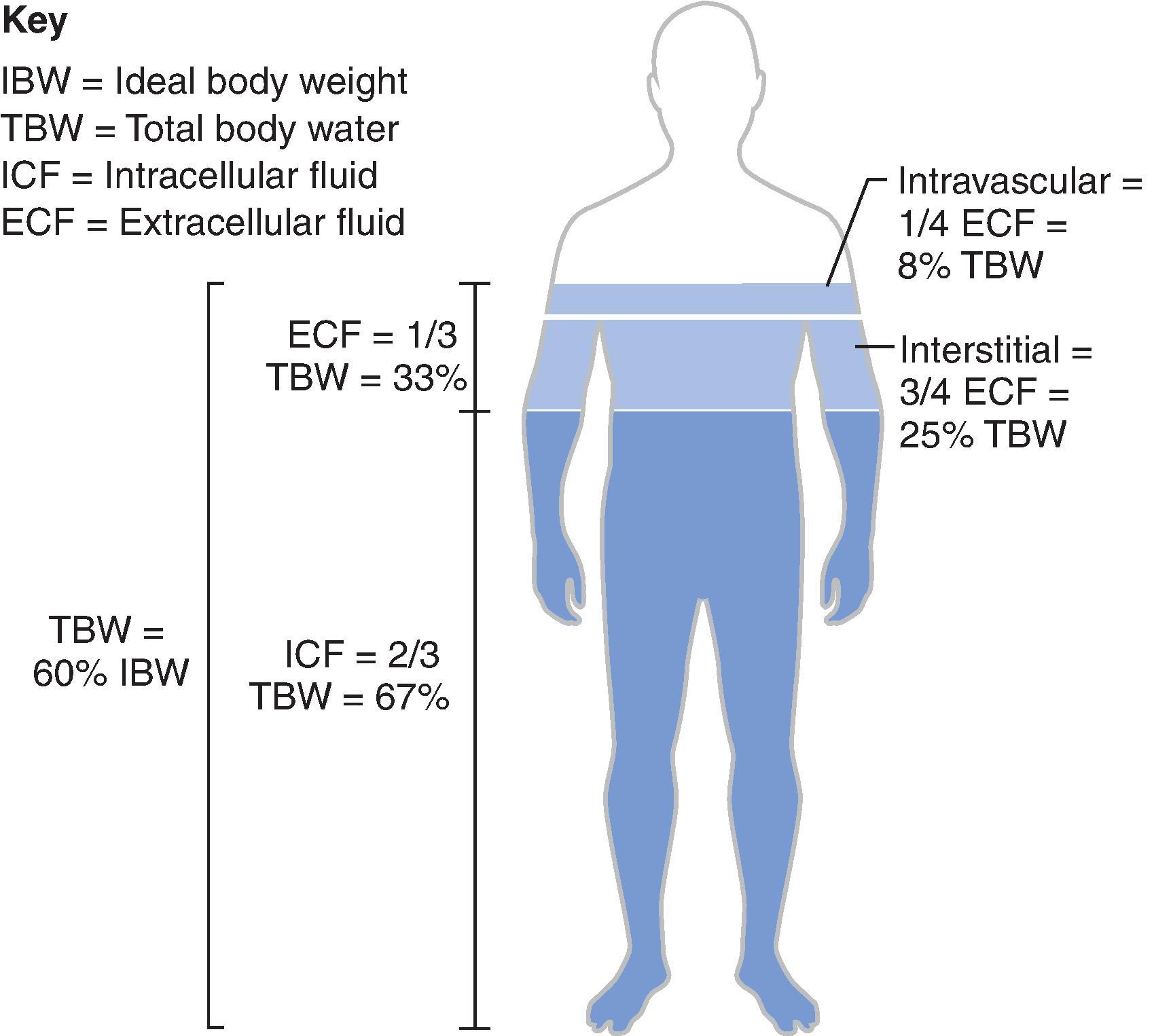
Water distributes throughout all body compartments, with two-thirds in the intracellular and one-third in the extracellular compartment. Three-quarters of the water in the extracellular compartment is located in the interstitial space, and one-quarter is in the intravascular space ( Fig. 8.2 ). Water is lost (or gained) in the same proportions as it is distributed throughout all body compartments. Pure water loss does not affect plasma volume status or hemodynamics significantly until very late because of the normal distribution of water throughout all body compartments. For example, for every 1 L of water deficit, only approximately 80 mL is lost from the intravascular (plasma) compartment.
The incidence of hypernatremia in all hospitalized patients ranges from less than 1% to approximately 3%. However, in critically ill patients, the overall prevalence of hypernatremia ranges between 9% and 26% and is hospital acquired in approximately 80% of these cases. Hypernatremia present at the time of hospital admission is primarily a disease of older adults and of those with mental illness or impaired sensorium. Most patients with hypernatremia on admission to the hospital have concomitant infections. Hypernatremia that is present on hospital admission is generally treated earlier than hypernatremia that develops during the hospital course, most likely because of increased attention paid to individual laboratory values and volume status on hospital admission.
In contrast, hospital-acquired hypernatremia is typically seen in patients who are younger than those with hypernatremia on admission, with an age distribution similar to that of the general hospitalized population. Hospital-acquired hypernatremia is largely iatrogenic from inadequate and/or inappropriate fluid prescription and, therefore, is largely preventable. It results from a combination of decreased access to water, disease processes that may increase insensible losses or interfere with the thirst mechanism, and administration of loop diuretics. Approximately half of patients with hospital-acquired hypernatremia are intubated and, therefore, have no free access to water. Of the remainder, most have altered mental status.
Patients at highest risk for hospital-acquired hypernatremia are those at the extremes of age (infants and older adults), those with altered mental status, and those without access to water (i.e., intubated or debilitated patients). In addition to the impaired thirst and decreased urinary concentrating ability that accompany advanced age, older patients have a lower baseline TBW content, making smaller changes in water balance more clinically relevant.
Clinical symptoms related to hypernatremia can be attributed to cellular dehydration and shrinkage due to the loss of intracellular water. Loss of intracellular water occurs throughout the body, but the primary symptoms are neurologic. The severity of neurologic symptoms is more dependent on the rate of rise in serum [Na + ] than on the absolute value. Polyuria and polydipsia are frequently the presenting symptoms of diabetes insipidus (DI), with or without the presence of hypernatremia.
Neurologic symptoms comprise a continuum that begins with fatigue, lethargy, irritability, and confusion and progresses to seizures and coma. Additional symptoms of hypernatremia include anorexia, nausea, vomiting, and generalized muscle weakness. Altered mental status can be both a cause and an effect of hypernatremia, and consequently can be difficult to distinguish clinically. In addition, cellular dehydration and shrinkage can lead to rupture of cerebral veins because of traction, which results in focal intracerebral and subarachnoid hemorrhages; this occurs more often in infants than in adults.
Signs of hypernatremia depend, in part, on its cause and severity. Abnormal subclavicular and forearm skin turgor and altered sensorium are commonly found in patients with hypovolemic or euvolemic hypernatremia, whereas patients with hypervolemic hypernatremia typically have classic signs of volume overload, such as elevated neck veins and edema.
A sound understanding of the normal physiology of water and salt balance is integral to the understanding and management of dysnatremias. The intracellular and extracellular body compartments exist in osmotic equilibrium. The development of hypernatremia is most commonly the result of increased water losses combined with inadequate intake. Rarely does hypernatremia occur as a consequence of excessive sodium intake.
Regulation of plasma [Na + ] is determined by the regulation of P osm , of which plasma [Na + ] is the primary determinant. Normal P osm is between 285 and 295 mOsm/kg. If the P osm varies by 1% to 2% in either direction, physiologic mechanisms are in place to return the P osm to normal. In the case of hypernatremia or hyperosmolality, receptor cells in the hypothalamus detect increases in P osm ; in response, they stimulate thirst to increase water intake and simultaneously stimulate antidiuretic hormone (ADH) release to limit urinary water losses (by increasing water reabsorption in the collecting duct). Under normal conditions, the body is able to maintain the serum osmolality under tight control. The goal of “normonatremia” is to avoid changes in cellular volume and thereby prevent potential disruptions in cellular structure and function. The body’s normal physiologic defense against hypernatremia is twofold: an endogenous thirst stimulus and renal conservation of water.
As with other electrolyte disturbances, the pathophysiology of hypernatremia can be easily categorized into two phases—an initiation phase and a maintenance phase. Simply stated, the initiation, or generation, phase must be caused by a net water loss or, less commonly, a net sodium gain. For hypernatremia to exist as anything more than a transient state, there must be a maintenance phase, defined necessarily by inadequate water intake.
Water metabolism is primarily controlled by arginine vasopressin (AVP) or ADH, as it is commonly termed. ADH is produced in the hypothalamus (supraoptic and paraventricular nuclei) and is stored in and secreted by the posterior pituitary. ADH release can be stimulated by either increases in P osm or decreases in mean arterial pressure or blood volume. In the setting of hypernatremia, the primary stimulus for the release of ADH comes from osmoreceptors located in the hypothalamus. ADH acts on the vasopressin type 2 (V 2 ) receptors in the collecting duct to cause increased water reabsorption from the tubular lumen via insertion of aquaporin-2 channels ( Fig. 8.3 ).
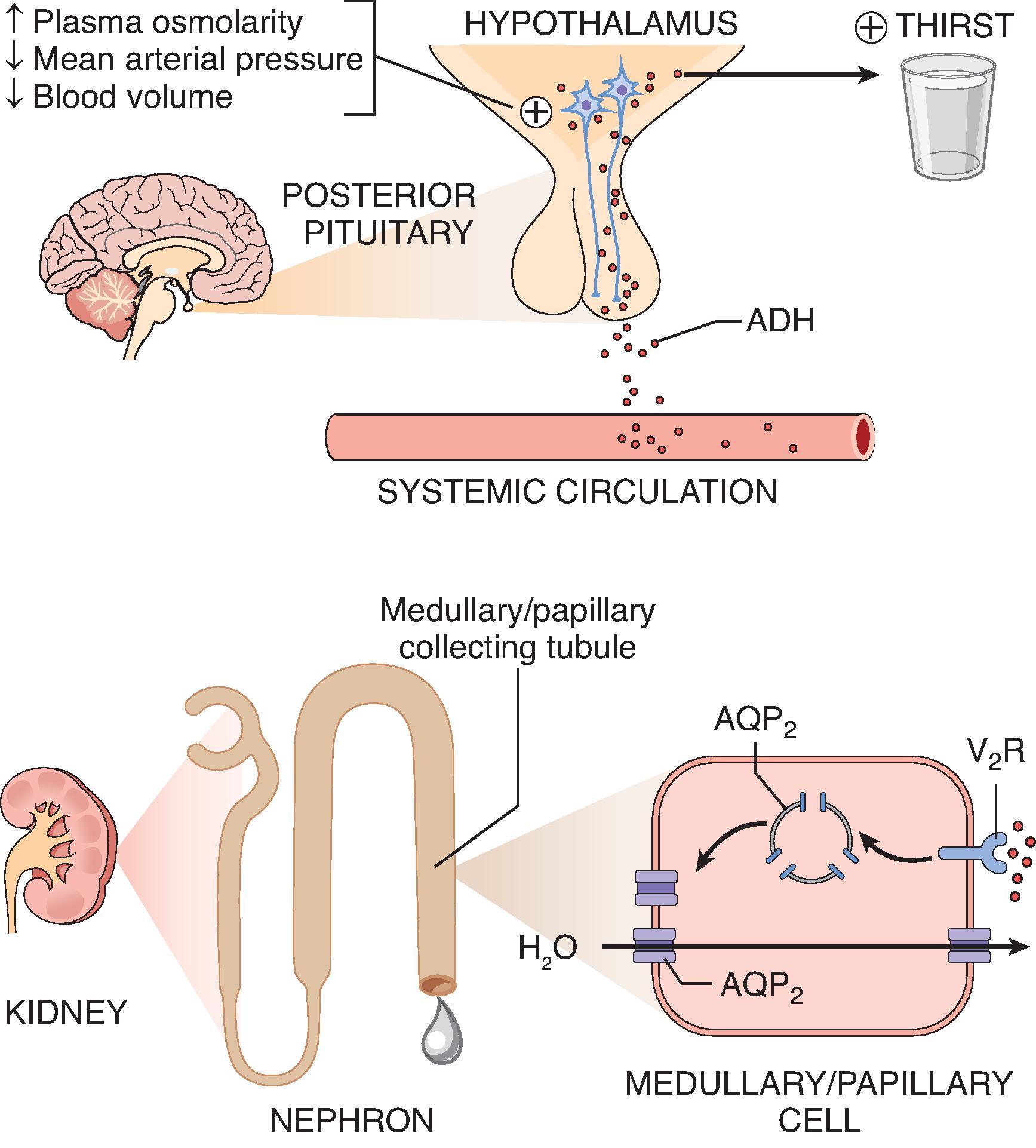
The kidney’s primary role in hypernatremia is to concentrate the urine maximally, preventing further loss of electrolyte-free fluid. For the kidney to do so, the following must be present: (1) a concentrated medullary interstitium, (2) ADH to insert aquaporin-2 channels into the apical membranes of the collecting duct, and (3) the ability of collecting duct cells to respond to ADH.
In a steady state, water intake must equal water output. Obligatory kidney water loss is directly dependent on solute excretion and urinary concentrating ability. Also recall that solute intake must equal solute excretion. Therefore, if a person has to excrete, for example, 700 mOsm of solute per day (primarily Na + , K + , and urea), and his or her urine concentration capability is impaired, as reflected by a maximum urinary osmolality (U osm ) of 100 mOsm/kg, then the minimum urine output requirement will be 7 L (see calculation below).
However, if the kidneys are able to concentrate the urine to a U osm of 700 mOsm/kg, urine output would need to be only 1 L.
Thirst, on the other hand, is an ADH-independent mechanism of defense against hypertonicity. Like ADH release, thirst is triggered by osmoreceptors located in the hypothalamus. The intense thirst stimulated by hypernatremia may be impaired or absent in patients with altered mental status or hypothalamic lesions and in older adults. It is important to note that patients with moderate to severe increases in electrolyte-free water losses may maintain eunatremia because of the powerful thirst mechanism. For example, a patient with nephrogenic DI may maintain a normal serum [Na + ] if given unlimited access to water but may develop marked hypernatremia in situations in which access to water is restricted (e.g., altered mental status, mechanical ventilation, nothing-by-mouth status during hospitalization).
Although ADH activity is a pivotal physiologic defense against hyperosmolality, only an increase in water intake can replace a water deficit. An increase in ADH activity in collecting tubules can only help to decrease ongoing water losses but cannot replace water that has already been lost. Therefore, both ADH-dependent and ADH-independent mechanisms are integral to the body’s efforts to protect against hypernatremia or hyperosmolality.
The brain has multiple defense mechanisms designed to protect it from the adverse effects of cellular dehydration. As the serum [Na + ] rises, water moves from the intracellular to the extracellular space to equalize the osmolalities in the body compartments. Almost immediately, there is an increase in the net leak of serum electrolytes (primarily Na + and K + ) into the intracellular space, which increases intracellular osmolality. In addition, there is an increased production of cerebrospinal fluid, with movement into the interstitial areas of the brain. Within approximately 24 hours, brain cells generate osmolytes or idiogenic osmoles (e.g., amino acids, trimethylamines, myoinositol) to increase intracellular osmolality to return water intracellularly. This process restores intracellular volume over a period of days, thereby decreasing the adverse clinical impact of hypernatremia (i.e., cellular dehydration). The increase in transcellular transport of electrolytes is somewhat transient because over time it interferes with normal cellular function. Idiogenic osmoles clearly serve a protective role, but their removal is also slow (days) when isotonicity has been reestablished. The clinical implications of slow removal of idiogenic osmoles from the perspective of hypernatremia correction varies based on patient population. Previously, recommendations for slow serum sodium correction due to assumed risk of cellular swelling and cerebral edema were based on pediatric data. However, a more recent prospective analysis of critically ill adult patients with hypernatremia demonstrated no association between rate of correction and cerebral edema, seizures, and/or alteration of consciousness. Therefore, in adult populations, more rapid hypernatremia correction is recommended.
Hypernatremia most commonly results from the combination of increased water loss and decreased water intake. Any clinical condition associated with increased water loss or decreased water intake predisposes to hypernatremia. In general, for hypernatremia to occur, the rate of water excretion must exceed that of water intake. Hypernatremia secondary to sodium loading, which is less common, is an exception to this basic principle.
Water losses occur via the genitourinary and gastrointestinal tracts, as well as via the skin and respiratory tract as insensible losses. DI and osmotic diuresis result in excess urinary loss of water, and excess water is lost via stool in severe diarrhea. Conditions that increase insensible losses include fever, burns, open wounds, and hyperventilation.
Although hypernatremia is due to an imbalance of water homeostasis, there may be a concomitant salt disturbance. After a thorough clinical history is obtained and a complete physical examination performed, the first decision point in the evaluation of any patient with hypernatremia is to determine the patient’s sodium balance or volume status (see Fig. 8.1 ). Hypernatremia can be seen in patients whose sodium balance is negative (hypovolemic), normal (euvolemic), or positive (hypervolemic), but in all cases a negative water balance is present. Determining the patient’s sodium balance is essential to planning the appropriate therapy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here