Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A carbonic dioxide arterial blood level (PCO 2 ) above 46 mmHg (6.1 kPa) is defined as hypercapnia. The increase in carbon dioxide (CO 2 ) partial pressure provokes a fall in blood pH, and its clinical features can be arterial hypertension, tachycardia, drowsiness, tachypnea, and skin rush, among other symptoms.
An increase in CO 2 production or a decrease in its excretion can generate hypercapnia; Table 1 details the possible etiologies. In the neurosurgical patient, hypoventilation is the main origin of a decrease in CO 2 excretion, leading to different degrees of hypercapnia. During an awake fiber optic intubation, hypercapnia episodes are frequently due to loss of respiratory efforts in a narcotized patient. Bradypnea, secondary to sedation, is a challenge during awake neurosurgery and is the most likely cause of hypercapnia. After posterior cranial fossa procedures, hypoventilation can appear as a consequence of surgical maneuvers besides brain stem structures. Following complex spine surgery in the prone position, edema can produce supraglottic airway obstruction with resulting hypercapnia.
| Reduced excretion of CO 2 |
|---|
|
| Increased production of CO 2 |
|
Most neurons are highly responsive to changes in pH of the surrounding interstitial fluids. Alkalosis greatly increases neuronal excitability; for instance, a rise in arterial blood pH from 7.4 to 7.8 or 8.0 often causes cerebral epileptic seizures because of increased excitability of some or all of the cerebral neurons. This can be demonstrated especially well by asking a person who is predisposed to epileptic seizures to overbreathe. The overbreathing blows off carbon dioxide and therefore elevates the pH of the blood momentarily, but even in this short time, it can often precipitate an epileptic attack.
Slight elevations of CO 2 cause direct neuronal cortical depression and increase the threshold for seizures. The application of 5% CO 2 has been proven to be effective and safe to suppress febrile seizures in children.
Higher levels of CO 2 (25–30%) stimulate subcortical hypothalamic centers, resulting in increased cortical excitability and seizures. This hyperexcitability is enhanced by adrenal cortical and medullary hormones released secondary to hypercapnia-induced stimulation of the hypothalamus. Further elevation of CO 2 with a fall in pH from 7.4 to below 7.0 usually causes an anesthetic-like state of cortical and subcortical depression. It causes a comatose state, such as in very severe diabetic or uremic acidosis, where coma virtually always develops.
CO 2 , not H+, crosses the blood–brain barrier (BBB) and the brain cell membrane affecting the cell metabolism. A sudden change in CO 2 causes a rapid change in cerebrospinal fluid (CSF) pH. Hypercapnia decreases cerebral vascular resistance, causing cerebral blood flow (CBF) to increase. The relationship between CBF and PCO 2 is linear from 20 to 100 mmHg with maximal vasodilatation at approximately 120 mmHg. For each mmHg increase in PCO 2 between 25 and 100 mmHg, CBF increases by 2–4%.
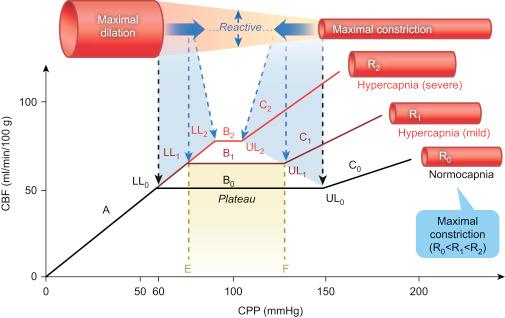
Cerebral autoregulation is a mechanism that maintains a stable CBF for a given cerebral metabolic rate in spite of fluctuation in cerebral perfusion pressure (CPP). It is visualized as a correlation plot of CBF (axis of ordinate) against CPP (axis of abscissas). The three key elements of the autoregulation curve are the lower limit (60 mmHg), the upper limit (150 mmHg), and the plateau (CBF = 50 mL/min/100 g). The lower and upper limits are the two sharp inflection points indicating the boundary of pressure-independent flow (the plateau) and the start of pressure–passive flow (see Figure 1 at normocapnia).
Multiple physiological processes are engaged in the regulation of CBF, but its execution relies on the cerebrovascular reactivity that provokes dilation to a decrease in CPP (arterial hypotension) and constriction to an increase in CPP (arterial hypertension).
However, cerebrovascular reactivity (CVR) is not exclusively linked to changes in pressure; changes in other physiological processes, notably CO 2 , can also rapidly alter cerebral vasomotor tone and thus regulate CBF.
Brain vasculature constriction, such as in response to hypocapnia, diminishes CBF and cerebral blood volume (CBV) and can lower intracranial pressure (ICP). The rapid response of hypocapnia in provoking decreases in high ICP values is extensively used during neurosurgical procedures; nevertheless, we need to keep in mind the high risk of brain ischemia that can provoke the lower CBF in response to the vasoconstriction.
Brain vasculature dilation, as in response to hypercapnia, increases CBV and can increase ICP.
If hypercapnia coexists with hypotension, the combined vasodilator effects on brain vasculature could shift the lower limit of autoregulation rightward, then higher arterial pressure values are needed in order to maintain an adequate CBF ( Figure 1 ).
On the opposite end, the dilation induced by hypercapnia could adversely affect the arterial hypertension-induced constriction response on brain vasculature, rendering a leftward shift of the upper limit. In this context, CBF will increase beyond the limits of autoregulation, with elevated arterial pressure levels that would have no effects in a normocapnia setting. In an animal study with dogs, Ekström-Jodal et al. showed that during normocapnia, the upper pressure limit of autoregulation (UPL) was maintained until a mean arterial pressure (MAP) value of 225 mmHg. During mild hypercapnia (PaCO 2 = 40–60 mmHg), this UPL had fallen to 150 mmHg and went down to a MAP value less than 125 mmHg, if PaCO 2 > 60 mmHg.
We recommend an extensive review on this topic published by Meng et al. showing that during hypercapnia, the plateau of the autoregulation curve is shifted upward and shortened, the lower limit is shifted rightward, and the upper limit is shifted leftward. The extent of these changes depends on the severity of hypercapnia. At severe hypercapnia, when cerebral resistance vessels are maximally dilated, the plateau is lost and the pressure–flow relationship is linear ( Figure 1 ).
Changing the arterial CO 2 levels in order to reduce CBF and ICP is a common practice in neuroanesthesia. Different studies have shown that inhalational and intravenous anesthetic agents have variable effects on CVR to CO 2 variations (CVR–CO 2 ), and the effects of anesthetic agents on CVR also vary with many physiological and pathological conditions. A qualitative systematic review on this topic has been published by Mariappan et al. ; they included 1356 citations and concluded that within the clinical anesthesia concentrations, CVR–CO 2 is maintained under both propofol and inhalational agents. Propofol lowers cerebral metabolic rate of oxygen consumption (CMRO 2 ) and has a net vasoconstriction effect. Relative CVR–CO 2 value is higher in the hypercapnia range. Inhalational agents also diminish CMRO 2 but have a net vasodilatation effect; relative CVR–CO 2 values is lower in the hypercapnia range when anesthetic agents are used. However, most of the available information is on nonneurosurgical patients and is difficult to extrapolate the results to our clinical target.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here