Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
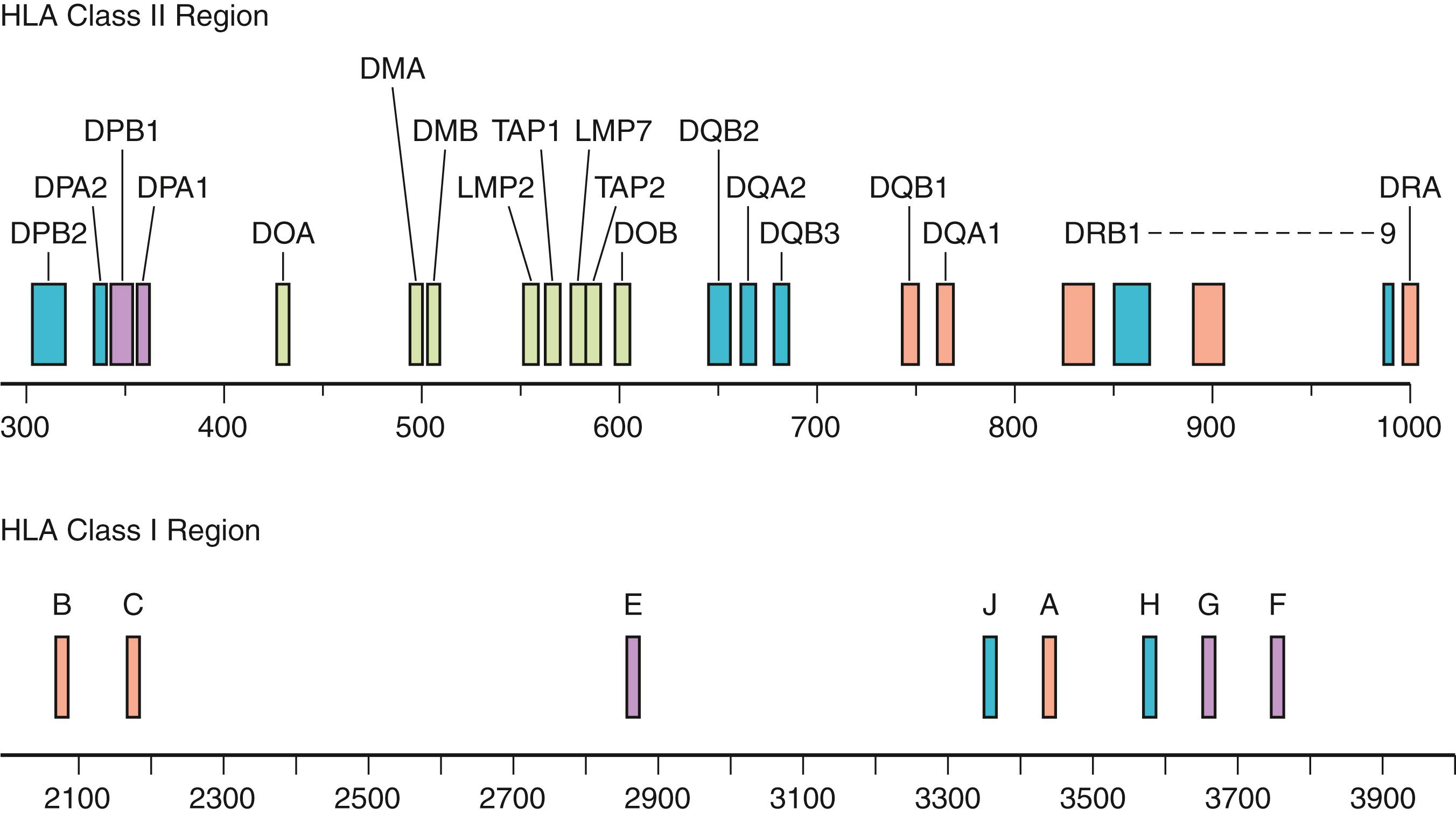
The major histocompatibility complex (MHC) is one of the most diverse and polymorphic genetic systems in humans. It consists of human leukocyte antigen (HLA) class I molecules (HLA-A, -B, and -C) and class II molecules (which include HLA-DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, -DPA1, and -DPB1). In total, there are over 20,000 unique HLA alleles.
MHC genes are closely linked and segregate into offspring according to classic Mendelian genetics.
Characterization of HLA alleles has been facilitated via molecular typing methods, including next generation, whole genome deoxyribonucleic acid (DNA) sequencing.
The concept of HLA matching varies as a function of the type of transplant. For solid organ transplantation, only HLA-A, -B, and -DR antigens are considered. Hence a “perfect match” for solid organ transplantation would be termed 6 of 6 match or 0-antigen mismatch. For stem cell transplantation, HLA-A, -B, -C, -DRB1, and -DQB1 are considered. However, individual HLA molecules are compared at the allele level. Thus individuals matched for stem cell transplantation would be termed 10 of 10 matched.
Patients can be sensitized to specific foreign HLA molecules through transfusion, pregnancy, or prior transplantation. Assessment of compatibility between the recipient and the possible donor includes testing for this sensitization.
The presence of donor-specific HLA antibodies may preclude or compromise a transplant. HLA antibody testing is performed using solid-phase, bead-based assays. Crossmatching may be either a physical test or a virtual assessment of immunologic compatibility.
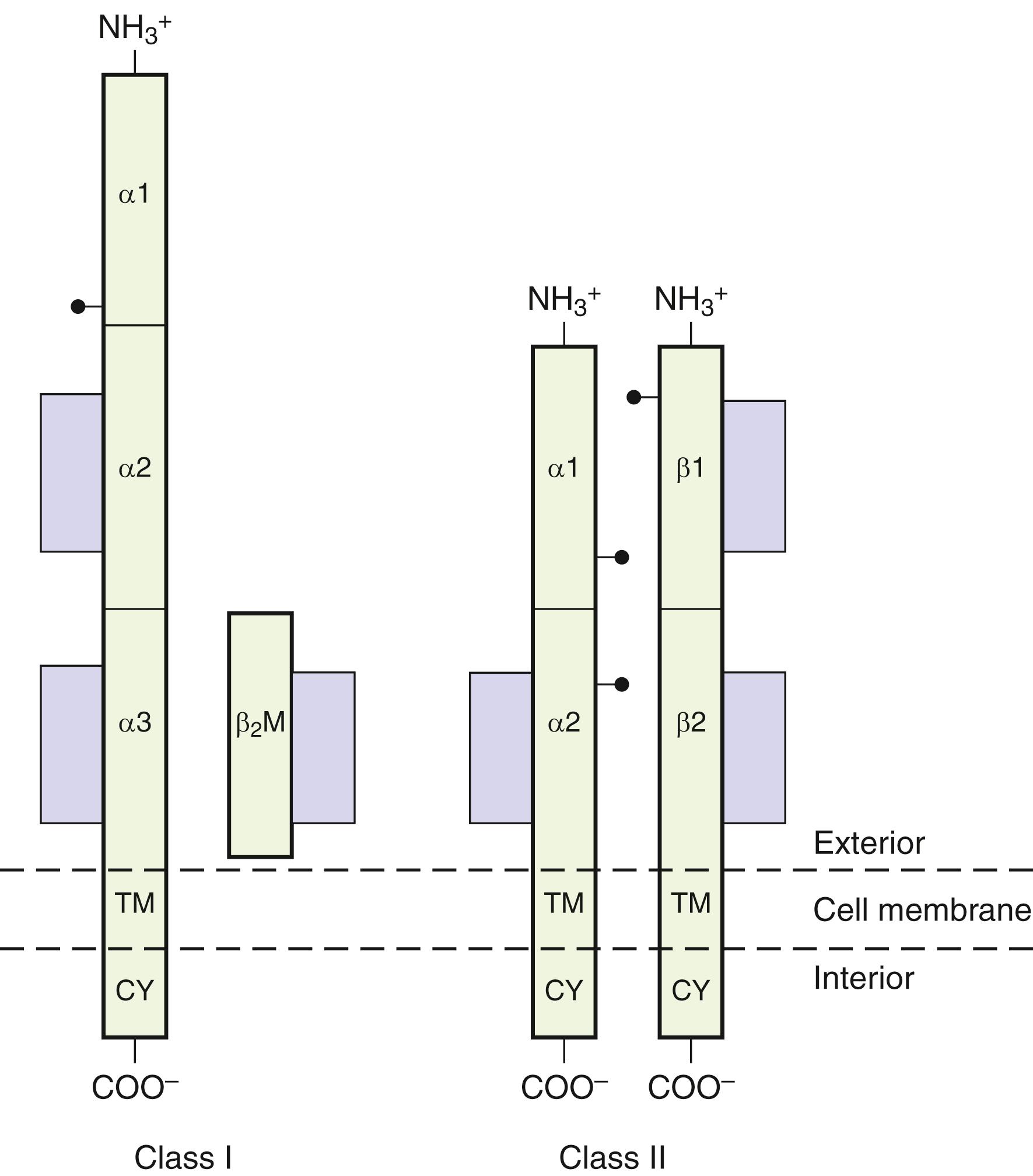
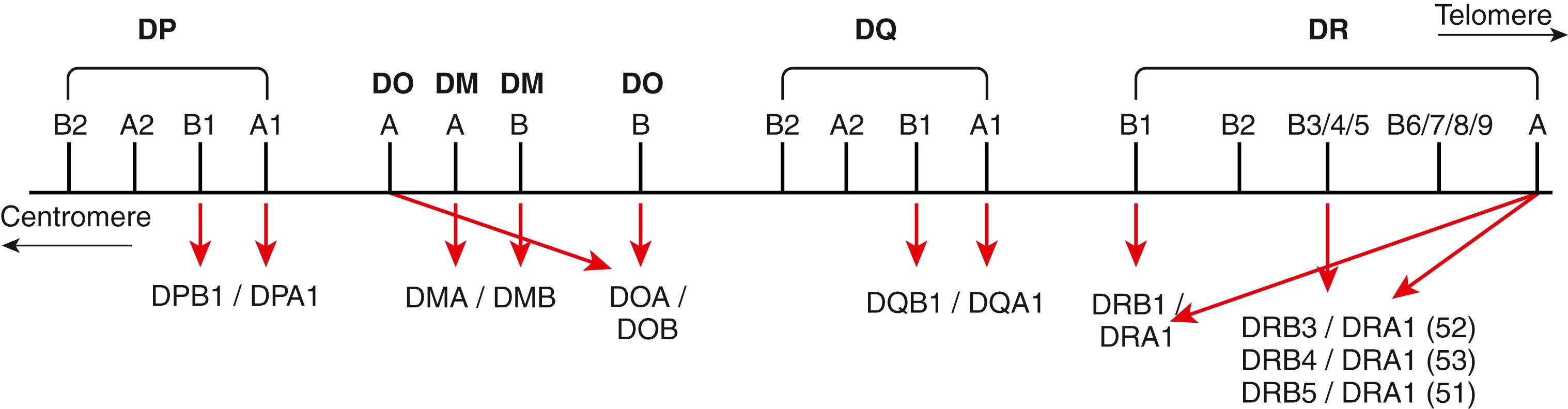
An individual’s survival depends on the ability of the immune system to recognize and respond to a multitude of foreign substances (antigens). Although this defense mechanism is basic to survival, this same defense system can have a negative impact when tissue is transplanted from one individual to another or when dysregulation triggers autoimmune disease. Genes of the MHC encode proteins, HLA class I and class II that are essential for immune recognition ( Fig. 50.1 ). In the human, class I molecules combine with β 2 microglobulin to form a stable heterodimer. Class I molecules include HLA-A, -B, and -C. For class II molecules alpha-beta heterodimers are formed via the combination of the nonpolymorphic DRA1 gene product with an HLA-DRB1 and/or HLA-DRB3, -DRB4, or -DRB5 gene products. HLA-DQA1 combines with HLADQB1, both of which are highly polymorphic, to form a stable DQ molecule. Lastly, HLA-DPA1 combines with HLA-DPB1, again both are highly polymorphic, to form a stable DP molecule. These molecules constitute the classic transplantation antigens. Other molecules encoded within the MHC are the class III molecules. These include the MHC-linked complement components (C2, C4, and Bf), 21-hydroxylase (CYP21), heat-shock protein 70 (HSP70), and tumor necrosis factor (TNF).
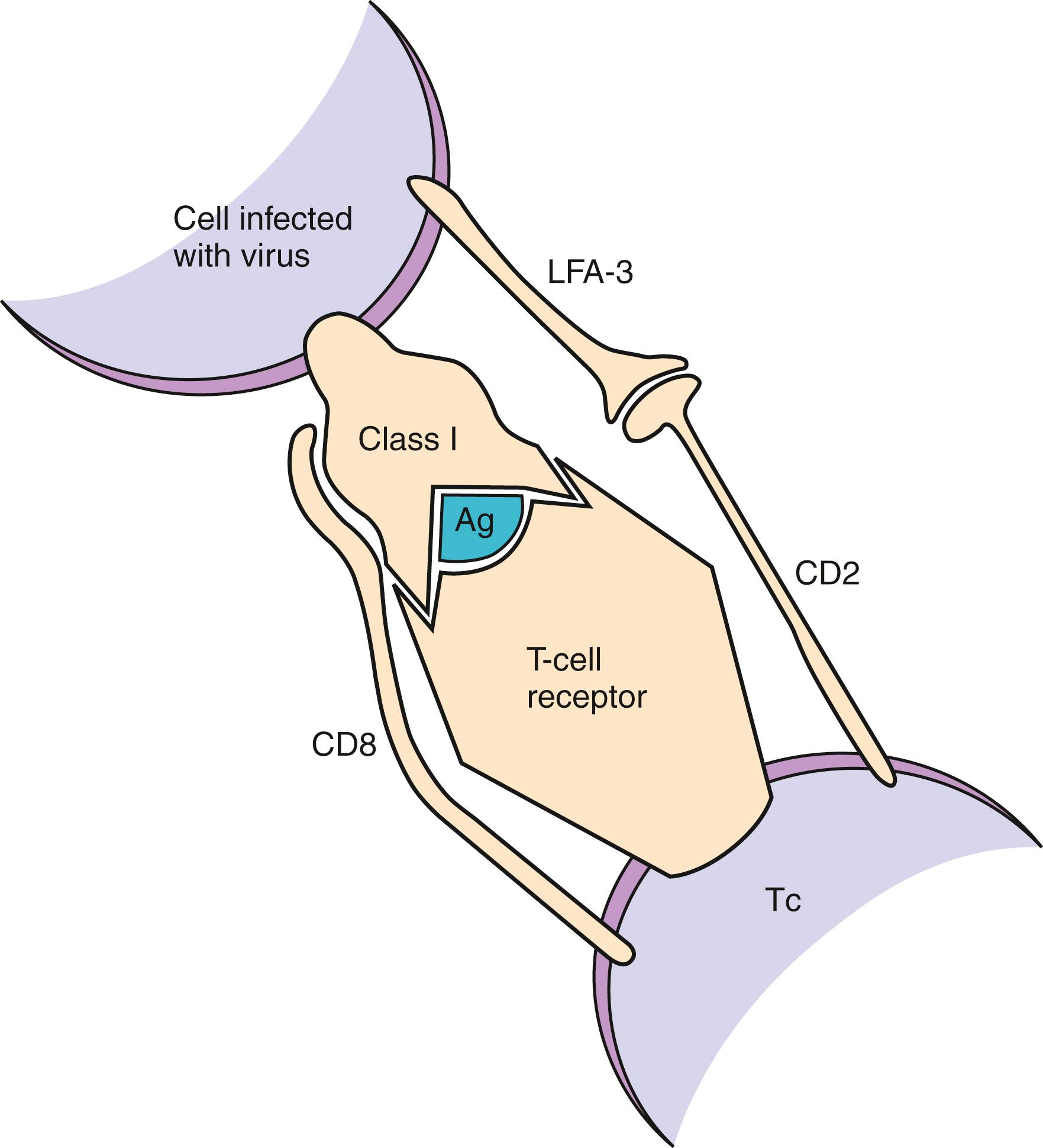
During an infection, the invading microorganism may infect or be engulfed by cells and then may be degraded into peptide fragments. Inside the cell (in the endoplasmic reticulum, HLA class I, and class II compartment), nascent HLA class I or class II molecules are loaded with peptide fragments derived from the microorganism. Once these fragments have bound to the HLA molecules and have translocated to the cell surface, receptors on T lymphocytes interact with the peptide/HLA complex (immune synapse), triggering the cellular then humoral immune responses ( Fig. 50.2 ). T-cell–specific surface molecules, CD4 and CD8, act to strengthen this cellular interaction and to transmit intracellular activation signals. Other cell surface molecules act to increase the affinity of cellular interactions (e.g., CD2 and its ligand, CD58 [LFA-3], or CD11a/CD18 [LFA-1] and its ligand, CD54 [ICAM-1]), and to transmit costimulatory signals (e.g., CD28 and its ligands, CD80/CD86 [B7], or CD40 and its ligand, CD154). The same interactions that occur between T-cell recognition of MHC class I or class II with its associated peptide in response to an invading pathogen also can occur between foreign HLA class I and class II molecules (alloantigens) and in recognition of self-antigens (autoantigens) (see Chapter 51 ).
The genetics, structure/function, nomenclature, and techniques for detection of human MHC gene products and antibodies against these products are discussed in this chapter. Also discussed is the evolving role of HLA matching for transplantation and the emergence of the virtual crossmatch. In addition to references listed at the end of the chapter, Table 50.1 presents helpful websites that provide both reference material and updates of clinical outcomes.
| Address | Information |
|---|---|
| www.ashi-hla.org | American Society for Histocompatibility and Immunogenetics, HLA typing standards |
| www.ebi.ac.uk/imgt/hla | HLA sequence database, tools for allele submission and sequence manipulation |
| www.anthonynolan.org.uk/hig | World Health Organization nomenclature information |
| www.ihiw18.org | 18th International Histocompatibility Workshop |
| www.bethematch.org | National Marrow Donor Program |
| www.bloodcell.transplant.hrsa.gov | Bone Marrow and Cord Blood Donation and Transplantation, HRSA |
| www.wmda.org | Bone Marrow Donors Worldwide |
| www.ebmt.org | World Marrow Donor Association |
| www.cibmtr.org | Center for International Blood & Marrow Research |
| www.ctstransplant.org | Collaborative Transplant Study |
| www.unos.org | United Network for Organ Sharing |
| www.optn.transplant.hrsa.gov | Organ Procurement and Transplant Network |
| www.nhgri.nih.gov | National Human Genome Research Institute |
| www.srtr.org | Scientific Registry of Transplant Recipients |
| www.allelefrequencies.net | Allele Frequency Net Database |
Mendel’s first law, the law of segregation, is based on the principle that hereditary traits are determined by factors that are distributed to progeny. In any one individual, these factors, or genes as they are now called, are present on chromosomes ( Chapter 70 ). Each gene may have multiple forms (i.e., alleles). Because humans are diploid, two genes are included per pair of homologous chromosomes. In meiosis, the chromosomes segregate randomly during formation of the gametes (ova or sperm) so that only one of each pair of chromosomes (i.e., a haploid number) is transmitted by any given gamete. The double, or diploid, number of chromosomes is restored when the male and female gamete fuse to form the zygote. Thus, for a trait determined by one gene, there will always be four possible genetic combinations in the offspring, each with equal probability of occurrence. These laws of segregation and random assortment apply to genes of the MHC system. However, in contrast to other genetic systems, HLA genes are codominant, meaning there is equal expression (with some exceptions) of gene products from both haplotypes. Following are definitions for genetic terms that will be used in this chapter ( ; www.nhgri.nih.gov ):
Gene: Unit factor of inheritance
Chromosome: Carrier of the unit factors of inheritance
Locus: Position of a gene on a chromosome
Allele: An alternative form of a gene at a single locus
Homozygous: Having identical alleles at a locus, one on each chromosome
Heterozygous: Having different alleles at a locus, one on each chromosome
Codominance: The state in which the allele at each locus expresses its characteristic effect equally in the heterozygote
Genotype: Genetic composition of an organism or individual
Phenotype: Observable characteristics produced by the genes
Polymorphic: Having two or more common distinct genotypes maintained in a population
Homologous chromosomes: The two members of a chromosome pair that have corresponding gene loci, one derived from each parent
Crossing over: Exchange of segments between homologous chromosomes; also termed recombination.
Allo-(antigen, graft): Refers to antigenic differences between individuals of a single species
Auto-(antigen, graft): Refers to tissue or antigens of the same individual (i.e., self)
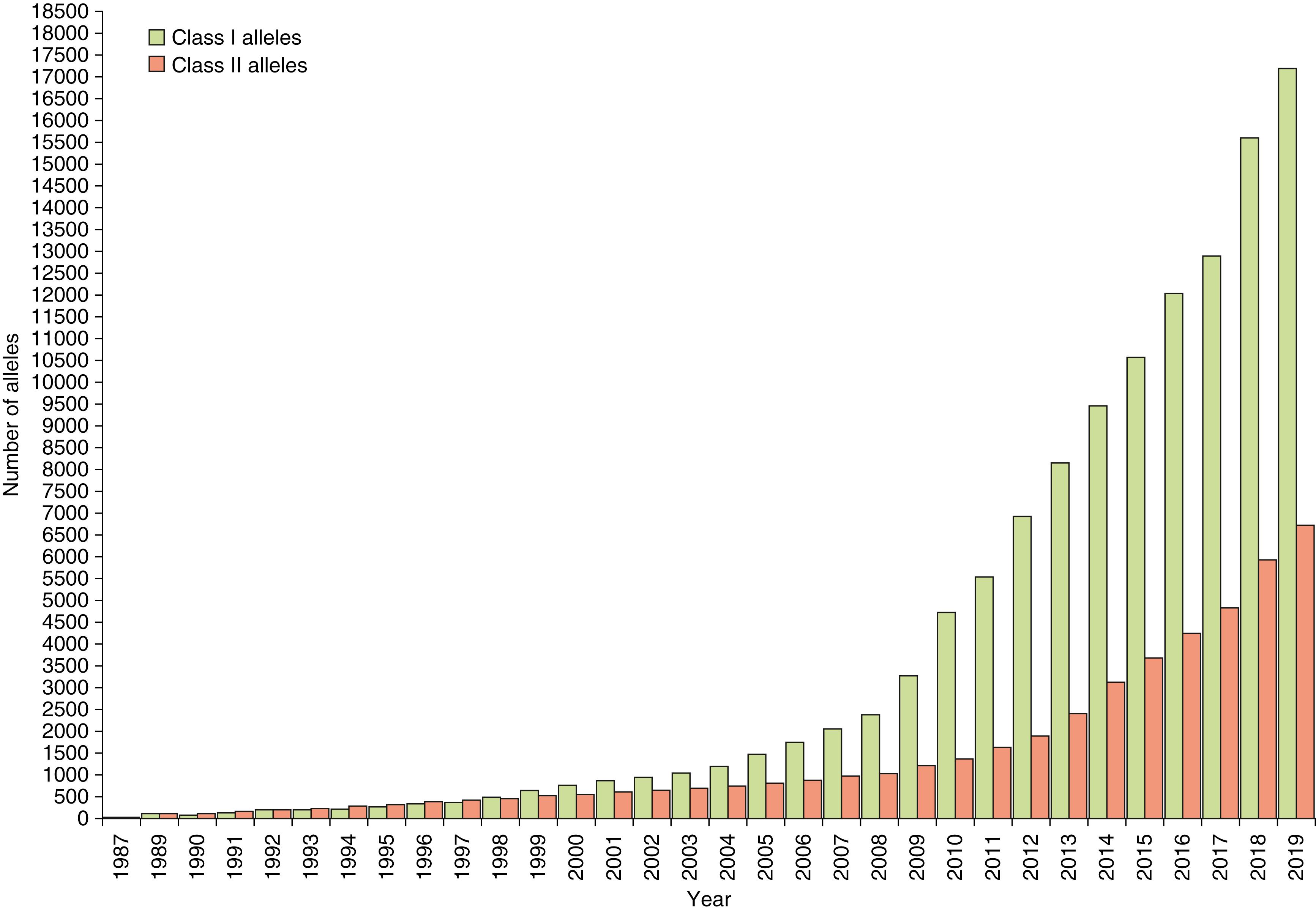
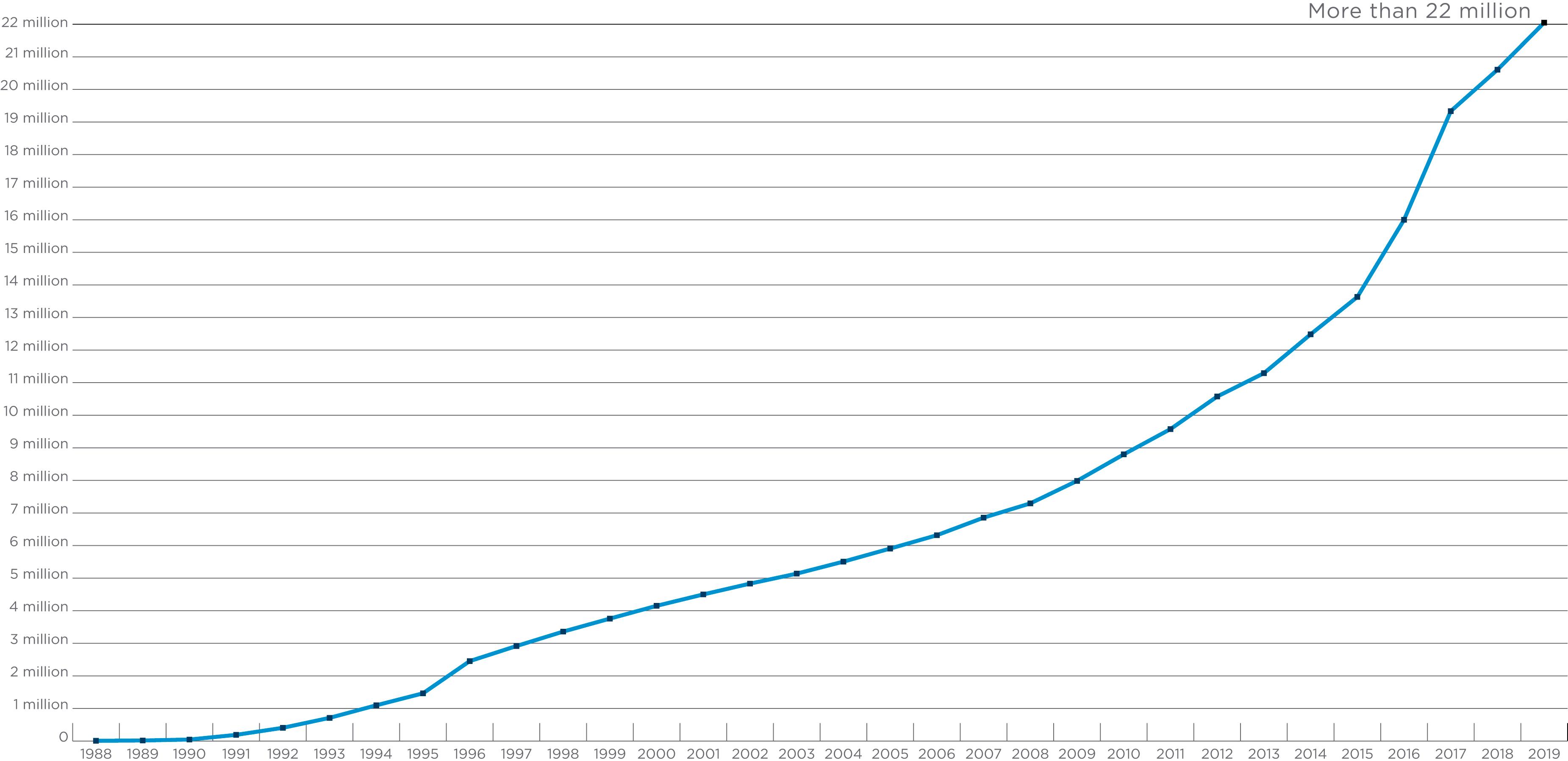
The major histocompatibility gene complex in humans is located on the short arm of chromosome 6 (i.e., 6p21). It spans ∼4000 kilobases (kb) and includes 224 identified gene loci, of which 121 are predicted to be expressed ( ). Approximately 40% of the expressed genes are estimated to have an immune system function ( ). MHC genes are located in six subregions: HLA-A, HLA-C, HLA-B (class I genes) and HLA-DR (DRB1, DRB3,4,5), HLA-DQ (DQA1, DQB1), and HLA-DP (DPB1, DPA1) (class II genes) (see Fig. 50.1 ). Each subregion encodes a minimum of one cell surface glycoprotein. β 2 microglobulin, encoded on chromosome 15 (15q21.1), contributes to the final tertiary structure of class I molecules. Class II region genes have additional complexities that will be discussed later. With only one exception, DRA1, the MHC gene products are highly polymorphic; that is, each MHC gene has multiple expressed alleles in the human population. Indeed, the MHC genes are the most polymorphic loci known in humans ( ; https://www.ebi.ac.uk/ipd/imgt/hla/ ) ( Table 50.2 ). Because MHC genes specify molecules that play an important role in the immune response, diversity is believed to be essential to survival of the species. As the majority of HLA typing methods involve testing at the DNA level, we can now appreciate the enormous diversity that resides within the MHC. As of September 2019, more than 23,000 HLA alleles have been identified that code for more than 16,000 unique proteins ( http://hla.alleles.org/nomenclature/stats.html ) ( Fig. 50.3 ). Among the MHC genes, the HLA-B locus presents the greatest variability with over 6500 alleles coding for more than 4400 unique proteins. As a result, the HLA B-locus is considered the most polymorphic genetic locus in the entire human genome.
| HLA-A Antigens | HLA-A Alleles b | HLA-DQ Antigens | HLA-DQB1 Alleles c |
|---|---|---|---|
| A1 | A∗01:01:01:01-01:319 | DQ1 | |
| A2 | A∗02:01:01:01–∗02:877 | DQ2 | DQB1∗02:01:01–∗02:99 |
| A203 | A∗02:03 | DQ3 | |
| A210 | A∗02:10 | DQ4 | DQB1∗04:01:01:01–∗04:73N |
| A3 | A∗03:01:01:01–∗03:369 | DQ5(1) | DQB1∗05:01:01:01–∗05:99 |
| A9 | DQ6(1) | DQB1∗06:01:01:01–∗06:99:03 | |
| A10 | DQ7(3) | ||
| A11 | A∗11:01:01:01–∗11:369 | DQ8(3) | DQB1∗03:01:01:01–∗03:99Q |
| A19 | DQ9(3) | ||
| A23(9) | A∗23:01:01:01–∗23:96 | ||
| A24(9) | A∗24:02:01:01–∗24:470 | ||
| A2403 | A∗24:03 | ||
| A25(10) | A∗25:01:01:01–∗25:63 | ||
| A26(10) | A∗26:01:01:01–∗26:190 | ||
| A28 | |||
| A29(19) | A∗29:01:01:01–∗29:136 | ||
| A30(19) | A∗30:01:01:01–∗30:158N | ||
| A31(19) | A∗31:01:02:01–∗31.160 | ||
| A32(19) | A∗32:01:01:01–∗32:131 | ||
| A33(19) | A∗33:01:01:01–∗33:191 | ||
| A34(10) | A∗34:01:01:01–∗34:23 | ||
| A36 | A∗36:01 | ||
| A43 | A∗43:01 | ||
| A66(10) | A∗66:01:01:01–∗66:37 | ||
| A68(28) | A∗38:01:01:01–∗68:221 | ||
| A69(28) | A∗69:01:01:01:–∗69:06 | ||
| A74(19) | A∗74:01:01:01:–∗74:36 | ||
| A80 | A∗80:01:01:01–∗80:06 | ||
a Each column of the table is independent. Numbers in parentheses indicate the broad serologic specificity. The serologic type may be listed without the broad specificity or molecular equivalents. For example, both A23(9) and A23 are correct designations. A9, A10, and A19 are no longer recognized as a distinct antigens and therefore do not have a molecular equivalents.
b HLA-A alleles specify HLA-A antigens. The antigen A1 is specified by either A∗01:01 through A∗01:319 alleles.
c HLA-DQA1 and HLA-DQB1 alleles specify HLA-DQ antigens. The antigen DQ5 (1) is specified by several different DQA1 and DQB1 combinations, including (1) DQA1∗01:01 and DQB1∗05:01 and (2) DQA1∗01:01 and DQB1∗05:02 alleles. In the past, serologic split designations were given to specificities that appeared to identify the antigen specified by a single HLA allele (e.g., A2 splits A203, A210; B7 splits B703; B39 splits B3901 and B3902). It is now recognized that other alleles may also encode antigens that carry these serologic specificities, and the WHO Nomenclature Committee has recommended that these splits be discontinued.
The genes of the MHC can be found on the short arm of chromosome 6 (6p). The map order and positioning of the MHC genes were initially determined by meiotic linkage analyses (i.e., studies of crossing over in families) and later by molecular biology techniques, including gene cloning, DNA sequencing, and pulsed field gel electrophoresis. The map order of genes within the MHC is HLA-A, -C, -B, -DR, -DQ, and -DP, with HLA-A being the most distal to the centromere (see Fig. 50.1 ).
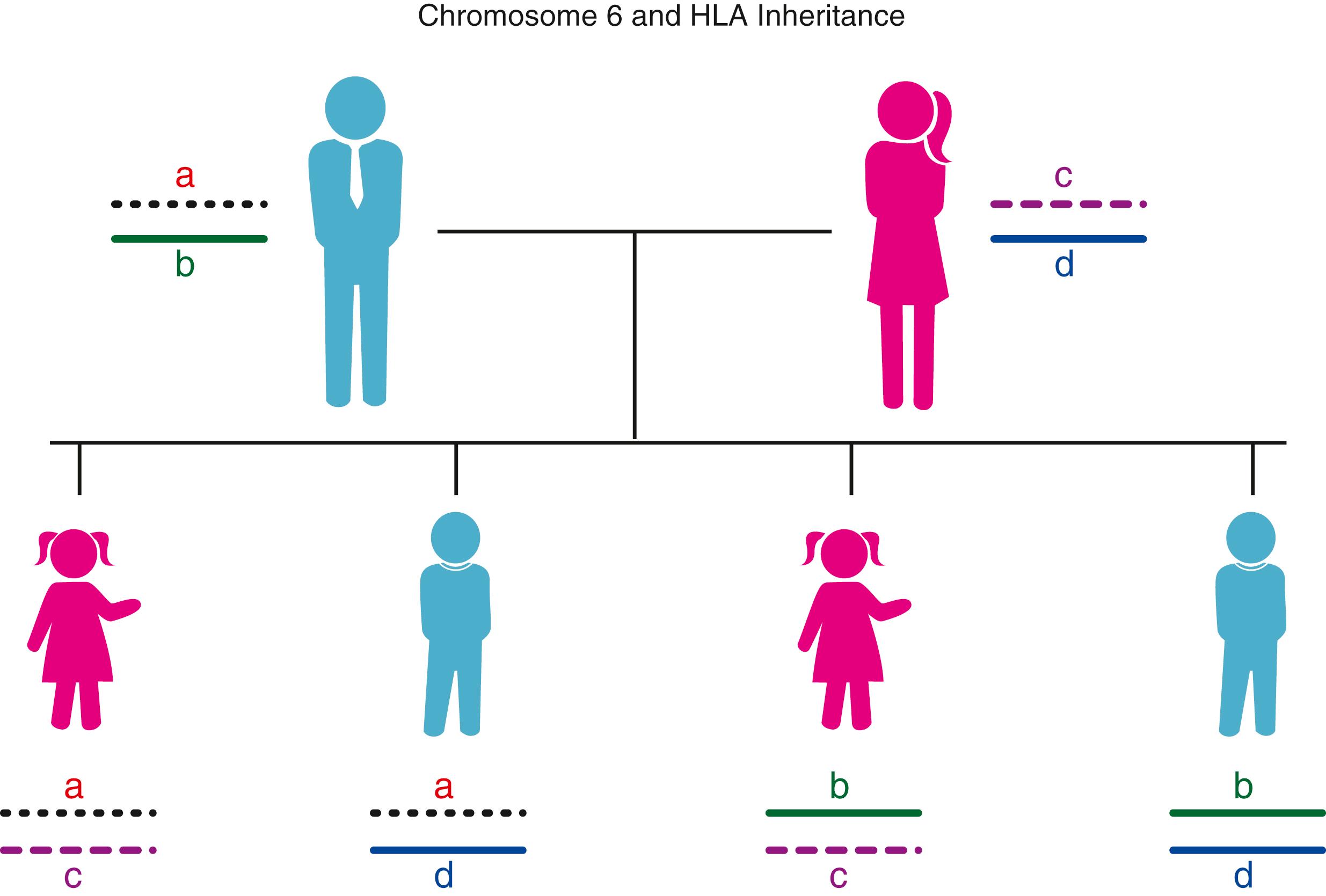
The MHC genes are closely linked; that is, they segregate en bloc to the offspring. The complex of linked genes that resides on one pair of homologous chromosomes and that segregates en bloc to the offspring is termed a haplotype . Each individual inherits two MHC haplotypes—one from each parent—and thus has two alleles for each of the genes. The inheritance of the MHC genes follows the rules of segregation set down by Mendel, and the alleles at each locus exhibit codominant expression. Within a family, each child inherits one MHC haplotype from the mother and one from the father. By convention, the paternal haplotypes are designated “a” and “b”, and the maternal haplotypes “c” and “d”. Thus there are four possible MHC genotypes in the offspring: “a/c”, “a/d”, “b/c”, and “b/d” ( Fig. 50.4 ). Because the chance of inheriting a given haplotype is random, the probability of occurrence of any one of the four genotypes is one in four for each offspring. In a family with five children, at least two of the children will be HLA identical assuming no crossover haplotypes have occurred. Although the MHC genes are closely linked, families have been reported in which a crossover has occurred. The frequency of crossing over between two linked genes was thought to be a measure of the distance separating the genes; however, molecular data have suggested the existence of recombination “hot” spots in the intervening DNA that can increase or decrease the likelihood of recombination during meiosis ( ).
The observation that alleles at different genetic loci occur in the population on the same haplotype significantly more frequently than would be expected on the basis of chance alone is called linkage disequilibrium. The expected frequency of two alleles (f 1 , f 2 ) is the product of the gene frequency of each allele in that population (f expected = f 1 × f 2 ). The observed frequency (fo) is determined from family studies within the same population. When the f observed is significantly greater than f expected , the two genes are said to be linked. Linkage disequilibrium is a hallmark of the human MHC and extends from HLA-A through HLA-DP. However, the linkage between DQ and DP can vary depending upon the HLA haplotype ( ). The best known example of linkage disequilibrium is the A1, Cw7, B8, DR17(3), DR52, DQ2 haplotype in Caucasians, which is observed approximately four times more frequently than expected. The significance of linkage disequilibrium as it applies to immune competence and disease is discussed under “Extended Haplotypes” in Chapter 51 .
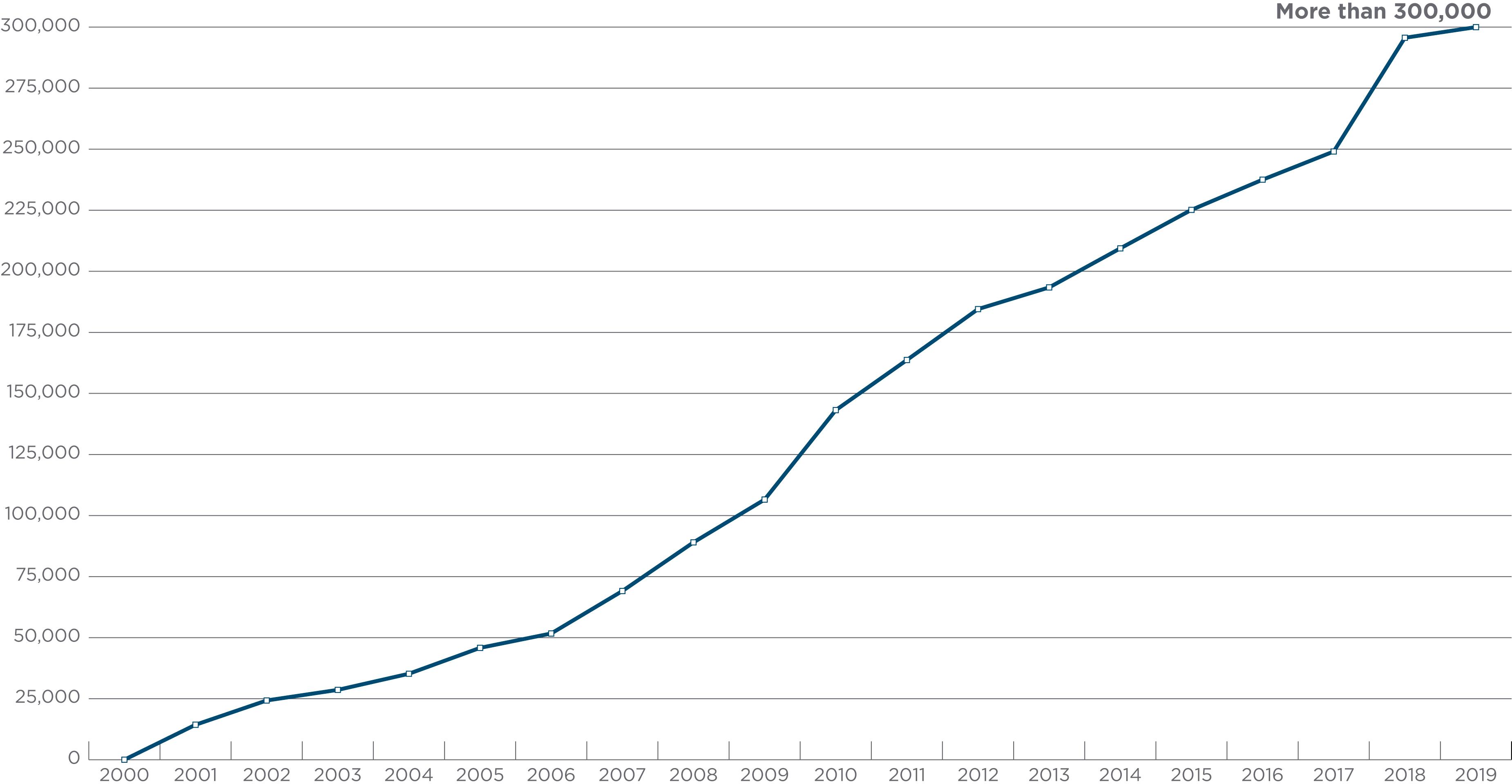
Accumulated data from the study of the world population groups ( ; ; ; ) demonstrate that the frequencies of HLA alleles differ significantly among ethnic population groups. MHC haplotypes and linkage disequilibrium of alleles also differ. These observations were taken into account when unrelated allograft hematopoietic progenitor cell donor registries and banks such as the US National Marrow Donor Program (NMDP) were established ( ). Both allele and haplotype frequencies dictate the number of volunteers required to find an HLA-matched unrelated donor for any patient. Some patients (e.g., those with rare or unusual HLA alleles or haplotypes) cannot find closely matched unrelated donors from among the more than 36 million volunteers listed in the World Marrow Donor Association registry as of 2019 ( www.wmda.org ) and more than 20 million in the “Be The Match” registry ( Fig. 50.5 ). Currently, patients are more likely to find a match not only because the number of stem cell donors has increased, but the number of umbilical cord blood units available for transplantation has also increased ( Fig. 50.6 ). It appears that cord blood transplantation may be more tolerant of HLA mismatches; this may be a result of reexposure to noninherited maternal antigens (NIMAs) found in the graft ( ) or the immune naivety of cord blood cells. Lastly, the growing success in the use of progenitor cells from partially HLA-mismatched donors may allow transplantation in patients for whom close matches cannot be found (see Chapter 39 ).
The HLA-A and -B molecules were the first MHC molecules to be described in humans ( ; ). Because these molecules were defined by antibody responses to white blood cells (WBCs), they were called human leukocyte antigens. Antibodies directed against human WBCs were observed as early as the 1920s, but it was not until the 1950s that a systematic study began. In 1952, Dausset convincingly demonstrated the existence of leukocyte antibodies (leukoagglutinins). Because these leukoagglutinins did not react with leukocytes from the antibody producer but did react with a percentage of leukocytes from red cell group O unrelated individuals, he suggested that leukoagglutinins were alloantibodies. Shortly thereafter, Payne and colleagues (1964) reported that sera from patients who had febrile nonhemolytic blood transfusion reactions frequently contained leukoagglutinins that demonstrated allospecificity. In 1958, Dausset described the first HLA alloantigen MAC (now HLA-A2) and showed it to be genetically inherited.
Originally, leukocyte specificities were thought to be the products of a single locus. A two-locus model, each locus with multiple alleles, was established through HLA studies in families by the identification of recombination events that separated the two allelic series. These two allelic series were called the first, or LA (now HLA-A), and the second, or “four” series (now HLA-B). These names were derived from descriptions of the LA 1, 2, 3 allelic series by Payne in 1967, and of the 4a, 4b allelic series by van Rood in 1969. A third locus was first proposed in 1970; however, it was not confirmed until 1975, when a family with a recombination between HLA-B and the new locus was identified. This third locus was designated HLA-C following the 1975 International Histocompatibility Workshop. was awarded the Nobel Prize for Medicine in 1980 for his original work on the human HLA system.
The classical class I molecules, termed HLA-A, HLA-B, and HLA-C in the human, are heterodimers consisting of a transmembrane glycosylated polypeptide (heavy chain, 44-kDa) noncovalently associated with β 2 microglobulin (12-kDa) ( Fig. 50.7 ) ( ). The heavy chain of the class I molecule spans the cell membrane and is oriented with its amino-terminus on the outside of the cell. β 2 microglobulin is associated with the extracellular region of the heavy chain and is necessary for cell surface expression. The extracellular region of the class I heavy chain is divided into three domains designated α1, α2, and α3, each consisting of about 90 amino acid residues. The amino-terminal α 1 domain contains a glycosylation site at the asparagine residue in position 86. The transmembrane segment of approximately 24 amino acids is mostly hydrophobic, whereas the intracellular carboxy-terminal segment of the molecule consists mainly of hydrophilic residues with a cluster of basic residues adjacent to the cytoplasmic surface of the cell membrane.
The polymorphisms of class I molecules (see Table 50.2 and Fig. 50.4 ) were initially defined by the use of (1) class I–specific antibodies (alloantisera and monoclonal antibodies) that bind to the HLA molecules, (2) cytotoxic T lymphocytes (CTLs) that recognize and kill in response to stimulation by foreign or allogeneic class I molecules in vitro, and (3) isoelectric focusing of isolated class I molecules. More recently, polymerase chain reaction (PCR)–based DNA typing of class I and II alleles and nucleotide sequencing of class I and II alleles represent the primary methods for identifying an individual’s HLA repertoire.
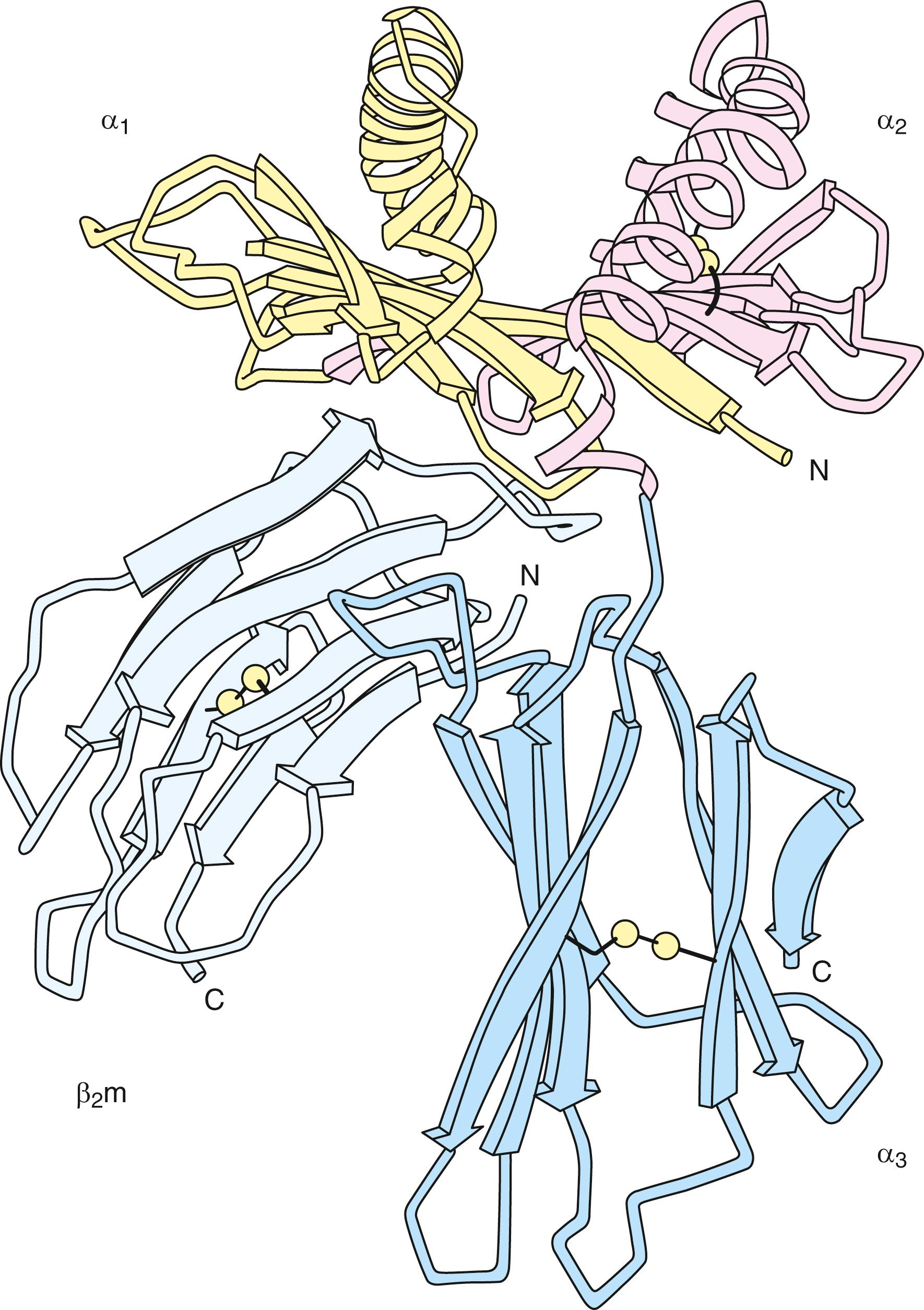
Fresh insights into the structure of the HLA molecule came when the three-dimensional structure of the class I molecule was defined using x-ray crystallography ( ). The structure of the extracellular portion is shown in Figure 50.8 . The molecule consists of two pairs of structurally similar domains: α1 has the same tertiary conformation as α 2 , and α 3 and β 2 microglobulin have similar tertiary conformations. The α 3 and β 2 microglobulin domains are each composed of two antiparallel β-pleated sheets—one with four strands and one with three strands, connected by a disulfide bond. The α 3 and α 2 domains interact with one another through these β-pleated sheets, and their structure closely resembles that described for an immunoglobulin constant region domain. The α 1 and α 2 domains are paired to form an eight-strand β-pleated sheet. This sheet is topped by two α-helices forming a groove at the top of the molecule. This groove is the site for binding of antigenic peptide fragments by the class I molecule (see Fig. 50.8 ). The sides and bottom of the groove are formed by side chains of the amino acids that constitute the helices and β-pleated sheets. Many of the amino acids that line this groove are polymorphic, creating allele-specific differences in antigen-binding specificity among the different class I allelic products ( ). Other residues in the helical regions of the groove interact with the T-cell receptor during recognition of the class I antigenic fragment complex by a T lymphocyte (see Fig. 50.2 ).
The HLA heavy chains are encoded within the MHC on chromosome 6 (see Fig. 50.1 ) ( ) and are highly polymorphic, whereas β 2 microglobulin is encoded on chromosome 15 and is not known to be polymorphic in humans. The typical class I gene is encoded by eight exons. The first exon encodes the 5′ untranslated region and a hydrophobic leader peptide. Exons 2 to 4 encode the three extracellular domains; exon 5 encodes the transmembrane region. The sixth and seventh exons encode the cytoplasmic region, and the eighth exon encodes the 3′ untranslated region, including the poly(A) addition site. Intervening sequences (termed introns ) between the exons are transcribed into ribonucleic acid (RNA) but are removed during messenger RNA (mRNA) splicing.
HLA class I molecules are expressed as transmembrane glycoproteins on the surface of many cell types. However, the level of surface expression can vary extensively ( ). The resting level of class I molecules is highest on lymphoid cells, with HLA-C expression being ∼10 times less than that of HLA-A or -B ( ). This is a result of inefficient association and a mutated cis -regulatory element of transcriptional control found in region I of the HLA-C promoter (enhancer A) that reduces its expression ( ). Differential expression of HLA molecules appears to reflect the level and antigenic specificity of the allogeneic sensitization seen in patients awaiting kidney transplantation because these patients are subjected to multiple random blood transfusions while on dialysis; additionally, posttransplant, donor-specific antibodies (DSAs) are more prevalent against HLA-A or -B or both HLA-A and -B, and far fewer DSAs are rejected by only HLA-Cw donor-specific antibodies; class I molecules are undetectable on the membranes of certain other cell types such as brain cells, muscle cells, and sperm cells. Sequence elements located upstream of class I genes bind regulatory factors that control the cell surface expression of class I molecules. During an immune response, the cell surface expression of class I genes can be upregulated by binding of cytokine (e.g., interferon-γ and TNF) to upstream regulatory elements. Tumors and certain viruses (e.g., human immunodeficiency virus) can suppress class I expression ( ).
The HLA-A, -B, and -C molecules play key roles in an immune response. First, in the adaptive immune response, class I molecules bind peptides derived from proteins degraded or synthesized in the cytosol and display them on the cell surface for interrogation by the antigen receptors on T lymphocytes (see Chapter 46 ). Presentation of antigen by class I molecules allows T cells to detect and mount a response to foreign material (e.g., a virus, abnormal proteins from a malignant cell). In the cytosol, intracellular antigens (including self-proteins and viral or abnormal proteins) are broken down into peptide fragments ( ). These peptide fragments are transported to the endoplasmic reticulum, where they bind to the groove at the top of newly synthesized class I molecules and then are carried to the cell surface ( ; ) (see Fig. 50.2 ). Four genes, located in the class II region of the MHC ( Fig. 50.9 ; see also Fig. 50.1 ) are involved in this process. Two of these genes ( LMP2 and LMP7 ) encode components of the proteosome complex, a macromolecular structure that degrades proteins within the cytosol ( ). The other two genes ( TAP1 and TAP2 ) encode components of peptide transporters that move peptides from the cytosol into the endoplasmic reticulum ( ). CD8 + CTLs recognize the processed peptide in conjunction with the class I molecule ( ). In the experimental system used to dissect this mechanism, CTLs were generated by in vitro stimulation with virally infected autologous cells. Recognition and lysis of the target cell were specific for the priming virus strain. Recognition also was affected by allelic polymorphism of class I molecules because only virally infected target cells sharing the appropriate class I allelic product(s) with the responder were lysed ( , ). The latter requirement is termed MHC restriction. Zinkernagel and his collaborator Doherty were awarded the Nobel Prize for Medicine in 1996 in recognition of the significance of the concept of MHC restriction.
Second, surface expression of class I molecules has a protective function in the innate immune response by preventing target cell lysis effected by natural killer (NK) cells and their killer cell activating and inhibitory receptors (KIR). Unlike CTLs, NK cells do not require activation through the recognition of peptide bound to the class I molecule but can lyse and destroy target cells lacking classical HLA class I molecule expression on the cell surface. Through this innate mechanism, NK cells play an important role in surveillance against viruses and tumor cells that downregulate expression of the class I molecule to avoid recognition by CTLs ( ; ).
There are two groups of NK receptors: (1) the C-type lectin receptor CD94/NKG2, whose genes reside on chromosome 12, and (2) the killer cell immunoglobulin-like receptors (KIRs) encoded by genes on chromosome 19. NK cell function appears to be regulated by a balance between positive signaling receptors that initiate and inhibitory receptors that suppress cell activation. Both groups of receptors recognize HLA class I ligands. For example, the KIR2D receptors differentiate between HLA-C ligands based on the presence of specific amino acid residues at codons 77 and 80 (i.e., asn77lys80 vs. ser77asn80). Another NK receptor, KIR3D, recognizes the HLA-Bw4/Bw6 polymorphism. A third NK receptor, CD94/NKG2, recognizes the HLA-E molecule complexed with the HLA leader peptide from some of the allelic products of HLA-A, -B, -C, and -G molecules ( ).
NK cells can specifically recognize and lyse allogeneic cells that do not express the specific class I molecules expressed by the effector cell (i.e., “missing self”) ( ). This could occur even in apparently HLA-matched transplant pairs where one member of the pair is homozygous and the other is heterozygous (e.g., donor: Bw4 and Bw6; recipient: Bw6). Thus Bw4-specific NK cells from the donor might lyse cells without Bw4 from the recipient in a hematopoietic progenitor cell graft. This response may be unidirectional in some settings. In the example, the donor does not lack any HLA molecule present in the recipient; thus the recipient NK cells will not detect any missing self. Because NK cells are relatively radioresistant and make up a subpopulation of about 10% to 15% of peripheral blood lymphocytes, the NK response could be substantial; however, the extent of the role of NK activity in affecting transplant outcome is not known at present ( ). Since then, based on new knowledge of NK cell function, several donor selection strategies have been used in an attempt to improve the outcome of unrelated donor transplantation ( ). These strategies include (a) the receptor-ligand model, (b) the missing ligand model or donor-recipient KIR ligand mismatch, (c) natural cytotoxicity model, and (d) the KIR gene-gene model or donor selection based on B haplotype content. These strategies have yielded a variety of outcomes that may depend on the type of malignancy; for example, donor selection based on the KIR gene-gene content of donor NK cell receptor has been shown to improve survival in acute myelogenous leukemia (AML) but not acute lymphoblastic leukemia (ALL) ( ). Also, haploidentical NK expanded cell transplantation using the KIR-HLA ligand mismatched model was used to successfully treat children with AML using a low-intensity protocol ( ).
As many as 20 additional nonclassical or class Ib genes have been identified within the class I region of chromosome 6 by gene cloning ( ). Many of these are pseudogenes; however, several encode and express class I–like mRNA and/or molecules HLA-E, -F, and -G. These molecules may be key mediators of both adaptive and innate immune responses ( ). Their genes are homologous to classical class I genes in structure, and the polypeptides encoded by these loci also associate with β 2 microglobulin. However, just as a high level of allelic polymorphism is a hallmark of the HLA-A, -B, and -C loci, a low level of polymorphism of the HLA-E, -F, and -G loci is a defining feature of these genes. In addition, by comparison with classical class I molecules, the cell surface expression of nonclassical class I molecules is low, and the distribution of these class Ib molecules on specific cell types varies.
HLA-G is expressed principally on extravillous cytotrophoblast cells at the fetal/maternal interface, where it is thought to play a role in maternofetal tolerance ( ). HLA-G, as a ligand for at least three NK and other cell inhibitory receptors, protects the invading placental tissue from the cytolytic action of NK cells. Although HLA-G has the capability to bind and present processed antigen (i.e., peptides) to T cells, the diversity of peptides bound by HLA-G appears to be less than the diversity of peptides bound by classical class I molecules. Regulation of expression of HLA-G also differs from that of classical class I genes, as the gene contains no interferon response signal; thus it is not interferon inducible. It has been hypothesized that the soluble forms may act as specific immunosuppressors during pregnancy ( ). Recent evidence supports this hypothesis. HLA-G transcripts are present in great numbers in placental extravillous membranes in the first trimester, but at term transcript numbers decline sharply in keeping with the theory that HLA-G provides protection for the allogeneic embryo from maternal NK cell surveillance ( ). This putative role raises the possibility that HLA-G may play a role in tolerance for transplanted tissues. Along these lines, it is interesting to note that liver-kidney transplant recipients with high concentrations of HLA-G in their sera had low numbers of acute rejection episodes and therefore may require less immunosuppressive therapy ( ).
HLA-E may play a role in protecting a target cell from NK cell–mediated lysis. HLA-E molecules bind a set of almost identical hydrophobic peptides derived from the leader sequences of the classical class I and HLA-G heavy chains. HLA-E displays these bound peptides at the cell surface for recognition by NK cells, providing a check for the integrity of the antigen-presenting pathway ( , ; ). Skin grafting in a transgenic mouse model suggests that HLA-E may be recognized as a transplantation antigen ( ). Recent studies suggest that host NK cells may be able to recognize HLA-E on allogeneic cells and possibly tolerate it, even when it is bound to donor peptides. With this finding comes the potential for exploiting this tolerance in pursuit of preventing NK cell–mediated graft rejection and graft-versus-host disease (GVHD) ( ).
The role of HLA-F is unknown. Computer modeling predicts that certain amino acid residues of HLA-F could contribute to a putative peptide-binding groove, consistent with a role in antigen presentation by HLA-F ( ). To date, HLA-F appears to be normally expressed only on the surfaces of extravillous trophoblasts that have invaded the maternal decidua, and has also been found on the surfaces of lymphoblastic cell lines infected with Epstein-Barr virus ( ).
The class II molecules in humans were first recognized by their ability to stimulate allogeneic T cells in the mixed leukocyte culture (MLC). During the 1975 International Histocompatibility Workshop, the MLC was used to define the HLA-D allelic series ( ). Because MLCs required 7 days before results could be obtained, rapid serologic detection of HLA-D was sought. In 1975, it was determined that alloantisera contained antibodies reactive with molecules closely associated with the specificities previously identified as HLA-D by cellular techniques ( ). Following the 1977 International Histocompatibility Workshop, these serologic specificities were termed DR, for D-related specificities, because HLA-D and HLA-DR were observed to be associated but not identical to each other. Serologic testing coupled with genetic and immunochemical studies identified additional class II molecules: DR52, DR53, DR51, and DQ. Cellular techniques identified still another class II molecule in the late 1970s ( ). This molecule (now termed HLA-DP ) was initially described as a secondary B-cell antigen (SB) because it was usually weak or undetectable in a primary MLC and required secondary phase stimulation in culture for detection. Subsequently, the DNA coding sequences and locations of these genes within the MHC were further characterized using molecular biology techniques ( ).
The class II HLA-DR, -DQ, and -DP molecules are heterodimers consisting of two noncovalently associated transmembrane glycoproteins: an α chain (33–35 kDa) and a β chain (26–28 kDa) ( ). Both polypeptide chains span the cell membrane and are oriented with their amino-termini on the outside of the cell. The extracellular regions of the α and β polypeptides are each divided into two domains, designated α 1 and α 2 and β 1 and β 2 , and each domain consists of approximately 90 amino acid residues. The α chain has two carbohydrate moieties, one high-mannose and one complex-type glycan, at amino acids 78 and 118, respectively. β chains have one complex-type oligosaccharide at amino acid 19. The amino-terminal α 1 and β 1 domains of α and β chains contain the polymorphic residues, while the membrane proximal α 2 and β 2 domains are highly conserved and are homologous to immunoglobulin constant region domains. A region of approximately 12 amino acids in length connects the second extracellular domain to the hydrophobic transmembrane region (23 amino acids) and a small intracytoplasmic domain (8–15 amino acids).
Based on x-ray crystallography, the class II molecule is similar in structure to the class I molecule ( ) (see Fig. 50.8 ). In the class II molecule, the α 1 and β 1 domains of the α and β chains form an eight-stranded β-pleated sheet topped by two α helices to form the antigen-binding groove at the top of the molecule. The α 2 and β 2 domains each form two antiparallel β-pleated sheets that support the groove at the top.
Similar to the class I molecules, the class II molecules are highly polymorphic (see Table 50.2 and Fig. 50.4 ) ( , ; ). Class II polymorphisms have been defined by (1) class II–specific antibodies (alloantisera and monoclonal antibodies), which bind to class II molecules preferentially recognizing polymorphisms in the α helices; (2) alloproliferative T cells that recognize and proliferate in response to foreign class II molecules in vitro (the MLC); (3) two-dimensional gel electrophoresis of isolated class II molecules; (4) hybridization of restriction endonuclease digests of DNA-encoding class II genes using locus-specific probes (a technique that detects restriction fragment length polymorphism); (5) PCR-based DNA typing of class II alleles; and (6) direct nucleotide sequencing of class II genes. Most of the immunologically relevant class II polymorphism arises from amino acid sequence differences localized in the α 1 and β 1 domains of the two polypeptide chains. Similar to class I, amino acid polymorphisms that lie within the peptide-binding groove of class II influence the peptide-binding specificities for the each of the different class II allelic products. It is important to note here that for HLA-DQ and -DP, the α and β chains are highly polymorphic; in contrast, the α chain that associates with DRB1, DRB3, DRB4, and DRB5 is essentially nonpolymorphic.
In contrast to the class I molecules, both the α and β chains of class II molecules are encoded within a 1100-kb section of the MHC region (see Figs. 50.1 and 50.9 ) ( ). The class II region includes three subregions—DR, DQ, and DP—each of which encodes at least one expressed A (encoding an α chain) and B (encoding a β chain) gene. Sequence comparisons suggest that both class I and class II genes arose through a successive series of gene duplications during the evolution of this gene complex. The original duplication in the class II region most likely gave rise to primordial A and B genes. More recent gene duplications generated the DR, DQ, and DP subregions containing multiple genes.
A typical A gene contains five exons encoding (1) the 5′ untranslated region and leader sequence; (2) the α 1 domain; (3) the α 2 domain; (4) the connecting peptide, transmembrane region, cytoplasmic tail, and a portion of the 3′ untranslated region; and (5) the remainder of the 3′ untranslated region, including the poly(A) addition signal. A typical β chain gene is similar to the α chain gene but has an extra exon for the cytoplasmic tail.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here