Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews human leukocyte antigen (HLA) and human neutrophil antigen (HNA) systems. A general background of the structure, function, and nomenclature of both systems and their relevance in clinical hematology is presented. Analysis of HLA gene products is applied in clinical settings (1) to select compatible donor-recipient pairs for transplantation, (2) to select HLA-compatible single-donor platelet products for thrombocytopenic patients refractory to standard transfusion of random pooled platelets, (3) to screen for genetic factors that may contribute to the prevalence of diseases, and (4) for forensic purposes in which the identity of individuals may contribute to solving legal disputes or criminal investigations. In addition, we discuss new applications that have broadened the relevance of HLA in the area of immune pathology. HLA phenotypes determine the suitability of patients for epitope-specific immunization. Tetrameric HLA/epitope complexes (tHLA) allow enumeration of antigen-specific T-cell responses. Furthermore, molecular identification of T-cell epitopes associated with distinct diseases and characterization of the communication between immune effector cells through HLA-HLA ligand interactions extend the relevance of HLA to biologic fields. These biologic fields encompass natural killer (NK) and cytotoxic T-cell function, antigen recognition in the context of infection, autoimmunity, the graft-versus-neoplasia (GVN) effect, and autologous cancer rejection. Finally, the recognition that polymorphism extends to other protein families relevant to immune pathology including cytokines, their receptors, and killer cell–inhibitory receptors has broadened the significance of immunogenetics beyond HLA. Thus, this chapter emphasizes the importance of viewing human pathologic conditions through the kaleidoscopic complexity of human polymorphism.
HLAs comprise a family of genes clustered in the short arm of chromosome 6 as the human version of the major histocompatibility complex (MHC), initially identified in mice as responsible for graft rejection between genetically unrelated strains (transplantation antigens). Credit for the description of the human MHC goes to three individuals. In 1952, Jean Dausset observed that the serum of individuals who had received several transfusions contained hemagglutinins specific for donors’ leukocytes. In 1958, Rose Payne noted that the only requirement for the development of hemagglutinins against leukocytes was a history of previous transfusion or pregnancy and concluded that these antibodies were directed against antigens on the surface of circulating leukocytes. This conclusion was concomitantly and independently confirmed by Jon van Rood, who observed that multiple pregnancies immunize mothers against leukocytes leaked from the fetus into the mother’s circulation. Based on these discoveries, the term human leukocyte antigen was subsequently adopted. It should be clarified, however, that this historical name is misleading. HLA molecule expression is not limited to leukocytes nor do they display, in natural conditions, antigenic behavior. In fact, several are expressed by most somatic cells, and, rather than being antigens, they chaperone protein bioproducts to the cell surface for recognition by T cells. There is, however, some substance to the name, because HLAs, by virtue of being densely packed on the cell surface, are exposed to recognition in a foreign environment, such as allotransplantation or xenoinfusion performed to induce anti-HLA antibodies as diagnostic reagents.
HLA genes constitute a string of coding sequences that regulate the expression of molecules with similar but not identical function. Residing in a region that spans approximately 4000 kilobases of the short arm of chromosome 6 ( Fig. 114.1 ), HLA contains several genes and pseudogenes characterized by sequence homology and functional similarity. Of them, 47 are officially recognized by the World Health Organization (WHO) Nomenclature Committee and include classic HLA class I and class II genes associated with antigen processing, such as PSMB8 and PSMB9 (proteasomal units) or TAP1 and TAP2 associated with peptide transport. Both HLA and HLA-associated genes can be physically grouped into three subregions according to chromosomal location. In the centromeric to telomeric direction, the first is HLA class II region comprising the α-and β-chains of HLA-DR, -DQ, -DP, -DM, and -DO as well as TAP and PSMB. Sandwiched between the class II and class I region, the class III region encodes for functionally unrelated genes such as complement components, heat shock proteins, and the tumor necrosis factor. The reason for their genetic link to the HLA complex is unknown, but their immunologic function seems more than coincidental. The class I region is most telomeric and includes HLA-A, -B, and -C loci; the nonclassic HLA-E, -F, and -G loci; and several pseudogenes.
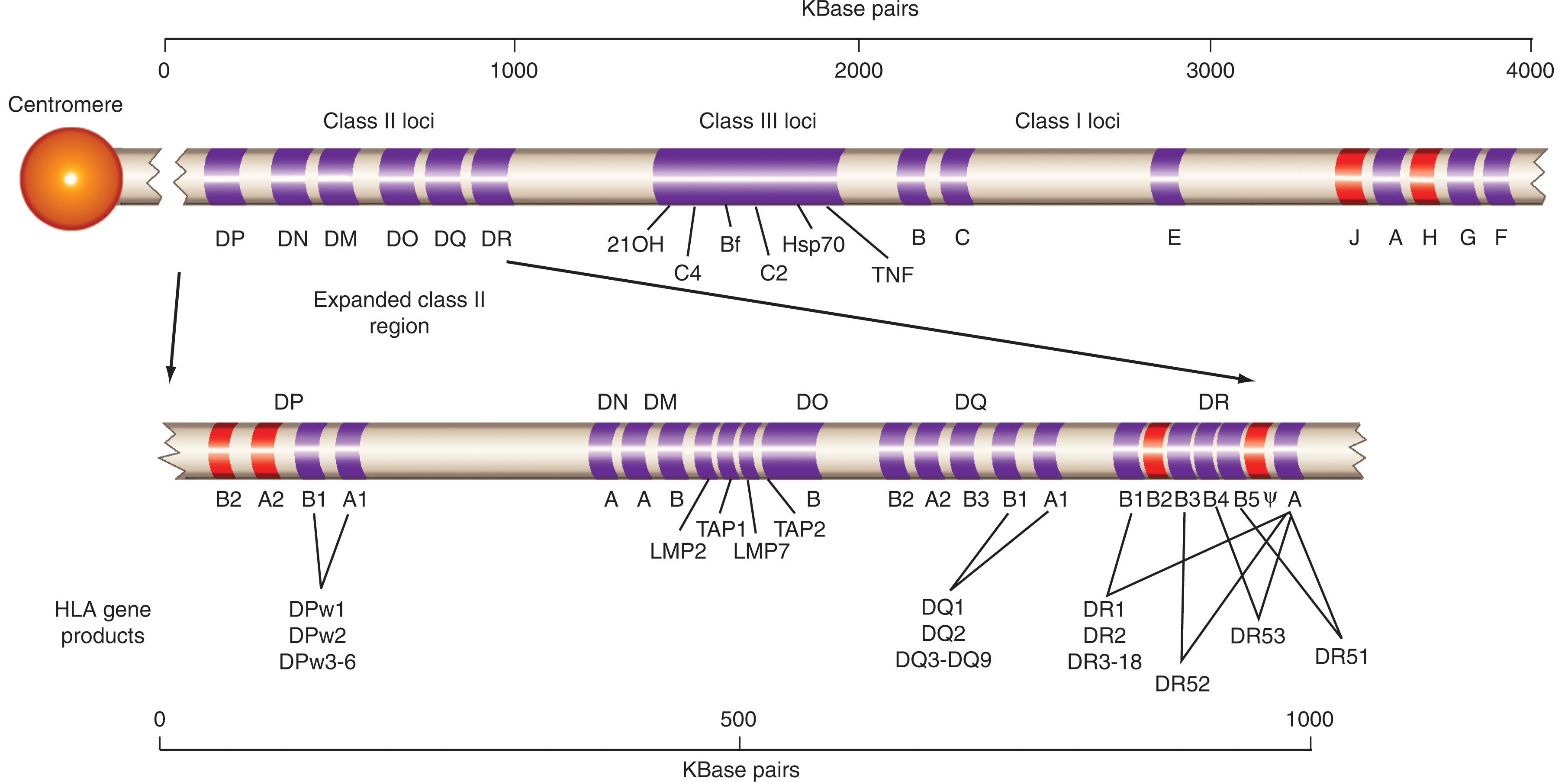
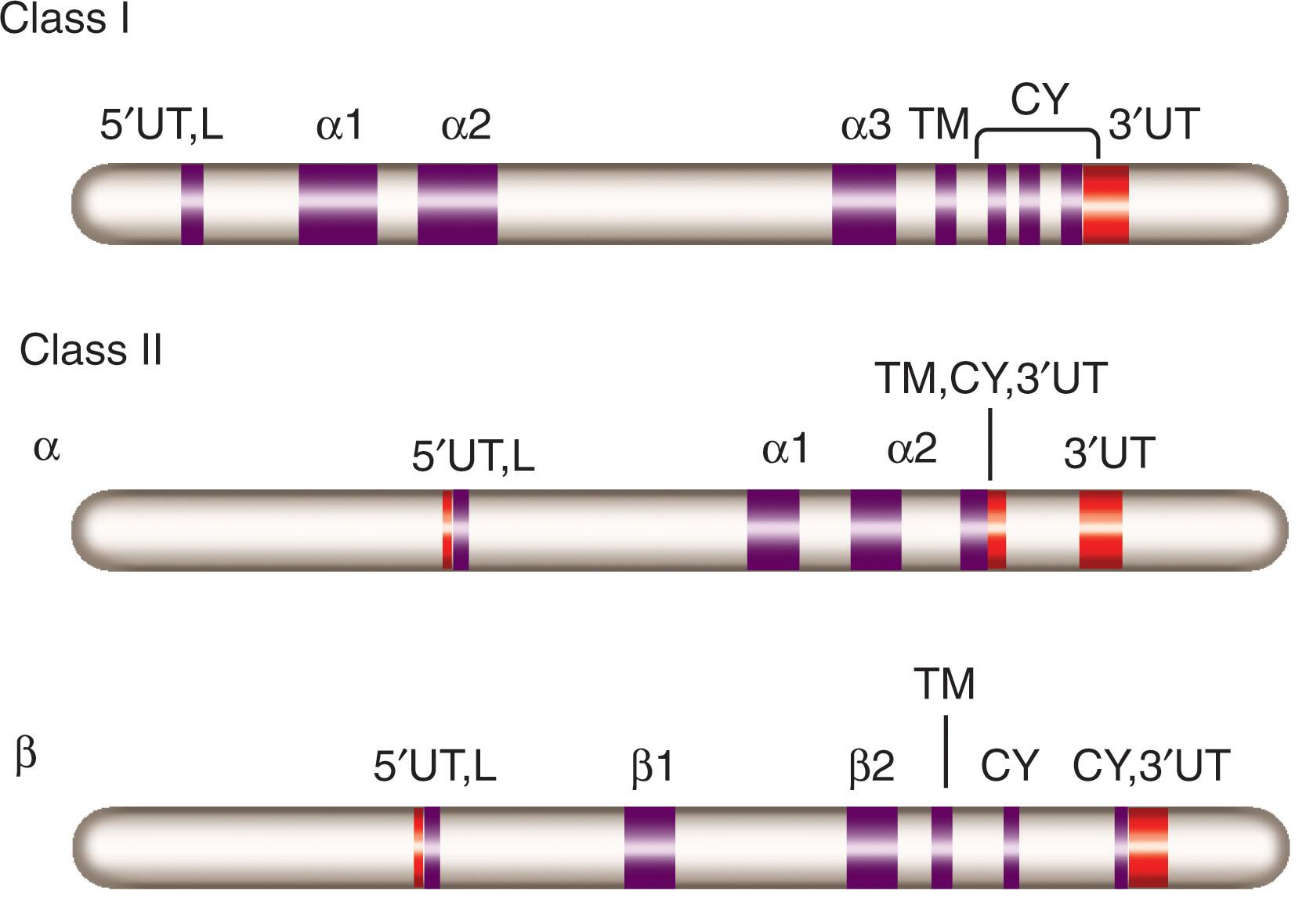
General terminology separates HLA genes into classic and nonclassic. Classic HLA genes have been well characterized and are clearly associated with the presentation of antigens to immune cells. They are further subdivided into class I (HLA-A, -B, -C) and class II (HLA-DR, -DQ, and -DP). In general, HLA class I and II genes have very similar structure and function. They contain six to eight exons coding for functionally distinct domains ( Fig. 114.2 ). The first exon encodes a leader sequence; the following exons (exons 2 to 4) are highly polymorphic and encode extracellular domains responsible for peptide binding and T cell–antigen receptor (TCR) engagement. Because they are exposed on the cell surface, these domains are also responsible for alloreactivity. The last exons encode a conserved transmembrane and small intracellular domains whose function is unclear.
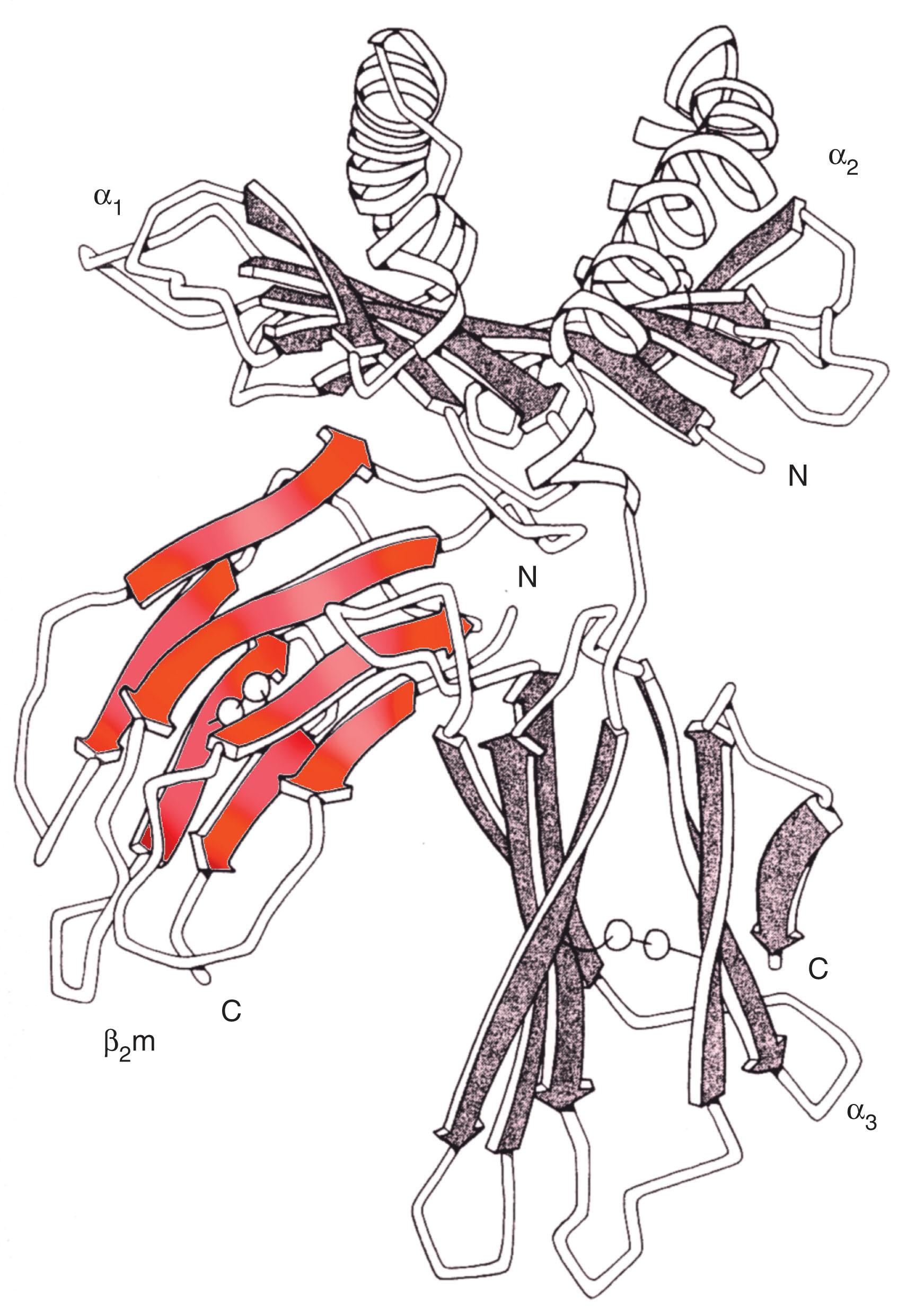
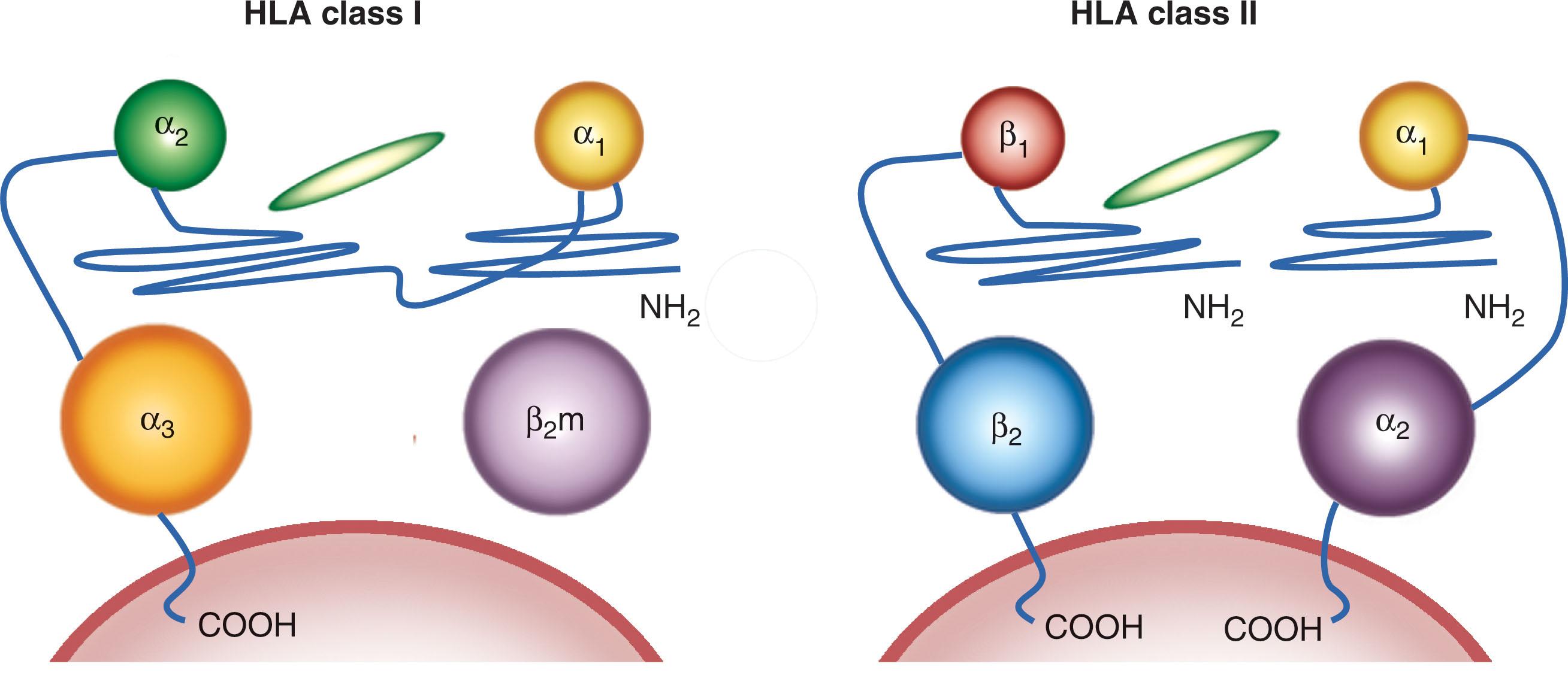
Only the heavy chain of HLA class I is encoded in the MHC region. Genes encoding HLA-A, -B, and-C contain three exons coding for α 1 , α 2 , and α 3 extracytoplasmic domains, one transmembrane, and three cytoplasmic domains ( Fig. 114.3 ). The associated class I light chain, β 2 -microglobulin, is encoded on chromosome 15. By contrast, the HLA class II molecule is composed of a heterodimer of an α-chain and a β-chain encoded in the MHC region. Although the genetics are different, the protein product is structurally similar to HLA class I, with two helices resulting in the antigen-presenting part of the molecule ( Fig. 114.4 ).
All DR molecules use DRA for α-chains but can use alleles coded by DRB1, DRB3, DRB4, or DRB5 for β-chains. DP, DQ, DM, and DO molecules are the product of DPA1 and DPB1, DQA1 and DQB1, DMA and DMB, and DOA and DOB genes, respectively. The HLA DRB1 locus is expressed in all HLA haplotypes (the set of HLA alleles derived from the same parental chromosome and therefore genetically linked). However, only one other HLA DR locus is present in each individual chromosome. Thus, each haplotype can have either DRB5 (DR1 haplotype); DRB3, -B4, or -B5 loci (DR2 haplotype); DRB4 locus (DR4 and DR7 haplotype); or none (DR8 and DR10 haplotype).
Because of their proximity within a short chromosomal distance, HLA genes are inherited en bloc from each parent unless a recombinant event occurs. Thus each HLA haplotype behaves as a unit and is transmitted through generations according to mendelian principles. Because there are four possible genotypes (two from each parent), the probability of genotypic identity between two siblings is 25%. Most HLA phenotypically identical siblings are also HLA genotypically identical, because the genetic pool of derivation is restricted to the parents. In 2% of cases, recombinant HLA haplotypes (a set of genes derived partially from two chromosomes through recombination) deviate from this rule.
The occurrence of HLA haplotypes within a population with a frequency higher than expected from the prevalence of individual alleles is called linkage disequilibrium . In large populations, gene frequencies achieve equilibrium within a few generations unless selective pressure influences individuals’ survival and mating capacity (Hardy-Weinberg principle). In equilibrium, gene prevalence is maintained based solely on its frequency. Thus, assuming that there were 18, 36, and 8 alleles for the HLA-A, -B, and -C loci, respectively (number of alleles known when this example was described ), theoretically, 18 × 36 × 8=5184 HLA class I allelic combinations or haplotypes would be possible. Adding HLA class II genes to the calculation yields an astronomical number, making the identification of two HLA-matched individuals unlikely. However, individual alleles occur with different frequency, and an allele that occurs with high frequency is predominant in a given population, such as HLA-A2 in persons of European ancestry and A24 or A11 in Asians. Because predominant alleles come with the related haplotype, most theoretical permutations never occur, and the chances of identifying matched individuals are much higher than theoretically possible.
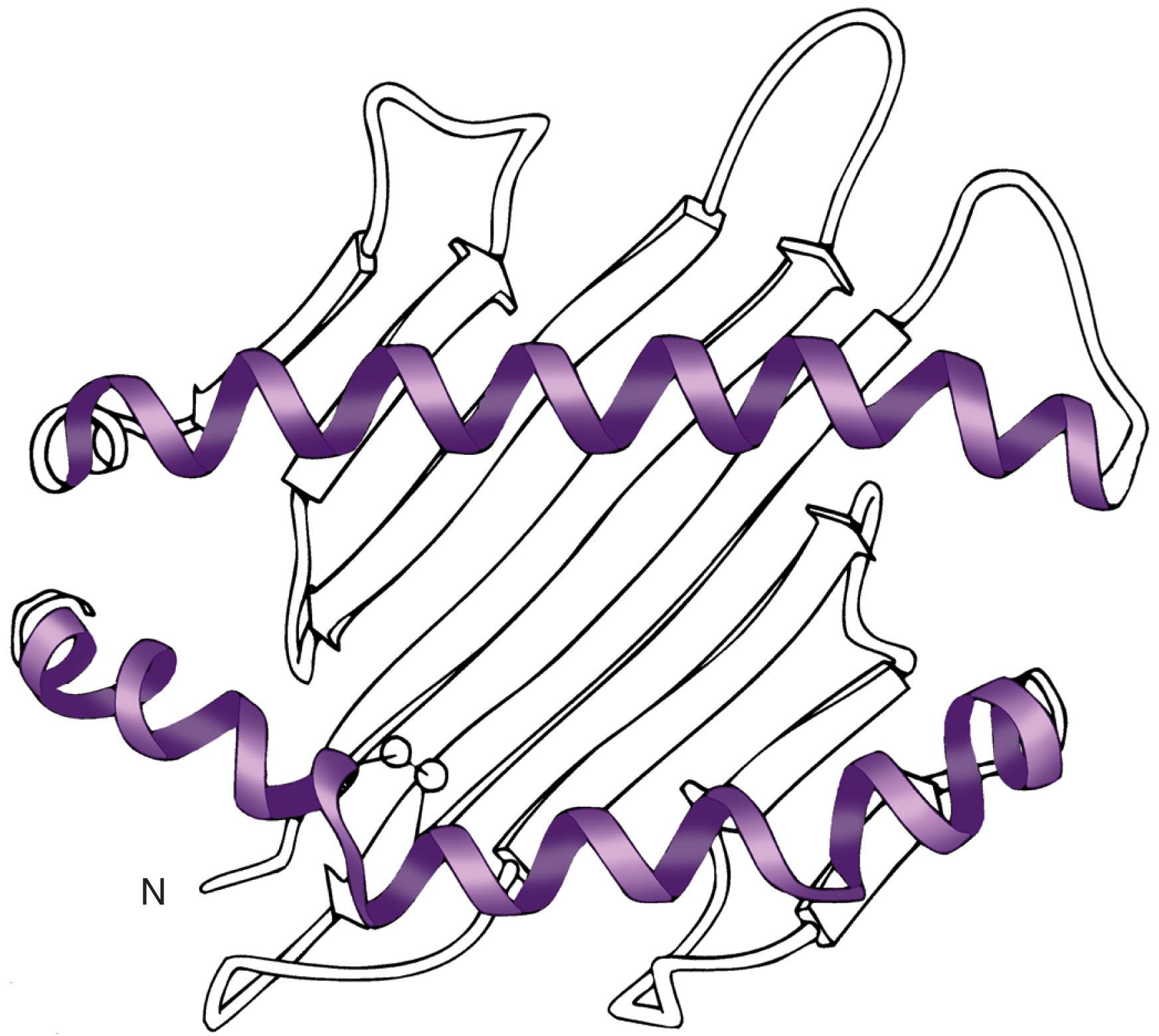
The structure of HLA molecules and their relationship with their natural ligand, the TCR, has been well characterized by crystallography. HLA molecules are heterodimer glycoproteins belonging to the immunoglobulin superfamily with common features (see Fig. 114.4 ). This includes two α-helical domains protruding toward the extracellular milieu. Between them lies a flat surface formed by β-sheet structures that contributes to the formation of a groove accommodating peptides generated from intracellular (HLA class I) or extracellular proteins (HLA class II) ( Fig. 114.5 ). The helices/peptide complex is exposed for TCR recognition. Because HLA polymorphism is clustered within the α-helixes and β-sheets domains, peptides display variable affinity for distinct HLA alleles. It has been proposed that a given peptide can bind to closely related alleles, and HLA superfamilies with similar binding characteristics have been described. However, peptide binding to related but distinct HLA alleles is associated with conformational dissimilarity caused by differential interaction with variant residues in the binding groove. The TCR interaction with HLA required for productive engagement spans a surface including the peptide and portions of the α- and β-helix. This double requirement of interaction between the TCR and the HLA/peptide complex represents the structural basis for HLA restriction. Degenerate and promiscuous TCR recognition of peptides presented by distinct HLA alleles within the same superfamily has also been described. Although this concept holds in general, several exceptions can be expected because single amino acid variants in the HLA molecule may disallow binding of a peptide or may not be permissive to TCR engagement.
The binding affinity of a given peptide for an HLA allele can be predicted through algorithms that compile available information to identify amino acid residues that fit distinct pockets of the HLA groove. Several algorithms implement this information with experimental testing based on the refolding capability of HLA heavy chains exposed to known peptide sequences in the presence of β 2 -microglobulin and/or their dissociation rates (see www.bimas.dcrt.nih.gov or www.uni-tuebingen.de/uni/kxi ). This is based on the principle that the affinity of a peptide for a given HLA promotes the stability of the noncovalent assembly of heavy chain with β 2 -microglobulin. Finally, peptide binding can be shown by direct elutriation from purified HLA heavy chains.
Beyond the interactive component of the HLA molecule with the TCR, other domains act as coreceptors for the TCR. CD8 + cytotoxic T cells bind to the α 3 -domain of HLA class I through CD8, whereas CD4 + T cells interact with HLA class II–specific domains. Although the coreceptor-TCR interaction is not an absolute requirement in most cases, it determines HLA class I or II restriction of individual T cells. Specific to HLA class I is the assembly with β 2 -microglobulin on stabilization in the presence of high-affinity peptides derived from the cleavage of intracellular proteins (endogenous pathway of antigen presentation). Most peptides derive from degradation of self-protein in the cytosol by proteasomes (PSMB) and other proteases. Soluble peptides (9 to 10 amino acids long) are then chaperoned into the endosomal compartment by transporter molecules associated with antigen processing (TAP1 and TAP2). Within this compartment, they bind to heavy chains according to each individual’s HLA phenotype. The binding and stability of the HLA-peptide complex depends on the affinity of each peptide for a particular allele. If the stability is sufficient, the peptide-loaded HLA migrates to the cell surface. Intracellular pathogens produce proteins that are also degraded by proteasomes into peptides that compete with self-peptides for binding to HLA class I molecules. Thus, the function of the endogenous pathway of antigen presentation is to provide information to the extracellular compartment of intracellular events. In physiologic conditions, only self-peptides are presented, and therefore minimal interactions occur with circulating T cells. During infection, pathogen-derived peptides signal cellular infection to T cells, whereas antibodies that cannot cross the cell membrane remain insensitive to intracellular pathogens. Several pathogens, such as cytomegalovirus, can interfere with this process as well as with reduction of HLA/peptide density on the cell surface and, consequently, diminished T-cell recognition. This escape mechanism can be counteracted by the host through increased susceptibility of virally infected cells to NK cell-mediated cytolysis. Reciprocally, viruses can evade NK cells and escape recognition by modulating HLA expression.
HLA class II molecules bind to longer peptides derived from the metabolism of molecules endocytosed from the extracellular compartment (exogenous pathway of antigen presentation). This is a specialized process used by professional antigen-presenting cells, such as macrophages and dendritic cells. HLA class II molecules are assembled within the endosomal compartment where they are composed of a heterotrimer. This includes the α- and β-chains plus a short invariant chain that stabilizes the molecule by occupying its groove while chaperoning its migration to the endosomal compartment where exogenous antigen is processed. Upon uptake, the exogenous antigen undergoes limited proteolysis in the membrane-bound acidic endosomal compartment (MHC class II peptide-loading compartment, MIIC). Upon entering the MIIC, the pre-HLA class II complex is degraded, and antigenic peptides are loaded with the help of the nonclassic HLA-DM molecule.
There are genetic and structural differences between the two classes of HLA alleles. The most striking difference is functional, because HLA class I molecules are expressed by most nucleated cells with the exception of germinal cells, whereas HLA class II molecules are expressed mainly by specialized antigen-presenting cells. HLA class I molecules are also expressed by platelets. They are responsible for refractoriness after multiple transfusions and sufficiently, though minimally, expressed by reticulocytes to be targets of alloantibodies during hemolytic transfusion reactions. HLA class II proteins are expressed constitutively by cells associated with the initiation of the immune response, such as monocytes, macrophages, and B cells, or by immune cells activated by cytokines during inflammation. Thus HLA class I molecules instruct the host about the condition of individual cells as potential targets for CD8 + cytotoxic T cells responsible for clearing the organisms of altered cells. HLA class II molecules initiate immune responses by taking up pathogen components and presenting them to CD4 + helper T cells, which can in turn initiate humoral and facilitate cellular immune responses.
Expression of HLA alleles is sensitive to environmental conditions and can be modulated by cytokines among which interferons play a major role. This is particularly important for HLA-B and HLA-C normally expressed at a lower density than HLA-A but more sensitive to cytokine induction. In addition, HLA class II molecules can be expressed by most cells following cytokine stimulation. Thus the ability of cells to present antigens in association with HLA is strongly influenced by the surrounding environment. This might explain why chronic inflammation induced by alloreactions during graft-versus-host disease (GVHD) may facilitate the presentation of tumor-specific antigens by tumor cells with consequent development of the GVN effect. In addition, it might explain how systemic administration of proinflammatory cytokines such as interleukin-2 may stimulate immune responses by increasing the antigen-presenting ability of cells within the tumor microenvironment.
A striking characteristic of the HLA system is its extreme polymorphism. By chance, most individuals are heterozygote and therefore carry two different alleles for each HLA gene that, being codominant, are equally expressed on the cell surface. Because everybody carries three HLA class I (-A,-B, and -C) and three HLA class II (-DR, -DQ, and -DP) genes, most individuals (with the exception of homozygotes) express six different HLA class I and six different HLA class II molecules on the surface of their cells. This has important functional implications, because HLA polymorphism is clustered in domains of the HLA molecule associated peptide binding and interaction with the TCR. Thus, most individuals have a broad repertoire of molecules capable of presenting different pathogen components to immune cells. Therefore, it is believed that HLA polymorphism improves the chances of a given species of surviving infection. This paradigm is difficult to demonstrate in human pathologic conditions in which the natural history of infectious diseases rarely correlates with HLA phenotype. An exception is the strong association between HLA-B*5701 and lack of progression to acquired immunodeficiency syndrome of individuals infected with human immunodeficiency virus (HIV). Associations have been observed between HLA phenotype and predisposition for nasopharyngeal carcinoma. This is of particular interest because nasopharyngeal carcinoma is a virally induced cancer against which T cells can mediate immune surveillance. HLA associations have also been described with less consistency for other virally driven tumors, such as cervical carcinoma, or immunogenic tumors, such as melanoma.
The difficulty in demonstrating conclusive associations between infectious disease and HLA in humans could be caused by the successful implementation of antigen presentation through a broad repertoire of HLA genes, which could compensate for each other in limitations in antigen presentation. Chickens carry a single MHC locus, and their susceptibility to infection appears to be clearly related to MHC. For instance, Kaufman et al. have shown that chickens carrying a particular Bf (the only MHC class I gene in chickens) allele are fully protected from Rous virus-induced sarcomas because this allele (B-f12) can bind several antigenic peptides from its proteins. On the contrary, chickens homozygous for B-f4 are killed by the same infection because this allele cannot bind peptides from sarcoma virus proteins.
HLA polymorphism is the basis of alloimmunization because most individuals are likely to have different HLA molecules on the surface of their cells. Hundreds of HLA alleles have been identified through high-resolution typing, making the chances of two individuals having identical HLA phenotypes extremely low. Thus partial mismatches are commonly accepted in transplantation cases, which are at the root of hyperacute, acute, and chronic rejections. Hyperacute rejection is caused by preformed antibodies against donor HLA alleles in patients presensitized by multiple transfusions. Acute and chronic rejection result from a combination of humoral and cellular immune reactivity toward HLA alleles of the donor. In addition to allosensitization, HLA alleles can mediate GVHD, whereby hematopoietic cells derived from the grafted tissue recognize and reject the host tissues. They do this by identifying polymorphisms of intracellular proteins of the host (minor histocompatibility antigens [mHags]) presented in association with donor-recipient matched HLA alleles.
Besides the three ubiquitously expressed highly polymorphic classic HLA class I molecules, humans encode three relatively conserved nonclassic, selectively expressed (HLA-E, -F, and -G) MHC class I genes (also known as MHC-Ib). These evolved at different rates in primates reflecting differential involvement in the modulation of immune responses. In addition, these molecules are characterized by unique patterns of transcription, protein structure, and immunologic function.
The MHC class I–related chain genes (MICA and MICB) are located within the MHC region and are characterized by high polymorphism (more than 50 alleles so far identified). The molecules encoded by these genes do not appear to bind peptides or associate with β 2 -microglobulin. Their polymorphic variants are not concentrated around the peptide-binding groove, yet they seem to have functional significance, because most mutations are nonsynonymous, suggesting selective pressure as a driving force. Their tissue distribution is restricted to epithelial and endothelial cells and fibroblasts. It appears that MIC genes modulate the function of NK and CD8 + T cells by binding the NKG2D stimulating receptor. Also, MIC genes have been implicated in transplant rejection because alloantibodies against them are often found in transplant recipients that may exert complement-mediated cytotoxicity against endothelial cells from the graft.
Other unusual MHC-like molecules are present in the genome and have disparate functions, including presentation of lipid antigens (CD1), transport of immunoglobulins (Fc receptor), and regulation of iron metabolism (hemochromatosis gene product). Contrary to classic MHC class I genes that are constitutively expressed, nonclassic MHC and MIC gene expression depends on stimulation by proinflammatory cytokines. In addition, two nonclassic MHC class II proteins (HLA-DM and HLA-DO) have been described that function as mediators of peptide exchange by stabilizing empty MHC class II molecules. Finally, it is possible that several nonclassic MHC molecules whose function is to present peptides to lymphocytes may be present throughout the genome. Because of their limited polymorphism, however, these genes may have evolved to serve specialized presentation functions.
Characterized by low polymorphism, the regulation of HLA-G expression follows a nonclassic behavior. Aberrant cytokine-responsive regulatory sequences may be responsible for its predominant expression by trophoblasts that do not express other HLA proteins. It may also account for its low levels in a variety of human tissues. Lack of responsiveness to common immunostimulatory pathways (NF-κB, interferon-γ, or CIITA) is most pronounced in HLA-G cells and is shared by other nonclassic MHC such as HLA-E. Also, HLA-G is expressed in a variety of cancers. It is hard to know what the relevance of HLA-G expression is because it can occur in various membrane-bound or soluble isoforms with distinct functional characteristics. Functional isoforms that include the α-1 and α-2 domains bind and present peptides from cytoplasmic proteins. Because of the minimal polymorphism, however, the repertoire of peptides presented is likely to be limited, suggesting that peptide binding is necessary to stabilize the molecule rather than being involved in antigen presentation.
Functionally, HLA-G is thought to modulate the function of NK cells through interactions with their inhibitory receptors. In addition, the HLA-G leader sequence contains a peptide that can bind and stabilize the expression of HLA-E, which in turn inhibits NK cells. Because the HLA-G–derived leader peptide has the strongest affinity for HLA-E (among all HLA class I molecules), it is likely that HLA-G is a powerful direct and indirect inhibitor of NK cells, reducing the risk for cardiac rejection or inducing immune escape of cancer cells. Although much has been published about the immunoregulatory role of HLA-G, its true function remains mysterious, principally because of discordant findings reported by various groups. With the goal of achieving consensus, a workshop was recently organized to standardize methods of analysis of HLA-G.
HLA-E is minimally polymorphic, binds hydrophobic peptides from other HLA class I leader sequences, and interacts with CD94/NKG2 lectin-like receptors present predominately on NK and partially on CD8 + T cells. The peptide binding is highly specific and stabilizes the HLA-E protein, allowing its migration to the cell surface. Thus surface density of HLA-E is an indirect reflection of the number of HLA class I alleles expressed by a cell. The interaction of HLA-E with CD94/NKG2 protects HLA-E–expressing cells from killing. Cells damaged by viral infection or neoplastic degeneration may lose HLA class I expression. As a backup mechanism of protection, reduced HLA class I expression results in decreased expression of HLA-E, leading to vulnerability to NK cells. Some viruses express mimic peptides that bind and stabilize HLA-E so that, although classic MHC molecules are downregulated, HLA-E expression is maintained, allowing the pathogen to simultaneously escape CD8 + T- and NK-cell killing.
The function of HLA-F remains enigmatic. Its transcriptional regulation is closest to classic HLA molecules in that it can be induced by NF-κB, interferon regulatory factor-1, and class II trans-activator. However, contrary to classic HLA molecules, HLA-F is predominantly empty, mostly intracellular, with a restricted pattern of expression. Its tissue distribution appears to be limited to B cells, and therefore it is mostly found in lymphatic organs. Structural studies suggest that HLA-F is a peptide-binding molecule and may reach the cell surface under favorable conditions when a suitable peptide is present. Once on the cell surface, HLA-F may interact with the effector-cell receptors IL-T2 and IL-T4, as suggested by HLA-F tetrameric complexes–binding studies. Thus it is possible that in specific yet unknown conditions, HLA-F may modulate the function of immune effector cells similarly to HLA-E and HLA-G.
Although this chapter is dedicated to HLA, it would be incomplete without mentioning the increasingly recognized polymorphisms of other immune modulators. The significance of non-HLA polymorphism is evidenced by the development of GVHD in the presence of HLA identical matching among relatives. As recently summarized, three general areas of polymorphism are being investigated: NK cell–receptor genes, minor histocompatibility antigens, and cytokines. Over the last decade, much progress has been made in identifying the mechanisms of action of NK cells. A major breakthrough was made in the discovery of HLA class I-specific inhibitory receptors and in the role that they play in the regulation of NK function with consequent effects on the eradication of hematologic malignancies, prevention of graft rejection, or induction of GVHD. NK cells recognize HLA molecules via killer immunoglobulin-like receptors (KIRs). The regulation of NK cell function by KIRs is further discussed in Chapter 12 . KIRs are glycoproteins encoded by at least 17 different genes located on chromosome 19q13.4. All human KIR genes derive from a gene encoding three immunoglobulin (Ig)-like domains (D0, D1, and D2) and a long cytoplasmic tail. However, the KIRs genes are diverse and may encode either two or three Ig-like domains and either a long or short cytoplasmic tail ( Table 114.1 ). The long cytoplasmic tails contain one or two immunoreceptor tyrosine-based inhibition motifs. Although KIR molecules with long cytoplasmic tails inhibit NK cytotoxicity, those with short tails do not. The names given to KIR genes are based on the molecule that they encode. The first digit corresponds to the number of Ig-like domains in the molecule and a D denotes domain. The D is followed by either an L for long cytoplasmic tail or S for short cytoplasmic tail or p for a pseudogene. The last digit indicates the number of the KIR gene.
| KIR | HLA Class I Allele | Amino Acid Sequence Motif |
|---|---|---|
| P58.1 (KIR2DL1) | HLA-C2, -C4, -C5, -C6 | Asn 77, Lys 80 |
| P58.2 (KIR2DL2/3) | HLA-C1, -C3, -C7, -C8 | Ser 77, Asn 80 |
| P70 (KIR3DL1) | HLA-Bw4 public specificity | Aa 77–83 |
| P140 (KIR3DL2) | HLA-A3, -A11 | |
| (KIR2DL4) | HLA-G |
Expression of KIR in individual NK cells is complex because NK cells may express several members of the KIR family. The number of KIR genes in each haplotype varies among individuals. The most common haplotype is known as group A, which is made up of six genes (2DL1, 2DL2 or 2DL3, 3DL1, 3DL2, 2DS4, and 2DL4). Various KIR genes can recognize different HLA-A, HLA-B, and HLA-C molecules. HLA-C antigens can be divided into two groups based on polymorphisms at amino acid positions 77 and 80 of their class I heavy chains. One group has asparagine (Asn) at position 77 and lysine (Lys) at 80, and the other has serine (Ser) at 77 and Asn at 80. Some KIRs recognize HLA-C antigens with Asn 77 and Lys 80, whereas other KIRs recognize HLA-C antigens with Ser 77 and Lys 80. The polymorphism at position 80 is most important. Another group of KIR reacts with HLA-B antigens that carry specific combinations of amino acids at positions 77 and 83 of the heavy chain that forms HLA-Bw4. Because a single KIR can interact with multiple HLA class I alleles, KIR recognition of HLA class I molecules is degenerate. Another NK inhibitory receptor (CD94-NKG2A) recognizes the nonclassical HLA-E molecule. In addition, each of the KIR genes is extensively polymorphic.
Because the genes for KIR, HLA, and CD94-NKG2A are located in separate chromosomes, they segregate independently, and consequently individuals can carry genes for KIR for which there is no correspondent HLA ligand. Because HLA-E is expressed in all individuals, NK cells that bear the CD94-NKG2A receptor are not alloreactive. Because the specificity of KIR for their ligands is broad and each individual carries several KIRs, it is likely that in most cases all NK cells of a given person express at least one KIR that is specific for a self-HLA class I allele. Thus in autologous settings, NK cells kill only aberrant cells that have lost HLA class I expression. By contrast, NK cells can kill allogeneic cells that do not express HLA class I alleles recognized by their KIR. Thus, by knowing the KIR repertoire of a given transplant recipient and the HLA type of the donor, it would theoretically be possible to predict the likelihood of an NK-mediated alloreaction. Importantly, it appears that alloreactive NK cells undergo proliferation on exposure to the stimulatory cells and therefore can preferentially expand in the presence of allogeneic tissue. NK cells also express activating receptors that are responsible for their lytic activity. Although the identity of the ligands for these receptors has not been identified, it is possible that they are expressed primarily by activated or proliferating cells. It is therefore possible that during the inflammatory process induced in allogeneic conditions, normal cells can become activated by cytokines and express ligands, which are responsible for NK activation in the absence of HLA class I molecules reactive with the inhibitory receptors. The relevance of KIR in transplantation has been well studied in the context of haploidentical hematopoietic transplantation. In this case, several combinations are possible: NK cells from the graft express KIRs that do not interact with the donor’s HLA (graft-versus-host alloreactivity). It seems that the presence of graft-versus-host reactive NK cells associated with incompatibilities between donor and recipient (especially HLA-C families) has favorable effects on the outcome of acute myeloid leukemia. Alternatively, a good match may be present between graft NK and host HLA as well as between host NK and graft HLA. In such a case, no alloreactivity occurs. Finally, the graft’s HLA type may be unsuitable for the host’s NK repertoire, and the host’s reactivity may lead to graft rejection. Alloreactive grafted NK cells seem to prevent GVHD while inducing GVN.
Like HLA, KIR genes are polymorphic, and their variability is clustered in positions likely to affect the overall structure of the molecule. The relevance of KIR gene polymorphism in the outcome of bone marrow transplantation (BMT) is unclear. It appears that the risk for GVHD is highest in the context of unrelated BMT when the recipient KIR genotype is included in the donor KIR genotype. These results show that compatibility between KIR genotypes themselves may influence the outcome of BMT.
The minor histocompatibility antigens (mHags) are represented by polymorphic molecules whose peptides containing variant sequences are presented by HLA alleles. They have been shown to be targets of cytotoxic T cells, which can lyse leukemia cells. In addition, some mHags are selectively expressed by neoplastic cells. At present, little is known about the identity of mHag epitopes in the context of various HLA types and their significance in the development of GVHD and GVN.
Cytokines are another large family of molecules associated with antigen recognition, graft rejection, and GVHD. Their polymorphism is becoming an important area of investigation in the context of transplantation, autoimmunity, and cancer. Polymorphic sites reside in regulatory regions so that genetic variants are associated with high or low production of a given cytokine rather than differences in its function. A website compiles information about cytokine polymorphism ( www.bris.ac.uk/Depts/PathAndMicro/services/GAI/cytokine4.htm ). Although no consensus has been achieved yet, several studies have shown associations between various cytokine genotypes, the propensity for disease, and transplant outcome. These studies have been summarized elsewhere. A strong association was recently noted between a low interleukin-10 producer genotype and a tendency to develop melanoma and prostate cancer.
The history of the HLA system nomenclature was summarized by Sir Walter Bodmer who, together with Ruggero Ceppellini, was primarily involved in its development. It began as HL-A, for human leukocyte locus A . With the recognition that HLA molecules are encoded by more than one locus, the A came to stand for antigen , and a locus designation was added after HLA (i.e., HLA-A, -B, -C, -D, etc.) . From then on, the WHO has updated nomenclature on a quarterly basis. The most recent update was assigned in January 2015, as seen in Table 114.2 . At present, two systems are used. An immunologically defined nomenclature is based on the identification of HLA antigens on the surface of leukocytes. Therefore, HLA phenotypes described by immunologic methods are conventionally called HLA antigens . The second system is based on the molecular identification of nucleotide sequences in genomic deoxyribonucleic acid (DNA), and results are conventionally referred to as HLA alleles . Because molecular typing has a higher resolution, it has gained increasing popularity.
| Name | Previous Equivalents | Molecular Characteristics | No. of Alleles January (2015) |
|---|---|---|---|
| HLA-A | – | Class I α-chain | 3107 |
| HLA-B | – | Class I α-chain | 3887 |
| HLA-C | – | Class I α-chain | 2623 |
| HLA-E | E, “6.2” | Associated with class I 6.2-kBa Hind III fragment | 17 |
| HLA-F | F, “5.4” | Associated with class I 5.4-kBa Hind III fragment | 22 |
| HLA-G | G, “6.0” | Associated with class I 6.0-kBa Hind III fragment | 50 |
| HLA-H | H, AR, “12.4”, HLA-54 | Pseudogene association with class I 5.4-kBa Hind III fragment | 12 |
| HLA-J | cda 12, HLA-59 | Pseudogene association with class I 5.9-kBa Hind III fragment | 9 |
| HLA-K | HLA-70 | Pseudogene association with class I 7.0-kBa Hind III fragment | 6 |
| HLA-L | HLA-92 | Pseudogene association with class I 9.2-kBa Hind III fragment | 5 |
| HLA-X | – | Class I gene fragment | 0 |
| HLA-DRA | DRα | DR α-chain | 7 |
| HLA-DRB1 | DRβI, DR1B | DR β 1 determining specificity for DR1, DR2, DR3, etc. | 1726 |
| HLA-DRB2 | DRβII2 | Pseudogene with DRβ-like sequence | 1 |
| HLA-DRB3 | DRβIII, DR3B | DR β3 determining DR52, Dw24, w25, -26 specificity | 59 |
| HLA-DRB4 | DRβIV, DR4B | DR β4 determining DR53 specificity | 15 |
| HLA-DRB5 | DRβV, DR5B | DR β5 determining DR52, Dw24, w25, -26 specificity | 21 |
| HLA-DRB6 | DRBX, DRBσ | Pseudogene found in DR1, DR2, and DR10 haplotypes | 3 |
| HLA-DRB7 | DRBψ1 | Pseudogene found in DR4, DR7, and DR9 haplotypes | 2 |
| HLA-DRB8 | DRBψ2 | Pseudogene found in DR4, DR7, and DR9 haplotypes | 1 |
| HLA-DRB9 | M 4.2 β exon | Pseudogene isolated fragment | 1 |
| HLA-DQA1 | DQα1, DQ1A | DQ α-chain as expressed | 54 |
| HLA-DQB1 | DQβ1, DQ1B | DQ β-chain as expressed | 780 |
| HLA-DOA | DNA,DZα, DOα | DO α-chain | 12 |
| HLA-DOB | DOβ | DO β-chain | 13 |
| HLA-DMA | RING 6 | DM α-chain | 7 |
| HLA-DMB | RING 7 | DM β-chain | 13 |
| HLA-DPA1 | DPα1, DP1A | DP α-chain as expressed | 33 |
| HLA-DPB1 | DPβ1, DP1B | DP β-chain as expressed | 520 |
| TAP1 | ABCB2, RING4 | ABC (ATP-binding cassette) transporter | 12 |
| TAP2 | ABCB3, RING 11 | ABC (ATP-binding cassette) transporter | 12 |
| MICA | PERB 11.1 | Class I chain–related gene | 101 |
| MICB | PERB 11.2 | Class I chain–related gene 18 | 41 |
Immunologically defined nomenclature follows this rule: HLA separated by a hyphen from a capital letter identifying the locus encoding distinct HLA class I (-A, -B, -C) or class II (-DR, -DQ, -DP) antigens. The letter is followed by a number that identifies a serologic family of alleles sharing epitopes recognized by alloantibodies or alloreactive cytotoxic T cells. With improved understanding of the molecular genetics of the HLA region, various appendages have been removed from the HLA nomenclature. For instance, the letter w was used to indicate a provisional assignment, and this has been discontinued, but it is occasionally added to HLA-C antigen nomenclature to distinguish it from complement genes; DP and DW also maintained this letter to reinforce the dependency of their immune identification predominantly through cellular techniques. Finally, HLA-Bw4 and -Bw6 retain the w to emphasize that the public epitopes are shared by several HLA-B and some HLA-antigens. More recently, a bridge between immunologic and molecular nomenclature has been proposed whereby HLA antigens that encompass a single gene product can be assigned a two-digit numeric extension corresponding to the molecular nomenclature for that allele.
In 1987, the 10th International Histocompatibility Workshop recommended a sequence-based nomenclature to describe alleles not distinguishable by immunologic methods. Since then, the number of HLA alleles has rapidly increased. As of January 2015, a total of 13,023 alleles for HLA exist. This is a drastic increase from the original numbers established in 2003. Other designations are summarized in Table 114.2 . HLA designates molecules belonging to the human MHC followed by the locus (-A, -B, etc). Alleles are then identified after an asterisk (*). Each HLA allele name has a unique number corresponding to up to four sets of digits separated by colons. The length of the allele designation is dependent on the sequence of the allele and that of its nearest relative. All alleles receive at least a four-digit name, which corresponds to the first two sets of digits; longer names are only assigned when necessary. The digits before the first colon describe the type, which often corresponds to the serological antigen carried by an allotype. The next set of digits is used to list the subtypes, numbers being assigned in the order in which DNA sequences have been determined. Alleles whose numbers differ in the two sets of digits must differ in one or more nucleotide substitutions that change the amino acid sequence of the encoded protein. Alleles that differ only by synonymous nucleotide substitutions (also called silent or non-coding substitutions) within the coding sequence are distinguished by the use of the third set of digits. Alleles that only differ by sequence polymorphisms in the introns or in the 5′ or 3′ untranslated regions that flank the exons and introns are distinguished by the use of the fourth set of digits. A four-digit number is used in which the first two digits refer to the original serologic family (e.g., HLA-A2 serologically would be HLA-A*02). Often numbers are missing because an original assignment was revoked (e.g., this is why there is no HLA-A*24:01). When silent mutations are identified (variation in nucleotide sequence that does not translate into changes in amino acid sequence), the name of the allele remains identical, but another two digits are added to designate a variant that has no functional significance. New sequences are submitted to European Molecular Biology Laboratory (EMBL; www.ebi.ac.uk/Submissions/index.html ), GenBank ( www.ncbi.nlm.nih.gov/Genbank/index.html ), or DNA Data Bank of Japan (DDBJ; http://www.ddbj.nig.ac.jp/submission-e.html ).
Requirements for new allele naming were described by Marsh et al. The WHO Nomenclature Committee also made recommendations about naming alleles with aberrant expression, such as HLA-G isoforms and KIR. Some alleles are identifiable at the genomic level but are not translated into protein (pseudogenes). These are indicated by the addition of an N (for null) following the numerical designation of the allele. Mutations inducing a reduction of expression are marked by L (low expression). An S denotes alleles expressed as soluble secreted molecules, such as HLA-B*44:02:01:02 S characterized by an intronic variant that disallows the expression of the transmembrane domain of the HLA molecule and is therefore only in soluble form. Differential splicing of HLA-G leads to production of membrane-bound and soluble forms, which are respectively denoted by a lowercase m or s before HLA. Limited cytoplasmic expression is denoted by C and aberrant expression by A . Finally, KIR polymorphism will be classified by a new system that is in preparation. A nomenclature system for cytokine polymorphism has not yet been developed.
Originally HLA typing was done primarily in support of transplantation or transfusion needs with the purpose of identifying histocompatibility through the best match between donor and recipient. Allosensitization of recipients previously exposed to heterologous cell products is tested by identifying alloreactive antibodies in serum, and crossmatch procedures are performed to grade compatibility of candidate donor-recipient pairs. In addition, HLA testing has been applied to identify links between a given disease and the genetic makeup of its carriers. Strong associations are exemplified by birdshot uveitis (a disease occurring exclusively in HLA-A29 individuals), type 1 diabetes and other autoimmune diseases, or long-term survival of HIV-infected individuals. These studies evaluated the role that genetic background may have played over environmental factors. HLA associations are thought to be caused by the differential ability of distinct alleles to present immunogenic epitopes or by the close linkage to the HLA class III region where potent immunomodulators such as tumor necrosis factor-α are located. It has also been suggested that HLA associations may be predictors of immune responsiveness of cancer to immune therapy. However, such associations have remained quite difficult to reproduce. HLA typing is requested for enrollment of patients into and interpretation of immunization protocols, because specific HLA-epitope combinations require high-resolution typing.
Serologic testing takes advantage of fetomaternal sensitization. In mammals, the progeny carries a full haplotype of paternal origin, and pregnant women may develop antibodies against the paternal haplotype. Maternal serum samples are collected at term and characterized by testing their ability to kill HLA-bearing repository cell lines of known phenotype in the presence of complement (complement-dependent cytotoxicity [CDC]). CDC is used for HLA typing by exposing circulating cells expressing HLA class I (most cells) and class II (predominantly B cells) antigens from the individual (to be typed to previously characterized sera or monoclonal antibodies). Conversely, already typed repository cell lines are used in CDC to identify alloantibodies in sera of sensitized individuals. The fraction of cell lines killed by the sera roughly grades the intensity of allosensitization or panel reactive antibody (PRA) reactivity. Some antibodies activate complement and kill with poor efficiency, known as the cytotoxicity-negative absorption-positive (CYNAP) phenomenon . As judged by cytotoxicity testing, CYNAP may underestimate allosensitization. By modifying CDC with the addition of antihuman antibodies suitable for complement activation, CYNAP can be circumvented (augmented CDC). In this case, however, relatively innocuous antibodies can cause overestimation of clinically relevant allosensitization.
Other methods identify alloantibodies, including immobilization of HLA molecules on solid surfaces to capture soluble antibodies and flow cytometry using a spectrum of microbeads loaded with known HLA alleles. An interlaboratory comparison of serum screening for HLA-antibody determination suggested that enzyme-linked immunosorbent assay and flow cytometry yield higher PRA activity values compared with CDC or augmented CDC. However, the study suggested a lack of consistency among participant laboratories, leaving unsolved the question of which method most accurately defines clinically relevant allosensitization, and a panel of various methods may be most informative.
CDC is declining in interest in the United States because most laboratories are switching to easier-to-handle and higher-resolution molecular methods. However, immunologic methods remain valuable to characterize functional aspects of HLA because molecular methods cannot define whether an HLA allele is expressed nor, at least until recently, grade allosensitization. Thus it is likely that immunologic methods will continue to complement molecular methods in the future.
The usefulness of conventional serologic typing of HLA antigens has been limited by the availability of allele-specific sera. Most importantly, because antibodies identify structural differences on the surface of HLA molecules, variants caused by nucleotide polymorphism in nonexposed areas such as the peptide-binding groove of the HLA heavy chain are not detectable. However, these differences are of functional significance because they determine the specificity and affinity of peptide binding and T-cell recognition of self and allogeneic target cells. DNA-based typing directly determines the sequence and provides higher resolution typing.
DNA-based typing can be performed with polymerase chain reaction (PCR)-based methods, among which are sequence-specific primer, sequence-specific oligonucleotide probe, and DNA sequencing. Molecular typing using sequence-specific oligonucleotide primers or probes provides higher level typing than serological typing, and DNA sequencing provides allele-level typing. The rich nature of HLA has led to proportionally increasing complexity of the assays used to cover all possible alleles. As a consequence, accurate HLA typing for donor and recipient matching in transplantation has become increasingly complex and burdensome. In addition, because of the important role that HLA molecules play in antigen presentation and the stringency of the relationship between epitope and associated HLA allele, high-resolution typing is increasingly requested for appropriate enrollment of patients into immunization protocols aimed at the enhancement of T-cell responses. Therefore high-resolution HLA typing is increasingly in demand in clinical and experimental settings.
Although oligonucleotide-based methods could theoretically discriminate any known polymorphic site, they have two major limitations. First, they require a specific PCR reaction for each allele investigated. Because each individual has only two alleles for each locus, a disproportionately large number of PCR reactions must be performed to cover all possible polymorphisms to identify the two borne by the individual tested. Because both methods are based on specific interactions with known oligonucleotide sequences unique to a particular allele, they cannot identify unknown polymorphisms unless the variation occurs within the region spanned by one of the oligonucleotides used in the assay. Because of these limitations, definitive typing methods that yield conclusive information about the identity of the alleles typed using DNA sequence-based typing have been developed.
Sequence-based typing is being performed using Sanger sequencing and next generation sequencing. Most methods begin with PCR amplification of the HLA locus of interest. Sanger sequencing involves chain termination sequencing techniques and does not allow the separation of sequences of maternally and paternally derived alleles. Sanger sequencing cannot distinguish cis / trans polymorphisms since both alleles are sequenced in one reaction and it is difficult to determine if two variants are from the same chromosome ( cis ) or from the opposite chromosome ( trans ). Therefore, some combinations of HLA class I and class II alleles result in ambiguous allele combinations that require additional testing for resolution. Sanger sequencing instruments analyze only one sample at a time, which limits sample throughput and further limits the usefulness of this technology.
Next-generation sequencing allows for the parallel sequencing of multiple single strands of DNA. This allows for the determination of the sequence of both maternal and paternal HLA sequences, which reduces ambiguities. It also provides information on HLA genes not previously sequenced, allowing for the identification of new alleles. Next-generation sequencing instruments permit the analysis of many samples at one time, allowing for high throughput analysis and reduces costs. A number of next-generation sequencing technologies and instruments are available.
High-resolution methods yield high-resolution information of an individual’s HLA type. However, the wealth of information is counterbalanced by increased difficulty in identifying suitable HLA alleles during donor-recipient pairing or accrual into immunization protocols restricted to specific HLA-epitope combinations. Thus, at present, clinicians are faced with the daunting task of applying high-resolution typing results of unclear relevance to clinical settings.
Any cell-containing product transfused or transplanted between different individuals should be compatible in an ideal situation. Yet, in most cases, histocompatibility is not prospectively sought. Thus patients with multiple exposures to blood products often become reactive to various antigens, including HLA. Transplant candidates often develop prior alloreactivity following transfusion of platelet concentrates contaminated with leukocytes, even though the incidence of allosensitization is much less because of leukodepletion of blood products. Alloreactivity must be documented before transplantation because alloreactive patients can still undergo transplantation, provided that the donor has no mismatched HLA antigens reacting with the patient’s antibodies. Patients who have received repeated platelet transfusions may become allosensitized and consequently refractory to further transfusions unless HLA-compatible platelets are used. Obviously, the best compatibility consists of identical matching. It is often impossible to identify a perfectly matched unrelated donor, particularly in the case of rare HLA types. Thus other strategies are adopted to identify the best possible match or compatible mismatch. Selection of unrelated donor-recipient pairs is carried out through typing with serologic, cellular, and molecular methods. The chances of identifying compatible donors based on full or partial HLA matching have become increasingly low with the increasing resolution of the typing methods. To broaden compatibility, matching criteria of donor-recipient pairs are based on shared public epitopes assigned to cross-reactive groups (CREGs) or shared amino acid polymorphisms defined through sequence information ( Table 114.3 ). The preexistence of alloantibodies restricts the identification of compatible donors even further. Highly sensitized recipients with PRA activity exceeding 85% of tested specificities (generally between 30 and 60) represent a particularly challenging group. An alternative approach to the exclusion of alloreactive determinants is the inclusion of acceptable antigen mismatches expressed in a panel of cells that give negative reactions with the recipient sera, which extends the repertoire of possible donors. All of these are fundamental tools for the identification of nonrelated, not fully matched donor-recipient pairs. Unfortunately, even these compromises often fail to identify a suitable match.
| Major Cross-Reactive Group | Public Epitope | Associated Private Epitopes | Approximate Epitope Frequency (%) a |
|---|---|---|---|
| 1 C | 1p | A1, 3, 9 (23, 24), 11, 29, 30, 31, 36, 80 | 79 |
| 10p | A10 (25, 26, 34, 43, 66), 11, 28 (68, 69), 32, 33, 74 | ||
| 2 C | 28p | A2, 28 (68, 69), 9, 17 | 70 |
| 9p | A2, 28 (68, 69), 9 (23, 24) | ||
| 17p | A2, B17 (57, 58) | ||
| 5 C | 5p | B5 (51, 52), 18, 35, 53, 78 | 50 |
| 21p | B5 (51, 52), 15 (62, 63, 75, 76, 77), 17 (57, 58), 21 (49, 50), 35, 53, 70 (71, 72), 73, 74, 78 | ||
| 7 C | 7p | B7, 8, 41, 42, 48, 81 | 54 |
| 22p | B7, 22 (54, 55, 56), 27, 42, 46 | ||
| 27p | B7, 13, 27, 40 (60, 61), 47 | ||
| 8 C | 8p | B8, 14 (64, 65), 16 (38, 39), 18 | 38 |
| 12 C | 12p | B12 (44, 45), 13, 21 (49, 50), 40 (60, 61), 41 | 44 |
| Bw4 | Bw4 | B13, 27, 37, 38, 47, 49, 51, 52, 53, 57, 58, 59, 63, 77, A24, 25, 32 | 79 |
| Bw6 | Bw6 | B7, 8, 18, 35, 39, 41, 42, 45, 46, 48, 50, 54, 55, 56, 60, 61, 62, 64, 65, 67, 71, 72, 73, 75, 76, 78, 81 | 87 |
Duquesnoy described a molecularly based algorithm to identify histocompatible pairs called HLAMatchmaker. This method focuses on the structural basis of HLA class I polymorphism so that compatible HLA mismatches can be identified without extensive serum screening. This algorithm is based on the principle that short amino acid sequences (triplets) characterizing polymorphic sites of the HLA molecules are critical components of allosensitizing epitopes. Such amino acids reside in the α-helices and β-loops of the heavy chain. Because each HLA molecule expresses a characteristic string of these determinants, it is possible to characterize each molecule according to the linear sequence of amino acid triplets present on its surface. Based on the reasonable assumption that none of the triplets present in the HLA repertoire of the recipient is self-immunogenic, it is possible through a process of electronic recombination to identify donors with HLA alleles different from the recipient’s but containing exclusively shared triplets. These HLA alleles will be compatible because they do not contain any epitope that is absent in the recipient.
In theory, a large number of triplets could occur if polymorphisms were randomly distributed. However, most HLA molecules span conserved domains, and only a total of 142 different polymorphic triplets designate serologically defined HLA-A, -B, and -C antigens. Triplet polymorphism can occur in 30 locations on HLA-A, 27 in HLA-B, and 19 in HLA-C chains. Because the HLAMatchmaker algorithm includes interlocus comparison, it is possible to accumulate the information into a single database. Among the 142 polymorphic triplets, 29 are polymorphic for one class I locus but monomorphic for another class I locus. Such polymorphic triplets cannot be immunogenic because they are always present on the patient’s own HLA antigens, whereas the remaining 113 triplets have immunogenic potential. With this algorithm, it is possible to significantly broaden the number of molecularly matched HLA alleles and significantly increase the chances of identifying a compatible donor, particularly in those cases in which the recipient has a rare HLA phenotype. In addition, HLAMatchmaker considers triplets that are present in the panel cells that give negative reactions with the recipient’s serum. These negative panel cells can be expected to share antigens with the patient’s, but other HLA antigens may be present and contain mismatched triplets apparently not immunogenic for that patient. Such triplets are therefore acceptable and can be added to the algorithm for the identification of possible donors. Thus HLAMatchmaker assesses HLA compatibility at a molecular level by determining whether or not a triplet in a given position of a mismatched HLA antigen is also found in the same position in any of the recipient’s own HLA-A, HLA-B, and HLA-C molecules. A shared triplet in the same position on a mismatched HLA antigen cannot elicit a specific antibody response in that patient. Preliminary verification of the algorithm in a series of high-PRA renal patients suggested that this is a proper strategy, at least in highly sensitized renal transplantation candidates waiting for unrelated donors. HLAMatchmaker is also effective at selecting an optimal HLA-typed platelet component for alloimmunized thrombocytopenic patients.
A new version of HLAMatchmaker considers so-called eplets and is based on the structural definitions of functional epitopes on well-characterized protein antigens that have been complexed with antibody. Eplets represent amino acid residue configurations within a 3- to 3.5-Å radius of each polymorphic residue on the HLA molecular surface. Many eplets correspond to triplets, but eplets represents a more complete repertoire of structurally defined epitopes.
By no means are HLA molecules antigenic in physiologic condition (with the exception of maternofetal alloimmunization). However, because of their high density on the surface of cells, they can become highly immunogenic in the nonphysiologic event in which cells from different individuals are exposed to another person’s immune system. The mechanism or mechanisms leading to allosensitization are believed to follow two pathways. The first pathway mimics the one followed during most immune reactions in which antigen is uptaken by antigen-presenting cells and presented to autologous lymphocytes (indirect pathway). In this case, the donor’s HLA molecules are processed into peptides through the exogenous pathway of antigen presentation and presented to autologous T cells as linear peptides. This pathway is believed to be responsible for the development of alloantibodies as well as T-helper cell responses, but its role in the development of cytotoxic T-cell responses remains unclear. Because this pathway depends on the presentation of donor HLA molecules by recipient HLA alleles, it may explain why the humoral response to HLA class I allodeterminants correlates with the HLA phenotype of the responder. The indirect pathway of HLA allorecognition has been associated with allograft rejection. Because the function of HLA molecules is to present antigenic determinants to T cells, it could be easily envisioned how minor changes in their structure could be misinterpreted as antigenic epitopes. Intact HLA molecules residing on the surface of donor cells are a perfect target for T-cell-mediated allorecognition (direct pathway) either through the direct cytotoxic effect of T cells against target cells or by the activation of helper T cells through HLA class II engagement, which leads to stimulation of antibody-mediated immune responses.
Humoral responses mediated most frequently by IgM are predominant in the sensitization to infrequent allogeneic exposure because they require smaller amounts of antigenic material. T-cell responses become more manifest in the context of transplantation in which the persistence of the allogeneic stimulus allows the expansion and sustenance of alloreactive cytotoxic T cells. Antibodies and TCR have different requirements for their engagement, which means epitopes recognized by T cells and antibodies are different. Antibodies require interaction with a small structure, including a limited number of amino acids; thus, any sequence combination on the surface of an HLA allele not present in the individual exposed to the alloreaction may represent an epitope. T cells have much lower binding affinity for their ligand and require a complete interaction with the peptide as well as the α and β helices of the HLA class I heavy chain. Although several B-cell epitopes recognized by antibodies can be identified in a given HLA molecule, generally the whole HLA molecule is necessary for T cell–dependent allorecognition. Definition and topographic mapping of epitopes defined by serologic or cellular methods has revealed distinct regions of hypervariability in the α-1 and α-2 domains of the class I heavy chains and in the α-1 and β-1 domains of class II molecules. Two types of antibody-defined epitopes can be identified according to their frequency among HLA alleles. Private epitopes are almost but not totally unique for a single serologically defined HLA antigen and are used for typing. These epitopes are generally shared by all molecular alleles present in that given family, and fine differences among alleles within a general family cannot be distinguished by antibodies. Public epitopes are more widely distributed and cluster distinct serologic families into groups. These epitopes bear an immunodominant character. Immune sera that identify public epitopes have been considered predictive of major CREGs with the idea that alloreactivity among patients belonging to the same CREG may be less likely (see Table 114.3 ). The predictive value of CREGs in transplant outcome or platelet transfusion results, however, remains to be demonstrated.
Not all subjects who have been exposed to alloantigens develop alloantibodies, and in fact exposure to low doses of donor-specific HLA antigens through donor transfusion may have a beneficial effect on graft survival. Obviously, the degree of compatibility in the context of allosensitization may vary according to the degree of mismatch between donor and recipient. In addition, alloantibodies are one aspect of alloreaction that does not take into account cellular responses. These have been more difficult to document, although they are likely to play an important role in the context of acute transplant rejection. Several hypotheses have been discussed about the reason or reasons for the capriciousness of allosensitization, including the presence of regulatory immune responses or cytokine-mediated immunosuppression. Currently the mechanism modulating the quality and quantity of alloimmunity remains elusive, and different aspects of this algorithm are discussed ad hoc in this chapter, with particular attention to molecularly defined algorithms for the prediction of histocompatibility.
Clearly, HLA matching is beneficial in patients undergoing renal transplantation. An analysis of more than 150,000 recipients receiving transplants in different centers participating in the Collaborative Transplant Study showed that a complete mismatch (6 HLA-A+B+DR) had a 17% lower survival expectation than no mismatch ( P < .0001). Matching was particularly beneficial in patients with highly reactive preformed alloantibodies. The same study suggested that high-resolution matching based on molecular typing improved graft survival. Similar results were observed in cases of cardiac transplantation in which HLA matching yielded significantly better results ( P < .0001). This is particularly important because donor hearts are currently not allocated according to HLA matches in most centers. In cases of liver transplantation, HLA matching was not beneficial.
Donor-specific hyporesponsiveness has been particularly well documented in the context of renal allotransplantation and may limit the need for immunosuppression. A recent randomized study suggested that pretransplant donor transfusions improved the survival of cadaver kidney grafts in patients receiving modern immunosuppressive regimens, but the mechanism remains unclear. Although most centers currently do not implement deliberate blood transfusions, the usefulness of this approach needs to be investigated further.
Approximately 5% to 10% of platelet transfusions are given to patients who have been previously exposed to HLA class I–expressing heterologous cells and are reactive to HLA antigens. Such patients are refractory to random-donor platelets and must be given HLA-matched or semi-matched platelet pheresis components. However, a provision of HLA-matched platelets does not always improve platelet recovery and survival. Possibly, the ineffective platelet transfusion is in part caused by unrecognized HLA mismatches between the donor and recipient resulting from the low-resolution methods used for typing, and higher resolution methods have been advocated. It is currently controversial whether or not molecularly based HLA typing confers an advantage over serologic typing, and this principle was recently questioned in the context of hematopoietic cell transplantation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here