Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
acute liver failure
bile acid synthetic defect
congenital disorders of glycosylation
coenzyme A
fatty acid oxidation
mitochondrial DNA
nuclear DNA
ornithine transcarbamylase
periodic acid–Schiff
progressive familial intrahepatic cholestasis
perinatal hemochromatosis
Reye syndrome
urea cycle disorder
Zellweger syndrome spectrum
Metabolic liver diseases result from genetically determined malfunction of enzymes, transporters, structural proteins, or organelles that are critical for normal liver function. Although the term metabolic disease may be extended to include transient functional disturbances that are precipitated by factors such as infections, drugs, pregnancy, and systemic diseases that may stress subclinically compromised physiologic pathways (synergistic heterozygosity), this chapter deals only with the former. Genetic disorders of canalicular bile secretion are considered in detail in Chapter 29B .
Metabolic diseases may affect the liver only or may involve other organs as well ( Table 7.1 ). When a metabolic disease in the liver is suspected, a liver biopsy is often performed to establish the diagnosis; interpretation of such a biopsy is an exercise in both morphology and context, being most effectively performed in concert with the clinician caring for the patient. In some diseases, morphologic findings are specific. In others, the histology and electron microscopy are distinctive and diagnostic for a particular group of metabolic diseases, significantly narrowing the differential diagnosis. Often, the microscopic findings may serve as a guide to selection of appropriate biochemical and/or genetic and molecular tests. Modern laboratory methods increasingly provide alternative approaches to a definitive diagnosis of metabolic disorders such as identification of diagnostic metabolites in body fluids or by molecular analysis of DNA from blood leukocytes or cultured fibroblasts. This process can be focused by the findings on a liver biopsy. The aim of this chapter is to provide an approach to pathologists who evaluate liver biopsy specimens in patients who are suspected to have a metabolic disease. For more exhaustive coverage of the subject, the reader is encouraged to consult other sources.
| Pattern | Diseases |
|---|---|
| Isolated hepatomegaly | Glycogen storage disease, types I, VI, VIII, and IX; cholesterol ester storage disease |
| Reticuloendothelial pattern: liver, spleen, bone marrow, lymph nodes | Gaucher type B disease; Niemann-Pick disease, all types; Farber disease |
| Generalized, including central nervous system | Mucopolysaccharidoses I, II, and III; GM1 gangliosidosis; Niemann-Pick disease, types A and C; glycogen storage disease IIa |
| Liver fibrosis prominent | Tyrosinemia, type IV glycogen storage disease, alpha-1 antitrypsin deficiency, Gaucher disease, bile acid synthetic defects, perinatal hemochromatosis |
| No liver involvement/no hepatomegaly | Fabry disease, urea cycle defects |
| Liver, brain, muscle, heart, gastrointestinal | Mitochondriopathy |
| Liver, muscle, heart | Fatty acid oxidation defects |
| Liver, brain, adrenal, malabsorption | Peroxisomal disease |
The clinical circumstances that raise suspicion for liver disease are well known and typically neither exclude nor strongly suggest an underlying metabolic disease. However, in patients with one or more of the conditions listed in Box 7.1 , the probability of a metabolic liver disease is increased.
Persistent neonatal cholestasis
Asymptomatic hepatomegaly
Multiorgan enlargement in a pattern that suggests reticuloendothelial system involvement in the absence of signs of portal hypertension
Multiorgan dysfunction in a pattern that suggests a disorder of energy metabolism (involvement of heart, skeletal muscle, brain, gastrointestinal tract)
Protein intolerance or avoidance
Unexplained episodes of hypoglycemia, precipitated by “trivial” intercurrent viral syndromes
Intolerance of caloric deprivation with inability to form ketones during fasting
Unexplained metabolic acidosis with elevated lactate, lactate-to-pyruvate ratio, or both
Organic acidemia, unusual pattern
Prominent itching
Xanthoma formation
If clinical suspicion for a metabolic liver disease is high, at least two cores of liver tissue should be obtained. A surgical biopsy specimen is almost always sufficient, but cautery must be avoided or enzyme studies may be useless. One core of liver tissue or one slice of a surgical biopsy specimen must be frozen in anticipation of need for biochemical or genetic analysis. The decision about use of frozen liver tissue is best delayed until light microscopy and electron microscopy are completed, unless the clinical information or laboratory values clearly indicate a particular metabolic disease. The value of electron microscopy is severely limited if part of the diagnostic biopsy is not fixed in a suitable fixative such as glutaraldehyde immediately on receipt of the fresh specimen; 2 mm of a typical needle core suffices for this purpose and is sufficient to provide two or three resin blocks that need not be processed further if deemed unnecessary. When clinical suspicion of a metabolic disease/storage disease is supported (or not eliminated) by light microscopy, it is very helpful to then perform electron microscopy to survey the organelles for structural abnormalities and identify any accumulated material and its subcellular location before deciding how to proceed. For example, when light microscopy identifies features of glycogen storage disorder and study of liver ultrastructure confirms that excess nonlysosomal glycogen displaces other organelles to the periphery in most hepatocytes, it is then appropriate to commit the frozen sample to measurement of the glycogen concentration and to screen for glycolytic enzyme defects. In a similar vein, when unusual inclusions or small vesicles accumulate in cytoplasm of hepatocytes or Kupffer cells, and clinical context supports suspicion of a metabolic disease, the ultrastructure of the stored material helps narrow the differential diagnosis and screening for lysosomal storage disorders may result in a specific diagnosis.
The following stains on paraffin sections of a diagnostic liver specimen are routine: hematoxylin and eosin, Masson trichrome, reticulin, and periodic acid–Schiff (PAS) with and without prior diastase digestion. Stains that prove useful only in certain contexts, but are nonetheless often routinely used, are Prussian blue for hemosiderin deposition or rhodanine for copper complexes in secondary lysosomes. Other stains may be used to detect a stored material as ceroid (Sudan black, Ziehl-Neelsen), mucopolysaccharide (Alcian blue, colloidal iron), phospholipid (Sudan black), or specific immunostains may be used to identify particular proteins such as alpha-1 antitrypsin or fibrinogen or proteins specific to organelles such as mitochondria or lysosomes.
A useful liver biopsy report requires systematic assessment of the histologic features in liver tissue. The structural components in a liver specimen to be commented on in the report are sample quality; adequacy in terms of the number of portal areas and central veins; status of portal and lobular architecture; the bile ducts and vessels in the portal tracts; the hepatocytes; the sinusoidal lining cells, especially the Kupffer cells; and the sinusoidal/hepatic venous drainage system. Pathologic changes to be specifically mentioned are altered lobular architecture, the pattern and extent of lobular collapse or fibrosis, and the presence or absence of each of the following: cellular infiltrates with specification of location and cell types; changes at the limiting plate (portal-lobular interface); the general condition of hepatocytes and Kupffer cells with note of any unusual appearance or abnormal expansion of cytoplasm or displacement of organelle-containing cytoplasm by an unexpected substance, patterns of degeneration, and/or necrosis; canalicular or cytoplasmic bile stasis; and other pigment deposits.
A small number of metabolic diseases of the liver are functional disorders with minimal or absent histologic changes and no capacity to cause long-term liver injury. Examples of metabolic diseases that consistently show absence of morphologic abnormalities on a liver biopsy are listed in Box 7.2 .
Phenylketonuria
Cystinosis
Urea cycle defects a
a The histology depends on the stage of the disease at the time of biopsy.
( eSlide 7.3 )
Aminoacidopathies a
Portal and lobular inflammation (ie, a hepatitic pattern) is inconspicuous or even absent in many metabolic disorders that involve the liver. The explanation for this, even in the face of slowly progressive fibrosis, is incomplete but probably involves the absence of toxic or inflammogenic metabolic by-products or intermediaries. Harmful effects, in some diseases are easily compensated by replacement of injured cells or organelles without permanent injury to the liver. On the other hand, some metabolic diseases of the liver are commonly accompanied by inflammatory changes that may be brisk, mimicking chronic viral hepatitis, drug-induced hepatitis, or autoimmune hepatitis. The common denominator in metabolic disorders consistently presenting as “hepatitis” seems to be accumulation of toxic metabolites that cause hepatocyte necrosis or sufficient metabolic stress, making these cells vulnerable to insults that might ordinarily be tolerated without harm. Moreover, it is possible that inflammation incidental to intercurrent events such as viremia, sepsis, systemic inflammatory disease, or drug exposure may be more injurious to the liver of a patient with a primary metabolic disease. Examples of typically nonhepatic and commonly hepatitic metabolic liver diseases and their propensity to evolve to cirrhosis are listed in Table 7.2 .
| Metabolic Disease | Inflammation | Fibrosis |
|---|---|---|
| Cystic fibrosis | Focal cholangiopathy | Focal, biliary ( eSlide 10.1 , eSlide 10.2 ) |
| Glycogenosis, types I, II, III, VI, VIII, and IX | Absent or mild | Absent or mild ( eSlide 7.9 , eSlide 7.10 ) |
| Glycogenosis, type IV | Mild | Cirrhosis |
| Mucopolysaccharidoses | Absent | Absent |
| Sphingolipidoses | Minimal to absent | Mild, progressive |
| Niemann-Pick disease, type C | Present | Progressive; cirrhosis |
| Gaucher disease | Minimal to absent | Mild, progressive ( eSlide 7.6 ) |
| Cholesterol ester storage disease | Minimal to absent | Mild, slowly progressive storage disease |
| Urea cycle defects | Minimal or absent | Minimal or absent |
| Citrin deficiency | Minimal or absent | Progressive |
| Bile acid synthetic defects | Minimal to mild | Progression varies with defect; acute liver failure in infants |
| PFIC1: variable phenotype | Usually absent | Slowly progressive ( eSlide 29B.1 ) |
| PFIC2: variable phenotype | Mild | Rapid or slow progression ( eSlide 29B.2 , eSlide 29B.3 ) |
| PFIC3: highly variable phenotype | Absent to moderate | Absent, mild or biliary cirrhosis ( eSlide 29B.4 ) |
| Fatty acid oxidation disorder | Absent | Absent |
| Mitochondriopathy | Mild to moderate | Fibrosis is variable; acute liver failure in infants; cirrhosis |
| Alpha-1 antitrypsin deficiency (PiZZ) | Variable | Progressive fibrosis is unpredictable; cirrhosis ( eSlide 9.1 ) |
| Tyrosinemia | May be prominent | Acute liver failure in infants; cirrhosis ( eSlide 7.1 ) |
| Wilson disease | May be prominent | Progressive fibrosis; acute liver failure; cirrhosis ( eSlide 8.1 , eSlide 8.2 , eSlide 8.3 , eSlide 8.4 ) |
Although many metabolic diseases in the liver are not associated with cholestasis, lobular cholestasis and “cholate-stasis” regularly occur in four classes of metabolic diseases ( Table 7.3 ). The first group is diverse wherein the metabolic disorder causes loss of substantial numbers of hepatocytes as a result of necrosis or extensive giant cell transformation with disruption of the canalicular network, resulting in liver failure with nonobstructive cholestasis ( Fig. 7.1 ). Examples are tyrosinemia ( eSlide 7.1 ), alpha-1 antitrypsin storage disease, perinatal iron storage disorder ( eSlide 5.6 ), galactosemia, and mitochondriopathies. The second and third groups include disorders in which the transport or synthesis of bile acids is abnormal ( eSlide 29B.2, eSlide 29B.3 ); serum gamma glutamyl transferase is typically normal in these conditions (see Fig. 7.1 ). The fourth group includes diseases that are characterized by progressive cholangiopathy affecting bile ducts such as Alagille syndrome ( eSlide 5.7 ), Zellweger syndrome, cystic fibrosis ( eSlide 10.1, eSlide 10.2 ), and progressive familial intrahepatic cholestasis type 3 (PFIC3) ( eSlide 29B.4 ); serum gamma glutamyl transferase is typically elevated.
| Disorders that cause hepatocyte necrosis or extensive giant cell transformation of hepatocytes | Tyrosinemia, alpha-1 antitrypsin storage disorder, a perinatal iron storage disorder, galactosemia, and mitochondriopathies |
| Disorders of bile acid synthesis | 3-hydroxysteroid dehydrogenase deficiency, 5-beta reductase deficiency |
| Disorders of bile acid transport | PFIC1, PFIC2 ( eSlide 29B.1 , eSlide 29B.2 , eSlide 29B.3 ) |
| Progressive cholangiopathies | Alagille syndrome ( eSlide 5.7 ), a Zellweger syndrome, a alpha-1 antitrypsin deficiency, a cystic fibrosis ( eSlide 10.1 , eSlide 10.2 ), PFIC3 ( eSlide 29B.4 ) |
a These diseases may show paucity of interlobular bile ducts (alpha-1 antitrypsin deficiency rarely).
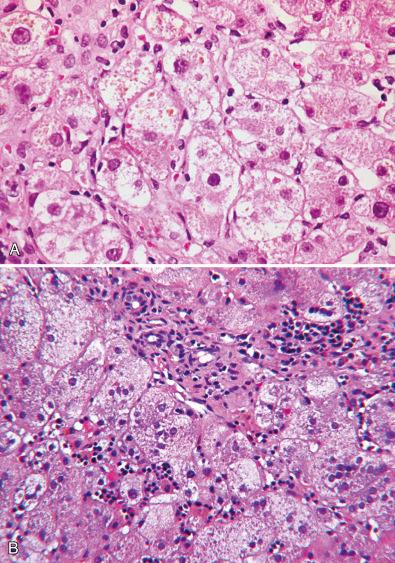
Paucity of interlobular bile ducts occurs regularly in Alagille syndrome and much less commonly in alpha-1 antitrypsin storage disease. Paucity has been reported rarely in a host of other metabolic, genetic, and acquired diseases such as PFIC2, bile acid synthetic defects (BASDs), congenital panhypopituitarism, or conditions such as Zellweger syndrome, Down syndrome, and arthrogryposis-renal-cholestasis syndrome. Paucity may be due to bile duct destruction with or without sclerosis, or developmental delay; the basis for paucity in metabolic diseases is poorly understood.
It is always challenging to interpret increases in the number of bile duct or bile ductlike structures in portal areas. This common interpretative problem has important implications for the distinction between metabolic diseases that are primary hepatocellular diseases with progressive liver fibrosis from true metabolic cholangiopathies such as PFIC3 and differentiation of both categories from obstructive cholangiopathies. Central to this problem is the need for the pathologist to appreciate the ductular reaction and the varied contexts in which it may be seen. This reaction, in addition to being a major component of the reaction to mechanical obstruction of large bile ducts, is a common nonspecific response to epithelial injury of the ductules (cholangioles), which are structures located at or near the margins of portal areas. The nonspecific ductular reaction, highlighted by a keratin immunostain, intensifies, in conjunction with progressive periportal fibrosis, in a wide variety of acute and chronic or persistent liver injuries such as drug reactions, or autoimmune, alloimmune, and chronic viral hepatitis, as well as in obstructive cholangiopathies of various types, biliary atresia in particular, even as the interlobular bile ducts disappear in the latter condition. Interlobular bile ducts may be selectively highlighted by K19 and EMA immunostains, but location in the interior of a portal zone and a patent lumen are more helpful features.
In the absence of proliferative changes in the interlobular and septal bile ducts, the ductular reaction should not be interpreted as evidence of an obstructive cholangiopathy. Similarly, the frequent association of the ductular reaction with a collar of polymorphonuclear leukocytes is not sufficient by itself to support a diagnosis of ascending cholangitis.
Ductular reactions are typically absent or mild early in the course of most types of metabolic liver disease but may be observed in alpha-1 antitrypsin deficiency and in the relatively more hepatotoxic BASDs, and they may be extremely prominent in PFIC3 ( Fig. 7.2 ). The epithelium of bile ducts of large and intermediate caliber and of interlobular bile ducts is not commonly involved in metabolic diseases of the liver. Exceptions include cystic fibrosis; some lysosomal storage diseases (type II glycogenosis, GM1 gangliosidosis, mucolipidosis); and perinatal hemochromatosis (PH) ( eSlide 5.6 ), in which hemosiderin deposits in duct epithelium may be observed as part of the general deposition of hemosiderin in extrahepatic parenchymal cells.
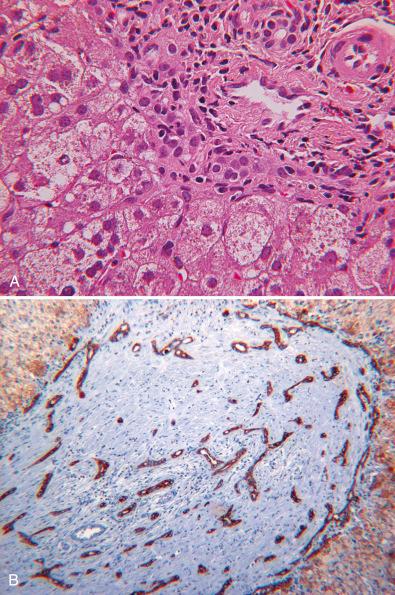
Fatty change in the liver of infants and children may either be acquired or be a manifestation of an underlying genetic metabolic disease ( Box 7.3 ). Accumulation of lipid in hepatocytes without inflammation, hepatocyte necrosis or other abnormalities of liver cells, or evidence of fibrosis is called simple steatosis ( Fig. 7.3 ). Transient hepatocyte steatosis readily occurs in infants and children during periods of temporary caloric deprivation and may also occur during a metabolic crisis in a patient with an underlying metabolic disorder such as a urea cycle defect (UCD) or an aminoacidopathy (see Fig. 7.3 ). There are no known pathologic consequences. Simple steatosis is also common at autopsy of critically ill children and adults who die in hospital intensive care units, as well as in a minority of infants who are diagnosed with sudden infant death syndrome. In both groups, lipid droplets are of small to medium size, often panlobular, and do not displace the hepatocyte nuclei. Because lipid has been extracted during processing, it can be presumptively identified only in paraffin sections based on the sharp interface with surrounding hepatocyte cytoplasm. Storage lysosomes may have a similar sharp interface by light microscopy, but a limiting membrane characterizes the ultrastructure.
Simple steatosis, transient
Macrovesicular steatosis, persisting
Dietary deficiency of protein (eg, poverty, avoidance)
Protein malabsorption (eg, cystic fibrosis, enteropathy)
Glycogen storage disease, types I and III
Galactosemia
Fructose aldolase deficiency (hereditary fructosemia)
Carnitine-acylcarnitine translocase deficiency
Urea cycle defects
Citrin deficiency (type II citrullinemia)
Secondary to hypertriglyceridemia
Chronic hepatitis C: nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
Diabetes mellitus
Drug toxicity (eg, methotrexate)
Phytotoxin (eg, Senecio alkaloids, Amanita phalloides toxin, aflatoxin)
Chemical toxins (eg, elemental phosphorus, carbon tetrachloride)
Macrovesicular and microvesicular steatosis
Fatty acid oxidation defects
Mitochondriopathy (eg, mDNA depletion)
Aminoacidopathy (eg, isovaleric acidemia)
Drug toxicity (eg, valproic acid–induced liver failure, tetracycline)
Microvesicular steatosis, transient, postviral (epidemic Reye syndrome)
mDNA, mitochondrial DNA.
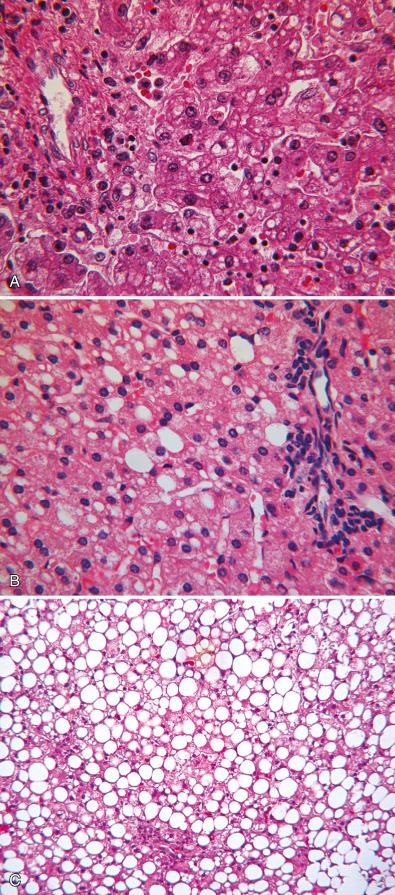
Chronic protein deficiency caused by inadequate diet, malabsorption (as in cystic fibrosis), or avoidance (as in UCD) results in fatty change in hepatocytes characterized by large vacuoles of neutral lipid that displace the nucleus to the periphery ( Fig. 7.4 ). This change is commonly limited to periportal hepatocytes (zone 1) but may be panlobular. Because coalescence of lipid into visible medium-size and large-size vacuoles is a dynamic process, hepatocytes containing medium-size and large-size lipid droplets often coexist. It is the predominant form that provides a clue, albeit an imperfect one, to the tempo and the underlying cause of lipid accumulation. Paradoxically, the dietary imbalances now prevalent in western societies have produced an epidemic of obesity and metabolic stress that cause steatosis accompanied by portal and lobular inflammation, hepatocyte degeneration and reactive changes in the ultrastructure of the endoplasmic reticulum and in mitochondria. The prevalence of this acquired fatty liver disease in the pediatric age group adversely affects the reliability of morphologic criteria previously considered useful for recognition of specific liver diseases.
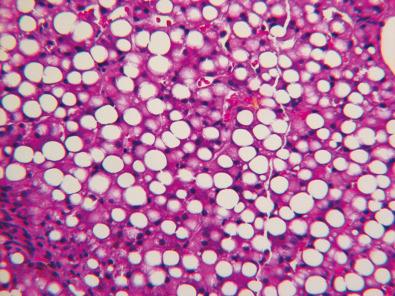
More than a dozen defects in fatty acid oxidation (FAO) have been described in the past two decades. Mortality is high, particularly during the first year of life and often in early infancy. Hepatic presentations with hepatomegaly and nonketotic hypoglycemia are most common, but true hepatic failure is rare. Cardiomyopathic and myopathic presentations are less common.
Medium-chain acyl-coenzyme A (CoA) dehydrogenase deficiency, the most common of the defects in FAO, presents as an acute metabolic crisis, often with nonketotic hypoglycemia, usually precipitated by a febrile illness that causes loss of appetite, exposing the inability to metabolize lipid during brief periods of starvation. FAO disorders may mimic epidemic Reye syndrome (RS) in clinical presentation, but morphologic manifestations differ. Long-chain acyl-CoA dehydrogenase deficiency ( Fig. 7.5 ) may be associated with progressive liver fibrosis or with cardiomyopathy, myopathy, or pigmentary retinopathy. Less common are FAO or lipid transport defects that result in a metabolic crisis in the immediate newborn period before feeding is initiated, as in the infantile forms of carnitine palmitoyltransferase 2 deficiency (see Fig. 7.5 ) and carnitine-acylcarnitine translocase deficiency. Most cases of acute fatty liver of pregnancy result from recessively inherited FAO defects in the fetus. The most common of these is a mitochondrial trifunctional protein defect that impairs oxidation of long-chain fatty acids.
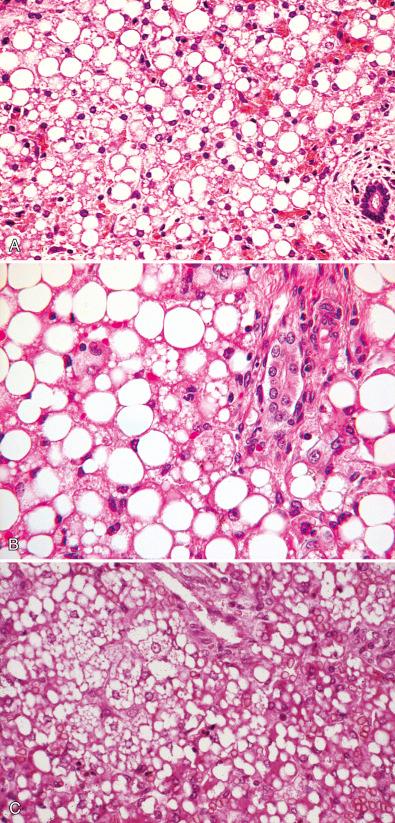
Several FAO defects, including medium-chain acyl-CoA dehydrogenase, long-chain acyl-CoA dehydrogenase, carnitine transporter defect, and carnitine translocase defect, have been implicated in a small number of sudden deaths in infancy and childhood.
On the basis of observations in liver biopsies and autopsy material, infants and children with defective FAO accumulate neutral lipid in organs most dependent on FAO such as the liver, heart, proximal renal tubules, and type 1 skeletal muscle fibers. The lipid accumulation occurs predominantly in the form of microvesicular steatosis in which the lipid vacuoles indent but do not displace the nucleus (see Fig. 7.5 ); large-droplet lipid is usually, but not always, a minor component. In medium-chain acyl-CoA dehydrogenase, the accumulation of lipid in hepatocytes may be transient and may abate or disappear without permanent liver injury when homeostasis is restored. A useful protocol to identify the underlying FAO defect at autopsy is available. Analysis of urine for acyl and acylcarnitine compounds and establishment of a fibroblast culture are essential supplements to appropriate tissue samples when clinical suspicion is high and these studies have not been initiated before death.
Prominent lipid vacuoles in hepatocytes of a child with undiagnosed liver disease do not necessarily indicate a primary disorder of the lipid utilization pathway. Hepatic steatosis may coexist as a confounding variable in many metabolic disorders, either because of poor nutrition or because the defect directly interferes with lipid processing or utilization. Fortunately, in such cases, the lipid accumulation usually does not dominate histologic changes of the primary disorder in the liver, although in rare instances this may be the case. In some aminoacidopathies such as isovaleric acidemia, hepatocyte lipid accumulation may be prominent at the time of a metabolic crisis. Large vacuolar lipid accumulation in hepatocytes is common in type I and type III glycogenosis and rarely is so extensive as to obscure the diagnostic histologic features of glycogen excess (see Fig. 7.3 ).
Ultrastructural changes in mitochondria in FAO disorders have been described but not thoroughly characterized. Observations to date suggest that ultrastructure of mitochondria in FAO are not distinctive in comparison to mitochondrial alterations in primary disorders of electron transport, mitochondrial DNA (mDNA) depletion, and epidemic RS.
Many metabolic liver diseases that commonly present in infancy are routinely included in the lengthy differential diagnosis of neonatal hepatitis, but it is helpful to know that with the following exceptions, prominent steatosis is unusual in most disorders subsumed under this convenient rubric. The more common exceptions include the following metabolic diseases: galactosemia, hereditary fructose intolerance, hepatic mitochondriopathies, UCDs, citrin deficiency, and lysinuric protein intolerance; in these disorders, lipid vacuoles of variable size accumulate in hepatocytes and may be accompanied by mild portal inflammation, progressive portal fibrosis, and liver failure.
Diagnosis is usually based on the plasma acylcarnitine profile determined by fast atom bombardment mass spectrometry from Guthrie card bloodspots, urine acylcarnitine and organic acid profiles, and studies performed on cultured fibroblasts.
The liver, as well as the brain, skeletal muscle, heart, and other organs, may be involved in systemic or organ-limited mitochondriopathies caused either by nuclear DNA (nDNA) or mDNA mutations/deletions, which result in deficiencies of one particular component of the electron transport system or a reduction in multiple electron transport activities. The liver may be clinically involved alone or in combination with the brain, skeletal muscle, or gastrointestinal tract. Mitochondrial hepatopathy in infants typically progresses to liver failure.
The most common basis for liver failure attributed to mitochondriopathy is mDNA depletion caused by mutations in nuclear genes that control mDNA processing, resulting in reduced activity of electron transport proteins that are coded by mDNA. Examples of mutations in nuclear genes or nuclear-coded proteins that are reported to cause hepatic or hepatocerebral mitochondriopathy are deoxyguanosine kinase, POLG1 , and MPV17 . The phenotypic spectrum of MPV17 mutations includes Navajo neurohepatopathy.
It is now apparent that most, if not all, of the valproic acid–associated cases of acute liver failure in infants or children with seizures have an underlying mitochondriopathy that affects both the liver and brain. Patients with underlying mitochondriopathy are exceptionally vulnerable to valproic acid because it interferes with mitochondrial oxidation of fatty acids. Similarly, it is now clear that most cases of Alpers-Huttenlocher syndrome are mitochondriopathies in which brain involvement dominates the early clinical presentation. The frequent but not invariable association of liver and brain mitochondriopathy is the basis for the warning to avoid liver transplants in infants or children with neurologic disease that is not secondary to liver failure.
Mitochondriopathy arising from hepatotoxicity of nucleoside reverse transcriptase inhibitors that inhibit DNA polymerase gamma may cause steatosis and lactic acidemia. These complications of therapy for chronic viral infections have not been reported in children, perhaps because long-term exposure is a requirement.
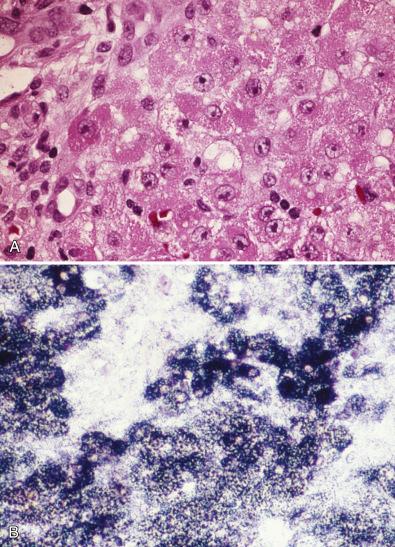
Light microscopic abnormalities that point to a primary disorder of mitochondria include patchy, sometimes extensive microvesicular and macrovesicular steatosis, intralobular cholestasis, swollen granular red hepatocytes that contain excessive numbers of mitochondria, scattered necrotic hepatocytes, foci of intralobular regeneration and collapse, and mixed portal/lobular inflammation accompanied by progressive portal fibrosis ( Fig. 7.6 ). In our experience, application of oxidative enzyme histochemistry to cryostat sections of liver may be helpful in recognition of mitochondriopathy. Selective reduction or hyperintensity of reaction within individual hepatocytes, or loss of activity of cytochrome oxidase coupled with preservation of succinic dehydrogenase, has been reported. Individual hepatocytes that contain increased numbers of mitochondria typically contain excessive succinic dehydrogenase activity. As the morphologic phenotype is expanded by descriptions of new variants and new entities in which mDNA depletion is a common feature, it is becoming clear that microscopic findings may not always be so distinctive or may be limited to steatosis only. Furthermore, the light microscopic and ultrastructural changes in hepatic mitochondriopathies before onset of acute liver failure are not well-characterized and possibly may be absent at earlier stages of the disease.
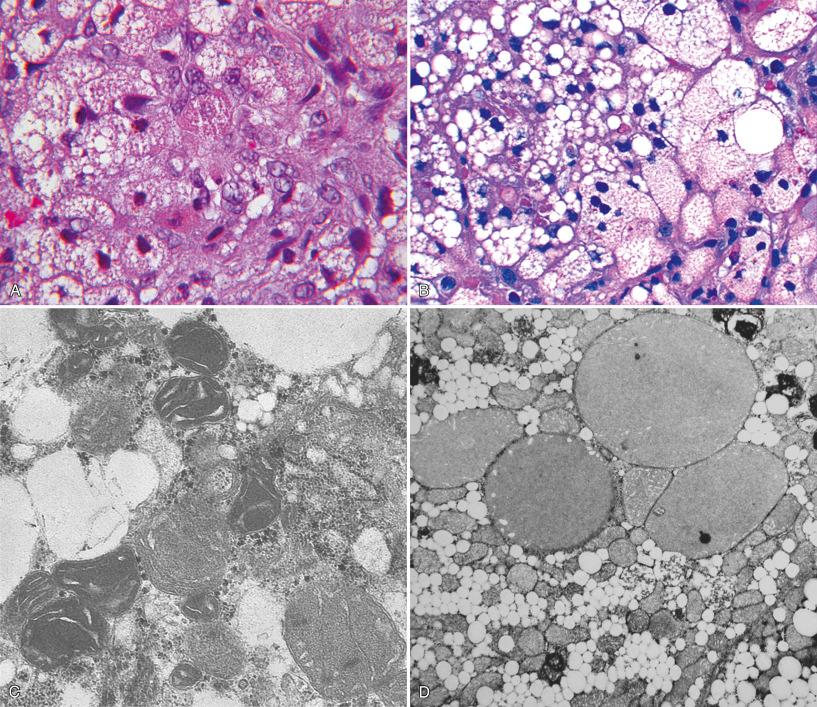
Distinctive mitochondrial abnormalities have been observed by electron microscopy in patients with proven mitochondriopathy (see Fig. 7.6 ). Similar morphologic changes have been observed in acute liver failure associated with what formerly were called idiosyncratic drug reactions to valproic acid used to treat seizures in infants or in older children ( Fig. 7.7 ). Many of these patients may have an unrecognized mitochondriopathy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here