Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term histiocyte refers to a bone marrow–derived progenitor cell that differentiates, depending on the cytokine and growth factor milieu, into a dendritic antigen-presenting cell (Langerhans cell, dermal dendrocyte) or a phagocytic cell (tissue macrophage). Histologic subclassification of disorders of histiocytes can be performed based on the morphologic and immunophenotypic attributes of these differentiated descendent cells, but a diagnosis of disease entities often requires clinicopathologic correlation.
Langerhans cells (LHCs) are cutaneous antigen-presenting cells; they are CD1a-positive, S100 protein–positive, CD68-negative dendritic cells, with characteristic tennis racquet–shaped Birbeck granules seen on electron microscopy. The term indeterminate is used for mononuclear cells, which share some, but not all, of the classic characteristics of LHCs. Similar to LHCs, they are immunoreactive for antibodies to CD1a and S100P but lack detectable Birbeck granules. Dermal dendrocytes are dermal mononuclear antigen-presenting cells typically immunopositive for factor XIIIa but negative for S100P and CD1a.
Dermal macrophages are phagocytic cells with mononuclear or multinuclear giant cell features. They may have a round, oval, scalloped, or fusiform shape. The cytoplasm may vary from foamy to eosinophilic. There may be multinucleated giant cells of the Touton type (ring of nuclei with peripheral lipid accumulation and an eosinophilic center) or foreign body type (multiple haphazardly arranged nuclei), or they may contain ground-glass cytoplasm. The largest group of non–LHC diseases is the xanthogranuloma family, which includes benign cephalic histiocytosis, juvenile xanthogranuloma (JXG), xanthoma disseminatum (XD), generalized eruptive histiocytosis (GEH), progressive nodular histiocytosis, and Erdheim-Chester disease (ECD). Other non-LHC diseases include multicentric reticulohistiocytosis (MRH), reticulohistiocytoma, and sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease).
Langerhans cell disease (LCD) refers herein to a clonal proliferative disease, not to reactive LHC hyperplasia because it may be seen in many infectious or allergic reactions. LHC proliferative disease is commonly referred to as LHC histiocytosis. In a recent study, nearly 60% of lesions of LHC histiocytosis were found to harbor the BRAFV600E mutation.
The clinical manifestations of LCD have historically been described as four separate disease entities. However, it is now recognized that each of these entities represents a clinical variant within a spectrum of findings found in this one disease process.
Typically young children
Disseminated disease
Cutaneous lesions common; may clinically simulate seborrheic dermatitis
Triad of lytic skull lesion, hypopituitarism, and diabetes insipidus
Cutaneous lesions in approximately one third of patients
Isolated granulomas, often in lung
Rare cutaneous involvement
Isolated or clustered cutaneous papules at any age
Letterer-Siwe disease represents a disseminated form of LCD that usually affects children in the first year of life, but it can be seen in any age, including adults. There are pinkish-yellow to red-brown coalescent scaly papules, plaques, and patches over seborrheic areas, including the scalp, groin, nasolabial folds, perioral area, and upper trunk. Less commonly, there are pustules, petechiae, erosions, or ulcerated nodules. The skin lesions are accompanied by systemic symptoms of fever, lymphadenopathy, weight loss, pancytopenia, and hepatosplenomegaly.
Hand-Schüller-Christian disease is more common in older children. The characteristic triad of features includes osteolytic skull lesions, hypopituitarism with diabetes insipidus, and exophthalmos. Skin lesions are seen in approximately one third of cases; there are papules and nodules, possibly eroded or ulcerated, in the intertriginous areas.
This form of LCD is more common in older children and adults and is characterized by bone lesions; skin involvement is rare.
This rare form is seen at birth or in neonates. Solitary or multiple papules, vesicles, or nodules, possibly ulcerated, are located on the head, trunk, palms, and soles. There is usually spontaneous resolution over weeks to months, possibly leaving behind areas of hyperpigmentation or hypopigmentation. However, congenital self-healing reticulohistiocytosis (CSHR) may progress toward a Letterer-Siwe clinical picture.
Many clinical presentations do not neatly fit into any of the “classic” variants of LCD, such as limited localized maculopapular cutaneous disease ( Fig. 15-1 ).
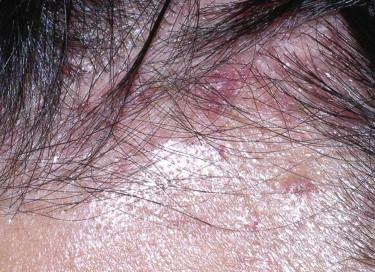
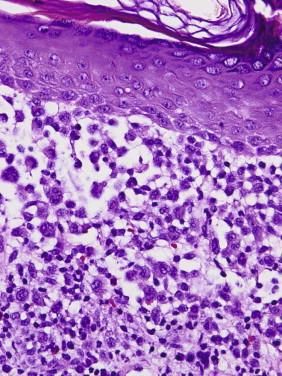
The common histologic feature is the accumulation of cells with abundant amphophilic or eosinophilic cytoplasm and large, vesicular folded, lobulated, or reniform nuclei without prominent nucleoli in the papillary dermis and epidermis ( Fig. 15-2 ) and, in nodular forms, in the reticular dermis. There are often accompanying nonhistiocytic inflammatory cells, including lymphocytes, eosinophils, and neutrophils. Occasionally, a more granulomatous pattern with occasional xanthomatous cells and multinucleated giant cells may be seen.
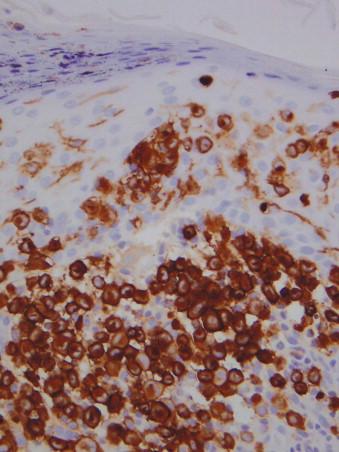
Langerhans cells are positive for CD1a, S100 protein, and Lanergin (CD207) ( Fig. 15-3 ). They are also positive for CD43 and CD4. They are negative for CD68.
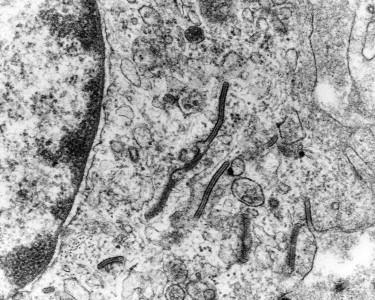
Electron microscopy demonstrates characteristic racquet-shaped or rocket-shaped Birbeck granules ( Fig. 15-4 ). Lesions of CSHR can also show myelinoid laminated inclusions, known as vermiform bodies. Electron microscopy is currently no longer part of the routine diagnostic workup of LCD.
Immunohistochemistry using the monoclonal antibody VE1 can help document the presence or absence of the mutant BRAFV600E protein. This may facilitate selection of patients with symptomatic systemic disease for treatment with vemurafenib. Immunoreactivity may be weak (generally weaker than in metastatic melanoma cells carrying the same mutation). Analysis by other methods (e.g., pyrosequencing or mass spectroscopy) may be necessary for confirmation of mutation status.
Langerhans cells may be confused with non-Langerhans histiocytes (e.g., foam-cell poor xanthogranuloma) or on occasion with a mast cell, immature or leukemic myeloid infiltrate, or even a melanocytic proliferation. Close attention to nuclear detail (reniform nucleus) is helpful. Immunostains allow for a ready distinction.
Reactive LHC hyperplasia as part of an allergic reaction, such as eczema, which may on occasion be florid, may be mistaken as LCD and lead to overtreatment. Whereas in LCD, there are typically dense clusters of LHCs, in reactive hyperplasia, LHCs tend to be more dispersed as solitary units, although there may be many of them in the dermis and epidermis admixed with lymphocytes and eosinophils. Careful attention to clinical history and presentation is important. One should hesitate, for example, to make a new diagnosis of LCD (histiocytosis) in an adult with a long history of eczema.
The disease course is variable and does not correlate with histology. Adverse prognostic features include age younger than 1 year (except in CSHR), organ dysfunction, and male gender. Complete remission occurs in 30%; the majority of patients have a chronic course. Systemic involvement may lead to severe morbidity and even death. Treatment strategies are related to the extent of disease. Patients with localized disease can be treated with topical nitrogen mustard, oral thalidomide, or ultraviolet light therapy. A variety of chemotherapy agents, steroids, and possibly bone marrow transplant are used in cases of systemic disease. Recent evidence suggests that patients with tumors harboring the BRAFV600E mutation respond well to treatment with vemurafenib.
It is postulated that the counterpart cell of this disorder is an immature LHC, that is, a dendritic cell before maturation in the lymph node.
This exceedingly rare disorder occurs in both children and adults of each gender. It generally presents as a solitary, soft, erythematous, ulcerated nodule or as crops of firm, red-brown papules that develop over several months or years. Extracutaneous involvement has been documented.
The dermis contains a well-circumscribed or diffuse infiltrate of histiocytes admixed with lymphocytic aggregates. The histiocytes have eosinophilic or granular cytoplasm.
The histiocytes are immunoreactive for CD68, S100 protein, and factor XIIIa and weakly or focally positive for CD1a. No Birbeck granules are detectable by electron microscopy.
This disorder should be distinguished from other histiocytoses (CSHR, JXG, GEH, MRH).
Solitary lesions can be treated with simple excision. Multiple lesions are usually stable or only slowly progressive, and treatment is usually not necessary.
This family of histiocytic tumors includes juvenile and adult xanthogranuloma and miscellaneous other non-LHC proliferations with histology similar to JXG (benign cephalic histiocytosis, XD, generalized eruptive histiocytoma).
Juvenile xanthogranuloma is the most common non-LHC histiocytosis. It is a normolipemic condition that presents congenitally in 15% to 30% of cases and within the first year of life in approximately 75% of cases. Identical lesions may also be seen in adults (adult xanthogranuloma). Skin lesions are more often solitary but can be multiple, and they consist of well-demarcated, rubbery, reddish brown to yellow asymptomatic papules measuring 2 to 5 mm. They are located mostly on the upper body but have been documented in skin of all sites. Less commonly, there are shiny, translucent, red to yellow nodules, sometimes with telangiectasias, measuring 5 to 20 mm. Giant forms measuring greater than 20 mm in diameter have been reported. There is a male predominance, especially in cases with multiple skin lesions. Mucous membrane and extracutaneous involvement can occur, with or without accompanying skin lesions. The eye is the most common extracutaneous site; ocular lesions occur in 1% to 10%, are usually unilateral, and may precede skin lesions. Systemic involvement may occur, affecting the oropharynx, orbit, nasal and paranasal sinus areas, central nervous system (CNS), liver, spleen, lungs, bone marrow, testis, breast, or kidney. Lesions of JXG may be associated with neurofibromatosis type I or with juvenile chronic myelogenous leukemia or juvenile myelomonocytic leukemia.
Most common form of non–Langerhans cell histiocytosis
The majority of patients are infants or children
May also present in adults (adult xanthogranuloma)
Usually skin of upper part of body
Eye
Other (e.g., visceral, bone) sites may be involved
Papular form (most common presentation)
Multiple small red, brown, or yellow papules
May be associated with café-au-lait spots of neurofibromatosis and juvenile chronic myeloid leukemia
Nodular form (less common)
One or a few lesions (1- to 2-cm nodules)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here