Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews aspects of Langerhans cell histiocytosis as a prototypical representative of the condition, and this is followed by consideration of the histiocytic and dendritic cell sarcomas and disseminated juvenile xanthogranuloma. Advances in these conditions since the previous edition are emphasized and the differential diagnostic points are highlighted.
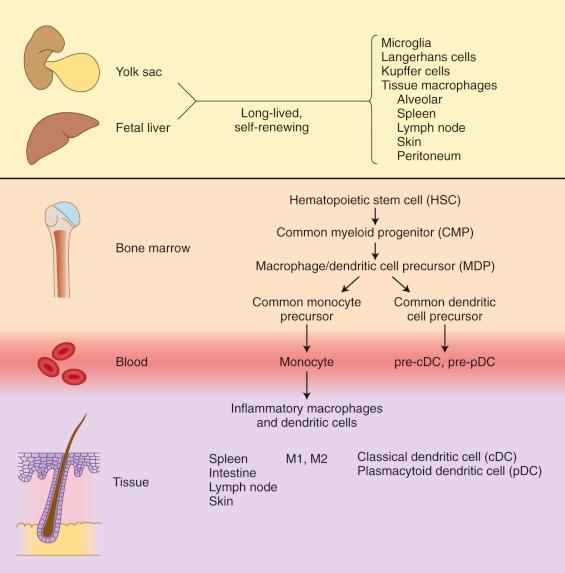
The nomenclature of the histiocytes is in a state of flux, and phenotypic distinctions between dendritic cells and macrophages are undergoing some change. The term histiocyte has come to be used as an umbrella term for all the cells of the mononuclear phagocytic system, macrophages and dendritic cells, but their complex and dynamic inter-relationships require updating in a fresh framework. Most information is derived from mouse models and still needs confirmation in humans, but the most recent findings suggest that adult macrophages are seeded to all sites in the fetus from yolk sac or fetal liver and are long-lived and self-renewing. Bone marrow–derived cells, on the other hand, contribute to replenishment of macrophages and dendritic cells during episodes of inflammation, tissue remodeling and repair, and tumor infiltration ( Fig. 53-1 ).
Langerhans cell histiocytosis (LCH) is a clonal neoplastic proliferation of Langerhans-type cells (LCH cells) that express CD1a, langerin (CD207), and S100 protein. It shows Birbeck granules by ultrastructural examination.
The disorder has a wide age range from the fetus to the elderly. The incidence is estimated at about 4 or 5 per million/year in childhood, with a peak incidence from 1 to 5 years and a male predilection ranging from 1.5:1 to 3.7:1. The incidence in adults is lower, at 1 or 2 per million/year. There is familial incidence of 10% in non-identical twins and 92% in identical twins, although vertical inheritance is rare. LCH can occur after acute lymphoblastic leukemias in children and shares the same molecular genetic signal of the leukemia in some. In adults, it may follow myeloid leukemias or follicular lymphoma, also sharing the genetic marker in some, suggesting transdifferentiation. There is increasing awareness of adult LCH, and an increased incidence of a second hematologic malignant neoplasm is claimed for adult LCH presenting in the skin.
There has been substantial recent change in the paradigm of LCH development. In the first instance, transcriptomes of LCH cells have been shown to share commonality with immature myeloid dendritic cells but not with epidermal Langerhans cells. The BRAF V600E mutation has been documented to be present in 38% to 64% of LCH and can also be identified with good correlation using the VG1 antibody. BRAF V600E was identified in circulating CD11c and CD14 cells and in bone marrow CD34 + cells in patients with active high-risk disease but was restricted to the lesional CD207 + cells in low-risk LCH, indicating a biologic distinction with a somatic mutation of progenitors in high-risk disease but mutation of tissue-restricted dendritic cells only in low-risk disease. BRAF V600E mutation was associated with a higher risk of disease recurrence but did not correlate with the age or clinical form of the condition, localized versus multifocal. Because LCH is neoplastic but not malignant and the BRAF V600E mutation is found in other benign conditions, it has been suggested that LCH be considered an “inflammatory myeloid neoplasm.” Signal-related kinases are activated in all instances of LCH and stain for phosphorylated MEK and phosphorylated ERK, and other mutations, ARAF and MAP2K1 , have been demonstrated in some cases with wild-type BRAF . All of these can affect cell proliferation and migration because of unopposed activation of the MEK-ERK pathway, and ERK activation due to the abnormal upstream signaling proteins may be common to all forms of LCH. Oncogene-induced senescence with cell cycle arrest and a resulting local inflammatory response is a postulated mechanism of LCH with kinase activation.
The Langerhans cell family of disorders spans a wide spectrum of clinical manifestations because of the wide age range, the variety of sites that can be involved, and the unpredictable clinical course. Congenital and neonatal disease can regress when it is localized, but multisystem disease still carries considerable mortality. Staging of the disease is important. Disease localized to a single site in low-risk organs, such as bone, skin, lymph node, thymus, pituitary, and thyroid, can be cured by excision and conservative treatment. Multisystem disease is more chronic, recurs soon, and often may lead to severe and disabling consequences. Multisystem disease that leads to dysfunction in the high-risk organs—liver, spleen, and bone marrow—increases the risk of death even with optimal current therapy.
Craniofacial lesions of the ear, eye, and oral region are regarded as high-risk lesions for the development of diabetes insipidus. Neurodegenerative disease is regarded as an irreversible late immune consequence of LCH, probably a paraneoplastic phenomenon. Pulmonary LCH in adults has major differences from other forms of LCH and has a very high association with smoking. Disseminated forms of LCH can be associated with a macrophage activation and hemophagocytic syndrome that accounts for some of the mortality.
For definitive diagnosis, all lesions require a population of LCH cells. The cells are generally large (15 to 25 µm), oval, and not “dendritic” like interspersed CD1a + inflammatory dendritic cells. They have a distinctive nucleus that displays a complex, folded pattern and often a “coffee bean” groove ( Box 53-1 ; Fig. 53-2, A ). There is a variable content of binucleated and multinucleated cells that also contain the folded LCH nucleus. The nuclear chromatin is finely dispersed; nucleoli are not prominent; and cytoplasm is generous, without granules. An intracytoplasmic ultrastructural feature is the Birbeck granule, a zipperlike structure with a bulbous end ( Fig. 53-2, B ). Eosinophils are often but not invariably present and can be prominent enough to produce microabscesses with Charcot-Leyden crystals. Small lymphocytes are interspersed or, more commonly, aggregated around the LCH cell lesions, but activated lymphoid cells and particularly plasma cells are not common features. Macrophages may be abundant, especially where necrosis is present, and giant osteoclast-type cells that lack the folded nuclear features are particularly notable in bone or paraosseous soft tissue lesions but can also be found in skin or lymph node lesions. Neutrophils are seen, usually in the presence of necrosis or fractures. On rare occasion, the LCH cells are deceptively spindled.
Large, oval, non-dendritic cell 10 to 25 µm in size
Complex grooved and folded nucleus
Surface CD1a, cytoplasmic and surface langerin (CD207), nuclear and cytoplasmic S100
Ultrastructural Birbeck granule (no longer required if CD207 is used)
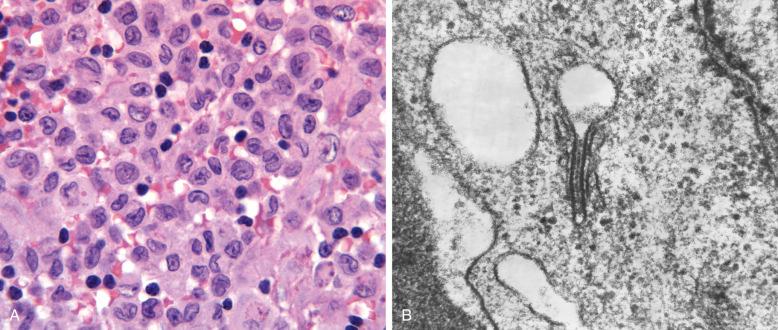
The nuclear features of LCH are bland and monotonous, even though mitosis can sometimes be brisk. Marked pleomorphism, frank cytologic atypia, and especially the finding of atypical mitoses are features of a Langerhans cell sarcoma. Ki67 content in double-stained CD1a or CD207 cells is below 10%, rarely higher.
LCH cells are characterized by the presence of CD1a in a surface and paranuclear pattern and langerin (CD207) that has granular cytoplasmic staining. Nuclear and cytoplasmic S100 is present ( Fig. 53-3 ). The search for the ultrastructural Birbeck granule has been superseded by this tightly diagnostic panel and is not required for the diagnosis of LCH. CD14 (and CD163) is demonstrable in some cases and was said to have some prognostic value, but this has not been validated. VE1, the antibody recognizing the BRAF V600E mutation, is co-expressed by the CD207 population but also by some CD14 + /CD207 − cells. LCH cells have a paranuclear dotlike staining for CD68 and HLA-DR; vimentin is strongly expressed, but cytoplasmic lysozyme is absent or very low. No CD15 or CD30 is present.
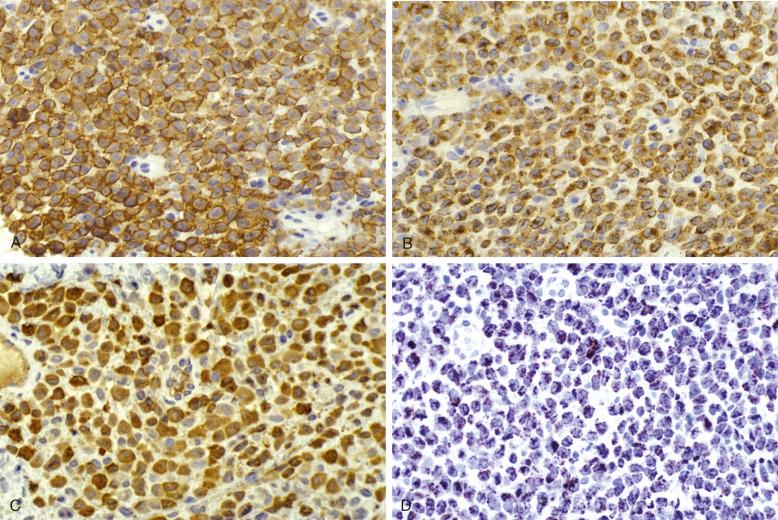
Numerous macrophages may be interspersed between LCH cells. The demonstration that the BRAF V600E mutation can be seen in CD14 + /CD207 − cells may mark LCH precursors in cases of systemic disease.
Fetal and neonatal disease can present as stillbirth, hydrops fetalis, or a “blueberry muffin” appearance, and placental involvement is documented ( Fig. 53-4 ). The disorder can be limited to the skin, but careful staging is necessary because 60% of neonatal cases were shown to have multisystem disease at presentation, most of them with “risk” organ involvement. Some instances of papular skin only involvement can regress spontaneously and are referred to as congenital self-healing reticulohistiocytosis or Hashimoto-Pritzker disease. Most of these lesions are LCH, but some are the congenital reticulohistiocytoma variant of juvenile xanthogranuloma. It has been suggested that instances of self-healing disease contained LCH cells that were more mature, CD14 − /CD86 + , and expressed E-cadherin, whereas systemic disease lacked E-cadherin and the LCH cells were more immature (CD14 + /CD86 − ), but this has not been validated. The BRAF V600E and V600D mutations have been identified in systemic and in benign congenital LCH but in blood and marrow precursors of systemic disease only.
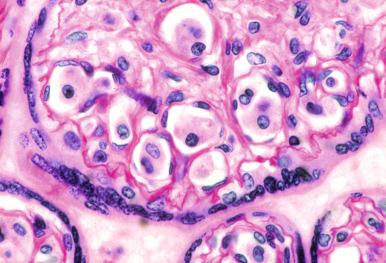
A problem with the diagnosis of congenital LCH is that not all cutaneous presentations are self-limited, and it is not predictable from the histopathology which children will have systemic disease. Careful staging is required because evidence of systemic involvement is usually present at the time of diagnosis. The recommendation is that the diagnosis of self-healing cutaneous LCH be made only in retrospect.
The true incidence of adult LCH is unknown but is estimated at 1 or 2 per million/year ; men are slightly more affected, and the mean age at onset is 33 to 35 years. The sites of involvement are similar to those in children, and 31% to 68% of adults have multisystem disease. Adult pulmonary disease is most commonly limited to the lung and has a very high association with smoking. Clonality was found in only 30% of lung lesions examined by human androgen receptor assay, suggesting that adult pulmonary LCH was a reactive, usually non-clonal reaction to smoking. BRAF V600E mutation has likewise been found in about 27% of cases in two studies. The diagnosis in adults rests on clinical and imaging findings confirmed by immunohistology for CD1a/CD207.
Unlike in children, however, Langerhans cell sarcoma is a differential consideration in all forms of adult LCH and should be excluded. A close association with Erdheim-Chester disease (ECD) is reported for LCH, both having frequent BRAF V600E mutations.
The sites most commonly involved in single-system disease are the skin, bone, lymph nodes, thymus, pituitary gland, and thyroid. Lung is commonly a single site in adults. Because skin is involved in most forms of multisystem disease, it is considered later. Diagnostic criteria do not vary by site, but local features can confound the diagnosis and are stressed.
Lytic lesions of the skull are the most distinctive, often associated with a soft tissue element that can impinge on the dura. Lesions of the temporal bones, facial bones, and sphenoid, ethmoid, and zygomatic constitute the “central nervous system (CNS) risk” bones that have a higher risk of diabetes insipidus or CNS involvement. The vertebrae, jaws, ribs, pelvic bones, and proximal long bones are typical sites of involvement, but small bones of the hands and feet are not. Pain at the site is the most common clinical presentation; however, proptosis can occur in orbital lesions, and temporal bone lesions present with chronic otitis or mastoiditis. Vertebral collapse, vertebra plana, can have its own clinical presentation, including neurologic deficits when the lesion presses on the cord. Loose teeth herald jaw involvement.
Plain film radiographs remain the basic imaging modality for the construction of the differential diagnosis of bone lesions, and computed tomography (CT) scan, magnetic resonance imaging (MRI), technetium scan, and positron emission tomography can have a place in enhanced diagnosis ( Figs. 53-5 and 53-6 ). Early lesions may raise the possibility of an aggressive process, such as a sarcoma, because the margins can be permeative, and cortical rupture with soft tissue extension is the rule. Involuting lesions undergo sclerosis and loss of margins but eventually reconstitute completely. The late lesions, because of their sclerosis, raise the differential of lower grade lesions, most notably chronic osteomyelitis.
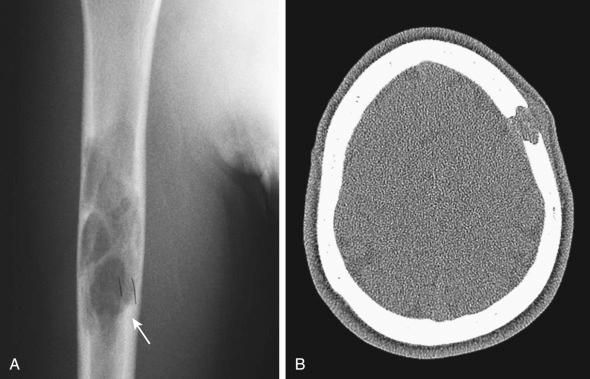
The diagnosis can be made on fine-needle aspiration cytology or tissue biopsy. The classic eosinophilic granuloma has a dominant population of oval histiocytes, with complex nuclei, that are positive for CD1a, CD207, and S100 with an interspersed population of osteoclast-type giant cells, eosinophils that can vary from few to overwhelming, phagocytic macrophages, and T lymphocytes. Plasma cells are generally sparse. Necrosis, hemorrhage, eosinophilic “abscesses,” and neutrophils can dominate the picture. CD1a is demonstrable on lesional cells by immunocytology or immunohistochemistry in a sharp, membranous pattern, with or without a cytoplasmic dot; CD207 survives decalcification and has a cytoplasmic pattern. S100 is of less diagnostic utility because of chondroid staining ( Fig. 53-6 ).
The clinical differential diagnosis in sites such as the skull, long bones, and vertebrae in the early phase will include high-grade lesions, Ewing's sarcoma family, osteosarcoma, neuroblastoma, Hodgkin's disease, and rarely, in young children, myofibromatosis. In late and involuting lesions, untangling the differential diagnosis can be more difficult. The LCH cells disappear as the lesion scars and may not be demonstrable on biopsy, resulting in failure to confirm the clinical and imaging suspicion. Extensive search can be rewarding. Bone lesions with pathologic fracture, necrosis, or hemorrhage can heal with an extensive xanthomatous macrophage component in which the LCH cells are not demonstrable and may be confused for fibrohistiocytic lesions. Plasma cells, rare in uncomplicated LCH, may be seen in complicated lesions after fracture, making distinction from chronic recurrent multifocal osteomyelitis difficult or impossible. Rare LCH lesions undergo aneurysmal bone cyst change ( Fig. 53-6 ). Rosai-Dorfman disease of bone is a rare mimicker compounded by the fact that its cells are also S100 + but morphologically distinct. Juvenile xanthogranuloma and Erdheim-Chester disease can affect bone, making the phenotypic distinction from LCH important.
Most single bone lesions require only biopsy and pain management, although curetting is widely practiced. Non-steroidal anti-inflammatory agents are thought to accelerate healing. Symptomatic lesions and those in vulnerable sites have been given a variety of therapies.
Lymph nodes can be the only site involved in LCH; they can be part of local involvement associated with adjacent bone or skin lesions and can be involved in systemic multiorgan disease. The clinical presentation is usually with asymptomatic swelling of the nodes, most commonly cervical, mediastinal, inguinal, axillary, or retroperitoneal. CT, MRI, and F-fluorodeoxyglucose positron emission tomography can define the extent of lymph node involvement and screen for bone and systemic lesions as part of the staging workup. The diagnosis is established by identifying the sinus pattern of nodal involvement by CD1a/CD207 + LCH cells ( Fig. 53-7 ). As the disease progresses, there is spillover into the paracortex, sparing the follicles but obscuring the sinus pattern. The sinus LCH cells retain their CD1a/CD207 phenotype, but the paracortical cells, although morphologically similar, appear to lose some of their CD1a and CD207 expression and acquire higher levels of surface HLA-DR. Caution should be exercised with CD207 alone because there is an endogenous population of langerin-positive, CD1a − cells in the medullary sinuses. Eosinophils, macrophages, giant cells, areas of necrosis, and hemosiderin can be present in varying amounts. There are no histologic features that distinguish node-only disease from systemic. The proliferative index of LCH cells can vary but is generally less than 10%. Distinction from Langerhans cell sarcoma with high mitotic count is an issue in adult cases. In multifocal or disseminated LCH, there can be an element of macrophage activation with or without hemophagocytosis, and this can obscure the LCH. Diagnosis is generally by node biopsy; fine-needle aspiration with immunophenotypic confirmation has been described ( Fig. 53-8 ), but it must be distinguished from dermatopathic lymphadenopathy ( Fig. 53-9 ).
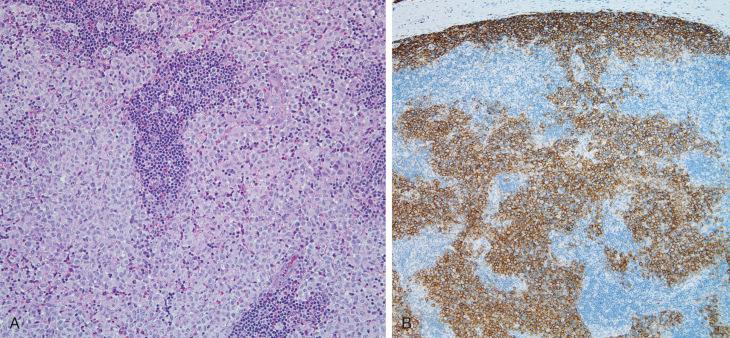
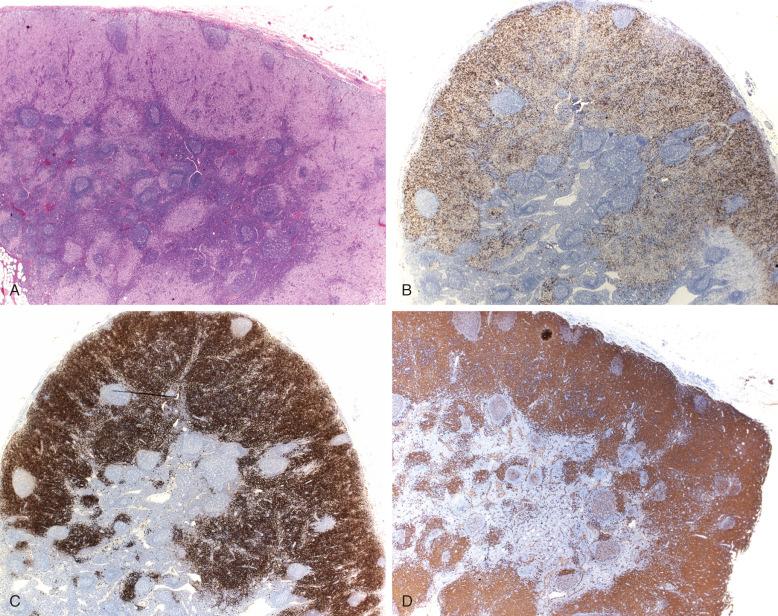
Although the differential diagnosis is wide ( Table 53-1 ), in practical terms, it resides between LCH and other histiocyte-rich lesions, such as dermatopathic lymphadenopathy, Kikuchi's disease, granulomatous lymphadenitis, and histiocyte-rich malignant disorders, such as the histiocyte-rich variant of anaplastic large cell lymphoma and some T-cell leukemias. The architectural clues, specifically the sinus pattern highlighted by lymph node biopsy, dispense with most potential confounders, but aspiration cytology requires greater caution. Langerhans cell sarcoma is more pleomorphic with a high mitotic rate, Ki67 content, and atypical mitoses.
| Site | Condition | Distinguishing Feature |
|---|---|---|
| Skin | Dermal dendritic cell hyperplasia | Perivascular CD1a + /CD207 − |
| Indeterminate cell lesion | Similar to Langerhans cell histiocytosis but CD1a + /CD207 − | |
| Juvenile xanthogranuloma family | CD1a − /CD207 − /S100 − /CD163 + /F13a + /fascin high | |
| Reticulohistiocytoma (epithelioid histiocytoma, juvenile xanthogranuloma) | Similar to juvenile xanthogranuloma | |
| Rosai-Dorfman disease | Large cell, pale nucleus, S100 + /fascin high/CD1a − /CD207 − | |
| Lymph node | Dermatopathic | No sinus involvement, S100 + /fascin high/CD1a + /langerin + |
| Histiocyte-rich lymphomas, leukemias, metastatic lesions | High interspersed macrophages/dendritic cells Lesional cells unstained CD14 + /CD68 + /CD163 + /S100 variable, CD1a low |
|
| Bone | Chronic recurrent multifocal osteomyelitis | CD1a − /CD207 − , plasma cells and CD163 macrophages |
| Benign fibroxanthomatous lesions | CD68 + /F13a − , fascin low | |
| Hodgkin's disease | Reed-Sternberg cells CD30 + /CD15 + , low interspersed CD1a + | |
| Brain | Histiocytic-rich lesions | CD14 + /CD68 + /CD163 + CD1a − /CD207 − |
There are reports of “LCH” occurring in the same node as a lymphoma, leukemia, or other tumors. None of these patients have had LCH elsewhere, at either presentation or follow-up. Microdissection has shown these lesions to be polyclonal, leading to the suggestion that they represent an exaggerated local hyperplasia. The BRAF mutation status of these LCH-like collections is unknown.
Isolated lymph node involvement with LCH, like single-site disease elsewhere, can be self-limited or responds to gentle therapy without consequence.
The thymus can be involved with LCH in three clinically different scenarios. Microscopic collections of LCH-type cells can be seen in incidental thymectomies and in patients with myasthenia gravis. These are likely to be local LCH-like hyperplasias and do not require treatment. Solitary thymic LCH most commonly presents as a cystic thymic mass and requires diagnostic differentiation from other cystic mediastinal masses, especially Hodgkin's lymphoma. Single-site thymic LCH can regress and requires no systemic therapy. The thymus can be involved in multisystem LCH and has even been associated with a higher death rate in this group that involves younger children. CD1a and langerin can be used to confirm thymic involvement when the histopathology is suggestive and the small CD1a + thymocytes and indigenous single CD207 + cells do not confound.
Like the thymus, the thyroid can have varied manifestations of LCH. The thyroid can be involved in multisystem LCH, and hypothyroidism due to LCH infiltration should be distinguished from that of hypothalamic-pituitary involvement. The thyroid can be a site of LCH-induced goiter as single-site disease. Diagnosis is made on fine-needle aspirate and confirmed with CD1a/langerin immunocytology, bearing in mind the following caveats. Surgery alone has been the mainstay of treatment. Clusters of hyperplastic Langerhans cells have often been reported in papillary thyroid carcinomas. Some of these may represent local Langerhans cell hyperplasia and not LCH, but there have also been many instances of papillary thyroid carcinoma in patients who have multifocal LCH. Because both thyroid papillary cancer and LCH can share the BRAF V600E mutation, further exploration of the association is warranted.
The lung can be involved in disseminated multivisceral LCH in young children, but adult-type pulmonary LCH is far more common and is usually a single-organ phenomenon, although local and cervical lymph node involvement sometimes occurs. Most patients with pulmonary LCH, greater than 90%, are smokers.
Pulmonary involvement is seen at diagnosis in 24% of children who have multisystem LCH; but in multivariate analysis, it was found not to be an independent prognostic variable, and lung involvement is no longer regarded as a “high risk” in multisystem LCH. Smoking can rapidly reinduce pulmonary LCH in adults who have had LCH in childhood. Clinical presentation in adults includes non-productive cough, dyspnea, or pneumothorax, although two thirds of patients are asymptomatic. Anatomically, the disease is strictly peribronchial and best demonstrated on high-resolution CT scan. Small nodules are generally symmetrical in the upper lobes, and the damage leads to interstitial fibrosis, cyst formation, and honeycombing, resulting in respiratory insufficiency in some.
Diagnosis is by transbronchial biopsy and confirmed with CD1a/CD207 immunostaining ( Fig. 53-10 ). Bronchoalveolar lavage with greater than 5% CD1a or langerin + cells has also been used in the appropriate clinical and imaging context. Once the disease has advanced with fibrosis and honeycombing, the LCH cells may no longer be demonstrable, and distinction from other causes of fibrosing cystic disease may be impossible.
Clonal analysis demonstrated non-clonality in 71% of adults with LCH, suggesting a hyperplastic response to smoking, and BRAF V600E analysis has confirmed that 75% of lung lesions do not have the mutation. Cessation of smoking is the mainstay of treatment in adults, with corticosteroids or systemic immune suppression for those who progress, with generally good prognosis. Lung transplantation has been used for advanced disease, with recurrence of LCH reported in 20%.
Skin may be the only site of Langerhans cell disease, or it can be part of more widespread involvement, being seen in half of all patients.
In children, the common sites of involvement are the scalp, flexural folds, and diaper area; petechiae are characteristic, and lesions may ulcerate. Adults present with reddish brown papules, nodules, or ulcers also on the scalp or flexures, but vulvar lesions are also noted.
An important differential diagnostic consideration is that chronic dermal perivascular inflammatory reactions may have a high content of CD1a + /CD207 − cells, most of which are small, spindly, or dendritic in shape. LCH, by contrast, is epidermotropic, filling the papillary dermis and infiltrating the epidermis ( Fig. 53-11 ). Parakeratosis, ulceration, and the presence of neutrophils may complicate the picture. LCH cells are large and oval and stain for the presence of CD1a in a membrane pattern and langerin in a cytoplasmic pattern. Langerin alone and langerin with CD1a are the preferred markers for LCH. The lesions may have a mixed population of T lymphocytes, eosinophils, and macrophages; multinucleated cells are uncommon.
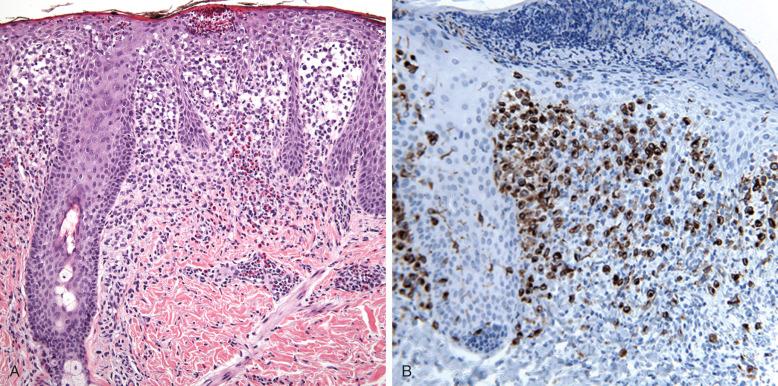
Langerhans cell disease in adults is said to have an association with a concurrent or later hematologic malignant neoplasm. Care should be taken to exclude immature myelomonocytic dermal infiltrates that contain CD1a + cells.
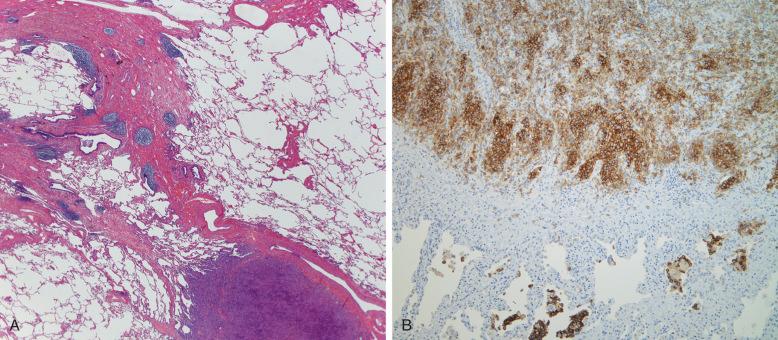
It is rare for a liver to be the sole site of involvement in LCH, but it is a site for multivisceral involvement mostly in childhood. In the early phases of disseminated LCH, there may be a transient hepatomegaly and hypoalbuminemia due to macrophage activation hypercytokinemia, but this does not constitute LCH liver “involvement.” Liver involvement with LCH presents as silent progression to cholestasis. LCH has a peculiar tropism for the larger bile ducts, and this can be demonstrated on imaging where biliary stenosis and dilations are accompanied by peribiliary changes ( Fig. 53-12 ). Enlarged hilar nodes may also be demonstrable. Features of sclerosing cholangitis appear and commonly progress to biliary cirrhosis; hence, rising gamma-glutamyltransferase (GGT) is a sensitive early marker. Intrahepatic infiltration is originally portal with bile duct involvement, but in advanced disease, lobular nodules may occur. Because the disease is focal, affecting larger bile ducts, biopsy of the liver often shows the obstructive features only; but on occasion, CD1a and langerin immunostains can reveal aggregates of LCH cells within the basement membrane of bile ducts. If a diagnosis of LCH has been established elsewhere, a rising bilirubin level and GGT is evidence of liver involvement. Once established, the cirrhosis may no longer have the LCH cells present. Recurrence after liver transplantation has been documented, as has an instance of systemic LCH after living donor transplantation.
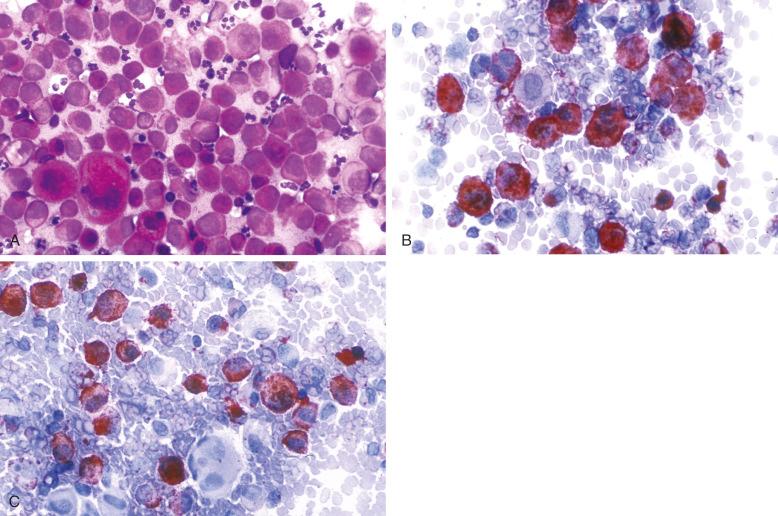
Patients with disseminated LCH may have anemia or even pancytopenia, but bone marrow examination in these cases reveals only small numbers of CD1a + cells by flow cytometry and is slightly more often positive by immunohistology. Even when cytopenias are present, it is unusual to find marrow replacement by CD1a + /langerin + LCH cells. It is not uncommon to find large numbers of CD68 + /CD163 + macrophages in these marrows ( Fig. 53-13 ). Berres and colleagues found BRAF V600 mutations in CD34 + bone marrow progenitor cells in patients with systemic disease, implying that LCH cells in the marrow may not express the full phenotype of CD1a + /langerin + . More work is needed to redefine criteria for marrow involvement with polymerase chain reaction analysis of marrow cells for BRAF V600E.
Macrophage activation and hemophagocytic syndrome are not uncommon in children who have disseminated LCH, and this is a possible mechanism, by the cytokinemia, for the cytopenias. Myelofibrosis and megakaryocytic dysplasia were common findings.
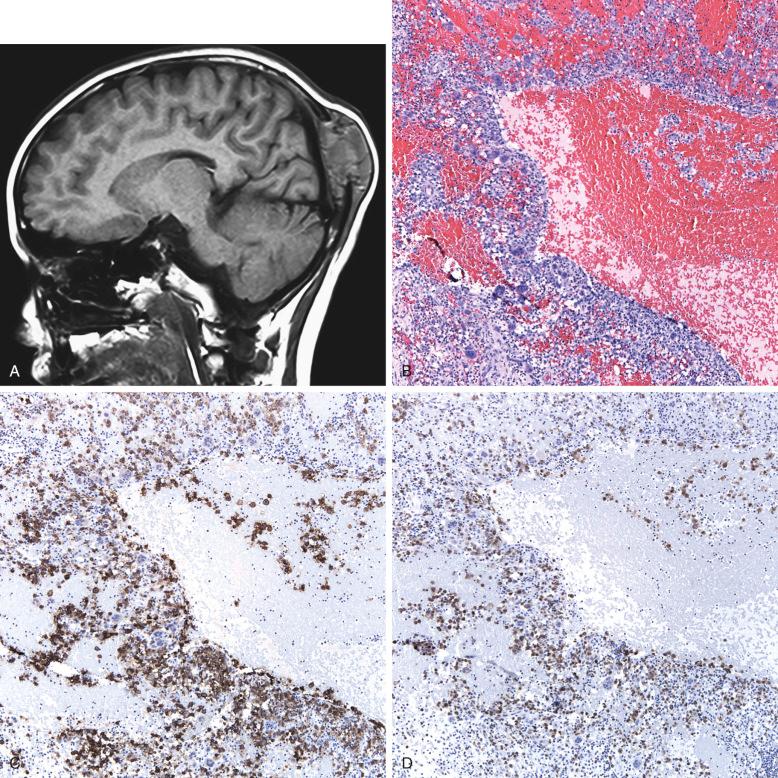
CNS involvement is dominated by the endocrine effects of hypothalamic and pituitary involvement, but a wide range of signs and symptoms can be due to mass or degenerative effects. The incidence of CNS involvement is 1% to 3% of patients with LCH, but the incidence of diabetes insipidus can be as high as 12%, and it is commonly a presenting feature. The presence of LCH elsewhere in the body and skull lesions of the temporal and orbital bones with intracranial extension is associated with a higher rate of CNS disease (CNS risk).
Three broad types of CNS LCH involvement are described; hypothalamic/pituitary, space occupying, and neurodegenerative. Hypothalamic-pituitary involvement is most common, but MRI reveals an unexpectedly high incidence of the pineal as well. Damage to the pituitary and pineal areas is due to direct infiltration with LCH cells, CD1a + , confirmed in many instances by biopsy.
Space-occupying lesions can be intracerebral, in the meninges, or within the choroid plexus. Disease confined to the brain is unusual, most often involving frontal or temporal lobes. Intracerebral lesions may have few LCH cells but have a relatively brisk inflammatory, macrophage, and astroglial component.
Early, fresh lesions are more likely to contain LCH cells. Space-occupying lesions that involve meninges or choroid plexus are characterized by a striking xanthomatous macrophage response, and at the time of surgery and biopsy, fibroxanthoma or juvenile xanthogranuloma may be suspected. These cells, however, do not express F13a.
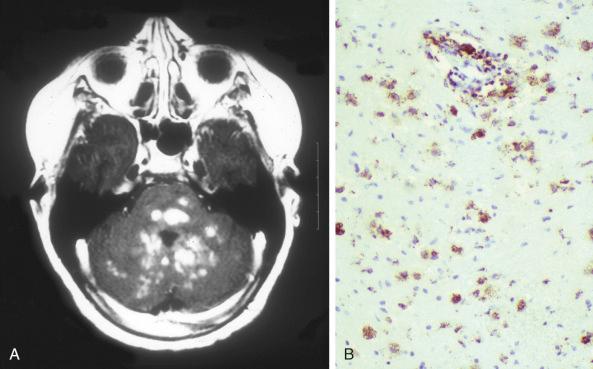
The third type of CNS involvement is the occurrence of neurodegenerative lesions, often late after diagnosis. About 2% to 3% of LCH patients develop cerebellar symptoms, and MRI reveals signal alterations simulating multisystem atrophy ( Fig. 53-14 ). These lesions do not have infiltrating LCH cells and are thought to be paraneoplastic in nature. There is thus no merit in biopsy to document LCH involvement in these lesions.
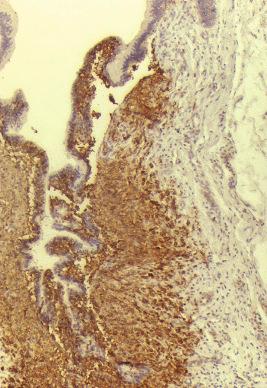
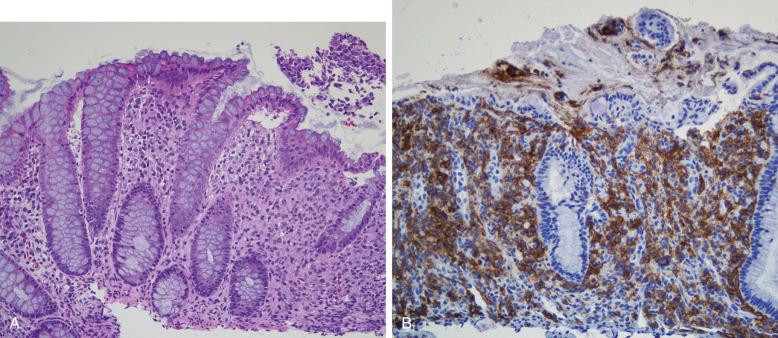
The gastrointestinal tract is most commonly involved as part of a disseminated systemic disease and may be a presenting site. Involvement is mucosal with patchy involvement of the lamina propria, sometimes polypoid. Bloody diarrhea and protein-losing enteropathy are described. Mass lesions in the stomach may cause obstructions. The diagnosis is confirmed by demonstrating the CD1a + /langerin + phenotype of the mucosal cells ( Fig. 53-15 ).
Splenomegaly of more than 3 cm below the costal margin at the midclavicular line, confirmed by ultrasound, is deemed to be splenic involvement by LCH and is regarded as a high “risk” site. Other causes of splenomegaly, such as hemophagocytic syndrome, liver disease, and extramedullary hematopoiesis, should be considered. Tissue or aspiration confirmation is exceptionally rare.
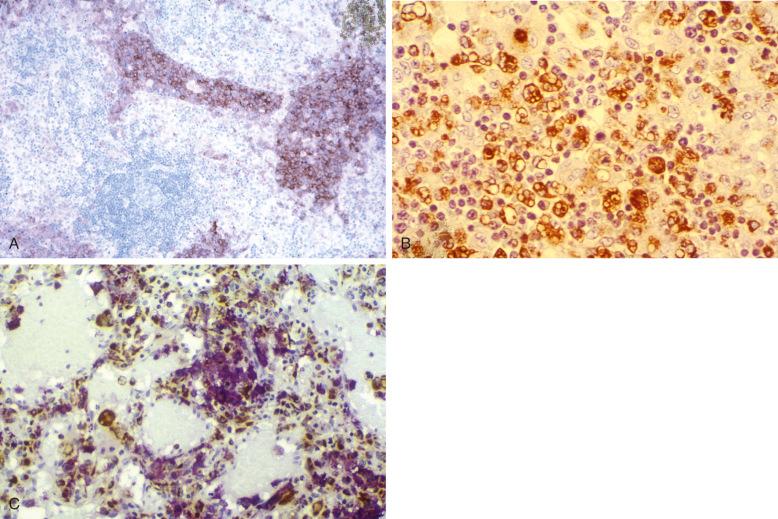
As already noted with LCH of the bone marrow and spleen, a vigorous macrophage response occurs at some sites. LCH, especially multifocal and disseminated disease, can be accompanied by varying degrees of macrophage activation. In its milder manifestations, increased numbers of large macrophages with or without hemophagocytosis can be seen in the bone marrow and other organs. The most extreme expression is a full-fledged hemophagocytic syndrome with hypercytokinemia that can be fatal if it is not treated ( Fig. 53-16 ). Note that CD163, used to demonstrate macrophages, is expressed by the lesional cells in some cases of LCH. The relationship between LCH cells and the macrophages with which they are associated will be clarified by exploring the mutations of the LCH cells and precursors.
Given the similar origin of the cell types involved and the local tissue effects, it should come as no surprise that some histiocytic lesions are “mixed,” with recognizable areas of one and the other. The association of Langerhans cell disease and Erdheim-Chester disease that share the BRAF V600E mutation has been recognized; both types of lesions may present simultaneously, or the Erdheim-Chester lesions may appear years later after the diagnosis of LCH. Similarly, Rosai-Dorfman disease and LCH can occur at separate sites in the same patient, but combined lesions have been seen. LCH and juvenile xanthogranuloma have been described in the same patient, but mixed lesions are also seen with areas of both.
Langerhans cell sarcoma is a high-grade neoplasm with overtly malignant cytologic features and the Langerhans cell phenotype.
The disease has no clear relationship to LCH despite a rare case report, the cases arising de novo. In a recent review of 53 cases from the literature, the age range was 7 to 88 years (mean age of 50 years), although younger patients are described. There was a 3 : 2 male predominance. The BRAF V600E mutation is documented in some.
Roughly equal numbers of patients have single-site disease or multifocal Langerhans cell sarcoma. The common sites for both are lymph node, skin, soft tissue, and bone marrow.
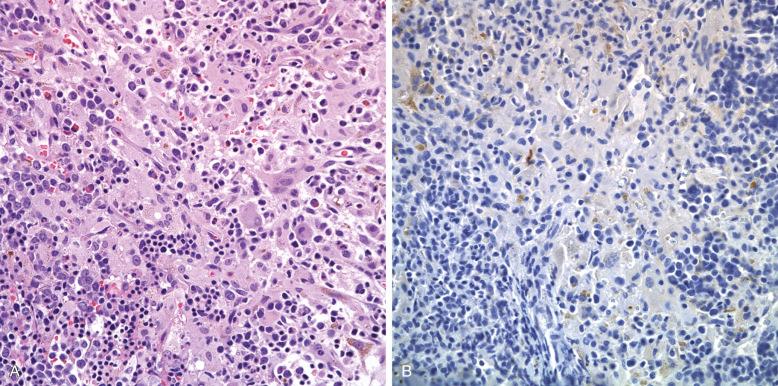
Unlike LCH, Langerhans cell sarcoma has generally overt high-grade cytologic features, but there is a range. The lesions are highly cellular with marked nuclear pleomorphism. The characteristic complex folding and grooving of Langerhans cell nuclei is seen, and there is moderately abundant cytoplasm. A few eosinophils can be interspersed ( Fig. 53-17 ). Mitosis is high, more than 50 per 10 high-power fields, and Ki67 in CD207 + cells is above 30%. When lymph nodes are involved, the pattern is that of sinus infiltration, as in LCH.
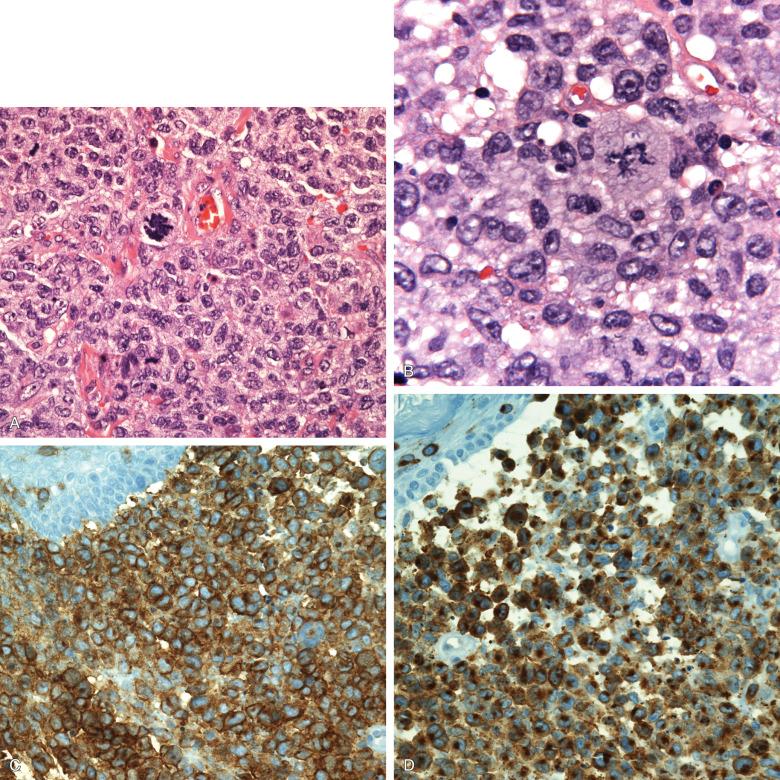
The phenotype, by definition, is that of the Langerhans cell, CD1a + , CD207 + , and S100 + . The number of cells exhibiting the full phenotype varies more than in LCH, and the markers can be progressively lost on recurrences. CD30 is rarely positive, and CD56 has been said to predict a poorer prognosis, but this has not been validated.
Tumors with Langerhans cell sarcoma features can occur after acute lymphoblastic leukemia or follicular lymphomas, sharing the molecular genetic signature of the lymphoma. Langerhans cell features may also occur in peripheral tumors of patients with monocytic leukemias or myeloproliferative syndromes and appear to be aberrant differentiation. This is different from the well-described occurrence of leukemia after etoposide therapy for LCH.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here