Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hepatitis A virus (HAV) is a positive-sense RNA virus and a member of the Picornaviridae family. The single-stranded RNA genome is approximately 7500 nucleotides long and contains a single open reading frame. The encoded polyprotein includes structural proteins for the 27–28 nm diameter capsid, nonstructural proteins with protease or polymerase activities, and other proteins with functions that have not been fully determined. ,
Although the pathogenesis of hepatitis A is not completely understood, a hallmark of HAV infection is noncytopathic replication. The absence of cytopathic changes in cell culture and the demonstration of HAV-directed natural killer and lymphokine-activated killer cells in vitro suggest that multiple mechanisms of cell-mediated immunity may be responsible for hepatocellular damage. Among infected people, HAV replicates in the liver and is excreted in bile. Quasi-enveloped virions (eHAV), which are infectious, have recently been shown to be extruded from infected hepatocytes without lysing these infected cells. The membranes of eHAV are stripped as it exits the biliary system, and nonenveloped HAV are thus found in high concentrations in stool. The incubation period averages 28 days (range, 15–50 days). Peak viral concentrations in stool and greatest infectivity are during the 2 weeks before until 1 week after symptom onset. Disease onset usually follows only after maximal stool shedding. Viremia occurs soon after infection and persists at least through the period of elevation of hepatic enzymes in serum. ,
HAV is a single serotype. Six HAV genotypes have been identified with unique geographic distribution; 4 genotypes are found in humans (I, II, II, IV). , Although systematic molecular surveillance is limited, genotype IA has historically been the most common genotype circulating in the US. More recently, genotype IB has predominated. In addition, genotype IIIA, considered rare in the US, has been increasingly detected.
Immunity resulting from HAV infection is lifelong. HAV infection does not result in chronic infection or chronic liver disease.
The principal mode of HAV transmission is from person to person by the fecal-oral route. Transmission occurs most commonly among close contacts, including household and sexual contacts of people infected with HAV. HAV also is transmitted by food contaminated by an infected handler or at any point during growing, processing, or distribution. Common-source food exposures are of increasing concern. Several outbreaks of hepatitis A have been associated with foods imported from hepatitis A–endemic countries. , , Transmission by contaminated water is rare and has been decreasing in the US since the improved implementation of ground water rules.
Bloodborne HAV transmission is rare but may occur due to transient viremia. On rare occasions, HAV infection has been transmitted by transfusion of blood or blood products collected from donors during the viremic phase of infection and through solid organ transplantation. , In the 1990s, outbreaks were reported in Europe and the US among patients who received factor VIII and factor IX concentrates. However, changes in viral inactivation procedures, introduction of hepatitis A vaccine, and improved donor screening have virtually eliminated the risk for HAV transmission from clotting factor concentrates. ,
In 32% of people with sporadic, community-acquired hepatitis A, no source of infection is identified. When a risk factor is identified, outbreak-associated cases are the most commonly reported, followed by cases associated with injection drug use. Vertical transmission to children from pregnant women who develop hepatitis A during the third trimester of pregnancy has been reported, but the risk appears to be low.
Because most young children have asymptomatic or unrecognized HAV infections, children formerly had a substantial role in the epidemiology of hepatitis A in the US by serving as sources of infection. Following the widespread implementation of universal childhood vaccination in 2006, the incidence of hepatitis A declined, and asymptomatic children no longer appear to be the primary source of infection for adults. , However, a significant proportion of adults were not exposed to HAV in childhood and remain unvaccinated and are susceptible to infection.
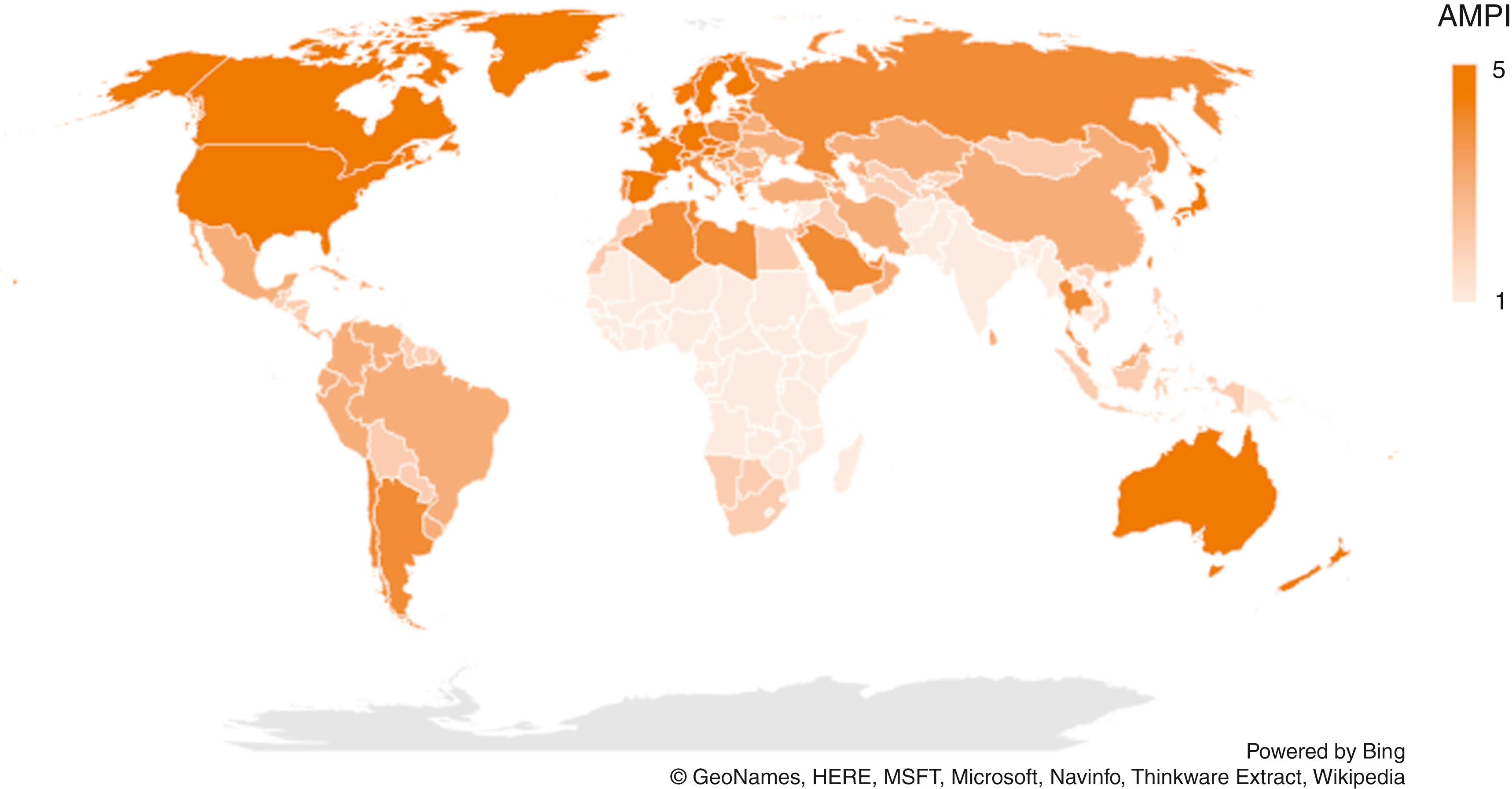
The relationship between risk for infection and likelihood of asymptomatic infection during childhood is important to understanding HAV transmission patterns worldwide and to developing vaccination policies. Level of economic development is correlated with global HAV transmission patterns ( Fig. 237.1 ). In areas where HAV seroprevalence is high among young children (e.g., parts of Asia, Africa, Central and South America), the lifetime risk of infection is greater than 90%, and infection occurs primarily in early childhood. , In these countries, asymptomatic infection predominates, the incidence of clinical hepatitis A generally is low, and outbreaks are rare because most adults are immune. However, adults who remain susceptible to infection, including travelers from other countries, are at high risk for HAV infection. In areas of moderate endemicity (e.g., Eastern Europe), HAV is not transmitted as readily because of better sanitation and living conditions, and the average age of infection is older than in areas of high endemicity. Paradoxically, the potential for large outbreaks can be increased (compared with highly endemic areas) because of the relatively larger pool of susceptible older children and adults who are more likely to develop symptomatic illness following HAV infection. Unimmunized travelers from well-resourced countries to these areas also are at risk. In some instances, countries undergoing rapid development accompanied by improvement in sanitation standards and clean water supplies have experienced declines in HAV seroprevalence among young children but increased HAV-associated morbidity and mortality among older children and adults. In most developed countries without universal vaccination policies, the incidence of both HAV infection and hepatitis A clinical disease is low but they continue to have cyclic, community-wide outbreaks that feature transmission among preschool and school-aged children and their adult contacts. , In developed countries with universal childhood vaccination policies, most cases and outbreaks occur among adults in defined risk groups, including travelers returning from endemic areas, men who have sex with men, and people who use drugs ( Table 237.1 )
| Type of Risk | Risk Category | Examples |
|---|---|---|
| Increased risk for HAV infection | Close personal contacts of persons with HAV infection b | Household contacts |
| Caretakers | ||
| Sexual contacts | ||
| Persons who anticipate close personal contact with an international adoptee | ||
| Occupational risk | Persons working with nonhuman primates | |
| Persons working with clinical or nonclinical material containing HAV in a research laboratory | ||
| Persons who use drugs | Persons who use injection or noninjection drugs (i.e., all those who use illegal drugs) | |
| Persons in settings where services to adults are provided | Group settings for persons with developmental disabilities | |
| Homeless shelters | ||
| Syringe services programs | ||
| Correctional facilities during outbreaks | ||
| International travelers | Persons traveling to or working in countries with high or intermediate HAV endemicity | |
| Increased risk for severe disease from HAV infection | Immunocompromised persons | Congenital or acquired immunodeficiency |
| HIV infection | ||
| Chronic renal failure, undergoing dialysis | ||
| Solid organ, bone marrow, or stem cell transplant recipients | ||
| Persons with diseases requiring treatment with immunosuppressive drugs/biologics (e.g., tumor necrosis alpha inhibitors), long-term systemic corticosteroids, radiation therapy | ||
| Persons with chronic liver disease | Hepatitis B virus infection | |
| Hepatitis C virus infection | ||
| Cirrhosis (any etiology) | ||
| Fatty liver disease (hepatic steatosis) | ||
| Alcoholic liver disease | ||
| Autoimmune hepatitis | ||
| Alanine aminotransferase or aspartate amino transferase level more than twice the upper limit of normal or persistently elevated for 6 months | ||
| Age | Adults aged >40 years |
a Not all risk categories include persons recommended for routine hepatitis A vaccination. Providers should assess the risk for HAV infection or severe disease from HAV infection when making decisions regarding the provision of postexposure prophylaxis or revaccination. Providers should consider vaccination in settings providing services to adults at risk for HAV infection.
b Excludes healthcare personnel using appropriate personal protective equipment.
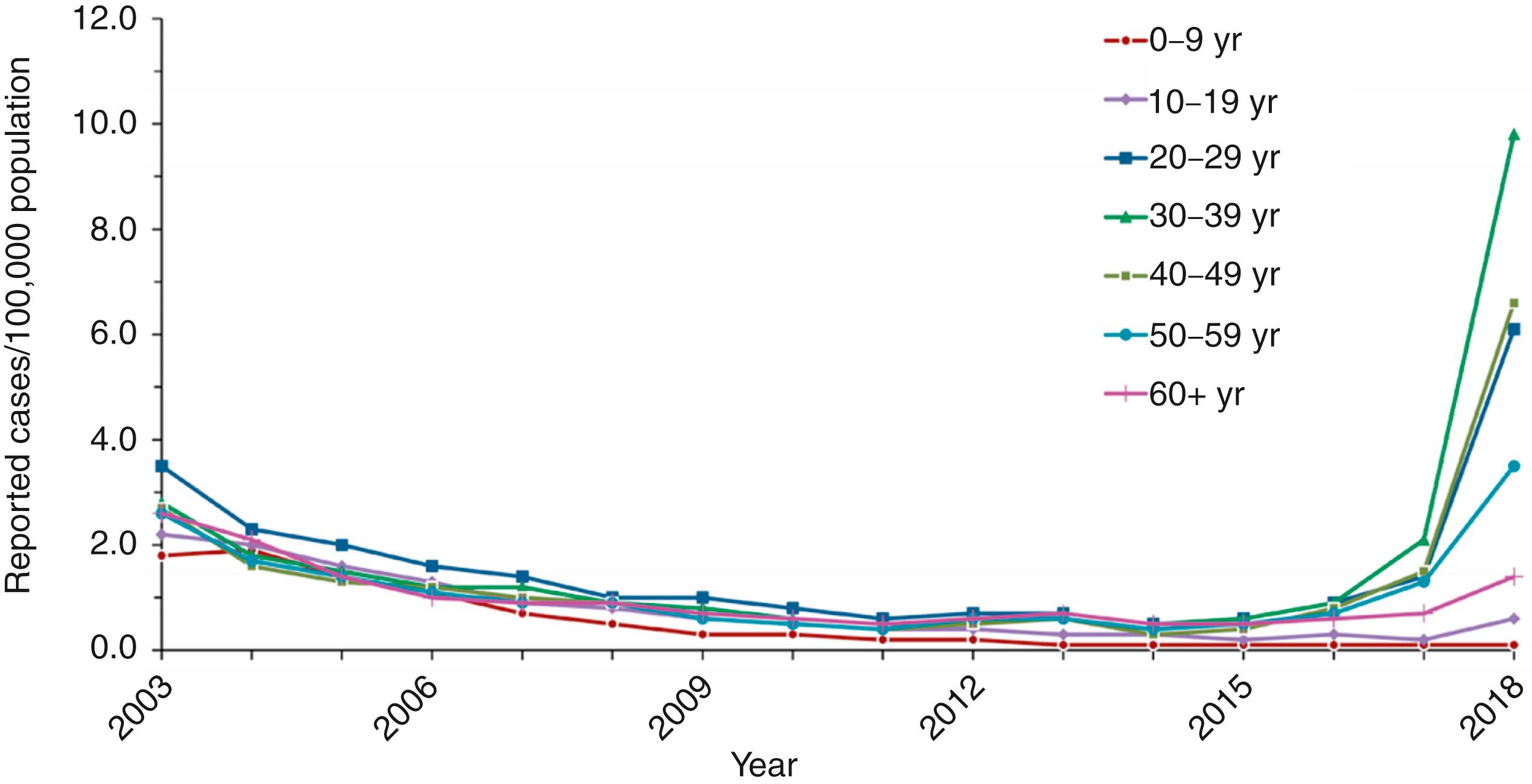
In the US, before hepatitis A vaccine was widely available, hepatitis A occurred in large, nationwide epidemics approximately every decade. Until 2001, the highest rates of HAV infection in the US were observed among children aged 5–14 years. The highest rates and the majority of cases occurred in the western and southwestern states. , , Incidence rates declined sharply after licensure of the first hepatitis A vaccines in 1995 and following implementation of targeted recommendations for childhood vaccination in states with high hepatitis A rates. , , In 2006, universal vaccination was recommended in all states, and incidence continued to fall in all age groups ( Fig. 237.2 ).
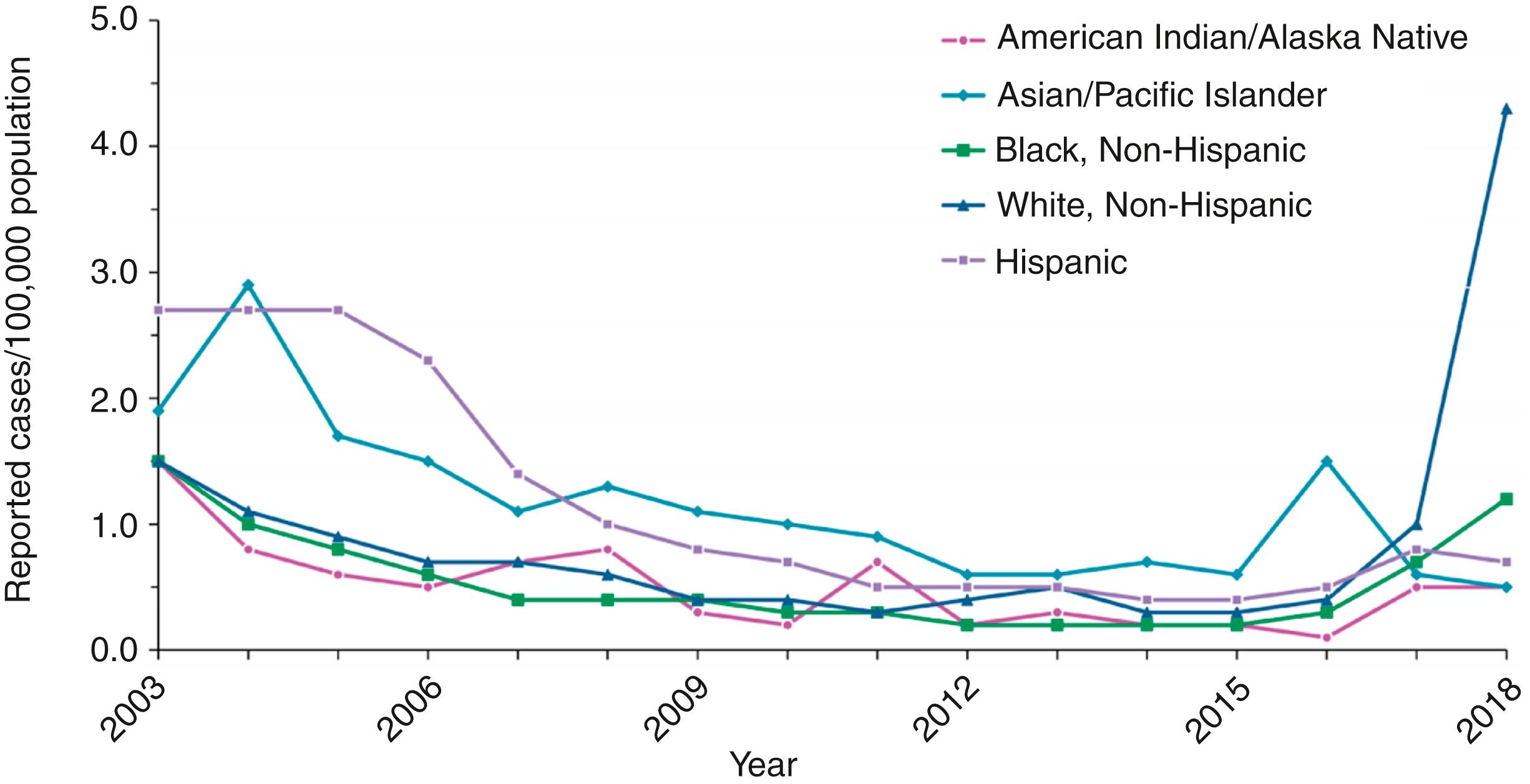
Hepatitis A rates have declined since vaccine introduction from 11.7 cases per 100,000 population in 1996 to 0.4 cases per 100,000 population in 2011, with a slight increase to 0.5 and 0.6 cases per 100,000 population in 2012 and 2013, respectively, and declined in 2014 and 2015 back to 0.4 cases per 100,000 population. , , Rates increased to 0.6 cases per 100,000 population and 1.0 per 100,000 population in 2016 and 2017, respectively. , Since the mid-2000s, regional, ethnic, and racial differences in incidence rates of hepatitis A have been eliminated, thereby indicating fundamental shifts in hepatitis A epidemiology ( Fig. 237.3 ). , Although there have been massive declines in HAV infections among children, the seroprevalence rate among adults has decreased, indicating increasing susceptibility, and average ages of hepatitis A hospitalizations and deaths have increased among adults. , , The likely reason for this finding is that the adults who were infected in childhood with subsequent immunity are aging out of the population to be replaced by adults who were too old to be vaccinated as children but who did not have the opportunity to be exposed to the virus and develop disease at an earlier age. From 2016–2020, outbreaks associated with contaminated foods and people who use drugs resulted in >31,000 cases of hepatitis A and almost exclusively impacted adults. , , ,
HAV infection can be inapparent (the patient is asymptomatic with no elevation in serum hepatic enzymes), subclinical (asymptomatic with elevation of hepatic enzymes), anicteric (symptomatic without jaundice), or icteric (symptomatic with jaundice). The likelihood of having symptoms with HAV infection is related to age. Most children aged <6 years have asymptomatic infection or mild, nonspecific symptoms with hepatitis A; <10% of children in this age group have jaundice. Among older children and adults, 76%–97% have symptoms, and 40%–70% of patients with symptoms are icteric.
When symptoms occur, most patients have an onset of low-grade fever, myalgia, anorexia, malaise, nausea, and vomiting. These symptoms are followed several days later by specific symptoms and signs of hepatic dysfunction, including dark urine, light-colored stools, jaundice, and scleral icterus. Many children (60%) and some adults (20%) have diarrhea. Some children (<20%) have upper respiratory tract symptoms (e.g., cough, coryza, sore throat).
Urticaria can occur at the onset of illness. Physical findings are variable and can include jaundice, scleral icterus, hepatomegaly, right upper quadrant tenderness, and splenomegaly. Abdominal ultrasonography showed edema of the gallbladder wall in approximately 50% of children with uncomplicated hepatitis A who were studied prospectively; transient ascites occurred in a few patients. The symptoms of hepatitis A last for several weeks on average and usually not longer than 2 months, although some people can have prolonged or relapsing signs and symptoms for up to 6 months. In immunocompromised people, more severe disease can occur, as well as prolonged viremia and fecal shedding.
HAV infection occasionally results in fulminant hepatitis and death. In addition, hepatitis A has several atypical manifestations, including relapsing hepatitis A, cholestatic hepatitis, autoimmune hepatitis, and extrahepatic symptoms. Of 348 children with acute liver failure from the US, Canada, and the UK who were entered into a registry, only 3 (0.9%) had acute hepatitis A.
Fulminant hepatitis A, characterized by the onset of severe liver injury and hepatic encephalopathy in patients with no known preexisting liver disease, is an infrequent occurrence. In 2016, the case-fatality rate among reported cases of hepatitis A in all age groups was approximately 0.7%. Host factors associated with a higher risk for fulminant hepatitis A include age >50 years and underlying chronic liver disease ( Table 237.1 ). Molecular studies have not shown an association between fulminant hepatitis A and any type of viral variant. Spontaneous recovery occurs in 30%–60% of people with fulminant HAV infection, and survivors generally regain full liver function. Prognosis is influenced by age, clotting factor level, stage of coma, and presence of renal disease. In approximately 10%–15% of patients with hepatitis A, relapsing hepatitis occurs, and approximately 20% of these patients have multiple relapses. These patients typically have another episode of hepatitis 1–4 months after the initial acute hepatitis. The relapse is accompanied by elevation of serum hepatic enzymes and persistence of IgM anti-HAV. Molecular studies have demonstrated the presence of HAV in stool specimens during relapse. Most patients recover completely within several weeks.
Cholestatic hepatitis occurs rarely following infection with HAV. Patients with this disorder have substantially elevated concentrations (>10 mg/dL) of total bilirubin and jaundice persisting for an extended period (in some cases >3 months). In cholestatic hepatitis, normal abdominal ultrasonographic findings are seen (compared with biliary duct dilation seen in obstructive jaundice).
Several case series have been described in which HAV infections in pediatric patients are followed by autoimmune chronic active hepatitis, in some instances requiring long-term corticosteroid therapy. Extrahepatic manifestations of hepatitis A include pruritus, arthralgia, cutaneous vasculitis, cryoglobulinemia, and hemophagocytic syndrome (anemia and thrombocytopenia, with hemophagocytosis apparent on biopsy of bone marrow). These manifestations are rare and resolve with resolution of hepatitis.
During HAV infection, inflammation of the liver is accompanied by abnormalities in serum hepatic enzymes, with increases in serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and γ-glutamyltranspeptidase. Usually during hepatitis A virus infection, ALT and AST values are between 200 and 5000 IU/L, with the ALT value higher than the AST value and alkaline phosphatase levels only mildly elevated. Elevations in ALT and AST can precede symptoms by a week or more and generally peak 3–10 days after the onset of symptoms ( Fig. 237.4 ).
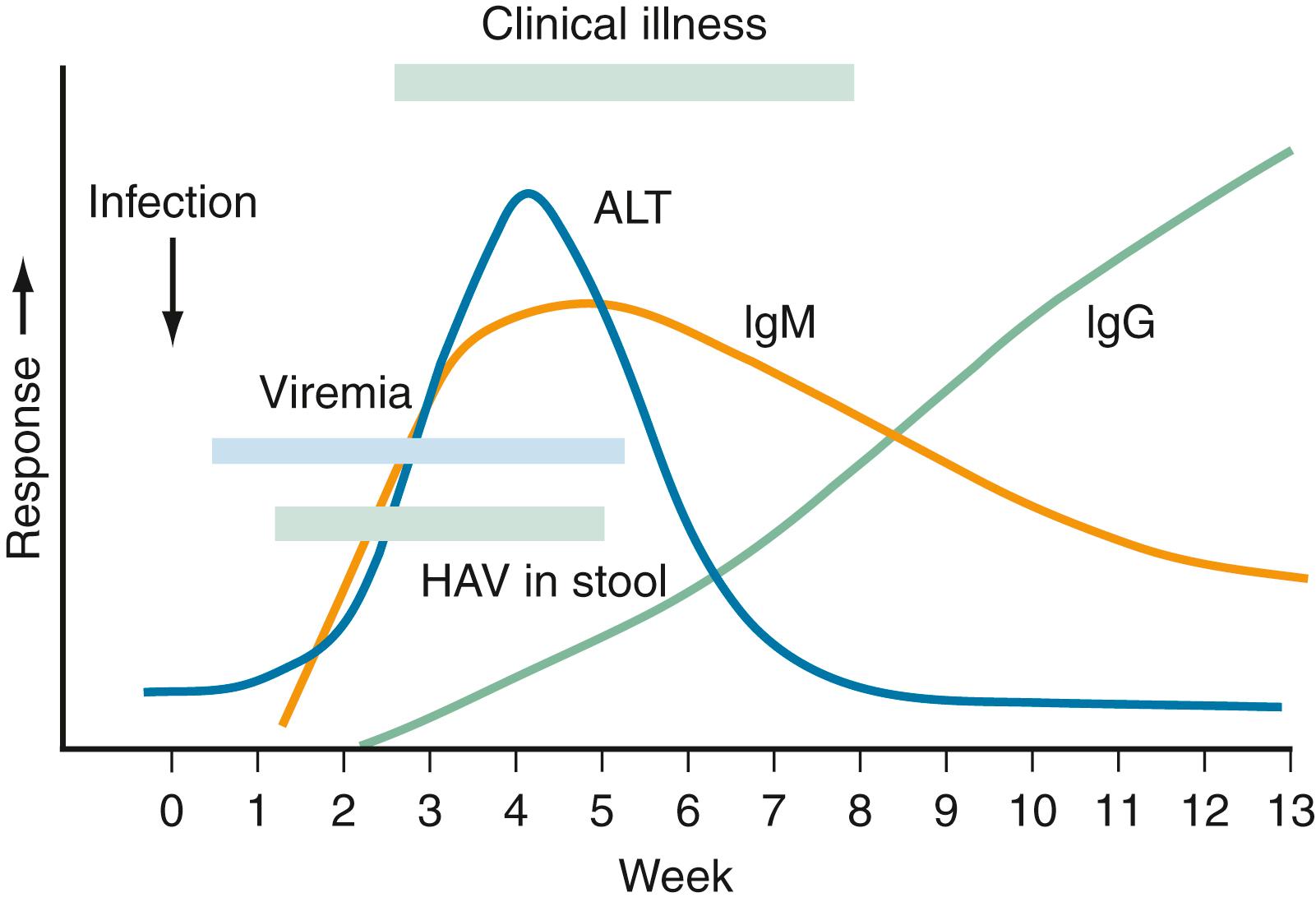
The specific diagnosis of hepatitis A relies on serologic testing in addition to the presence of symptoms. Virtually all people with acute hepatitis A have detectable immunoglobulin (Ig) M anti-HAV during the acute or early convalescent phase of infection ( Fig. 237.4 ). IgM anti-HAV generally disappears within 6 months after the onset of symptoms, although people who test positive for IgM anti-HAV >1 year after infection or with no known history of infection have been reported. , IgG anti-HAV, which appears in the convalescent phase of infection, remains detectable in serum for the person’s lifetime and confers protection against disease. Commercial enzyme immunoassays are available for detection of IgM anti-HAV and total anti-HAV in serum. IgM anti-HAV detection is necessary to make the diagnosis of active HAV infection.
Polymerase chain reaction assays can be used to detect HAV RNA in stool specimens and sera of people with HAV infection. , Based on epidemiologic studies, peak infectivity occurs during the 2 weeks before the onset of symptoms. For practical purposes, people infected with HAV can be assumed to be noninfectious 1 week after the onset of jaundice. Children may experience prolonged shedding of HAV for up to 10 weeks after clinical onset. After one outbreak, infected neonates shed HAV for up to 6 months. Usually, chronic fecal shedding of HAV does not occur; however, among patients with relapsing illness, recurrent shedding can be detected. Viremia is present shortly after infection and persists while liver enzymes remain elevated. , , , In immunocompromised patients, prolonged fecal shedding and viremia for more than 1 year have been reported.
Light microscopic examination of liver specimens in acute hepatitis A reveals pathologic features common to all forms of viral hepatitis, including hepatocellular necrosis, inflammatory infiltration of lymphocytes and other mononuclear cells, and regeneration of hepatocytes. The extent of involvement varies with the stage and severity of hepatitis and age of the patient. Liver biopsy typically is not indicated for the diagnosis or management of hepatitis A.
Treatment generally is supportive. Hospitalization may be necessary for patients who are dehydrated from nausea and vomiting or who have fulminant hepatitis A. No specific diet or restriction of activity is necessary. Medications that can cause hepatic damage or that are metabolized by the liver should be used with caution. For the infrequent presentations of cholestatic or relapsing hepatitis A, a short course of corticosteroid therapy has been reported as effective in limiting symptoms and hastening recovery, but no controlled trials have evaluated this approach. ,
Measures to prevent HAV infection include hand hygiene, provision of safe drinking water, adequate disposal of sanitary waste, and pre-exposure or post-exposure prophylaxis with hepatitis A vaccine ( Table 237.2 ). In addition, immunoglobulin (IG) may be co-administered in certain situations ( Table 237.2 ). The dosage of IG was updated in 2017 to improve protection against HAV ( Table 237.3 ). , ,
| Indication/Age Group | Risk Category/Health Status | Hepatitis A Vaccine | Immune Globulin a |
|---|---|---|---|
| Post-Exposure Prophylaxis | |||
| <12 months | Healthy | No | 0.1 mL/kg |
| 12 months–40 years | Healthy | 1 dose b | None |
| >40 years | Healthy | 1 dose b | 0.1 mL/kg c |
| ≥12 months | Immunocompromised or chronic liver disease | 1 dose b | 0.1 mL/kg d |
| ≥12 months | Vaccine contraindicated e | No | 0.1 mL/kg |
| Pre-Exposure Protection (e.g., Travel) f | |||
| <6 months | Healthy | No | 0.1–0.2 mL/kg g |
| 6–11 months | Healthy | 1 dose h | None |
| 12 months–40 years | Healthy | 1 dose i | None |
| >40 years | Healthy | 1 dose i | 0.1–0.2 mL/kg g , j |
| >6 months | Immunocompromised or chronic liver disease | 1 dose i | 0.1–0.2 mL/kg g , j |
| >6 months | Persons who elect not to receive vaccine or for whom vaccine is contraindicated | No | 0.1–0.2 mL/kg g |
a Measles, mumps, and rubella vaccine should not be administered for at least 2 weeks before and 6 months after administration of immune globulin.
b A second dose of hepatitis A vaccine is not required for post-exposure prophylaxis; however, for long-term immunity, the vaccination series should be completed with a second dose at least 6 months after the first dose.
c The provider’s risk assessment should determine the need for immune globulin administration. If the provider’s risk assessment determines that both vaccine and immune globulin are warranted, hepatitis A vaccine and immune globulin should be administered simultaneously at different anatomic sites.
d Vaccine and immune globulin should be administered simultaneously at different anatomic sites.
e Life-threatening allergic reaction to a previous dose of hepatitis A vaccine or allergy to any vaccine component.
f Immune globulin should be considered before travel for persons with special risk factors for either HAV infection or severe disease from HAV infection.
g 0.1 mL/kg for travel up to 1 month; 0.2 mL/kg for travel up to 2 months, 0.2mL/kg every 2 months for travel of ≥2 months’ duration.
h This dose should not be counted toward the routine 2-dose series, which should be initiated at age 12 months.
i For persons not previously vaccinated with hepatitis A vaccine, administer dose as soon as travel is considered, and complete series according to routine schedule.
| Indication | Time | Dose∗ | Route |
|---|---|---|---|
| Pre-exposure Prophylaxis | Up to 1 month duration of travel | 0.1 mL/kg | IM |
| Pre-exposure Prophylaxis | Up to 2 months duration of travel | 0.2 mL/kg | IM |
| Pre-exposure Prophylaxis | ≥2 months duration of travel | 0.2 mL/kg (repeat every 2 months) | IM |
| Post-exposure Prophylaxis | Within 2 weeks of exposure | 0.1 mL/kg | IM |
a The dosage of immune globulin is based on weight for all ages and does not have a maximum dose for protection against hepatitis A.
Hepatitis A vaccine is preferred over IG for pre-exposure and post-exposure prophylaxis because of induction of active immunity and longer protection, greater ease of administration, and often greater acceptability. In the US, HAVRIX (GlaxoSmithKline) was approved by the Food and Drug Administration in 1995 and VAQTA (Merck) was approved in 1996; both vaccines are licensed for people aged ≥12 months ( Table 237.4 ). In 2001, a combination hepatitis A and hepatitis B vaccine (TWINRIX, GlaxoSmithKline) was approved for patients aged ≥18 years. Inactivated hepatitis A vaccines are prepared by methods including growth in cell culture, purification by ultrafiltration or other methods, inactivation with formalin, and adsorption to an aluminum hydroxide adjuvant. , HAVRIX and VAQTA are available in pediatric and adult formulations and are licensed in a 2-dose series. If VAQTA is used, the second dose should be scheduled 6–18 months after the first. If HAVRIX is used, the second dose should be scheduled 6–12 months after the first. However, if the second dose of VAQTA is given >18 months (if HAVRIX is used, >12 months) after the first dose, no additional doses are required. The route of administration is intramuscular, in the anterolateral thigh for children aged <2 years and the deltoid muscle for children and adults. TWINRIX is licensed in a 3-dose series (0, 1, 6 months) or a 4-dose accelerated series (0, 7, 21–30 days and booster at 12 months). Monovalent hepatitis A vaccine may be used to complete hepatitis A vaccination begun with TWINRIX and vice versa.
| Vaccine | Trade Name (Manufacturer) | Age Group (yr) | Dosage | Route | Schedule | Booster |
|---|---|---|---|---|---|---|
| HepA, inactivated (2 doses) | Havrix (GlaxoSmithKline) | 1–18 | 0.5 mL (720 ELISA units inactivated HAV) | IM | 0, 6–12 months | None |
| ≥19 | 1 mL (1,440 ELISA units inactivated HAV) | IM | 0, 6–12 months | None | ||
| HepA, inactivated (2 doses) | Vaqta (Merck) | 1–18 | 0.5 mL (25 units HAV antigen) | IM | 0, 6–18 months | None |
| ≥19 | 1 mL (50 units HAV antigen) | IM | 0, 6–18 months | None | ||
| Combined HepA and HepB a (3 doses) | Twinrix (GlaxoSmithKline) | ≥18 (primary) | 1 mL (720 ELISA units inactivated HAV + 20 μ g HBsAg) | IM | 0, 1, 6 months | None |
| ≥18 (accelerated) | 1 mL (720 ELISA units inactivated HAV + 20 μ g HBsAg) | IM | 0, 7, 21–30 days | 12 months |
a Combined HepA and HepB vaccine (Twinrix) should not be used for postexposure prophylaxis.
Inactivated hepatitis A vaccines have been studied extensively in children and adults and have been found to be safe and highly immunogenic. One month after receiving the first dose, 97%–100% of persons aged 2–18 years had protective levels of antibody, and 1 month after receiving the second dose, 100% had protective levels. Although nearly 100% of patients will develop protective antibodies after receiving 2 doses, there are several factors associated with reduced immunogenicity. Response to vaccine may be lower in infants with passively acquired maternal antibody, immunosuppressed patients, and patients with advanced liver disease or recipients of liver transplants, and older adults. Final antibody concentrations in infants with passively acquired maternal antibody are one-third to one tenth of those of anti-HAV–negative infants who are vaccinated according to the same schedule. , However, after a booster dose administered 1–6 years later, most infants with passively acquired antibody do experience an anamnestic response. , , In most infants, passively acquired maternal antibody declines to undetectable levels by the age of 1 year. , For children who began hepatitis A vaccination at age >1 year, regardless of maternal anti-HAV status, hepatitis A vaccine is highly immunogenic. , Delayed administration of the second dose beyond 18 months does not appear to reduce immunogenicity. Available data indicate that the vaccines are safe and immunogenic in children <12 months of age who do not have passively acquired maternal antibody.
Inactivated hepatitis A vaccine is highly efficacious in preventing hepatitis A. In a large study conducted in Thailand among children aged 1–16 years, efficacy of the vaccine was 94% after 2 doses of vaccine administered 1 month apart. In another study conducted in New York among children aged 2–16 years with a different inactivated vaccine, efficacy was 100% starting 17 days after administration of 1 dose.
The duration of protection after vaccination is unknown; however, anti-HAV has been shown to persist in vaccine recipients for at least 22 years. Detectable antibodies are estimated to persist for 30 years or longer, based on mathematical modeling and anti-HAV kinetic studies.
In prelicensure clinical trials among children, the most frequently reported local reaction following hepatitis A vaccination was soreness at the injection site (15%–19%). , Systemic reactions that include fatigue, fever, diarrhea, and vomiting occurred in <5% of vaccine recipients. Post-licensure passive-surveillance identified serious adverse events in 6% of reports. Most (80%) reports involved hepatis A vaccine administered concomitantly with other vaccines during the same health care visit. The most frequently reported adverse events were fever, injection site reactions, and rash. , ,
In 1996, the Advisory Committee on Immunization Practices (ACIP) recommended hepatitis A vaccine for children aged ≥2 years in communities with high rates of HAV disease or for outbreak control. , Adult risk groups recommended for vaccination included people traveling to or working in countries that have high or intermediate endemicity of infection, men who have sex with men, people who use drugs, people who have occupational risk for infection, and people who have chronic liver disease. By 2004, first-dose coverage rates among children 24–35 months of age who were living in these states had reached 25%–50%. The impact of even limited routine vaccination of children on hepatitis A incidence was evidenced by historic reductions in incidence rates. , In 2006, the ACIP extended recommendations to routine vaccination of all children 12–23 months of age ( Box 237.1 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here