Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Heparin-induced thrombocytopenia (HIT) is the most important drug-induced immune-mediated cytopenia for several reasons. First, heparin is a widely used anticoagulant (see Chapter 143 ). Second, HIT is relatively common, occurring in approximately 1% to 3% of postoperative patients, and 0.2% to 0.5% of medical patients, who receive unfractionated heparin (UFH) derived from porcine intestine for 7 to 14 days. Third, HIT frequently causes life- and limb-threatening venous or arterial thrombosis. Fourth, there are several pitfalls of HIT management, including the potential for thrombocytopenia and/or thrombosis to worsen despite stopping heparin, the high risk for warfarin-associated microthrombosis (most often manifesting as venous limb gangrene), and the potential for failure of approved direct thrombin inhibitor (DTI) therapy because of the confounding of activated partial thromboplastin time (aPTT)–monitored dosing that results from HIT-associated coagulopathies. Finally, various off-label therapies are gaining acceptance for treating HIT, including parenteral anticoagulants (fondaparinux, danaparoid), oral agents (rivaroxaban, apixaban), and even high-dose intravenous immunoglobulin (IVIG) to interrupt HIT antibody-induced platelet activation.
HIT is caused by platelet-activating immunoglobulin G (IgG) antibodies that bind to multimolecular complexes of cationic platelet factor 4 (PF4)—a member of the CXC subfamily of chemokines—bound to (anionic) heparin through electrostatic interactions. Although anti-PF4/heparin antibodies are frequently triggered by heparin therapy, relatively few patients develop clinically evident HIT. Indeed, a major current problem with HIT is its “overdiagnosis” : only 5% to 15% of patients who are referred for antibody testing have a serologic profile that supports a diagnosis of HIT. The challenge for the clinician is to discern which of the (many) patients who develop thrombocytopenia in association with heparin therapy have HIT, a conundrum magnified by the observation that at most 50% of anti-PF4/heparin antibody-positive patients have “true” HIT, as indicated by the presence of platelet-activating IgG antibodies.
Table 131.1 lists risk factors for HIT. Patients at highest risk for HIT are those with multiple interacting risk factors (e.g., females given postoperative thromboprophylaxis with UFH for 2 weeks [frequency approximately 5%]). Even higher frequencies (approximately 10%) are reported in patients with ventricular assist devices who are receiving therapeutic doses of UFH. Ironically, even though the risk for HIT appears to be somewhat higher in women (odds ratio, 1.5 to 2.0), HIT is rare in pregnancy, particularly with the use of low-molecular-weight heparin (LMWH). The synthetic antithrombin-binding sulfated pentasaccharide, fondaparinux, although similarly immunizing as LMWH, is much less likely than LMWH to cause HIT, likely because fondaparinux does not usually increase the platelet-activating potential of HIT antibodies. Indeed, fondaparinux is an effective treatment for HIT (discussed later).
| Heparin type | Unfractionated > low-molecular-weight heparin > fondaparinux |
| Patient type | Postoperative (major > minor surgery) > medical > obstetric/pediatric |
| Dose a | Prophylactic dose > therapeutic dose > flushes |
| Duration | 11–14 days b > 5–10 days > 4 days or fewer |
| Sex | Female > male |
a Importance of heparin dose is uncertain because of confounding effect of patient type (e.g., postoperative patients tend to receive prophylactic-dose heparin whereas medical patients [e.g., with venous thromboembolism] are more likely to receive therapeutic-dose heparin); nevertheless, reported frequencies of heparin-induced thrombocytopenia (HIT) are relatively high in patients given postoperative prophylactic-dose heparin.
b Heparin exposure beyond 14 days does not usually increase the risk of HIT beyond that of an 11- to 14-day exposure.
Only a minority of patients who form anti-PF4/heparin antibodies following heparin treatment develop clinically evident HIT. The proportion of antibody-positive patients who develop HIT ranges from as high as one-third (e.g., postorthopedic surgery thromboprophylaxis with UFH [nowadays rarely used for this indication]) to as few as 1 in 50 (postcardiac surgery patients). Notably, the risk for HIT in postcardiac surgery patients who receive UFH thromboprophylaxis is only approximately 1% even though as many as 50% to 80% of patients develop detectable anti-PF4/heparin antibodies within 2 weeks of surgery. In general, those at the highest risk for HIT are the subgroup of patients whose anti-PF4/heparin antibodies evince strong platelet-activating properties in vitro. However, patient-dependent susceptibility factors are also important, because not all patients who form platelet-activating antibodies develop HIT.
Fig. 131.1 illustrates several features of HIT pathogenesis. Heparin binds reversibly and saturably to platelets and can weakly activate platelets in vitro through potentiation of α IIb β 3 -mediated outside-inside signaling. In general, the direct platelet-activating effects of heparin, as well as its immunogenicity, are proportional to its molecular mass and degree of sulfation, and associated ability to form ultra-large antigenic complexes, respectively; consequently, LMWH is less likely to cause HIT than UFH. Antigen complexes are formed at relatively narrow PF4:heparin molar ratios, suggesting that immunization could reflect attainment of particular immunizing ratios of PF4 and heparin in vivo (“stoichiometric model of immunization”). A unique laboratory characteristic of HIT is that high heparin concentrations (10 to 100 units/mL) inhibit platelet activation by the pathogenic IgG ; this laboratory feature is exploited in diagnostic testing for HIT (see Diagnosis). The high overall frequency of anti-PF4/heparin immunization could reflect innate immunity, given that PF4/heparin ultra-large antigenic complexes bind to “natural” IgM in blood, activating the classical pathway of complement, with the complement-coated PF4/heparin/IgM complexes binding to B-cells via the complement receptor CD21, triggering the immune response.
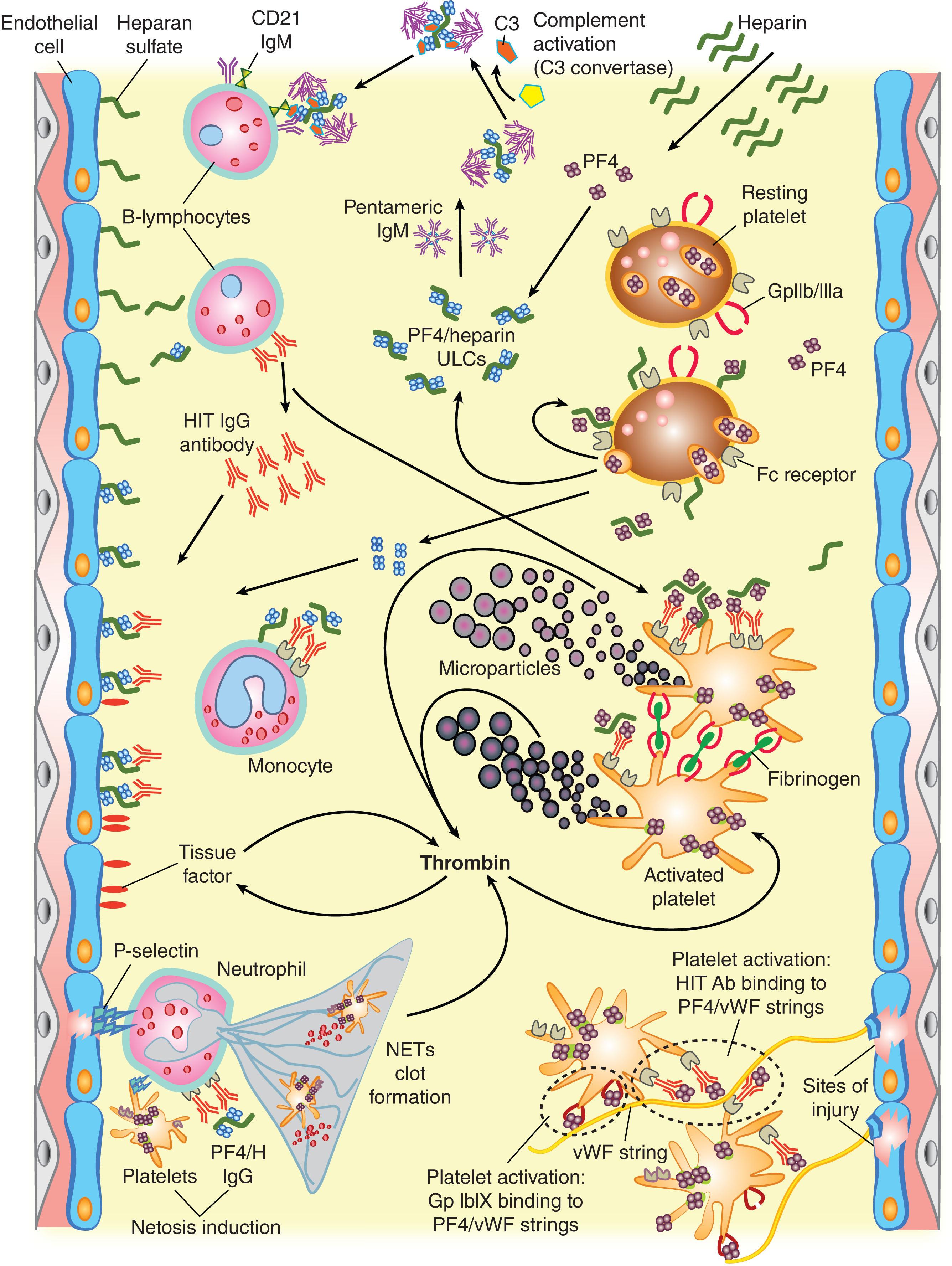
Platelet activation by HIT antibodies occurs because of platelet-activating HIT-IgG cross-link platelet FcγIIa receptors. This leads to “strong” platelet activation, including the formation of procoagulant platelet-derived microparticles. However, the high thrombotic risk of HIT likely reflects “pancellular” activation beyond platelets alone. This includes HIT antibody-induced activation of endothelium and monocytes (with cell surface expression of tissue factor) and activation of neutrophils (including resulting “netosis”). Moreover, injured vessels release von Willebrand factor (vWF) “strings” to which PF4 binds, resulting in additional platelet activation through interaction with HIT antibodies.
The pancellular activation results in excess thrombin generation, which contributes to the pathogenesis of some of the unusual sequelae of HIT, which can include thrombosis involving large veins and arteries, as well as microvascular thrombosis (e.g., warfarin-associated venous limb gangrene. HIT can also be complicated by overt (decompensated) disseminated intravascular coagulation (DIC).
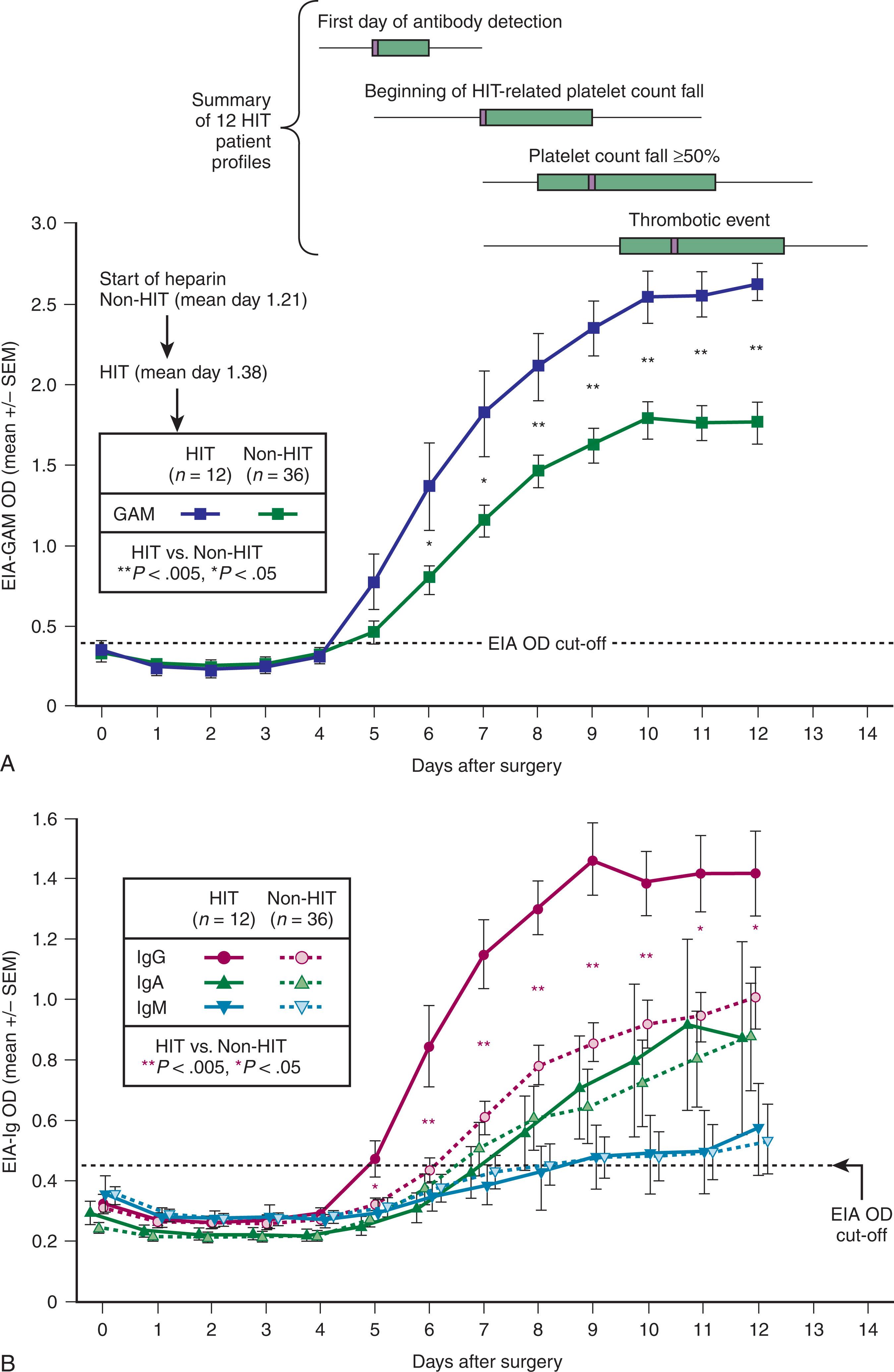
There is a characteristic timeline that underlies the HIT immune response ( Fig. 131.2 ). Formation of pathogenic IgG antibodies is surprisingly fast (detectable 4 to 5 days following initial heparin administration), even in patients who have never previously been exposed to heparin. Bacterial cell walls, which are negatively charged, bind PF4, and there is evidence that bacterial infection could be responsible for preimmunization against PF4-dependent antigens. This could explain both the high frequency of anti-PF4/heparin immunization and of clinical HIT (i.e., HIT could represent a misdirected antibacterial immune response). Although heparin-treated patients can form heparin-dependent antibodies of the IgM or IgA subclass, these appear unlikely to cause HIT.
As noted, thrombocytopenia develops in only a minority of patients who form HIT antibodies. The variable risk for HIT in different patient populations may reflect variations in the susceptibility of platelets to activation by HIT-IgG and/or differences in the levels and immunoglobulin class composition of HIT antibodies. Poorly defined clinical factors also influence the risk for HIT, which occurs more often in surgical patients than in medical or obstetric patients. Major trauma was more likely than minor trauma to be associated with HIT antibody formation and clinical HIT in one study. There is indirect evidence that the formation of stoichiometrically optimal complexes of PF4/heparin influences the risk for immunization. The clinical situation influences the type of HIT-associated thrombosis: venous thromboembolism typically develops in orthopedic patients with HIT, whereas thrombosis develops equally often in arteries and veins in cardiovascular patients with HIT. Genetic factors likely play a role, too: HIT-associated thrombosis is more likely to occur in patients who bear the arginine (R) allele within a platelet Fc receptor polymorphism (H131R), perhaps because this allele better allows for endogenous IgG2 subclass antibodies to inhibit the interaction of HIT antibodies with Fc receptors.
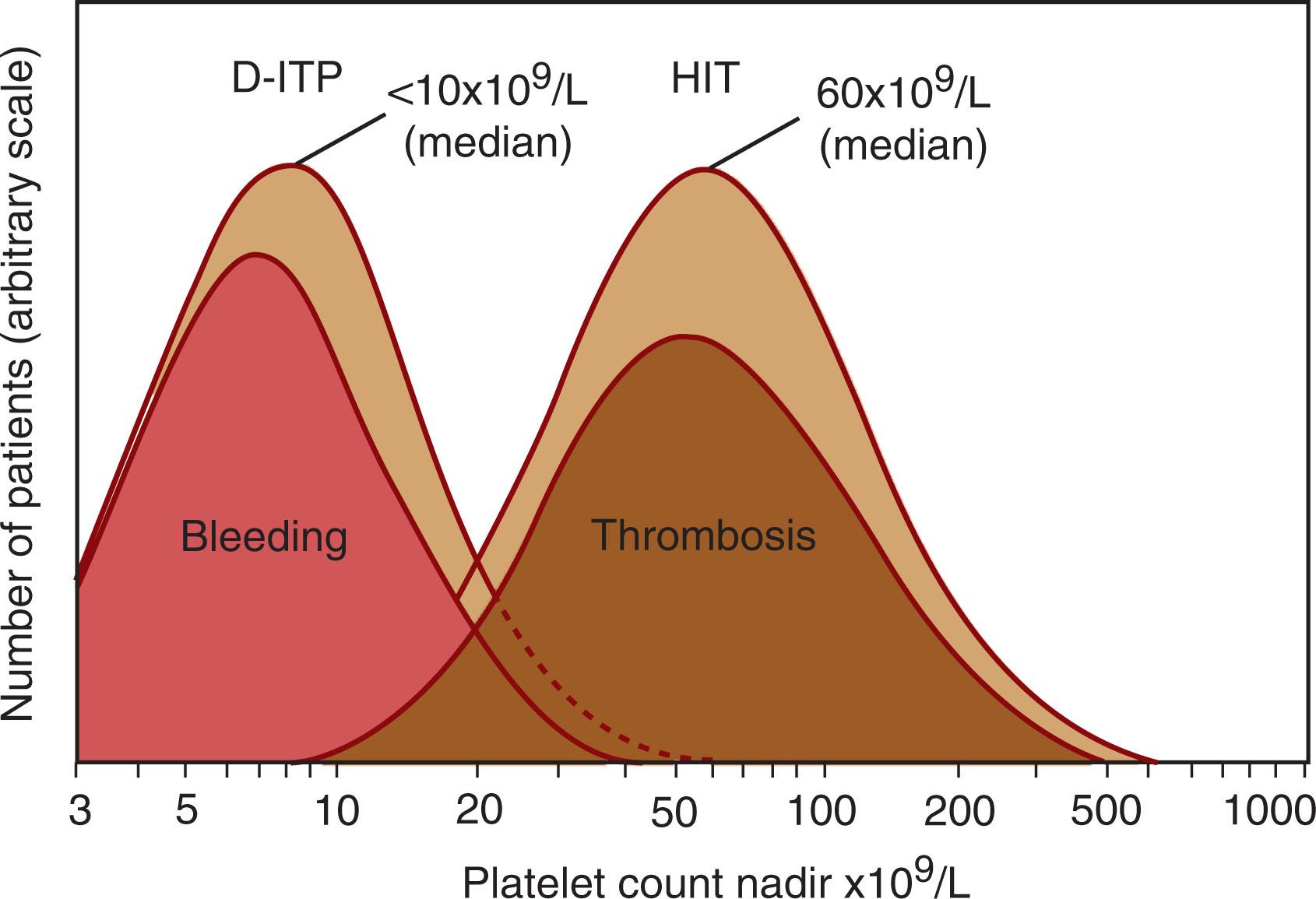
Most patients with HIT have moderate thrombocytopenia; The median platelet count nadir is approximately 60 to 70 × 10 9 /L; for 90% of patients, the platelet count nadir is greater than 20 × 10 9 /L ( Fig. 131.3 ). In the rare patient whose platelet count falls to less than 10 × 10 9 /L, there is often evidence of overt DIC, and red cell fragments and circulating normoblasts may be evident on examination of the blood smear. Thrombosis can occur in HIT patients even if there is a minimal decrease in the platelet count, and even if the platelet count nadir never falls below 150 × 10 9 /L. Neutrophil counts have also been reported to be elevated in patients with HIT, particularly those who develop thrombotic complications.
The platelet count fall usually begins 5 to 10 days after the initiation of an immunizing heparin exposure and while the patient continues to receive heparin (typical-onset HIT). Usually the immunizing trigger is heparin given during surgery (e.g., cardiac or vascular surgery) or that is started soon after surgery (e.g., postoperative thromboprophylaxis). Interestingly, starting LMWH thromboprophylaxis before elective surgery (as is more frequently done in Europe) is less likely to be associated with antibody formation compared with postoperative first-dose administration.
HIT is recognized in approximately one-quarter of patients when the platelet count fall occurs abruptly after restarting UFH or LMWH (rapid-onset HIT). Invariably, such patients have been exposed to heparin within the previous 5 to 100 days, because HIT antibodies are transient and are only detectable for several weeks after an immunizing heparin exposure. Consequently, rapid-onset HIT, which is caused by the administration of heparin to a patient with preformed HIT antibodies, is strongly associated with recent heparin exposure.
HIT is a highly prothrombotic condition (relative risk for thrombosis, ~15) : Approximately 40% to 70% of HIT patients (with diagnosis confirmed by both PF4-dependent immunoassay and platelet activation assay) develop thrombosis. Both venous (deep vein thrombosis and/or pulmonary embolism) and arterial (especially aortic and iliofemoral arterial thrombosis, ischemic stroke, or myocardial infarction) thrombosis can occur (venous:arterial thrombosis ratio, ~4:1). Other complications include cerebral venous sinus thrombosis, necrotizing skin lesions at the sites of subcutaneous heparin injection and acute inflammatory/cardiorespiratory (anaphylactoid) or transient memory disturbances after intravenous heparin bolus administration to sensitized persons. Unexplained abdominal/flank pain or hypotension in a patient with HIT suggests adrenal infarction (often with associated adrenal hemorrhage), which if bilateral can lead to acute adrenal failure; the adrenal necrosis results from adrenal vein thrombosis. Bowel infarction secondary to mesenteric artery thrombosis or mesenteric/portal vein thrombosis has also been reported.
Patients with HIT have an unusual predisposition to develop ischemic limb necrosis. Indeed, approximately 2% to 5% of such patients develop some degree of limb necrosis necessitating amputation. Historically, ischemic limb necrosis complicating HIT was linked to arterial thrombosis featuring platelet-rich clots. However, microvascular thrombosis in a limb with deep-vein thrombosis, known as venous limb gangrene, is the most common explanation for ischemic limb loss in HIT. Occasionally multiple limbs are involved. (See the box on Diagnostic Considerations in the Patient With Limb Ischemia and Thrombocytopenia . )
Concurrence of limb ischemia/necrosis and thrombocytopenia suggests one of several hematologic emergencies:
HIT. Occlusion of large lower-limb arteries by platelet-rich “white clots” is characteristic of HIT. The major clue is an otherwise unexplained platelet count fall that begins 5 or more days after the initiation of heparin, with an absence of arterial pulses. Urgent thromboembolectomy may be limb-sparing. Sensitive assays for HIT antibodies give strongly positive results. See subsequent entry for warfarin.
Warfarin-induced phlegmasia cerulea dolens/venous limb gangrene. Coumarin anticoagulants, such as warfarin, can lead to venous ischemia (phlegmasia cerulea dolens) or venous limb gangrene in patients with DIC caused by HIT or adenocarcinoma. Limb loss can occur even though the limb pulses are palpable.
Adenocarcinoma-associated DIC. Severe venous or arterial thrombosis can develop in patients with metastatic adenocarcinoma who have DIC. This often occurs within hours after stopping heparin. A clinical clue is an otherwise unexplained rise in platelet count that occurs with initial or repeated heparin therapy.
Sepsis-associated microvascular thrombosis. Acquired natural anticoagulant depletion (e.g., markedly reduced antithrombin or protein C levels), especially in the setting of acute ischemic hepatitis (“shock liver”), can complicate DIC associated with sepsis or cardiogenic shock, leading to bilateral acral limb ischemia or necrosis involving feet and (sometimes) fingers/hands (“symmetric peripheral gangrene”). These patients can be misdiagnosed as having HIT. Optimal treatment is unknown but can include heparin anticoagulation (with anti-factor Xa monitoring), antithrombin concentrates, and plasma infusion.
Septic embolism. Rarely, infective endocarditis or aneurysmal thrombosis leads to the constellation of thrombocytopenia and acute limb ischemia.
Antiphospholipid syndrome. Autoimmune thrombocytopenia and hypercoagulability can interact to produce acute limb ischemia and thrombocytopenia in patients with antiphospholipid syndrome (particularly, catastrophic antiphospholipid syndrome, or CAPS).
Recently, the term “autoimmune HIT” (aHIT) has been introduced into the HIT lexicon ( Table 131.2 ). The unifying concept is the detectability of HIT antibodies that activate platelets strongly in vitro even in the absence of heparin; the clinical correlate is frequently that of a patient whose platelet count fall occurs or persists despite absence (or discontinuation of) heparin.
| aHIT Syndrome | Definition |
|---|---|
| Delayed-onset HIT | HIT-related platelet count fall that begins or worsens after stopping heparin |
| Persisting HIT | HIT-related platelet count fall that requires prolonged time for platelet count recovery (at least 1 week a ) |
| Heparin “flush” HIT | HIT triggered by exposure only to small concentrations (“flushes”) of heparin |
| Spontaneous HIT syndrome | HIT without proximate heparin exposure; patients often have knee replacement surgery (without heparin exposure) or preceding infection |
| Fondaparinux-associated HIT | HIT caused by fondaparinux (most such patients have aHIT antibodies that further enhance platelet activation in the presence of fondaparinux (“cross-reactivity”) |
| Severe HIT with overt DIC | Some patients with severe HIT (marked thrombocytopenia, multiple thrombi, and/or overt DIC) who do not meet the above 5 definitions of aHIT have detectable aHIT antibodies |
a No accepted definition, but platelet count recovery time exceeding 1 or 2 weeks suggests the diagnosis of “persisting” HIT (also known as “refractory” HIT).
The first reported aHIT syndrome, “delayed-onset HIT,” indicates patients who develop thrombocytopenia that begins at least 5 days after a brief exposure to heparin or that worsens despite stopping heparin. Sometimes, thrombocytopenia persists for several weeks despite stopping heparin (“persisting” or “refractory” HIT). Other patients have developed HIT despite the sole exposure to heparin being heparin “flushes.” On exceptionally rare occasions, a transient prothrombotic disorder that resembles HIT clinically and serologically occurs without any apparent preceding heparin exposure; triggers of so-called “spontaneous HIT syndrome” include knee replacement surgery (perhaps because of the release of heparin-like glycosaminoglycans from knee cartilage) and infection. Some patients with fondaparinux-associated HIT have aHIT antibodies whose platelet-activating properties are enhanced in the presence of fondaparinux. Finally, some patients with multiple venous and/or arterial thrombi, or who have unusually low platelet counts with DIC, have a laboratory picture of aHIT. aHIT syndromes are important to recognize, as they have certain treatment implications, including the efficacy of adjunctive therapy with high-dose IVIG.
Only approximately 10% of patients who undergo laboratory investigations for clinically suspected HIT have a serologic profile that supports the diagnosis (i.e., detectable heparin-dependent, platelet-activating IgG antibodies that recognize PF4/heparin complexes). The differential diagnosis includes postoperative thrombocytopenia (hemodilution/platelet consumption), thrombocytopenia of critical illness, and septicemia, among others. Some non-HIT disorders mimic HIT so closely that they warrant the term pseudo-HIT. One example of pseudo-HIT is adenocarcinoma-associated DIC, in which the combination of progressive thrombocytopenia and severe venous limb ischemia/necrosis that occurs during the transitioning of patients from heparin (either UFH or LMWH) to warfarin may suggest a diagnosis of HIT. Another disorder that can mimic HIT is the antiphospholipid syndrome: here, thrombocytopenia, thrombosis, and a false-positive PF4-dependent enzyme immunoassay (EIA) caused by anti-PF4 (not anti-PF4/heparin) antibodies can be present. Recently, protamine (heparin)-induced thrombocytopenia as a potential explanation for post-cardiac surgery thrombocytopenia/thrombosis has been reported (see Chapter 130 ).
The pretest probability of HIT can be estimated using validated scoring systems. The 4Ts system represents a mnemonic that is based on T hrombocytopenia, T iming (of onset of thrombocytopenia or thrombosis), T hrombosis (or other clinical sequelae of HIT), and no o T her explanation for thrombocytopenia, with each of the 4Ts scoring as an integer of 0, 1, or 2 points (thus the maximum score is 8 points) ( Table 131.3 ). The presence of platelet-activating HIT antibodies is unlikely (<3%) with a low score (≤3 points), but relatively probable (approximately 65%) with a high score (≥6). An intermediate score (4 or 5) indicates a clinical profile compatible with HIT, but also with other disorders, such as sepsis; here the frequency of platelet-activating HIT antibodies is still only approximately 10% to 20%.
| Points (0, 1, or 2 for Each of Four Categories: Maximum Possible Score = 8) | |||
|---|---|---|---|
| 2 | 1 | 0 | |
| T hrombocytopenia (acute) | Platelet fall >50% (nadir ≥20 × 10 9 /L) and no surgery within preceding 3 days | Nadir, 10–19 × 10 9 /L; or any 30%–50% fall; or, >50% fall within 3 days of surgery | Nadir, <10 × 10 9 /L; or any <30% fall |
| T iming a of platelet count fall, thrombosis, or other sequelae | Clear onset between days 5–10; or ≤1 day (if prior heparin exposure in the past 5–30 days) | Consistent with day 5–10 fall, but not clear (e.g., missing platelet counts); or ≤1 day (heparin exposure within past 31–100 days); or platelet fall after day 10 | Platelet count fall begins in ≤4 days without recent heparin exposure |
| T hrombosis or other clinical sequelae | New proven thrombosis; skin necrosis b ; anaphylactoid reaction after IV heparin bolus; adrenal hemorrhage | Progressive or recurrent thrombosis; erythematous skin lesions b ; suspected thrombosis (awaiting confirmation with imaging) | None |
| O T her cause for thrombocytopenia is not evident | No other explanation for platelet count fall is evident | Possible other cause is evident | Definite other cause is present |
| Pretest probability score: 6 – 8 = HIGH; 4 – 5 = INTERMEDIATE; 0 – 3 = LOW | |||
a First day of immunizing heparin exposure is considered on day 0; immunizing heparin is usually that given during or soon after surgery (e.g., unfractionated heparin [UFH] during cardiac surgery is more immunogenic than UFH given during preceding acute coronary syndrome or with heart catheterization); the day the platelet count begins to fall is considered the day of onset of thrombocytopenia.
Another scoring system is called the HIT Expert Probability (HEP) score ( Table 131.4 ). In the initial evaluation, a HEP score of 4 points or higher indicated an approximately 50% probability of HIT, whereas a score of 3 points or lower was associated with only a 3% probability of HIT.
| Clinical Feature | Score |
|---|---|
|
|
|
−1 |
|
1 |
|
3 |
|
|
| For patients in whom typical-onset HIT is suspected | |
|
−2 |
|
2 |
|
3 |
|
2 |
|
−1 |
| For patients with previous heparin exposure in the last 100 days in whom rapid-onset HIT is suspected | |
|
2 |
|
−1 |
|
|
|
−2 |
|
2 |
|
|
| For patients in whom typical-onset HIT is suspected | |
|
3 |
|
2 |
| For patients in whom rapid-onset HIT is suspected | |
|
3 |
|
2 |
|
|
|
3 |
|
|
|
2 |
|
|
|
−1 |
|
|
|
−1 |
|
−2 |
|
−2 |
|
−2 |
|
−2 |
|
−1 |
|
3 |
It is unlikely that one scoring system offers major advantages over the other. In general, low scores in either system indicate a low probability of HIT, whereas high scores indicate approximately a 50 : 50 chance of HIT. Thus laboratory testing is crucial to establishing a diagnosis of HIT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here