Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Development of the human hemostatic system begins in utero and continues until well after birth. As a result, functional levels of many of the procoagulants, coagulation inhibitors, fibrinolytic components, and platelet-associated factors differ from those of older children and adults, with “adult” values often not reached until about 6 months of age. These unique aspects of fetal and neonatal coagulation were first fully documented in the pioneering work of Dr. Maureen Andrew in the 1980s and have been termed developmental hemostasis . A firm understanding of these differences, as well as access to the “normal” ranges of various hemostatic factors in infants at different gestational and postnatal ages, is critical to proper interpretation of laboratory tests performed on newborns and infants. Despite broad differences from adults in functional levels of hemostatic factors, healthy newborns rarely have difficulties with hemorrhage or thrombosis. Thus these differences in prothrombotic and antithrombotic factors are uniquely balanced in the neonate and fetus and represent a normal physiologic state. However, this system provides little reserve, which often contributes to significant morbidity in sick and preterm infants. In addition to these developmental aspects of hemostasis, many inherited bleeding and clotting disorders can present in the newborn period. This chapter describes the normal development of the hemostatic system and reviews disorders of hemostasis and thrombosis that can occur in the newborn period. Data tables listing normal ranges of hemostasis laboratory tests for term and preterm infants are included in the Appendix. For additional discussion of the investigation and management of neonatal hemostasis and thrombosis, see several recent evidence-based guidelines.
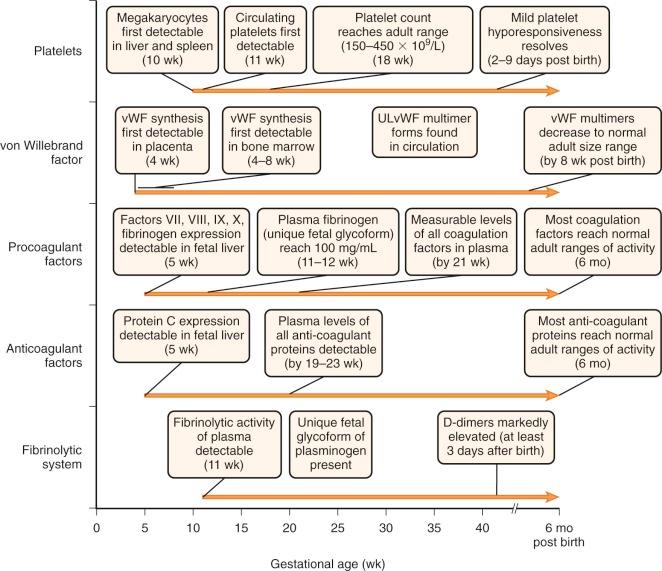
The biochemistry and function of the individual coagulation factors are reviewed in detail in Chapter 27 . Coagulation proteins do not effectively cross the placental barrier and are independently synthesized by the fetus. Messenger RNA (mRNA) transcripts for coagulation factors VII, VIII, IX, and X and fibrinogen are detectable at about 5 weeks of gestation in hepatocytes of the embryo ( Fig. 5-1 ). By 10 weeks of gestation, plasma protein levels of most factors are measurable but range from 10% of the normal adult level for factor IX to about 30% for most of the other coagulation factors. In general, these levels continue to increase gradually in parallel with gestational age ( Appendix Table A5-1 ) . However, with the exception of fibrinogen and factors V, VIII, and XIII, these levels remain considerably below the normal adult range, even at the time of birth for full-term infants. They then continue to gradually increase and reach the normal adult range by about 6 months of age ( Appendix Tables A5-2 and A5-3 ). Premature infants have an accelerated increase in levels after birth and, in general, “catch up” to the full-term infant range by 3 months' postnatal age ( Appendix Table A5-4 ). True reference ranges for extremely premature infants (<30 weeks' gestation) are not available, because the majority of these infants have postnatal complications. Appendix Table A5-5 provides reference ranges for older children. The nearly normal “adult” levels of fibrinogen and factors V and VIII in neonates make them useful markers for the investigation of possible consumptive coagulopathy or hemophilia A.
Regulation of fetal coagulation protein levels occurs by both transcriptional and posttranscriptional mechanisms. In addition, ratios of hepatocyte levels to plasma levels of many factors are relatively high in comparison with adults, suggesting that delayed hepatocyte release may also contribute to the relatively low coagulation factor levels in fetuses and newborns.
Qualitative differences in procoagulant proteins also exist between neonates and adults. Fibrinogen from fetuses has increased sialic acid content, increased phosphorus content, and different chromatographic profiles compared with fibrinogen from adults.
Like many of the procoagulant factors, functional levels of most coagulation inhibitors in fetuses and neonates are significantly lower than those in adults ( Appendix Tables A5-6 and A5-7 ; see also Appendix Table A5-3 ). Awareness of these differences is critical to proper interpretation of laboratory studies obtained during evaluation of neonatal thrombosis. Most coagulation inhibitors reach the adult range of activity by 6 months of age.
As reviewed in Chapter 27 , at least three mechanisms/pathways exist for inhibition of procoagulant activity: (1) cleavage of factors V and VIII by the protein C/S system; (2) direct inhibition of thrombin by antithrombin (AT), heparin cofactor, and α 2 -macroglobulin; and (3) inhibition of factor VIIa by the tissue factor pathway inhibitor (TFPI)/factor Xa complex. When thrombin binds to thrombomodulin on endothelial surfaces, it no longer cleaves fibrinogen but it activates protein C. Activated protein C, in complex with protein S, proteolytically inactivates factors V and VIII. At birth, plasma concentrations of protein C are quite low and remain decreased during the first 6 months of life. Neonates also have about a twofold higher amount of the single-chain form of protein C than adults do, and they may exhibit increased protein C glycosylation. Despite these alterations, there is no evidence that protein C from neonates differs functionally from that of adults when compared at equivalent concentrations.
In adults, protein S circulates in either a free form or in a form that is bound to the carrier protein C4b binding protein. In fetuses and neonates, circulating protein S is almost entirely of the free type because levels of C4b binding protein are extremely low. Thus the protein S activity-to-antigen ratio is greater for fetuses and neonates than for adults. Nonetheless, both total and free protein S levels are lower in fetuses and neonates. They rise gradually after birth, with the adult range of free protein S reached by 4 months and total protein S by about 10 months. The interaction of protein S with activated protein C in the plasma of newborns may be modulated by increased levels of α 2 -macroglobulin.
Healthy full-term newborns have lower functional AT levels than adults do, with an average of 0.55 U/mL based on several studies. This is most likely due to a reduced amount of absolute AT protein inasmuch as most studies have found no significant difference in the antigen-to–functional activity ratio or the immunoelectrophoretic or chromatographic properties of purified AT from neonates and adults. The reduced AT levels in neonates probably account, at least in part, for the relative heparin resistance seen in this age group. AT levels typically reach the adult range by 3 months of age in full-term healthy infants. Preterm infants with respiratory distress syndrome (RDS) have significantly lower levels of AT, typically less than 0.2 U/mL, and the AT present often has additional dysfunctional properties. Lower AT levels correlate with increased catheter-related thromboses, intracranial hemorrhages (ICH), and mortality rates.
Like AT, levels of heparin cofactor II are also substantially lower in infants than adults. A circulating fetal dermatan sulfate proteoglycan has been described that has natural anticoagulant properties mediated through heparin cofactor II. This fetal anticoagulant is also present in the plasma of pregnant women and is produced by the placenta. The length of time that it circulates in newborns is not known; however, it is still present during the first week of life in sick premature infants with RDS.
α 2 -Macroglobulin is a more important inhibitor of thrombin in plasma from newborns than in adults. It compensates, in part, for the low levels of AT and heparin cofactor in newborns, even in the presence of endothelial cell surfaces. However, the overall rate of thrombin inhibition is still lower in newborns than adults.
TFPI binds to factor VIIa/tissue factor complexes in a factor Xa calcium-dependent reaction that results in the inhibition of factor VIIa. After generation of small amounts of thrombin, TFPI prevents further generation of thrombin via tissue factor/factor VIIa. TFPI levels in cord plasma have been reported to be approximately 50% of adult values.
Factors II, VII, IX, and X and proteins C, S, and Z undergo posttranslational γ-carboxylation dependent on vitamin K (VK) of certain glutamic acid residues (see Chapter 27 ). Typically, 9 to 12 glutamic acid residues, often clustered within a 45–amino acid region referred to as the “Gla” domain, are modified. γ-Carboxylation of glutamic acids enhances calcium-dependent phospholipid interactions of the coagulation factors and helps regulate the coagulation cascade. Because of the association of relative VK deficiency and hemorrhagic disease of the newborn (HDN; see detailed description later), development of these VK-dependent factors in utero has been the focus of considerable research. During gestation, a steep gradient of VK concentration is maintained across the placenta, with fetal levels being 10% or less of maternal levels. This gradient is even further enhanced during the third trimester. Consequently, VK stores in newborns are low, as shown by low levels of VK in the cord blood and liver of aborted fetuses and increases in VK-dependent coagulation factor activity following VK administration to neonates. The teleologic explanation for maintenance of low fetal VK levels is not known. However, VK has been shown to promote DNA mutagenesis in vivo. For this reason, several investigators have suggested that maintenance of low fetal VK levels might be a mechanism to prevent chromosomal damage during the rapid cellular proliferation of embryogenesis. In addition to these low VK levels, deficiency of VK reductase activity (a rate-limiting step in the reaction) as a result of immaturity of the hepatocyte may contribute to inefficient γ-carboxylation of coagulation factors. In fact, undercarboxylated prothrombin and protein C have been detected in the cord and peripheral blood of up to 7% and 27% of healthy term infants, respectively, and these levels correlate with gestational age. Even when corrected for this relative VK deficiency, levels of VK-dependent factors are still considerably lower than the normal adult range because the values presented in Appendix Tables A5-1 to A5-4, A5-6, and A5-7 were measured in infants who received VK prophylaxis at birth.
VK-dependent glutamic acid carboxylation also occurs on proteins outside the coagulation system, many of which play roles in development. Such proteins include Gas-6, the ligand for the receptor kinases Sky and Axl; the bone growth–related and calcification-related factors osteocalcin and matrix Gla protein; the urine calcium binding protein nephrocalcin; and PRPG1 and PRPG2, two proline-rich predicted signaling proteins expressed in the central nervous system and endocrine tissues. Thus VK is predicted to have pleiotropic effects on development.
Although VK is maintained at low levels in the fetus, further deficiency of VK during gestation can reduce functional levels of the VK-dependent coagulation factors to an even greater extent. Maternal use of certain anticonvulsant medications, including phenytoin, phenobarbital, valproic acid, and carbamazepine, is associated with an increased risk of VK deficiency in the fetus and neonate. Fetal or neonatal bleeding has been linked to maternal use of these medications on rare occasions. Fetal VK deficiency can be prevented in such cases by antenatal oral administration of 10 mg of VK 1 daily to the mother beginning at 36 weeks' gestation.
Warfarin is a potent VK antagonist commonly used for long-term anticoagulation. It crosses the placenta and inhibits VK-dependent carboxylation in the fetus. Its use during pregnancy at 6 to 12 weeks of gestation is associated with an embryopathy syndrome consisting of limb deformities, nasal hypoplasia, ophthalmologic malformations, mental retardation, hypotonia, and ear abnormalities. Warfarin exposure during the second or third trimester is associated with fetal wastage and central nervous system anomalies. The developmental defects related to warfarin use during pregnancy are thought to be due to bleeding into the developing limb buds or effects on bone and cartilage growth from inhibition of bone-associated Gla proteins, or both.
The enzyme plasmin degrades polymerized fibrin and is responsible for clot dissolution. It is generated from its precursor plasminogen by two known activators, tissue plasminogen activator (tPA) and urokinase plasminogen activator. These activators, in turn, are inhibited by the plasminogen activator inhibitors PAI-1 and PAI-2. In addition, plasmin itself is inhibited by the circulating blood protein α 2 -antiplasmin. See Chapter 28 for a more detailed description of the fibrinolytic system. In newborns, plasminogen levels are only 50% of adult values, α 2 -antiplasmin levels are 80% of adult values, and plasma concentrations of PAI-1 and tPA are significantly greater than adult values ( Appendix Table A5-8 ). The increased levels of tPA and PAI-1 found on day 1 of life contrast markedly with values obtained from cord blood, which are significantly lower than adult levels. The discrepancy between newborn and cord plasma concentrations of tPA and PAI-1 may be explained by the enhanced release of both proteins from the endothelium shortly after birth. PAI-2 levels are detectable in cord blood but are significantly lower than they are in pregnant women. Unique glycoforms of plasminogen with an increased content of mannose and sialic acid exist in the fetus. This fetal form of plasminogen is less efficiently converted to plasmin by tPA compared with adult plasminogen. No significant differences exist in activation kinetics by urokinase plasminogen activator.
D-dimers are the breakdown product of fibrin mesh cross-linked by factor XIII. Neonates have markedly elevated D-dimer levels in comparison with older children and adults that persist for at least 3 days after birth ( Appendix Table A5-9 ). These high levels suggest activation of the coagulation system at birth or decreased clearance of D-dimers in neonates, or both.
von Willebrand factor (VWF) is a large protein that mediates adhesion of platelets to exposed subendothelium and is involved in platelet aggregation (see Chapter 26 ). Its synthesis can first be detected as early as 4 weeks of gestation in placental endothelial cells and by 4 to 8 weeks in bone marrow. In contrast to other coagulation factors, VWF functional levels are considerably elevated in neonates in comparison with the normal adult range (see Appendix Tables A5-2 and A5-5 ). They gradually decline to the normal adult range by about 3 to 6 months. It is important to keep these physiologically elevated levels in mind when using VWF as a marker of inflammation in infants.
VWF is released from endothelial cells and megakaryocytes as large multimers with molecular weights in the millions-of-dalton range. They are then cleaved into smaller multimers by the metalloproteinase ADAMTS-13. Unusually large VWF (ULVWF) multimers are VWF multimer forms larger than those found in normal plasma. These ULVWF multimers are 10 to 20 times more active in shear stress–induced platelet aggregation and bind more avidly to the extracellular matrix of fibroblasts than do the VWF multimer forms found in normal plasma. In older children and adults, the presence of ULVWF plasma forms is the hallmark of thrombotic thrombocytopenic purpura, a microangiopathic disorder. It is due to deficiency, acquired or congenital, of ADAMTS-13. ULVWF forms are consistently found in platelet-poor plasma (PPP) from fetuses less than 35 weeks' estimated gestational age (EGA) and most fetuses older than 35 weeks' EGA, as well as in umbilical cord blood. These forms are not seen in simultaneously sampled maternal PPP, indicating that the ULVWF forms are fetal specific. The ULVWF in fetal PPP is similar to the VWF directly released from endothelial cells. This similarity may be explained by the finding of low VWF cleaving protease (ADAMTS-13) activity in many neonates. Serial monitoring of PPP from neonates shows a gradual reduction in ULVWF to the normal size range within 8 weeks after birth. The presence of ULVWF during fetal development is probably physiologically relevant because plasma derived from umbilical cord blood shows increased shear stress–induced and ristocetin-induced VWF platelet-binding activity in comparison with plasma derived from adults, even when adult platelets are used in the assay. The enhanced hemostatic efficacy of ULVWF may therefore partially balance the low functional levels of other coagulation factors to achieve reliable hemostasis in the fetus and newborn.
In humans, megakaryocytes are first detectable in the liver and spleen by 10 weeks of gestation. By 30 weeks, megakaryocytes are present in bone marrow and actively contribute to thrombopoiesis. Circulating megakaryocyte progenitors are detectable in the blood of neonates, and their number decreases as a function of postconceptional age (gestational age plus days of life). Neonatal megakaryocytes have distinct properties compared with adult megakaryocytes, including increased thrombopoietin sensitivity, increased proliferation, lower ploidy, and increased cytoplasmic maturation for the degree of ploidy. The differences in megakaryocyte size resolve by about 4 years of life.
In humans, circulating platelets are first detected by 11 weeks' gestational age. Thereafter, the plasma platelet concentration rises rapidly and reaches the adult range of 150 to 450 × 10 9 /L by about 18 weeks of gestation. During the earliest phase of platelet production, mean platelet volume is larger than the adult range. However, platelet volume “normalizes” soon thereafter and reaches the adult range of 7 to 9 fL. It is possible that the larger platelets arise from a unique wave of “primitive” megakaryopoiesis that occurs early in gestation, as demonstrated in several mouse studies, but this mechanism remains to be determined in humans. Platelet survival has not been measured in healthy infants. In rabbits, survival of 111 In-oxine–labeled platelets is similar in adult and newborn rabbits. However, in humans, levels of reticulated platelets, which represent those recently released from megakaryocytes, are elevated about twentyfold in comparison with adults. Concentrations of thrombopoietin (TPO), the major cytokine for megakaryocyte progenitor proliferation and development, are increased about twofold in fetal versus adult plasma. These findings suggest possible increased platelet turnover in human fetuses versus adults.
Peripheral blood platelets from newborns contain similar numbers of platelet-specific granules as platelets from older children and adults; however, serotonin and adenosine diphosphate (ADP), which are stored in dense granules, are present at concentrations of less than 50% of adult values. Newborn platelets contain normal levels of the receptors glycoprotein Ib (GPIb) (part of the VWF binding complex), GPIa/IIa, P-selectin, and HPA-1a. Variable levels of GPIIb/IIIa have been reported, with some studies indicating significantly reduced expression. Functional epinephrine receptors are significantly diminished in newborn platelets.
Platelet aggregometry and other functional assays of neonatal and cord blood platelets have shown variable results, but most demonstrate a modest hyporesponsiveness to ADP, epinephrine, collagen, and thrombin when compared with platelets from older children and adults ( Fig. 5-2 ). The hyporesponsiveness spontaneously resolves by 2 to 9 days after birth in full-term infants. Extremely low birth weight neonates (<1 kg, gestation < 30 weeks) have markedly hyporesponsive platelets. This impaired platelet response may contribute to the propensity of extremely low birth weight neonates for ICH. The platelet hyporesponsiveness improves over the first 10 to 14 days after birth, but platelet reactivity does not reach adult levels even by day 14. Several explanations have been proposed to account for this hyporesponsiveness, including impaired calcium channel transport and signal transduction, decreased availability of α-adrenergic receptors because of either delayed receptor maturation or occupation by catecholamines released during birth, and the presence of inhibitory placenta-derived prostaglandins.
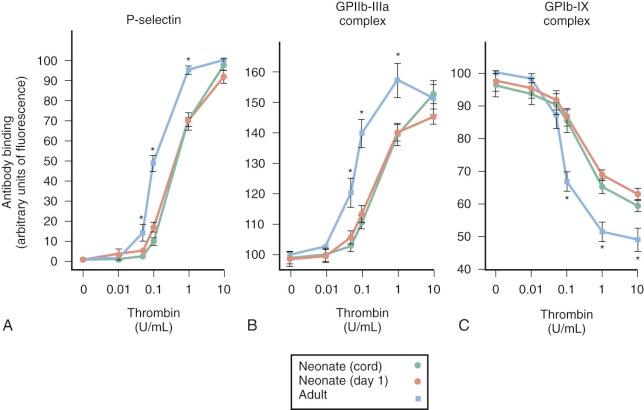
In contrast to the agonists just described, agglutination in response to low concentrations of ristocetin is significantly enhanced in fetal and newborn platelet-rich plasma in comparison with that of adults, probably partly because of high VWF levels and the presence of ULVWF forms in newborn plasma. In the end, the hyporesponsiveness to certain platelet agonists and the hyperresponsiveness to VWF appear to balance each other out inasmuch as most healthy term infants do not experience bleeding or thrombotic complications in the newborn period.
There is strong evidence that platelets are activated during the birth process. Cord plasma levels of thromboxane B 2 , β-thromboglobulin, and platelet factor 4 are increased; the granular content of cord platelets is decreased; and epinephrine receptor availability is reduced, perhaps as a result of occupation. The mechanisms of activation are probably multifactorial and include thermal changes, hypoxia, acidosis, adrenergic stimulation, and the thrombogenic effects of amniotic fluid.
The endothelium fulfills a complex role in hemostasis in that it prevents thrombotic complications under physiologic conditions and promotes fibrin formation when injured. One of the anticoagulant properties of endothelial cell surfaces is mediated by lipoxygenase and cyclooxygenase metabolites of unsaturated fatty acids. Prostacyclin (prostaglandin I 2 ) production by cord vessels exceeds that by blood vessels in adults. A second endothelial cell–mediated antithrombotic property is promotion of AT neutralization of thrombin by cell surface proteoglycans. Structurally, there is evidence that the vessel wall glycosaminoglycans of the young differ from those of adults. In a rabbit venous model, increased amounts of glycosaminoglycans are seen in the inferior venae cavae of rabbit pups as compared with adults. Similarly, in the rabbit arterial model, greater glycosaminoglycan-mediated AT activity is seen in rabbit pups than in adult rabbits.
Preeclampsia is a pregnancy-associated syndrome consisting of maternal hypertension and proteinuria. Alterations in maternal coagulation and fibrinolysis, including reduced AT and PAI-2 levels and increased tPA antigen, PAI-1 antigen, and fibrin degradation product levels, have been described in women with preterm preeclampsia. This suggests a state of enhanced thrombin generation and fibrinolysis in the maternal circulation. Analysis of cord blood from infants born to mothers with preeclampsia shows no statistical difference in the levels of these factors versus those of age-matched controls born to mothers without preeclampsia, thus indicating a probable protective effect of the placenta on the fetal circulation.
In summary, the salient differences in the hemostatic system of neonates and older children or adults are (1) decreased plasma concentrations of many of the procoagulant proteins, including factors II, VII, IX, X, XI, and XII, prekallikrein, and high–molecular-weight kininogens; (2) a unique fetal glycoform of fibrinogen; (3) decreased plasma concentrations of the coagulation inhibitors AT, heparin cofactor II, TFPI, protein C, and protein S, with a concomitantly slower rate of thrombin inhibition; (4) a unique glycoform of plasminogen that is less efficiently converted to plasmin by tPA; (5) markedly elevated D-dimer levels until at least 3 days after birth; (6) increased plasma VWF concentrations and elevated levels of circulating ULVWF multimers; (7) smaller and more proliferative megakaryocytes; and (8) modest, transient hyporesponsiveness of platelets to certain agonists such as collagen and epinephrine, but increased agglutination with low-dose ristocetin. In general, most of these differences resolve within the first 6 months of life.
Although acquired disorders are more commonly seen, severe forms of congenital factor deficiencies or platelet disorders can present in early infancy and should be seriously considered in otherwise healthy infants who are bleeding. About 15% to 30% of children with inherited bleeding disorders have hemorrhage in the neonatal period. In addition, a third of new cases of severe hemophilia represent new mutations and are therefore not accompanied by any antecedent family history.
The clinical manifestations of bleeding disorders are different in newborns than in older children and adults. Bleeding may appear as oozing from the umbilicus, bleeding into the scalp, large cephalhematomas, bleeding after circumcision, bleeding from peripheral phlebotomy sites, and bleeding into the skin. A small but important proportion of infants are seen with ICH as the first manifestation of their bleeding tendency. Sick infants can bleed from mucous membranes, the bladder, and sites of invasive procedures. Joint bleeding is rare. The most common causes of bleeding in healthy infants are thrombocytopenia secondary to transplacental passage of a maternal antiplatelet antibody, sepsis, VK deficiency, and congenital coagulation factor deficiencies.
In addition to a workup for sepsis, laboratory evaluation of infants with bleeding complications should include determination of the prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin clotting time (TCT), fibrinogen level, and platelet count. As discussed earlier, results need to be compared with the expected range of values for neonates because some may not reflect the normal adult range. It is also critical to apply age-specific reference ranges generated using the same analyzer and reagents because these can influence the measured absolute levels. Tests that provide exogenous AT should be avoided because they do not reflect the normal physiologic state of low AT levels in neonates. Values obtained from cord blood may be different from those obtained from neonatal peripheral blood samples. Abnormalities in test results usually prompt additional tests, such as specific factor assays. For a male child in whom hemophilia A or B is suspected, specific factor assays should be performed regardless of the APTT value. The PT, APTT, and TCT are not prolonged in those with factor XIII and α 2 -antiplasmin deficiency. Therefore levels of these factors must be measured directly if deficiencies are suspected.
Collection of samples for laboratory investigation of hemostatic defects in neonates, especially premature infants, presents particular challenges because of the patient's small size and increased hematocrit. Specialized microcollection tubes (1 mL) should be available in the neonatal unit. Blood sampling techniques should avoid contamination with intravenous fluids and heparin and should also avoid activating the coagulation system. Slow transit time and contact with plastic tubing are most likely to cause activation. All neonatal samples should be inspected for the presence of fibrin clumps before processing, and those containing clumps should not be used for analysis. Ideally, the volume of anticoagulant in the sample should be based on the volume of plasma, not the total volume of blood, because the increased hematocrit of neonates causes accentuated dilution of the coagulation factors if not taken into account. This is particularly important for neonates with very high hematocrit values, such as those with cyanotic congenital cardiac disease. Overfilled or underfilled samples should not be analyzed.
Screening tests of plasma clotting activity, including the PT, APTT, and TCT, are all prolonged during fetal development and in neonates when compared with the normal adult range (see Appendix Tables A5-1 to A5-3 ), probably because of physiologic “deficiencies” of the VK-dependent factors and contact factors and the presence of fetal glycoforms of fibrinogen. The absolute values for these tests, as well as specific coagulation factor tests, depend on the reagents and analyzer used. Appendix Table A5-3 provides reference ranges using the STA Compact analyzer and Diagnostica Stago reagents, a system currently used in many clinical laboratories. In contrast to some earlier systems, such as the ACL system, the STA Compact analyzer is not affected by plasma bilirubin levels, which are frequently elevated in neonates. Reference ranges using the Behring Coagulation System (BCS) and the Sysmex CA-1500 System with various reagents have also recently been published. The differences observed underscore the need for each clinical laboratory to establish its own normal age-related reference ranges.
The bleeding time has traditionally been used as an in vivo test of platelet function, particularly for testing adhesion to injured vasculature. However, it is subject to considerable operator-dependent variability and is not routinely used in newborns. Automated devices modified for newborns and children are available and have been standardized. In contrast to the prolonged coagulation screening tests, multiple studies have shown that bleeding times in infants during the first week of life are significantly shorter than those in adults. This somewhat paradoxic finding can be explained by several mechanisms, including higher plasma concentrations of VWF, enhanced function of VWF because of a disproportional increase in ULVWF multimers, large red cells, and high hematocrits.
The Platelet Function Analyzer 100 (PFA-100) system provides an in vitro method of assessing primary platelet-related hemostasis by measuring the time (closure time) required for a platelet plug to occlude a microscopic aperture cut into a membrane coated with collagen and either epinephrine or ADP. It uses the patient's own platelets and plasma. The PFA-100 system is attractive for use in neonates because of the small volume required, rapidity of testing, and standardization of testing cartridges. Like the bleeding time, most studies of neonates' peripheral or cord blood report PFA-100 closure times, for both epinephrine and ADP, that are shorter than those for older children and adults ( Appendix Table A5-10 ). This is probably due to similar mechanisms as described earlier for the shortened bleeding time. Ranges for premature infants have been reported and show an inverse relationship between ADP closure time and gestational age. Although the PFA-100 system may eventually become a standard screening test for platelet function in neonates, older children, and adults, its clinical utility has not yet been clearly established. Thus further studies will be required to determine the optimal method of assessing primary hemostasis in newborns and children, particularly premature infants.
For most hemostatic components, both severe and mild forms of deficiency can occur, with severe deficiencies often characterized by significant bleeding in newborns. Chapters 31 and 32 discuss hereditary factor deficiencies in detail.
Deficiencies of factors II, V, VII, XI, and XII, prekallikrein, and high–molecular-weight kininogen are rare autosomally inherited disorders, with consanguinity present in many families. Deficiencies of factor XII, prekallikrein, and high–molecular-weight kininogen do not result in hemorrhagic complications and thus are not considered further here. Deficiencies of factors VIII and IX are sex linked and the most common congenital bleeding disorders to present in newborns. Rarely, combined deficiencies of factors II, VII, and IX and/or factors V and VIII may also present in the neonatal period. Tests are available to diagnose most congenital factor deficiencies prenatally so that either termination of pregnancy or management of affected infants can be planned (see Chapters 31 and 32 ).
In the majority of newborns with congenital coagulation factor deficiencies, bleeding does not occur in the perinatal period unless a hemostatic challenge is present. On the other hand, unexplained bleeding in an otherwise healthy newborn should be carefully investigated because it may be due to a congenital coagulation factor deficiency.
ICH is rare in full-term newborns and usually occurs either spontaneously or after insults such as birth asphyxia or trauma, VK deficiency, or severe congenital factor deficiencies. Other risk factors include low birth weight, young gestational age, and ethnic background. The risk for ICH in children with severe hemophilia A or B ranges from 2% to 8%. The location of the ICH is most commonly the subarachnoid area, but subdural and parenchymal bleeding also occurs. Head ultrasound may not detect small parafalcine or subdural bleeding, so computed tomography or magnetic resonance imaging is the preferred modality when deficiency of a coagulation factor is suspected. Some infants require surgical intervention, and many have long-term neurologic deficits.
Full-term infants with unexplained ICH should be carefully evaluated for congenital or acquired hemostatic defects. Unfortunately, the diagnosis of ICH may be delayed because of the nonspecific nature of the early clinical findings, including poor feeding, lethargy, apnea, vomiting, and irritability. Further delays can result when secondary coagulopathies such as disseminated intravascular coagulation (DIC) occur or because of confusion related to the physiologically low levels of some coagulation factors in newborns. The more extreme clinical manifestations of ICH are usually recognized early and include seizures, meningismus, and a tense fontanelle.
Although severe deficiency of factor VIII is the most common cause of ICH from a coagulation factor deficiency, severe congenital deficiencies of fibrinogen and factors II, V, VII, VIII, IX, X, XI, and XIII can also cause ICH at birth. The incidence of ICH in newborns is unknown and is probably changing because of improvements in perinatal care. The widespread use of ultrasound, a safe modality for monitoring fetuses at risk, has resulted in the detection of ICH in utero. In utero factor replacement has been accomplished in several infants. Though less common than ICH, subgaleal bleeding with concurrent shock and DIC may be the initial manifestation of a congenital factor deficiency.
In newborns, the diagnosis of many congenital factor deficiencies based on plasma concentrations can be difficult because of neonates' physiologically low levels at birth (see Appendix Tables 5-2 and 5-3 ). Mild to moderate hereditary deficiencies of factors II, VII, IX, X, and XI result in plasma concentrations that may overlap with the normal neonatal range. In contrast, plasma concentrations resulting from either mild to moderate factor VIII deficiency or severe deficiency of factors V, VII, VIII, IX, X, and XIII can easily be distinguished from physiologic values. Prenatal diagnosis of most hereditary factor deficiencies is performed by amniocentesis or chorionic villus biopsy and is largely confined to severe hemophilia A and B, although deficiencies of factors V, VII, and XIII and VWF have also been diagnosed prenatally.
In the presence of active bleeding or a planned hemostatic challenge, the fundamental principle of management is to increase the plasma concentration of the deficient coagulation protein to a minimal hemostatic level. This target level varies, depending on the protein and nature of the hemostatic challenge (see Chapters 31 and 32 ). When possible, recombinant protein products or detergent-treated pooled donor preparations should be used to reduce the risk of infectious complications. Most products available for the treatment of older children and adults can be used in neonates. Although it has not been well studied, the half-lives and volumes of distribution of recombinant factor VIII (rFVIII) and recombinant factor IX (rFIX) appear to be similar in full-term newborns and older children, so standard initial dosing regimens can be used. However, it is critical to monitor trough levels closely and make appropriate dose modifications to ensure achievement of the proper target level. There are a few case reports of extremely premature infants (<1500 g or <30 weeks' EGA) having a slightly shortened half-life of factor VIII (about 6 hours vs. 8 to 12 hours in full-term infants and older children).
Fresh frozen plasma (FFP) should be given for bleeding emergencies in neonates in whom a coagulation defect is suspected but a specific factor deficiency has not yet been documented. There are case reports of a small number of infants (<4 months) treated with recombinant factor VIIa (rFVIIa) for catastrophic bleeding of varying causes. However, rFVIIa treatment of patients without congenital factor VII deficiency or hemophilia with high-titer inhibitors should be considered investigational at this time. Infants with known or suspected deficiencies in coagulation factors should receive vaccinations by the subcutaneous rather than the intramuscular route, and arterial puncture should be avoided when possible.
The optimal mode of delivery for infants of hemophilia carrier mothers has yet to be determined. However, normal vaginal delivery appears to be generally safe for many babies with hemophilia. Use of vacuum assistance is associated with an increased risk for significant cranial hematomas in newborns with hemophilia and should be avoided if possible.
Deficiency of fibrinogen is rare. Bleeding secondary to afibrinogenemia has been reported in newborns after circumcision or as umbilical stump bleeding and soft tissue hemorrhage, with some cases being fatal. Reported replacement therapies have included whole blood, cryoprecipitate, FFP, and fibrinogen concentrates. One fibrinogen concentrate (Haemocomplettan HS, Centeon/Aventis Behring) is available in Europe and North America.
Deficiency of prothrombin is very rare. Reported bleeding complications in newborns include gastrointestinal bleeding and ICH. Reviews of adult patients have reported bleeding after invasive events such as circumcision and venipuncture or as soft tissue hematomas. Although FFP can be used as initial therapy, factor II concentrate or prothrombin complex concentrate (PCC) is the preferred replacement product.
Bleeding as a result of severe factor V deficiency has been reported in newborns. Clinical manifestations include ICH, subdural hematoma, bleeding from the umbilical stump, gastric hemorrhage, and soft tissue hemorrhage. Replacement therapy includes whole blood and FFP. Although thrombotic complications occur in some patients with factor V deficiency, they have not been reported in newborns.
Severe factor VII deficiency (factor VII level <1%) usually causes significant bleeding equivalent to that seen in patients with severe hemophilia. Patients with factor VII levels greater than 5% generally have mild hemorrhagic episodes. The most commonly reported bleeding complication in newborns with congenital factor VII deficiency is ICH. In a review of 75 patients with factor VII deficiency, ICH was observed in 12 (16%). In 5 (42%) of these 12 patients, ICH occurred in the first week of life, with a fatal outcome. Congenital factor VII deficiency may occur in infants with Dubin-Johnson syndrome or Gilbert syndrome in certain populations. Bleeding episodes secondary to factor VII deficiency can be treated with FFP, PCC, purified factor VIIa concentrates, or, preferably, rFVIIa. Lower doses of rFVIIa (15 to 20 µg/kg) than those used for patients with severe hemophilia and high-titer inhibitors (90 µg/kg) are often sufficient to control bleeding in patients with factor VII deficiency. However, this has not been carefully defined in prospective clinical trials. Moreover, dosing has not been optimized for neonates and infants. Caution should be used in combining treatment with rFVIIa and PPC because thrombotic complications have been reported.
The severity of factor VIII deficiency is determined by the plasma concentration of factor VIII, with a level of less than 1% being severe, 1% to 5% being moderate, and 5% to 50% being mild. Severe factor VIII deficiency is the most common inherited bleeding disorder of the neonatal period. In addition, a small number of neonates with moderate and mild hemophilia are seen after a hemostatic challenge. Large cohort studies have revealed that approximately 10% of children with hemophilia have clinical symptoms in the neonatal period. In this group of factor VIII–deficient children experiencing bleeding in the neonatal period, approximately 50% of bleeding episodes occur after circumcision, with almost 20% of patients suffering intracranial bleeding; some deaths also occur. In contrast to older infants and children, bleeding into joints is extremely rare in neonates. Severe factor VIII deficiency can, on rare occasion, occur in female infants as a result of homozygosity or compound heterozygosity for an FVIII gene mutation or skewed lyonization. Management of bleeding secondary to factor VIII deficiency is discussed in detail in Chapter 31 .
Classification of the severity of factor IX deficiency is identical to that used for factor VIII deficiency. Diagnosis of milder forms of factor IX deficiency is complicated by physiologic levels of factor IX that can be as low as 0.15 U/mL and, in rare infants, by the potential for concurrent VK deficiency. Bleeding after circumcision and ICH can occur in neonates with factor IX deficiency. Management of bleeding secondary to factor IX deficiency is discussed in detail in Chapter 31 .
Severe factor X deficiency can be manifested as bleeding in the newborn period. ICH was present in a high proportion of reported cases, several of which were fatal. Additional sites of bleeding that have been noted include umbilical, gastrointestinal, and intraabdominal sites. Whole blood, FFP, factor X concentrate, and PCC have been used as replacement therapy.
Severe deficiency of factor XI is rare. It is different from other coagulation protein deficiencies in that bleeding symptoms are not necessarily tightly correlated with factor plasma concentrations. Bleeding complications as a result of severe factor XI deficiency were reported in two newborns. One bled after circumcision at the age of 3 days and had a factor XI level of 0.07 U/mL. In another newborn, factor XI deficiency with bilateral subdural hemorrhage was diagnosed prenatally. Either FFP or cryoprecipitate can be used for the treatment of factor XI–deficient patients if factor XI concentrate is not available.
Severe factor XIII deficiency is typically manifested at birth as bleeding from the umbilical stump or ICH. Other clinical manifestations of homozygous factor XIII deficiency include delayed wound healing, abnormal scar formation, and recurrent soft tissue hemorrhage with a tendency to form hemorrhagic cysts. ICH occurs even in the absence of trauma in approximately a third of all affected patients. Newborns with heterozygous factor XIII deficiency are not clinically affected.
FFP, cryoprecipitate, or factor XIII concentrates can be used for the treatment of factor XIII–deficient patients. Newborns with known factor XIII deficiency should receive a prophylactic regimen of factor XIII because of the high incidence of ICH. Plasma concentrations of factor XIII greater than 1% are effective, and the very long half-life of factor XIII permits once-per-month therapy. Therefore prophylactic replacement therapy consists of either small doses of FFP (2 to 3 mL/kg) administered every 4 to 6 weeks, cryoprecipitate at a dose of 1 bag/10 to 20 kg of weight every 3 to 6 weeks, or, preferably, factor XIII concentrate at a dose of 10 to 20 U/kg every 4 to 6 weeks, depending on the clinical situation and the preinfusion plasma concentration of factor XIII.
Hereditary deficiencies of two or more coagulation proteins have been reported for 16 different combinations of coagulation factors. Combined deficiency of factors V and VIII has been described in patients with mutations in the genes encoding LMAN1 (also called ERGIC-53) and MCFD2. Administration of FFP is usually the initial therapy. Subsequent treatment varies, depending on the specific factors affected.
HDN, as first described by Townsend in 1894, consists of hemorrhage on days 1 through 5 of life from multiple sites in otherwise healthy infants. Subsequently, a causal link between HDN and abnormal blood coagulation was established. Initial treatment of infants with HDN consisted of intravenous, intramuscular, or subcutaneous injection of blood or serum. Even at this very early time, the difficulty of separating treatment response from spontaneous improvement was recognized. Later, a randomized, controlled trial showed that intramuscular injection of blood was not helpful in preventing the abnormalities in blood coagulation that occurred during the first week of life. The link between VK deficiency and spontaneous hemorrhage was first recognized in chicks in 1929. The association between VK deficiency and HDN quickly followed, as subsequently did treatment of infants with HDN.
The next historical step was recognition of the link between decreased prothrombin activity and increased PT on days 2 to 4 of life in the absence of prophylactic VK. Prothrombin activity was observed to return to normal by days 5 to 7 of life. These observations led to the hypothesis that VK administered prophylactically could prevent HDN. There was uniform agreement that the prophylactic administration of VK to mothers or infants prevented the decrease in prothrombin activity during the first 3 to 4 days of life. On the basis of these studies, VK prophylaxis was widely recommended. Subsequently, the scientific basis for this policy became less clear for several reasons. First, plasma concentrations of other coagulation proteins in addition to prothrombin were shown to be low in newborns. Second, there was increasing recognition that bleeding in neonates was often not due to VK deficiency. Third, administration of high amounts (50 to 70 mg) of a water-soluble form of VK resulted in hemolytic anemia with kernicterus in some infants. Fourth, many clinicians suggested that VK prophylaxis was not needed for all healthy full-term infants. Consequently, VK prophylaxis was suspended in some countries, and recurrence of HDN ensued. Most of the controversy concerning prophylactic use of VK can be explained by the design of the trials and subsequent interpretation of their results. The best evidence comes from large randomized, placebo-controlled trials. Such trials have consistently shown a statistically significant benefit from VK prophylaxis in terms of clinical bleeding. No randomized, controlled trials with large enough sample size have shown that prophylactic VK does not prevent bleeding.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here