Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most of the bleeding that occurs during surgery or in association with trauma is mechanical and usually can be controlled. In contrast, nonsurgical bleeding most commonly is caused by the concomitant administration of antithrombotic medications, and less commonly by congenital or acquired defects of the hemostatic system. The vascular surgeon must understand the hemostatic pathways and the characteristics of the various antithrombotic agents in sufficient depth to prevent or arrest bleeding and restore hemostasis. In addition, vascular surgeons play an integral role in the surgical and medical management of patients who develop arterial and venous thromboembolism (VTE). This requires an in-depth knowledge of the myriad demographic; situational, inherited, and acquired risk factors for thrombosis, as well as a solid understanding of the various medical options available to alter the balance between thrombosis and hemostasis.
Hemostasis is the process by which bleeding from injured tissue is controlled. Although hemostasis is a dynamic process, it can be divided into several components: vessel response to injury, platelet activation, fibrin formation, fibrinolysis, and coagulation inhibition. Each component has numerous modulatory mechanisms.
When a vessel is injured, the interaction of humoral, neurogenic, and myogenic systems leads to temporary vasoconstriction in the muscular arteries and arterioles. Mechanisms for vasoconstriction may include the release of thromboxane A 2 (TXA 2 ) by activated platelets, endothelin by endothelial cells, bradykinin, and fibrinopeptide B. Vasoconstriction has less of a role in obtaining hemostasis in veins and venules.
In normal vessels, endothelial cells cover the luminal surface, forming a monolayer with tight cell-cell interaction. In their quiescent state, endothelial cells are actively antithrombotic. They synthesize and secrete several modulators that lead to vasodilation, decreased platelet aggregation, decreased levels of thrombin, factors Va and VIIIa, and factors IXa and Xa, by which an antithrombotic state is promoted. Specifically, prostacyclin and nitric oxide are potent vasodilators and inhibitors of platelet aggregation. Heparan sulfates accelerate the activity of antithrombin (AT), thereby inactivating thrombin. Thrombomodulin (TM) also inactivates thrombin by forming the thrombomodulin-thrombin complex, a potent activator of the natural anticoagulant protein C , which with the help of cofactor protein S, inactivates factors Va and VIIIa, leading to decreased thrombin and factor Xa levels. Tissue factor pathway inhibitor (TFPI), which is bound to the endothelial surface, strongly inhibits the extrinsic coagulation pathway after heparin administration. Tissue-type plasminogen activator (t-PA) and urokinase are synthesized, which bind fibrinogen and fibrin, increase plasmin, and promote fibrinolysis.
The endothelium also possesses substantial procoagulant activity and acts as a scaffold for hemostasis when stimulated after vessel injury. Tissue factor (thromboplastin, factor III) is a protein that is constitutively expressed by most cells; however, endothelial cells only express tissue factor when stimulated by agonists such as thrombin or endotoxin. Vessel injury causes endothelial denudation and activation, which results in the exposure of tissue factor to low circulating levels of activated factor VII in blood to form complexes that catalyze the conversion of factor IX to IXa and factor X to Xa, leading to thrombin formation.
Endothelial cells also synthesize and secrete von Willebrand factor (vWF), which is necessary for platelet adhesion to the vessel wall. This factor has binding sites for collagen, platelet glycoproteins (GPs) Ib and IIb/IIIa, and factor VIII. Factor VIII and vWF circulate together as a complex. Endothelial cells, in addition to the liver, synthesize factor V. Factors V and VIII are cleaved by thrombin into their activated states (Va and VIIIa) and then become integral components of membrane-bound complexes that accelerate the formation of thrombin and factor Xa ( Fig. 6.1 ). Endothelial cells also synthesize plasminogen activator inhibitor (PAI-1), which rapidly inactivates circulating t-PA.
Platelets are small, discoid-shaped, anuclear cells with an average circulatory life span of 8 to 12 days. There are usually 150,000 to 300,000 platelets/mL in human blood. Platelets are released as cytoplasmic fragments of megakaryocytes within bone marrow.
The platelet membrane is composed of a phospholipid bilayer, GPs, and proteins. Circulating proteins interact with the carbohydrate moieties of the GPs. Several surface receptors are known to exist. Some of the more common receptors bind thrombin, adenosine diphosphate (ADP), TXA 2 , fibrinogen, collagen, and vWF. Platelets contain three types of storage granules: (1) dense granules, which contain serotonin, ADP, adenosine triphosphate (ATP), and calcium; (2) α-granules, which contain coagulation proteins (high-molecular-weight kininogen [HMWK], fibrinogen, fibronectin, factor V, vWF, platelet factor 4), growth factors, and adhesion proteins (fibronectin, thrombospondin, P-selectin); and (3) lysosomes.
The initial stage of hemostasis, consisting of vasoconstriction and platelet plug formation, is termed primary hemostasis . Immediately after vascular injury, platelets adhere to the subendothelial matrix via proteins, such as collagen and vWF. vWF binds primarily to the GP Ib-IX-V complex and the GP IIb-IIIa complex, whereas collagen binds via the GP Ia-IIa complex and GP IV. Collagen-induced platelet activation results in platelet shape change and release of prothrombotic α- and dense granule contents. Granule release reactions further amplify platelet activation and aggregation via several proteins, including vWF, fibrinogen, and ADP.
Platelet activation is associated with numerous downstream signals, including protein kinase C activation, inositol triphosphate formation, intracellular calcium mobilization, and the generation of arachidonic acid. Arachidonic acid is then converted by cyclooxygenase-1 (COX-1) to prostaglandin endoperoxides (PGG 2 , PGH 2 ). PGG 2 is converted to TXA 2 by thromboxane synthetase. PGG 2 , PGH 2 , and TXA 2 stimulate further aggregation and platelet granule release.
Regardless of the agonist, the final common pathway for platelet aggregation involves a conformational change in the GP IIb-IIIa complex that leads to the reversible exposure of binding sites for fibrinogen, which allows fibrinogen to form bridges between adjacent platelets, forming the platelet aggregate.
Numerous medications inhibit platelet function at several steps in the pathway described previously. Aspirin irreversibly inhibits platelet COX-1, inhibiting TXA 2 -mediated platelet aggregation for the life of the platelet. Clopidogrel, ticagrelor, and prasugrel inhibit ADP-mediated platelet activation and aggregation. Novel GP IIb-IIIa inhibitors prevent platelet aggregation by blocking the binding of fibrinogen.
The platelet plug, which is required for normal hemostasis, de-aggregates unless thrombin is generated and fibrin stabilization of the plug occurs; this is known as secondary hemostasis . The formation of fibrin requires the interaction of platelet aggregates, endothelial cells, and plasma coagulation proteins.
Plasma coagulation proteins have been designated by the Roman numerals I through XIII (the letter “a” follows the Roman numeral when the factor has been activated), and all of these factors are synthesized in the liver. The hepatic synthesis of factors II, VII, IX, and X is vitamin K dependent. When vitamin K is not available, these factors are synthesized and released but are not biologically active.
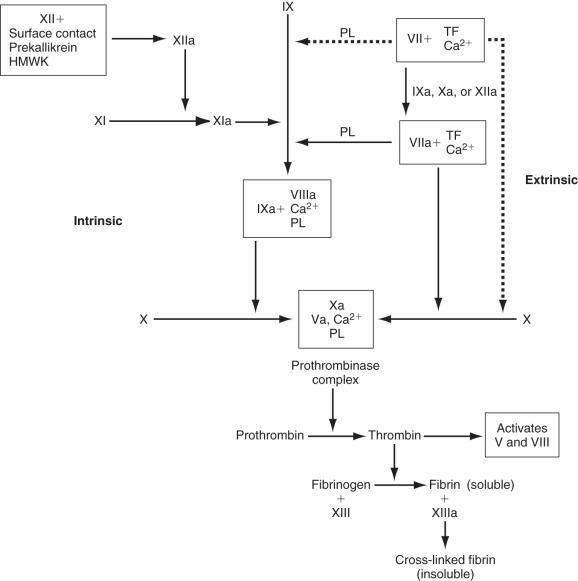
The sequence of enzymatic events leading to thrombin formation is the coagulation cascade (see Fig. 6.1 ). The intrinsic pathway is activated when plasma is exposed to a negatively charged surface such as subendothelium, collagen, or endotoxin. Factor XII is activated to XIIa by the interaction of HMWK, prekallikrein, and the negatively charged surface; however, the physiologic significance of factor XII activation is unclear because deficiencies in factor XII, HMWK, and prekallikrein are not associated with any clinical bleeding diatheses.
The extrinsic pathway is the more physiologic route for the generation of thrombin and fibrin. It is initiated by the exposure of tissue factor, which binds to low levels of circulating factor VIIa in the presence of calcium (TF-VIIa). This complex activates factor X and factor IX. Factor Xa alone does not generate thrombin efficiently; however, factors Xa and thrombin together can activate factors VII, V, and VIII. Factors Va and VIIIa are critical components of the prothrombinase and tenase complexes (see Fig. 6.1 ), respectively, which are 10 5 -fold to 10 6 -fold more active at generating thrombin than their serine protease factors acting independently.
Thrombin proteolytically cleaves peptides from the fibrinogen molecule, resulting in the polymerization of fibrin monomers to form a gel. Thrombin also activates factor XIII in a reaction that is greatly accelerated (>80-fold) by the presence of fibrin. Factor XIIIa covalently cross-links adjacent fibrin monomers, forming a stable clot that is more resistant to lysis by plasmin.
Several mechanisms have evolved to control the rate of thrombin and fibrin formation ( Fig. 6.2 ). AT is a serine protease inhibitor that is synthesized in the liver and endothelial cells. AT inhibits numerous coagulation factors, but its most important targets are thrombin and factor Xa. AT activity is enhanced at least 1000-fold whenever it binds to circulating heparin or endothelial-bound heparin-like molecules. After the AT–heparin complex binds to an activated coagulation factor, the heparin dissociates and continues to act as a catalyst for the formation of other AT–serine enzyme complexes.
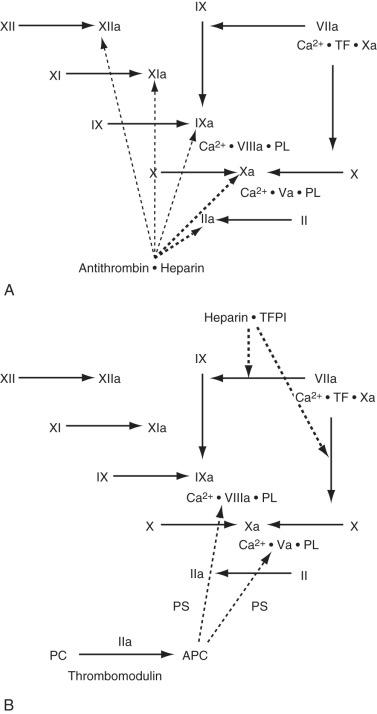
TFPI is an enzyme inhibitor synthesized by the endothelium and megakaryocytes. It binds to the TF-VIIa-Xa complex and inhibits the further activation of factors X and IX. TFPI is constitutively expressed on the endothelium, and its activity and antigen levels increase dramatically after the administration of heparin.
TM is a proteoglycan expressed on the surface of most endothelial cells that binds thrombin, causes a conformational change in the substrate binding site, and renders the thrombin molecule incapable of binding active coagulation factors. TM also accelerates the inactivation of thrombin by AT.
Protein C and protein S are synthesized by the liver, but protein S has also been found in endothelium and platelets. Activated protein C (APC) binds to protein S on the endothelial or platelet surface and cleaves several peptide bonds in factors Va and VIIIa, resulting in decreased formation of the prothrombinase and tenase complexes.
Heparin cofactor II is another specific thrombin inhibitor that forms a stable 1 : 1 complex with thrombin. Heparin, heparan-like molecules, and dermatan sulfate accelerate the activity of heparin cofactor II. Unlike AT, heparin cofactor II cannot inhibit other coagulation factors. The plasma concentration of heparin cofactor II (70 µg/L) is much lower than that of AT (150 mg/L), and it is unlikely that heparin cofactor II has a major role in the regulation of hemostasis.
Plasminogen, an inactive precursor synthesized in the liver, can be converted to plasmin by several plasminogen activators. Circulating t-PA does not activate plasminogen efficiently; however, both t-PA and plasminogen have high affinity for fibrin, which acts as a template for accelerated plasminogen activation (>1000-fold). Thus the primary role for t-PA–activated plasmin is the lysis of preexisting formed fibrin clots. Alternatively, exogenously administered t-PA may activate nonbound plasminogen, resulting in the release of free plasmin, leading to the limited breakdown of fibrinogen, platelet receptors, factor V, and factor VIII, leading to a systemic fibrinolytic state.
t-PA is commercially available in several forms. Recombinant human t-PA is the most widely used agent for peripheral vascular applications, and it is Food and Drug Administration (FDA) indicated for acute ischemic stroke, acute myocardial infarction, and massive pulmonary embolism. Tenecteplase is a recombinant variant of t-PA with amino acid substitutions at three sites, resulting in a longer half-life and a higher affinity for thrombus-bound plasminogen. Each step within the plasminogen activation system has a known inhibitor. PAI-1 is released by endothelial cells, platelets, and hepatocytes. This inhibitor efficiently inactivates t-PA and two-chain u-PA and performs other functions, including the inhibition of thrombin and smooth muscle cell migration. α 2 -Antiplasmin inactivates circulating plasmin more readily than it does fibrin-bound plasmin, thus decreasing overall systemic fibrinolysis.
A thorough history and physical examination will detect the majority of bleeding disorders preoperatively. Laboratory testing is warranted if a bleeding disorder is present or suspected. Careful questioning should distinguish a congenital bleeding disorder from an acquired one. Determining the pattern of inheritance can further aid in identifying a congenital deficiency. A history of bleeding problems beginning in childhood or at the beginning of menses implies an inherited bleeding disorder. A history of postoperative or spontaneous bleeding in a family member is important, because many patients with inherited disorders do not experience serious bleeding until challenged by an operative procedure or trauma. All patients should be asked about bleeding after tooth extraction, minor trauma, circumcision, and other surgical procedures.
An acquired hemostatic disorder should be suspected in adults who bleed during or after surgery or trauma, but who have no previous history of bleeding disorders; however, some patients with congenital disorders, such as von Willebrand disease, may not demonstrate a bleeding diathesis until challenged. Patients with liver disease are at increased risk for developing a coagulopathy during surgery, after trauma, and after massive transfusion. A detailed history of drug use is also important, because many drugs alter platelet function and predispose patients to bleeding complications.
Physical examination should include a thorough inspection for ecchymoses, petechiae, purpura, hemangiomas, jaundice, hematomas, and hemarthroses. Petechiae, ecchymoses, and mucocutaneous bleeding (epistaxis, gastrointestinal or genitourinary bleeding, menorrhagia) are more commonly associated with defects in primary hemostasis. Bleeding into deep tissues (hemarthroses, muscle, and retroperitoneal hematomas) tends to occur with defects in coagulation. Signs of hepatic insufficiency should be noted, because these patients may have decreased production of coagulation proteins. Patients with myeloproliferative disorders, some malignant neoplasms, collagen disorders, or renal insufficiency are at increased risk for bleeding complications.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here