Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Globally, neonatal encephalopathy precipitated by perinatal hypoxia-ischemia remains a common cause of brain injury. The incidence varies from 1 to 3 per 1000 to up to 25 per 1000 in developed and developing countries, respectively. Therapeutic hypothermia has been demonstrated to improve both survival and neurological morbidity ; however, there remains a significant burden of mortality and long-term neurological sequelae among survivors. Birth asphyxia, defined as a lack of blood flow or gas exchange to or from the fetus in the period immediately before, during, or after delivery, accounts for approximately 1 million deaths per year, , making it a leading cause of infant mortality. In addition, the approximately 1 million children who survive birth asphyxia yearly do so with chronic neuro-developmental morbidities, including cerebral palsy and intellectual and learning disabilities. Although the cellular and metabolic contributors to brain injury are complex, abnormal cerebral blood flow is an essential contributor to brain injury by this mechanism ( Figure 22.1 ). In this chapter we will review the interrelationship between the cardiovascular system and the brain, the impact of therapeutic hypothermia on hemodynamics, and management considerations specific to this patient population.
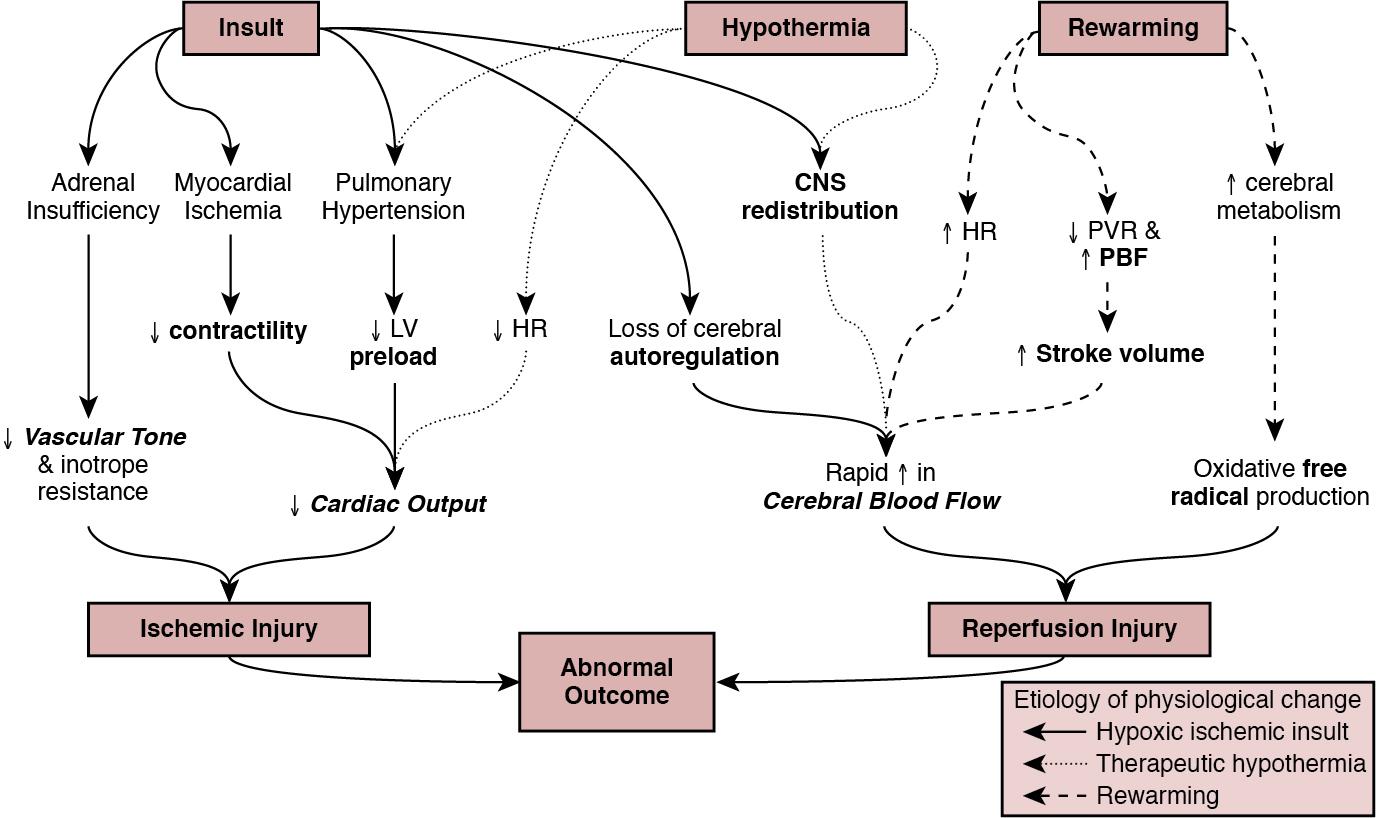
The primary determinants of fetal cerebral blood flow are cardiac output, arterial oxygen content, and blood pressure, as has been shown in a fetal lamb model. , Over the range of cerebral autoregulation, the fetal lamb maintains a constant cerebral blood flow despite a wide range of experimentally varied cardiac output (200–700 mL/min/kg). Adaptation to low cardiac output involves a complex mechanism that begins with a surge of catecholamines and an associated increase in systemic blood pressure. , This increase in blood pressure is associated with a redistribution of systemic blood flow to the brain. Simultaneously, there is an increase in pulmonary vascular resistance which acts to divert more blood from the lungs to essential organs, including the brain, coronary circulation, and adrenal glands. This is facilitated by an increase in right ventricular afterload, which diverts a greater proportion of placental flow from the right to left atrium, and therefore the brain and coronary circulation. When these and other compensatory mechanisms fail, brain injury may occur.
In healthy infants cerebral blood flow (CBF) remains constant over a relatively wide range of changes in systemic mean arterial blood pressure. In animal models the fetal autoregulatory curve differs from that of the adult. , The curve is narrower, particularly at the upper elbow of the curve, and importantly, the normal mean arterial blood pressure in the less mature animal is only marginally above the lower elbow of the autoregulatory curve. In human preterm and term infants the mean blood pressure value at the lower elbow of the autoregulatory curve, or the blood pressure threshold associated with neuronal dysfunction and then tissue injury are unknown.
Autoregulation is disrupted by hypoxia, hyperoxia, hypocarbia, hypercarbia, and/or acidosis. Severe disruption of autoregulation results in a pressure-passive cerebral circulation. In animal models the role of asphyxia-associated impaired CBF autoregulation in the pathogenesis of ischemic cerebral injury has been clearly described. If the same is true for humans, the clinical implications are of importance, as hypotension and decreased cardiac output result in decreases in CBF and thus secondary energy failure and neuronal injury. As expected, this process is more pronounced in the more vulnerable regions of the brain, such as the parasagittal cortex or periventricular white matter. However, in humans this link between cerebral ischemia and cardiovascular dysfunction is not yet clear, and thus it is not known whether appropriate cardiovascular support and stabilization of CBF improve outcomes in asphyxiated infants.
The effects of HI insult on the cardiovascular system are complex, and it is important to understand the principles of developmental cardiovascular physiology and pathophysiology in these sick infants.
Both the primary insult and the ongoing redistribution of blood flow can lead to reduced myocardial perfusion, potentially resulting in myocardial ischemia, especially in the sub-endocardial tissue and papillary muscles. Echocardiography studies have consistently demonstrated differences in various measures of wventricular function between patients with HIE and healthy controls, particularly using advanced measurement techniques such as tissue Doppler imaging and speckle tracking. , Structural and metabolic characteristics of the neonatal myocardium confer a greater vulnerability to the effects of hypoxia-ischemia. Specifically, the antioxidant capacity remains underdeveloped at the time of birth, despite upregulation toward late gestation, which primes the neonate for oxidative stress during postnatal resuscitation. In addition, ischemia interrupts the normal transition of cardiomyocytes from a glycolytic metabolic pathway utilizing lactic acid to the oxidation of fatty acids. This transition is required for efficient energy utilization and adaptation to the oxygen-rich environment. Thus its interruption may confer added vulnerability. Myocardial ischemia may be further complicated by diminished preload, asphyxia-induced autonomic dysfunction, and/or vasoplegia. Adequate coronary perfusion, which is often compromised due to hypotension following asphyxia, is critical for optimal cardiac function and may represent an additional metabolic disadvantage. Decreased myocardial contractility may also occur secondary to acidosis and hypoxia. ,
Approximately 25% of neonates with myocardial necrosis on autopsy have a clinical history of asphyxia, making it the most common single cause of perinatal myocardial ischemia. , Transient myocardial ischemia, as diagnosed by electrocardiogram and other non-specific findings, occurs in two-thirds of asphyxiated infants. Clinical features may or may not be externally evident ; however, both electrocardiography changes and abnormalities of cardiac enzymes have been linked to the severity of neurological outcomes. , , This supports the importance of monitoring for transient myocardial ischemia, even among so-called “stable” patients. These patients are often normotensive until they are no longer able to compensate and may present with acute cardiovascular collapse. Several factors point to the central role of myocardial ischemia in the pathogenesis of shock among HIE patients. These include higher cardiac troponin T compared to healthy controls and the association of elevation in this enzyme with low cardiac output and impaired coronary perfusion. That the burden of brain injury is greater among patients with concurrent cardiac dysfunction has also been suggested using echocardiography, and as modern evaluative tools become increasingly sophisticated, new associations will become relevant. This includes a recently reported relationship between right ventricular function at 24 hours postnatal age and both short- (i.e., death or abnormal brain magnetic resonance imaging) and long-term (survival with neurological impairment) outcomes.
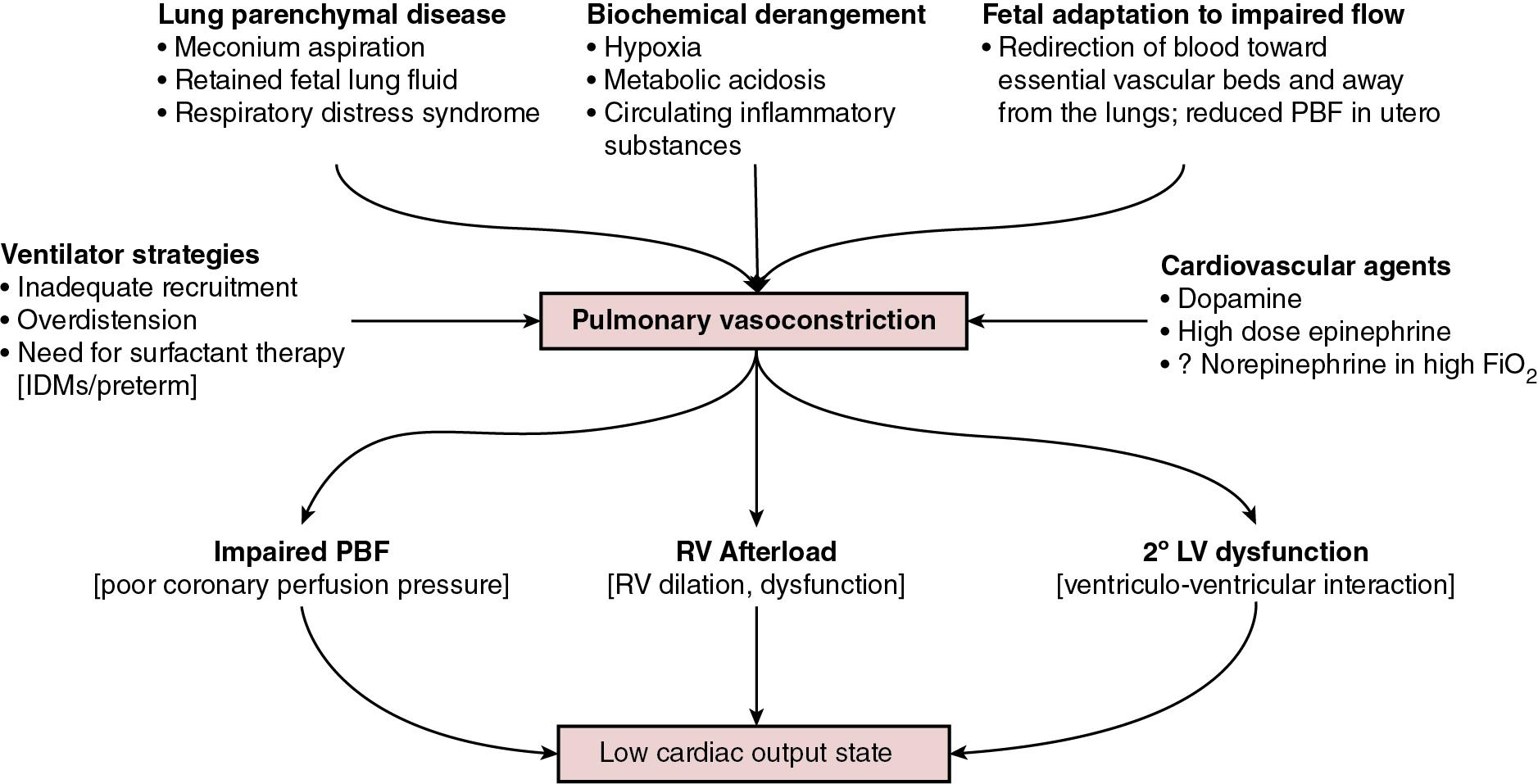
Although there are important effects of perinatal hypoxia-ischemia in the systemic circulation, the pulmonary vascular impact merits specific consideration. As previously mentioned, part of the fetal adaptive response to impaired CBF includes a redirection of pulmonary blood flow into essential systemic circulatory beds. This, coupled with the fact that neonatal resuscitation efforts are less effective at establishing functional residual capacity than a spontaneously breathing neonate, places the fetus with HIE at risk of failed postnatal transition. Additionally, hypoxia and acidosis are potent pulmonary vasoconstrictors. Finally, neonates with a hypoxic-ischemic injury often have associated morbidities such as meconium aspiration syndrome, lung parenchymal disease, and/or sepsis. These conditions negatively impact lung compliance and/or alter circulating inflammatory mediators, which may independently have a negative impact on cardiac function ( Figure 22.2 ). Pulmonary vasoconstriction has several important downstream effects. First , it produces an afterload stress on the right ventricle, which may exacerbate pre-existing myocardial dysfunction. Second, heterometric adaptation, or dilation, of the right ventricle and septal flattening negatively impact left ventricular function due to ventricular-ventricular interaction. Finally , impaired pulmonary blood flow occurs due to high resistance, compromised right ventricular systolic function, and systemic recirculation of right ventricular output via right-to-left ductal shunt when the ductus is open. This results in compromised left ventricular filling, low left ventricular output, and therefore further impairment of coronary perfusion pressure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here