Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The health of cells and tissues depends on the circulation of blood, which delivers oxygen and nutrients and removes wastes generated by cellular metabolism. Under normal conditions, as blood passes through capillary beds, there is little net movement of water and electrolytes into the tissues (discussed later). This balance is often disturbed by pathologic conditions that alter endothelial function, increase vascular hydrostatic pressure, or decrease plasma protein content, all of which promote edema —the accumulation of fluid in tissues resulting from a net movement of water into extravascular spaces. Depending on its severity and location, edema may have minimal or profound effects. In the lower extremities, it may only make one’s shoes feel snugger after a long, sedentary day; in the lungs, however, edema fluid can fill alveoli, causing life-threatening hypoxia.
The structural integrity of blood vessels is frequently compromised by trauma. Hemostasis is the process of blood clotting that follows blood vessel damage. Inadequate hemostasis results in hemorrhage (excessive bleeding), which may compromise tissue perfusion and, if massive and rapid, lead to hypotension, shock, and death. Conversely, inappropriate clotting (thrombosis) or migration of clots in the vasculature (embolism) may lead to blood vessel obstruction and ischemic cell death (infarction) . Importantly, thromboembolism underlies three major causes of morbidity and death: myocardial infarction, pulmonary embolism, and cerebrovascular accident (stroke).
With this as background, we begin our discussion of hemodynamic disorders with conditions that increase blood volumes, either locally or systemically.
Hyperemia and congestion both refer to an increase in blood volume within a tissue but have different underlying mechanisms. Hyperemia is an active process resulting from arteriolar dilation and increased blood inflow; it occurs at sites of inflammation and in exercising skeletal muscle. Hyperemic tissues are redder than normal because they are engorged with oxygenated blood. Congestion is a passive process resulting from impaired outflow of venous blood from a tissue. Congestion occurs systemically in cardiac failure and locally as a consequence of venous obstruction. Congested tissues have an abnormal blue-red color (cyanosis) that stems from the accumulation of deoxygenated hemoglobin. In long-standing chronic congestion, inadequate tissue perfusion and persistent hypoxia may lead to parenchymal cell death and secondary tissue fibrosis, and the elevated intravascular pressures may cause edema or rupture capillaries, producing focal hemorrhages.
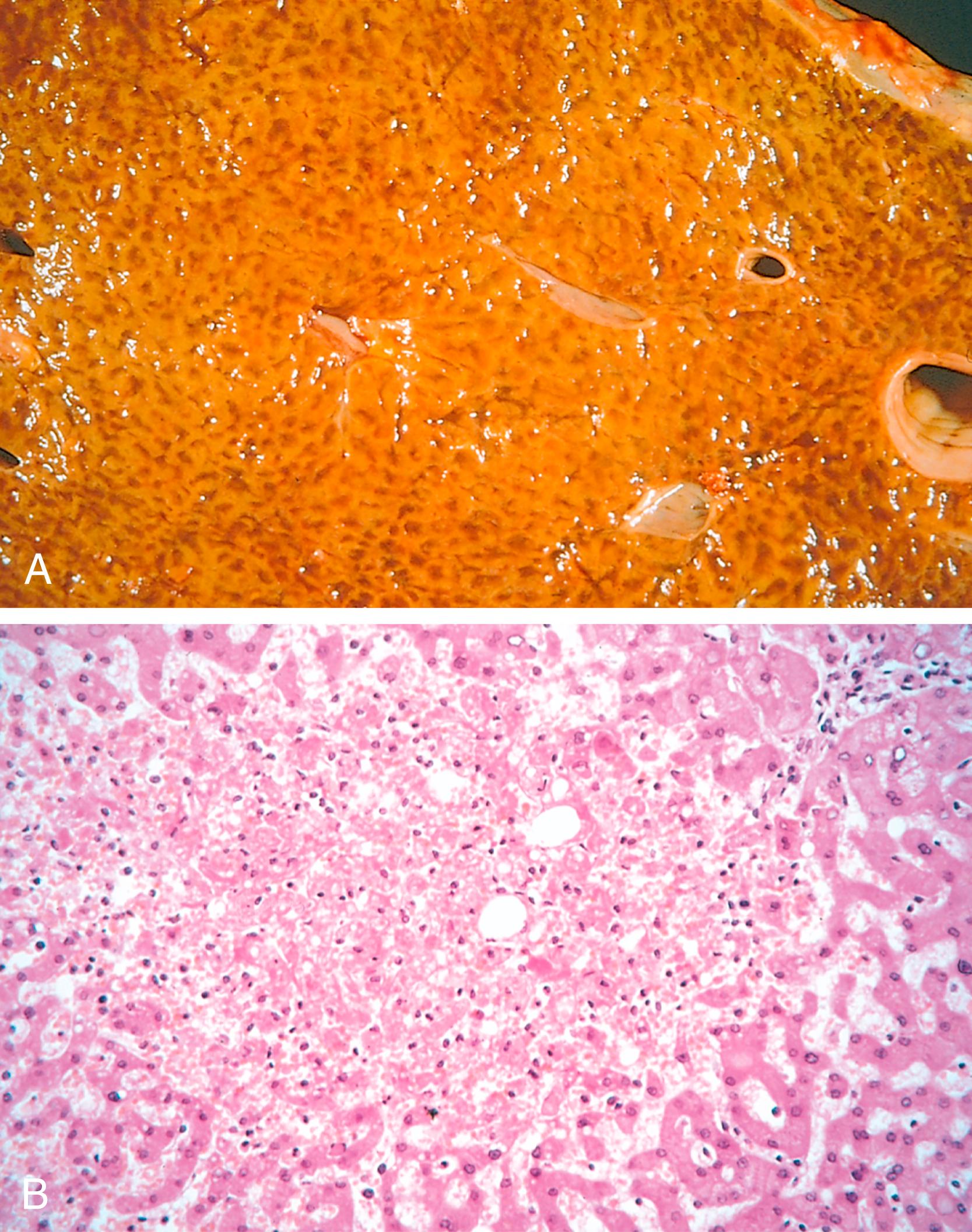
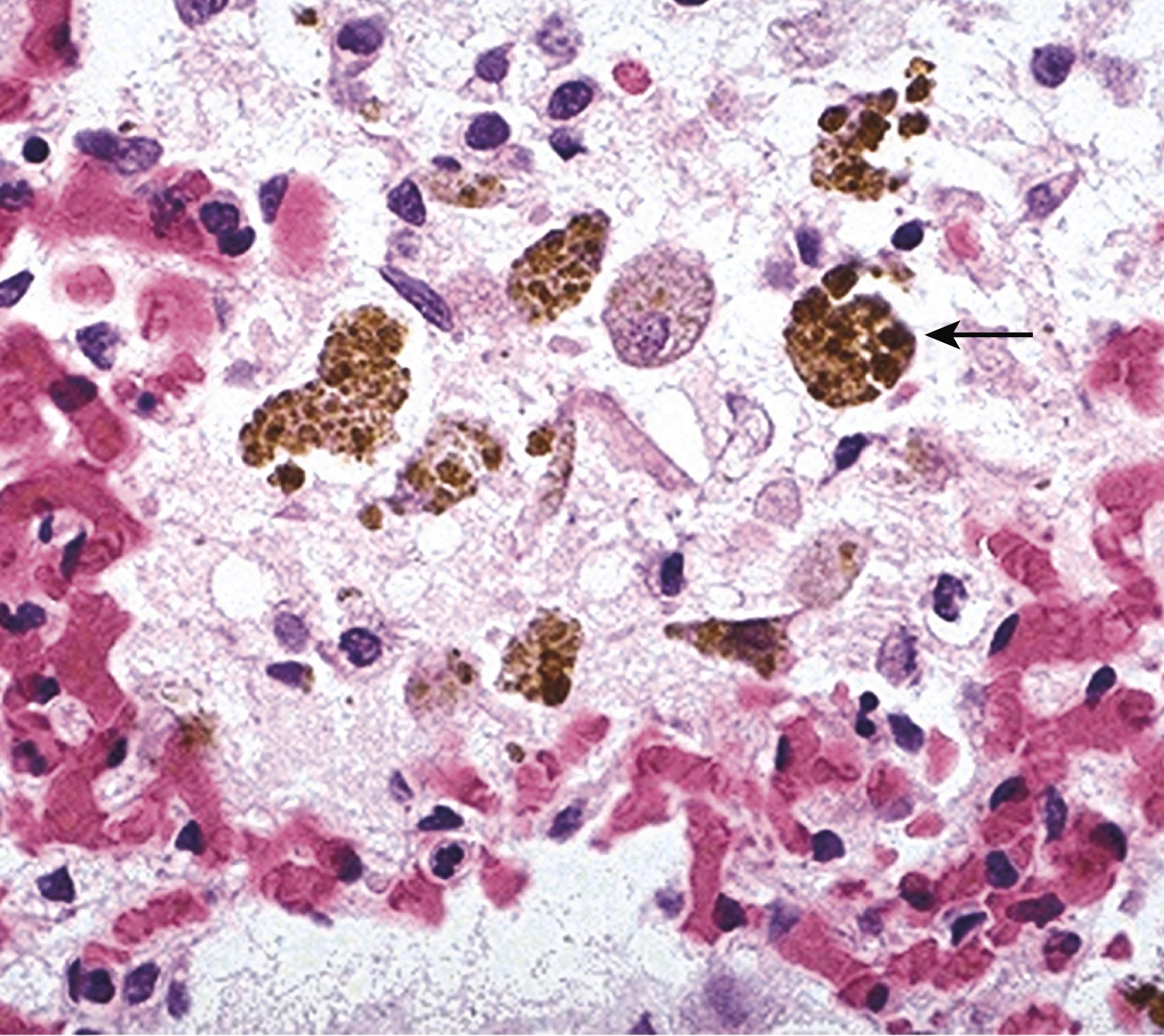
Cut surfaces of hyperemic or congested tissues feel wet and typically ooze blood. Microscopically, acute pulmonary congestion is marked by blood-engorged alveolar capillaries and variable alveolar septal edema and intraalveolar hemorrhage. In chronic pulmonary congestion, the septa become thickened and fibrotic and the alveolar spaces contain numerous macrophages laden with hemosiderin ( “heart failure cells,” eFig. 3.1 ) derived from phagocytosed red cells. In acute hepatic congestion, the central vein and sinusoids are distended with blood and centrally located hepatocytes may undergo necrosis. Periportal hepatocytes, which experience less severe hypoxia because of their proximity to hepatic arterioles, may develop fatty change. In chronic passive liver congestion, the central regions of the hepatic lobules are congested, red-brown, and slightly depressed (owing to necrosis and cell loss) and are accentuated by surrounding zones of tan, sometimes fatty, periportal hepatocytes (nutmeg liver) ( Fig. 3.1A , B).
Approximately 60% of lean body weight is water, two-thirds of which is intracellular. Most of the remaining water is found in tissues in the form of interstitial fluid; only 5% of the body’s water is in blood plasma. As noted earlier, edema is an accumulation of interstitial fluid within tissues. Extravascular fluid can also collect in body cavities, where it is often referred to as an effusion. Examples include effusions in the pleural cavity (hydrothorax), the pericardial cavity (hydropericardium), and the peritoneal cavity (hydroperitoneum, or ascites ). Anasarca is severe, generalized edema marked by profound swelling of subcutaneous tissues and accumulation of fluid in body cavities.
Table 3.1 lists the major causes of edema. In inflammation, edema is due to increased vascular permeability ( Chapter 2 ); the noninflammatory causes are described in the following discussion.
| Increased Hydrostatic Pressure |
| Impaired Venous Return |
| Congestive heart failure Constrictive pericarditis Liver cirrhosis Venous obstruction or compression
Lower extremity inactivity with prolonged dependency |
| Arteriolar Dilation |
| Heat Neurohumoral dysregulation |
| Reduced Plasma Osmotic Pressure (Hypoproteinemia) |
| Protein-losing glomerulopathies (nephrotic syndrome) Reduced protein synthesis (e.g., advanced liver disease) Malnutrition Protein-losing gastroenteropathy |
| Lymphatic Obstruction |
| Inflammatory Neoplastic Postsurgical Postirradiation |
| Sodium Retention |
| Excessive salt intake with renal insufficiency Decreased renal excretion of sodium
|
| Inflammation |
| Acute inflammation Chronic inflammation Angiogenesis |
Fluid movement between the vascular and interstitial spaces is governed mainly by two opposing forces: vascular hydrostatic pressure and colloid osmotic pressure produced by plasma proteins. Normally, the outflow of fluid produced by hydrostatic pressure at the arteriolar end of the microcirculation is nearly balanced by inflow at the venular end owing to osmotic pressure. The small net outflow of fluid into the interstitial space is drained by lymphatic vessels to the bloodstream by way of the thoracic duct, keeping the tissues “dry.” Either increased hydrostatic pressure or diminished colloid osmotic pressure will result in increased movement of water into the interstitium ( Fig. 3.2 ), and if the drainage capacity of the lymphatics is exceeded, edema results.
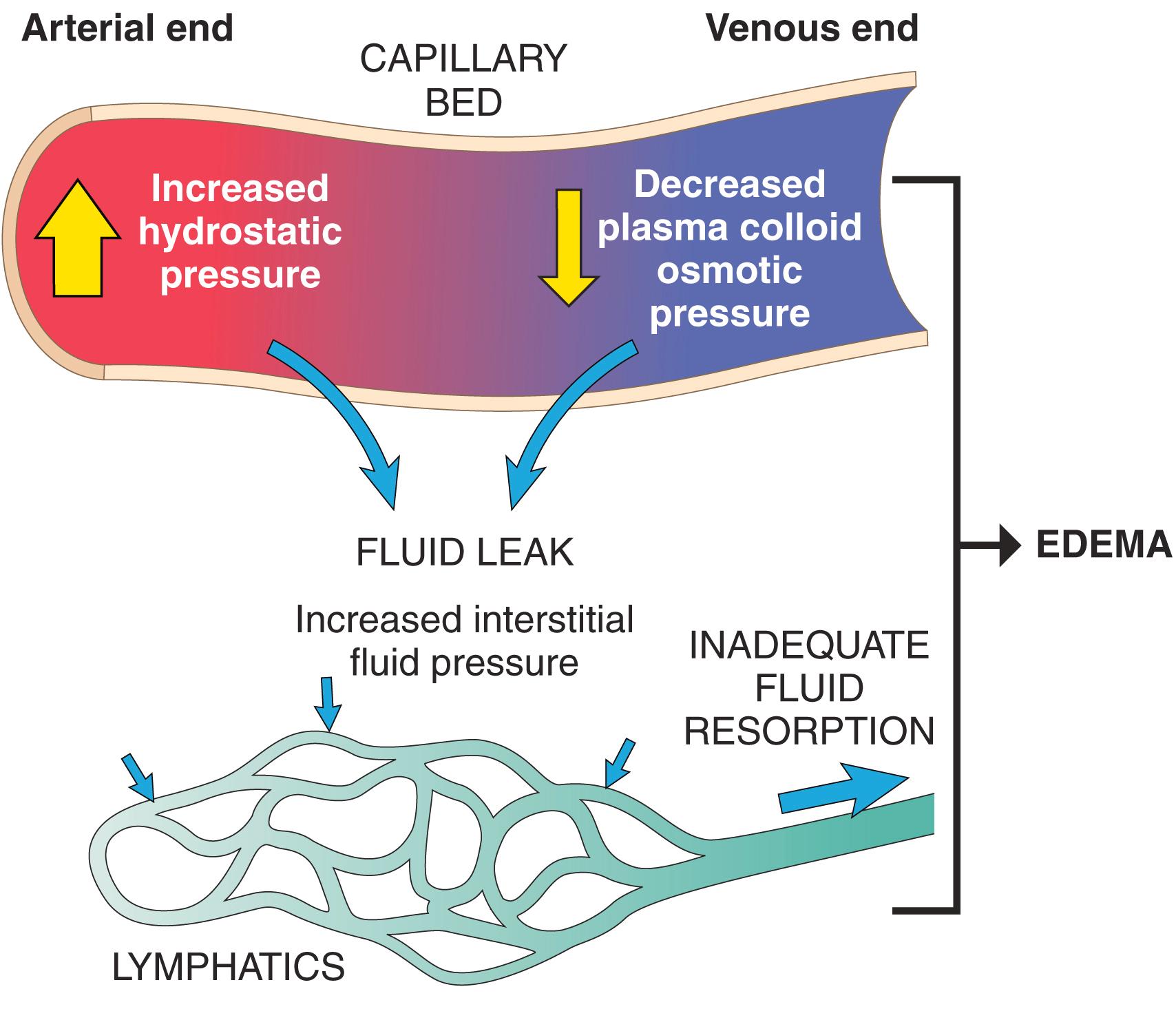
The edema fluid that accumulates due to high hydrostatic pressure or low colloid pressure typically is a protein-poor transudate ; by contrast, because of increased vascular permeability, inflammatory edema fluid is a protein-rich exudate . Next we discuss the various causes of edema.
Increases in hydrostatic pressure are mainly caused by disorders that impair venous return. For example, deep venous thrombosis in the lower extremity may cause edema restricted to the distal portion of the affected leg, whereas congestive heart failure ( Chapter 11 ) leads to a systemic increase in venous pressure and, often, widespread edema. Fig. 3.3 illustrates the mechanisms underlying the generalized edema that may be seen in the context of cardiac, renal, or hepatic failure. Several factors increase venous hydrostatic pressure in patients with congestive heart failure. Reduced cardiac output leads to pooling of blood in the venous circulation and increased capillary hydrostatic pressure. The reduction in cardiac output also results in hypoperfusion of the kidneys, triggering the renin-angiotensin-aldosterone axis and inducing sodium and water retention (secondary hyperaldosteronism). In patients with normal heart function, this adaptation increases cardiac filling and cardiac output, resulting in improved renal perfusion. However, the failing heart often cannot increase its output in response to increases in cardiac filling, and a vicious cycle of fluid retention, increased venous hydrostatic pressures, and worsening edema ensues. Unless cardiac output is restored or renal water retention is reduced (e.g., by salt restriction or treatment with diuretics or aldosterone antagonists), this downward spiral continues. Secondary hyperaldosteronism is also a feature of noncardiac generalized edema, which may also benefit from treatment with salt restriction, diuretics, and aldosterone antagonists.
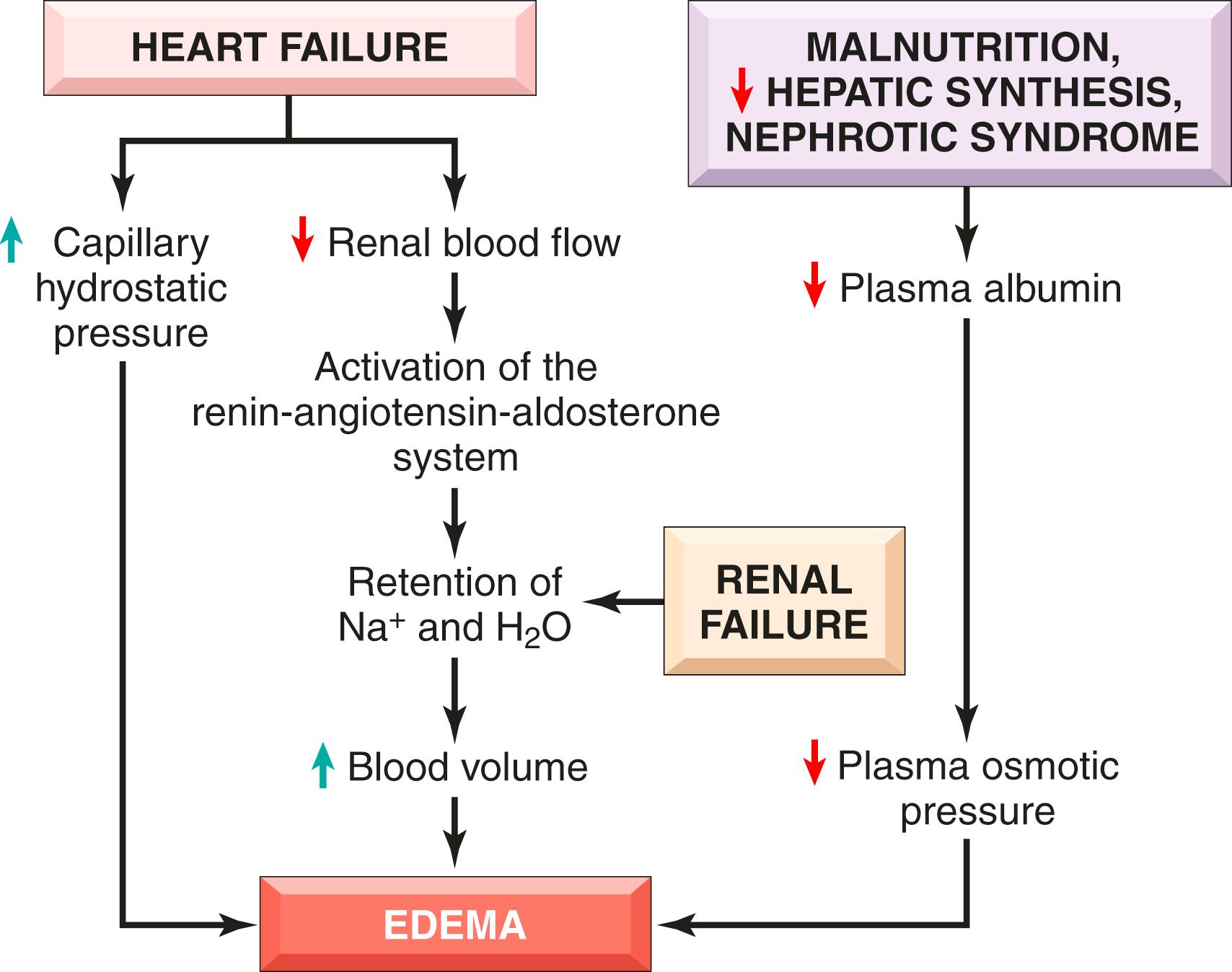
Reduced plasma albumin is a common feature of disorders in which edema is caused by decreases in colloid osmotic pressure. Normally, albumin accounts for almost half of the total plasma protein and is the biggest contributor to colloid osmotic pressure. Albumin levels fall if urinary loss increases or hepatic synthesis decreases.
Nephrotic syndrome is the most important cause of albuminuria. In diseases associated with nephrotic syndrome ( Chapter 12 ), damage to glomeruli allows albumin (and other plasma proteins) to pass into the urine.
Reduced albumin synthesis is seen in the setting of severe liver disease (e.g., cirrhosis) ( Chapter 14 ) and protein malnutrition ( Chapter 7 ).
Regardless of cause, low albumin levels lead to edema, reduced intravascular volume, renal hypoperfusion, and secondary hyperaldosteronism. Unfortunately, increased salt and water retention by the kidney not only fails to correct the plasma volume deficit but also exacerbates the edema, because the primary defect—low serum protein—persists.
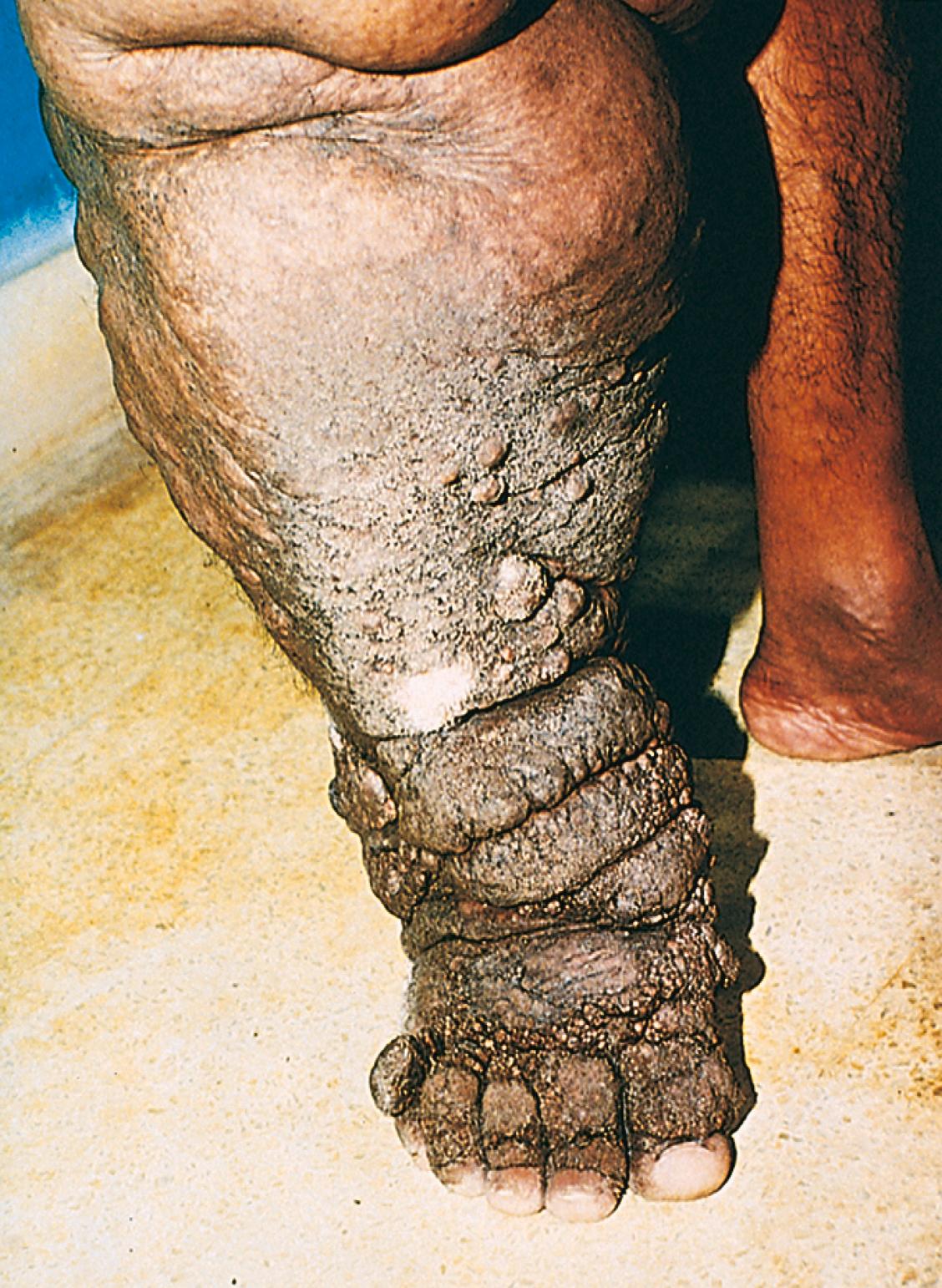
Edema may result from lymphatic obstruction that compromises resorption of fluid from interstitial spaces. Impaired lymphatic drainage and consequent lymphedema usually results from a localized obstruction caused by an inflammatory or neoplastic condition. For example, the parasitic infection filariasis can cause massive edema of the lower extremity and external genitalia (so-called “elephantiasis”) secondary to fibrosis of the inguinal lymphatics and lymph nodes ( eFig. 3.2 ). Infiltration and obstruction of superficial lymphatics by breast cancer may cause edema of the overlying skin; the characteristic finely pitted appearance of the skin of the affected breast is called peau d’orange (orange peel). Lymphedema may also occur as a complication of therapy, as in patients with breast cancer who undergo axillary lymph node resection and/or irradiation; both may disrupt and obstruct lymphatic drainage and cause severe lymphedema of the arm.
Excessive retention of salt (and associated water) can lead to edema by increasing hydrostatic pressure (owing to expansion of the intravascular volume) and reducing plasma osmotic pressure (because of decreased plasma protein concentration). Excessive salt and water retention are seen in a wide variety of diseases that compromise renal function, including poststreptococcal glomerulonephritis and acute renal failure ( Chapter 12 ).
Edema is most easily recognized on gross inspection; microscopic examination shows more subtle clearing and separation of the extracellular matrix (ECM) elements. Although any tissue can be involved, edema is most commonly encountered in the subcutaneous tissues, lungs, and brain.
Subcutaneous edema preferentially occurs in parts of the body positioned the greatest distance below the heart, where hydrostatic pressures are highest. Thus, edema is most pronounced in the legs with standing and the sacrum with recumbency, a relationship termed dependent edema. Finger pressure over edematous subcutaneous tissue displaces the interstitial fluid, leaving a finger-shaped depression (so-called pitting edema ). Edema resulting from renal dysfunction or nephrotic syndrome often manifests first in loose connective tissues (e.g., the eyelids, causing periorbital edema ). With pulmonary edema ( eFig. 3.3 ), the lungs are often two to three times their normal weight and when sectioned exude frothy, sometimes blood-tinged fluid consisting of a mixture of air, edema fluid, and extravasated red cells. Brain edema ( Chapter 21 ) can be localized (e.g., because of abscess or tumor) or generalized, depending on the nature and extent of the pathologic process or injury. With generalized edema, the sulci are narrowed as the gyri swell and become flattened against the skull.
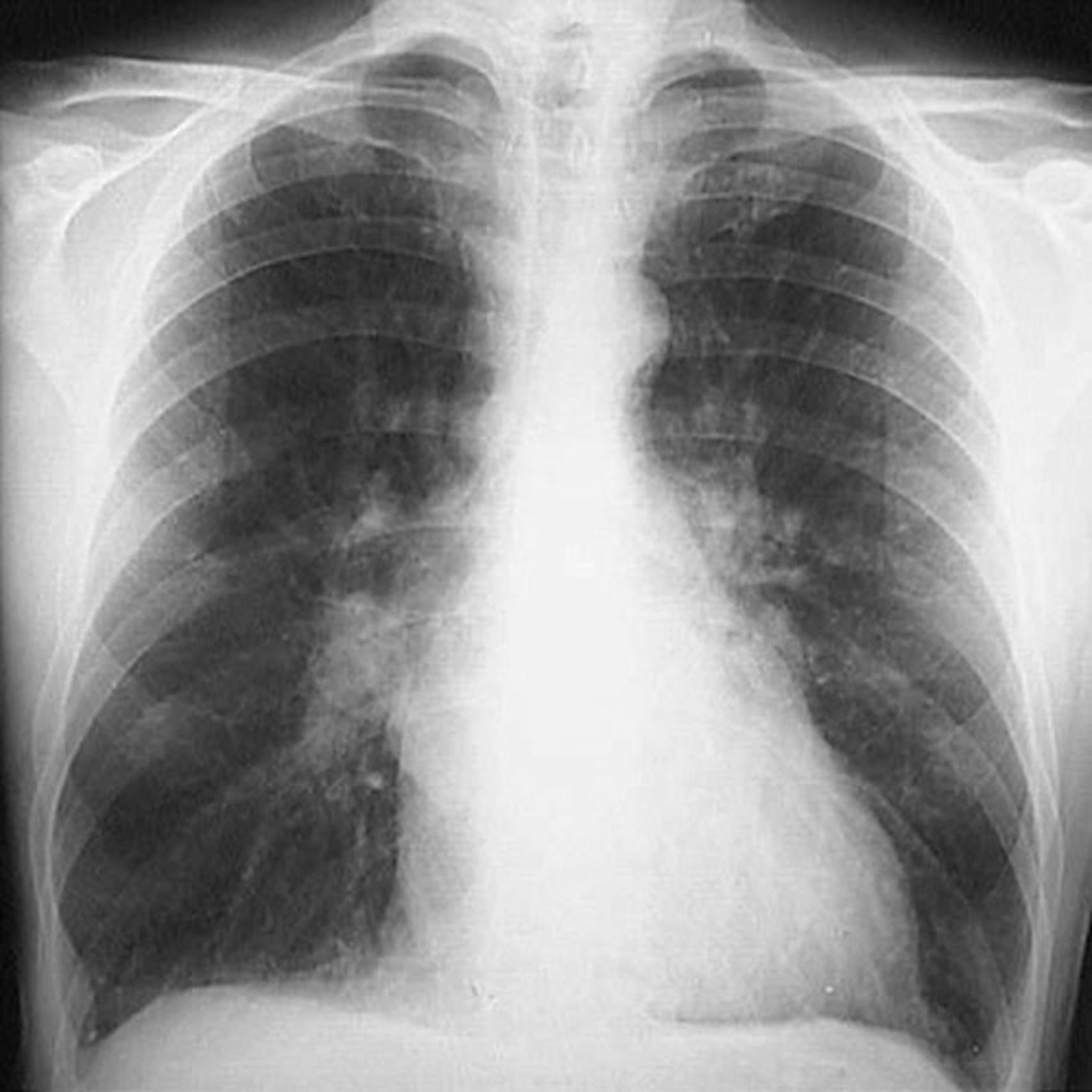
The effects of edema vary, ranging from merely annoying to rapidly fatal. Subcutaneous edema is important to recognize because it may often signal underlying cardiac or renal disease; if severe, it may also impair healing of cutaneous wounds and the clearance of skin infections. Pulmonary edema is a common clinical problem. It is seen most frequently with left ventricular failure but also may occur with renal failure, acute lung injury ( Chapter 11 ), and inflammatory and infectious disorders of the lung. It may cause death by interfering with normal ventilatory function, and alveolar edema fluid also creates a favorable environment for superimposed infection. Brain edema is life threatening: if the swelling is severe, the brain may herniate (extrude) through the foramen magnum. With increased intracranial pressure, the brain stem vascular supply may be compromised, leading to death due to injury to the medullary centers that control respiration and other vital functions ( Chapter 21 ).
Hemorrhage, defined as the extravasation of blood from vessels, results from damage to blood vessels and may be exacerbated by defects in blood clotting. As described earlier, capillary bleeding can occur in chronically congested tissues. Trauma, atherosclerosis, or inflammatory or neoplastic erosion of a vessel wall also may lead to hemorrhage, which may be massive if the affected vessel is a large vein or artery.
The risk of hemorrhage (often after a seemingly insignificant injury) is increased in a wide variety of disorders collectively called hemorrhagic diatheses . These have diverse causes, including inherited or acquired defects in vessel walls, platelets, or coagulation factors, all of which must function properly to ensure hemostasis. These are discussed in the next section. Here we focus on clinical features of hemorrhages, regardless of the cause.
Hemorrhage may be manifested by different appearances and consequences.
Hemorrhage may take the form of external bleeding or may accumulate within a tissue as a hematoma. These range in significance from trivial (e.g., a bruise) to fatal (e.g., a massive retroperitoneal hematoma caused by rupture of a dissecting aortic aneurysm) ( Chapter 8 ). Large bleeds into body cavities are described according to their location— hemothorax, hemopericardium, hemoperitoneum, or hemarthrosis (in joints). Large hemorrhages can occasionally result in jaundice as red cells and hemoglobin are broken down by macrophages.
Petechiae are minute (1 to 2 mm in diameter) hemorrhages into skin, mucous membranes, or serosal surfaces ( Fig. 3.4A ). Causes include low platelet counts (thrombocytopenia), defective platelet function, and loss of vascular wall support, as in vitamin C deficiency (scurvy, Chapter 7 ).
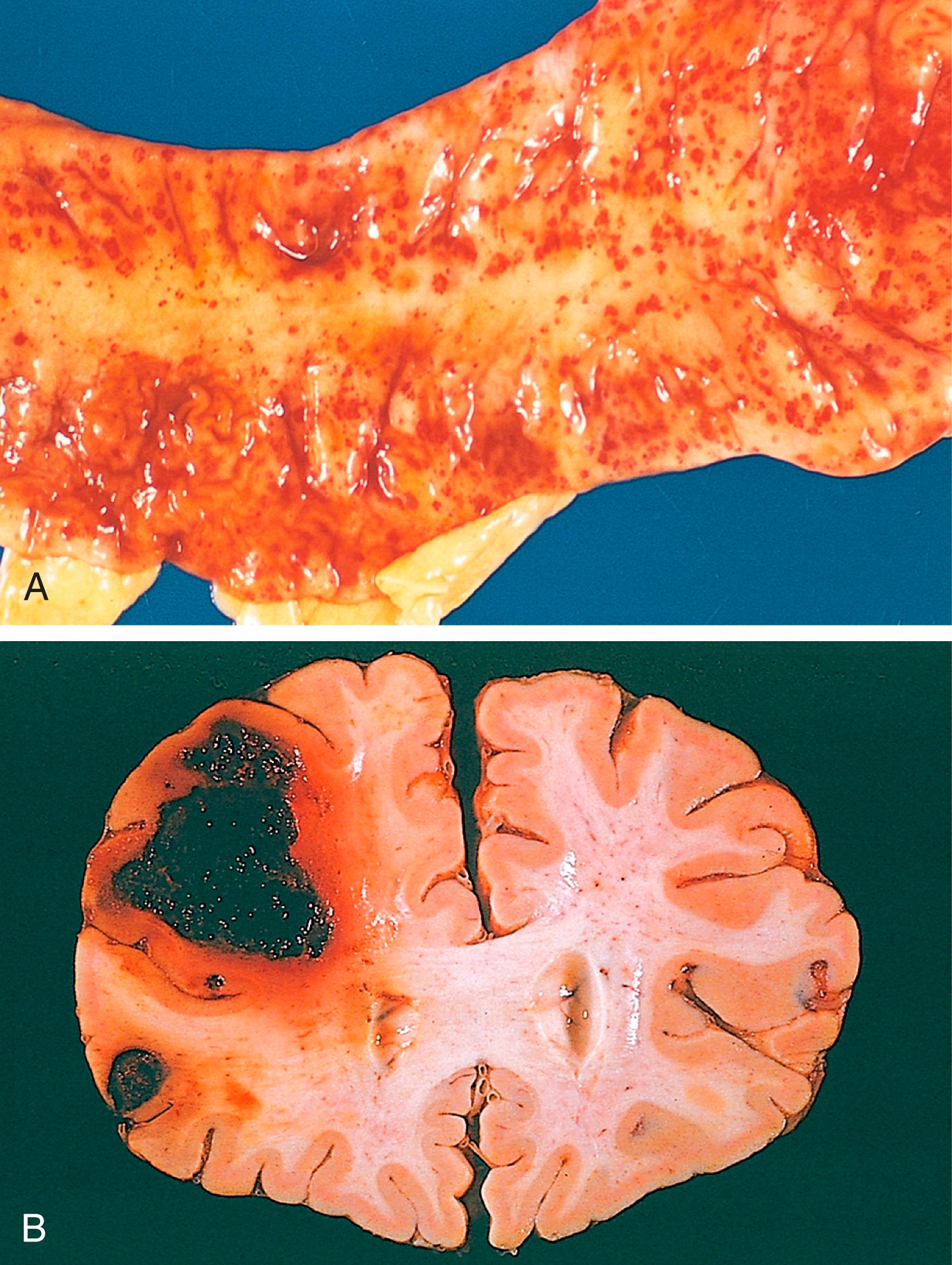
Purpura are slightly larger (3 to 5 mm) hemorrhages. Purpura can result from the same disorders that cause petechiae, as well as trauma, vascular inflammation (vasculitis), and increased vascular fragility.
Ecchymoses are larger (1 to 2 cm) subcutaneous hematomas (colloquially called “bruises”). Extravasated red cells are phagocytosed and degraded by macrophages; the characteristic color changes of a bruise result from the enzymatic conversion of hemoglobin (red-blue color) to bilirubin (blue-green color) and eventually hemosiderin (golden-brown).
The clinical impact of a hemorrhage depends on the volume of blood that is lost, the rate of bleeding, the location of the bleed, and the health of the individual affected. Rapid loss of up to 20% of the blood volume may be well tolerated in healthy adults yet cause cardiovascular decompensation in individuals with underlying heart or lung disease. Greater losses may cause hemorrhagic (hypovolemic) shock (discussed later) even in those who are healthy. A bleed that is relatively trivial in the subcutaneous tissues may cause death if located in the brain ( Fig. 3.4B ). Finally, chronic or recurrent external blood loss (e.g., due to peptic ulcer or menstrual bleeding) frequently leads to iron deficiency anemia owing to loss of the iron in hemoglobin. By contrast, iron is efficiently recycled from phagocytosed red cells, so internal bleeding (e.g., a hematoma) does not lead to iron deficiency.
Hemostasis is a process initiated by a traumatic vascular injury that leads to the formation of a blood clot. The pathologic counterpart of hemostasis is thrombosis, the formation of a clot (a thrombus) within vessels that have been damaged by a disease process. This discussion begins with hemostasis and its regulation, to be followed by causes and consequences of thrombosis.
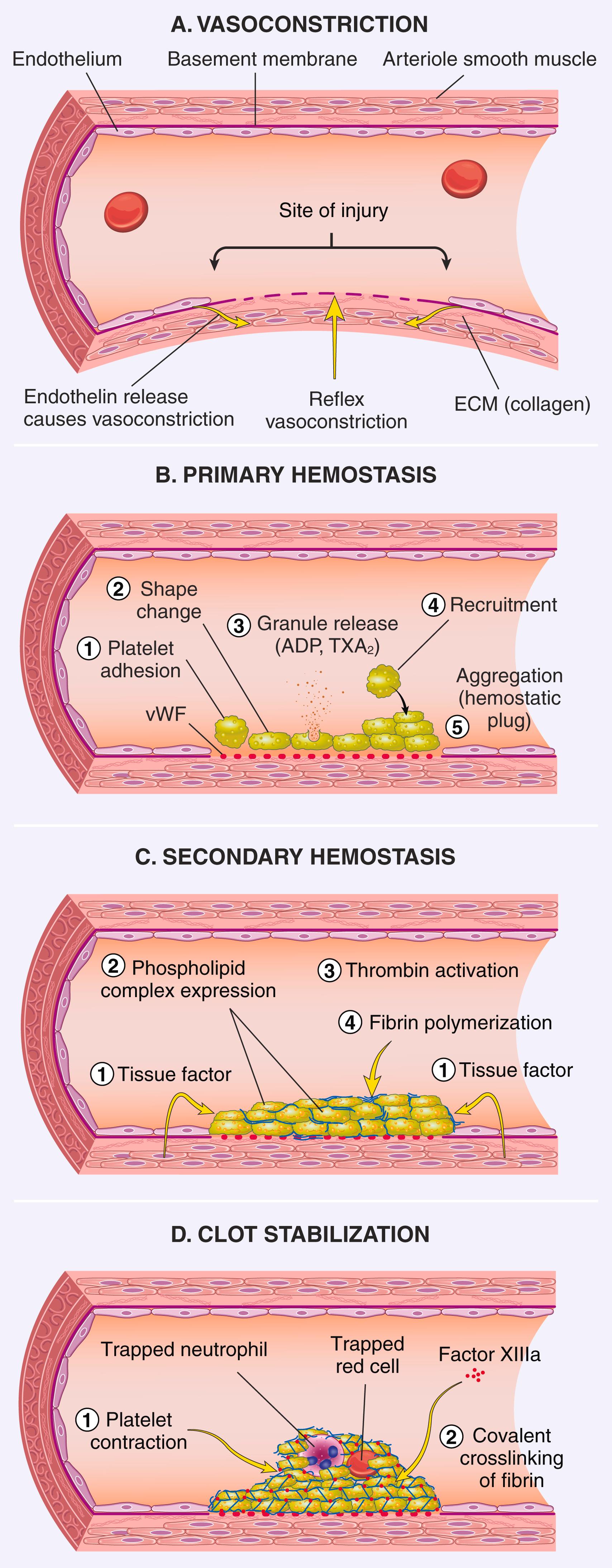
Hemostasis is a precisely orchestrated process involving platelets, clotting factors, and endothelium that occurs at the site of vascular injury and leads to the formation of a blood clot, which serves to prevent or limit the extent of bleeding. The general sequence of events leading to hemostasis at a site of vascular injury is shown in Fig. 3.5 .
Arteriolar vasoconstriction occurs immediately and markedly reduces blood flow to the injured area (see Fig. 3.5A ). It is mediated by neurogenic reflexes and augmented by the local secretion of factors such as endothelin, a potent endothelium-derived vasoconstrictor. This effect is transient, however, and bleeding resumes without the activation of platelets and coagulation factors.
Primary hemostasis: the formation of the platelet plug. Disruption of the endothelium exposes subendothelial collagen, which binds von Willebrand factor, a molecule that promotes platelet adherence and activation. Activated platelets undergo a dramatic shape change (from small, rounded discs to flat plates with spiky protrusions that markedly increase surface area) and release their secretory granules. Within minutes the secreted products recruit additional platelets, which aggregate to form a primary hemostatic plug (see Fig. 3.5B ).
Secondary hemostasis: deposition of fibrin. Vascular injury exposes tissue factor at the site of injury. Tissue factor is a membrane-bound procoagulant glycoprotein that is normally expressed by subendothelial cells in the vessel wall, such as smooth muscle cells and fibroblasts. Tissue factor binds and activates factor VII (see later), setting in motion a cascade of reactions that lead to thrombin generation. Thrombin cleaves circulating fibrinogen into insoluble fibrin , creating a fibrin meshwork, and is a potent activator of platelets, leading to additional platelet aggregation at the site of injury. This sequence, referred to as secondary hemostasis, consolidates the platelet plug (see Fig. 3.5C ).
Clot stabilization. Polymerized fibrin is crosslinked covalently by factor XIII and platelet aggregates contract, both of which contribute to the formation a solid permanent plug that prevents further bleeding (see Fig. 3.5D ). The size of the clot is limited by counterregulatory mechanisms (described later) that restrict clotting to the site of injury and eventually lead to clot resorption and tissue repair.
The integrity and function of endothelial cells determine whether clots form, propagate, or dissolve. Healthy endothelial cells express a variety of anticoagulant factors that inhibit platelet aggregation and coagulation and promote fibrinolysis; after endothelial injury or activation, however, this balance shifts to favor clotting (discussed later). Endothelium can be activated by microbial pathogens, hemodynamic forces, and a number of proinflammatory mediators, all of which may increase the risk of thrombosis. We will return to the procoagulant and anticoagulant actions of endothelium after a detailed discussion of the role of platelets and coagulation factors in hemostasis, following the scheme illustrated in Fig. 3.5 .
Platelets play a critical role in hemostasis by forming the primary plug that initially seals vascular defects and by providing a surface that binds and concentrates activated coagulation factors. Platelets are disc-shaped anucleate cell fragments that are shed from megakaryocytes in the bone marrow into the bloodstream. Their function depends on several glycoprotein receptors, a contractile cytoskeleton, and two types of cytoplasmic granules. α-Granules have the adhesion molecule P-selectin on their membranes ( Chapter 2 ) and contain coagulation factors such as fibrinogen, factor V, and vWF as well as protein factors involved in wound healing, such as fibronectin, platelet factor 4 (a heparin-binding chemokine), platelet-derived growth factor (PDGF), and transforming growth factor-β. Dense (or δ) granules contain adenosine diphosphate (ADP), adenosine triphosphate (ATP), polyphosphate, ionized calcium, serotonin, and epinephrine.
After a traumatic vascular injury, platelets encounter constituents of the subendothelial connective tissue, such as collagen and attached vWF, which is normally present here as well as in the plasma. On contact with these proteins, platelets undergo a sequence of reactions that lead to the formation of a platelet plug (see Fig. 3.5B ).
Platelet adhesion is mediated largely by interactions with vWF, which acts as a bridge between the platelet surface receptor glycoprotein Ib (GpIb) and exposed collagen ( Fig. 3.6 ). Notably, genetic deficiencies of vWF (von Willebrand disease, Chapter 10 ) or GpIb (Bernard-Soulier syndrome) result in bleeding disorders, attesting to the importance of these factors.
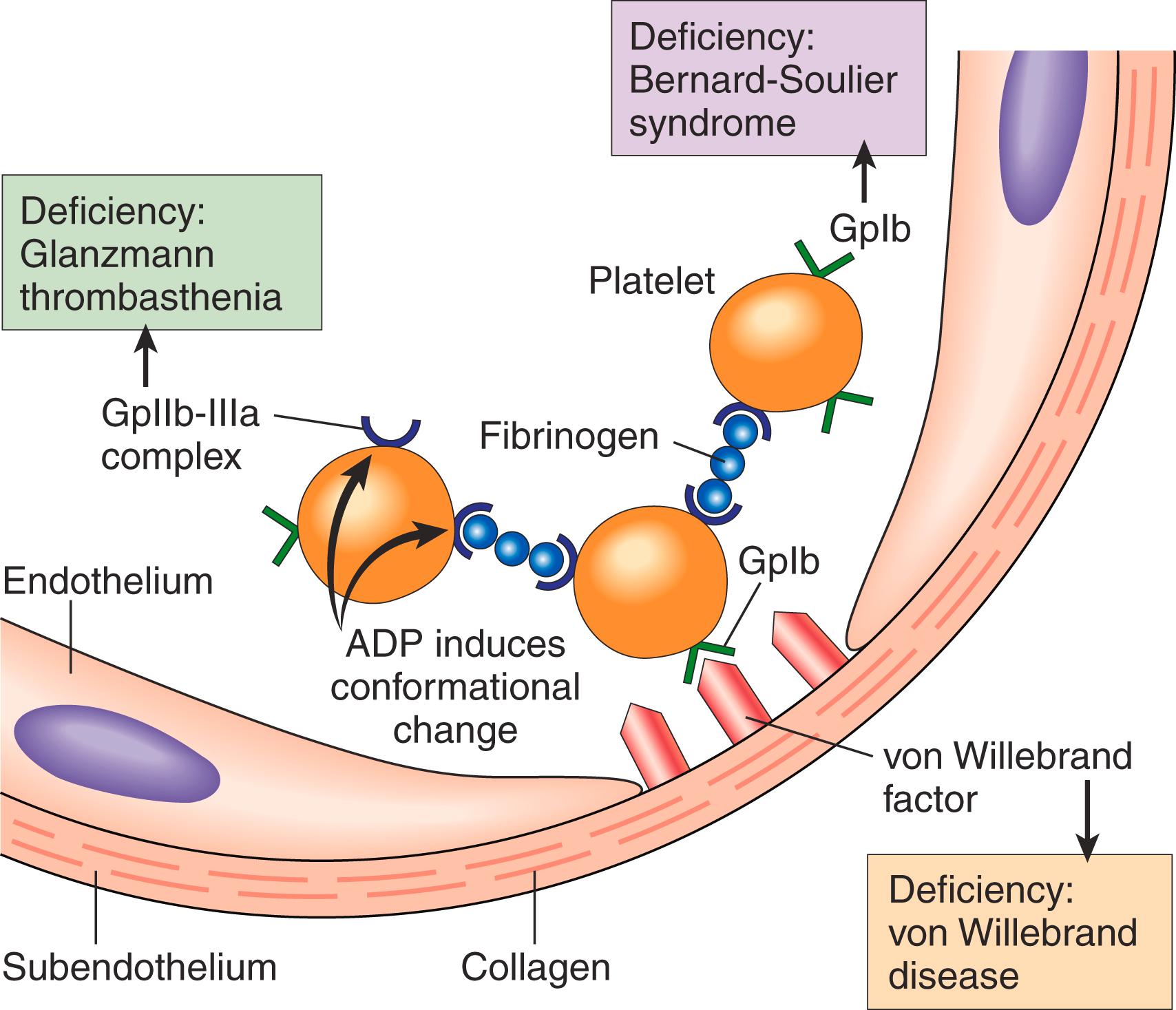
Platelets rapidly change shape following adhesion, converting from smooth discs to spiky “sea urchins” with greatly increased surface area. This change is accompanied by alterations in glycoprotein IIb/IIIa that increase its affinity for fibrinogen (see later), and by the translocation of negatively charged phospholipids (particularly phosphatidylserine) to the platelet surface. These phospholipids bind calcium and serve as nucleation sites for the assembly of coagulation factor complexes.
Secretion of granule contents (release reaction) occurs along with changes in shape; these two events are often referred to together as platelet activation. Platelet activation is triggered by a number of factors, including the coagulation factor thrombin and ADP. Thrombin activates platelets by proteolytically cleaving and switching on a special G-protein–coupled receptor referred to as a protease-activated receptor (PAR). ADP is a component of dense-body granules; thus, platelet activation and ADP release leads to additional rounds of platelet activation, a phenomenon referred to as recruitment. Activated platelets also produce the prostaglandin thromboxane A 2 (TxA 2 ), a potent inducer of platelet aggregation. Aspirin inhibits platelet aggregation and produces a mild bleeding defect by inhibiting cyclooxygenase, a platelet enzyme that is required for TxA 2 synthesis. It is thought that growth factors released from platelets such as PDGF contribute to the repair of the vessel wall following injury.
Platelet aggregation follows their activation. The conformational change in glycoprotein IIb/IIIa that occurs with platelet activation allows binding of fibrinogen, a large bivalent plasma protein that forms bridges between activated platelets, leading to their aggregation. Inherited deficiency of GpIIb-IIIa results in a bleeding disorder called Glanzmann thrombasthenia. The initial wave of aggregation is reversible, but concurrent activation of thrombin stabilizes the platelet plug by causing further platelet activation and aggregation and by promoting irreversible platelet contraction. Platelet contraction is dependent on the cytoskeleton and consolidates the aggregated platelets. In parallel, thrombin converts fibrinogen into insoluble fibrin and activates factor XIIIa, which covalently crosslinks fibrin, cementing the platelets in place and creating the definitive secondary hemostatic plug. Entrapped red cells and leukocytes are also found in hemostatic plugs, in part due to adherence of leukocytes to P-selectin expressed on activated platelets.
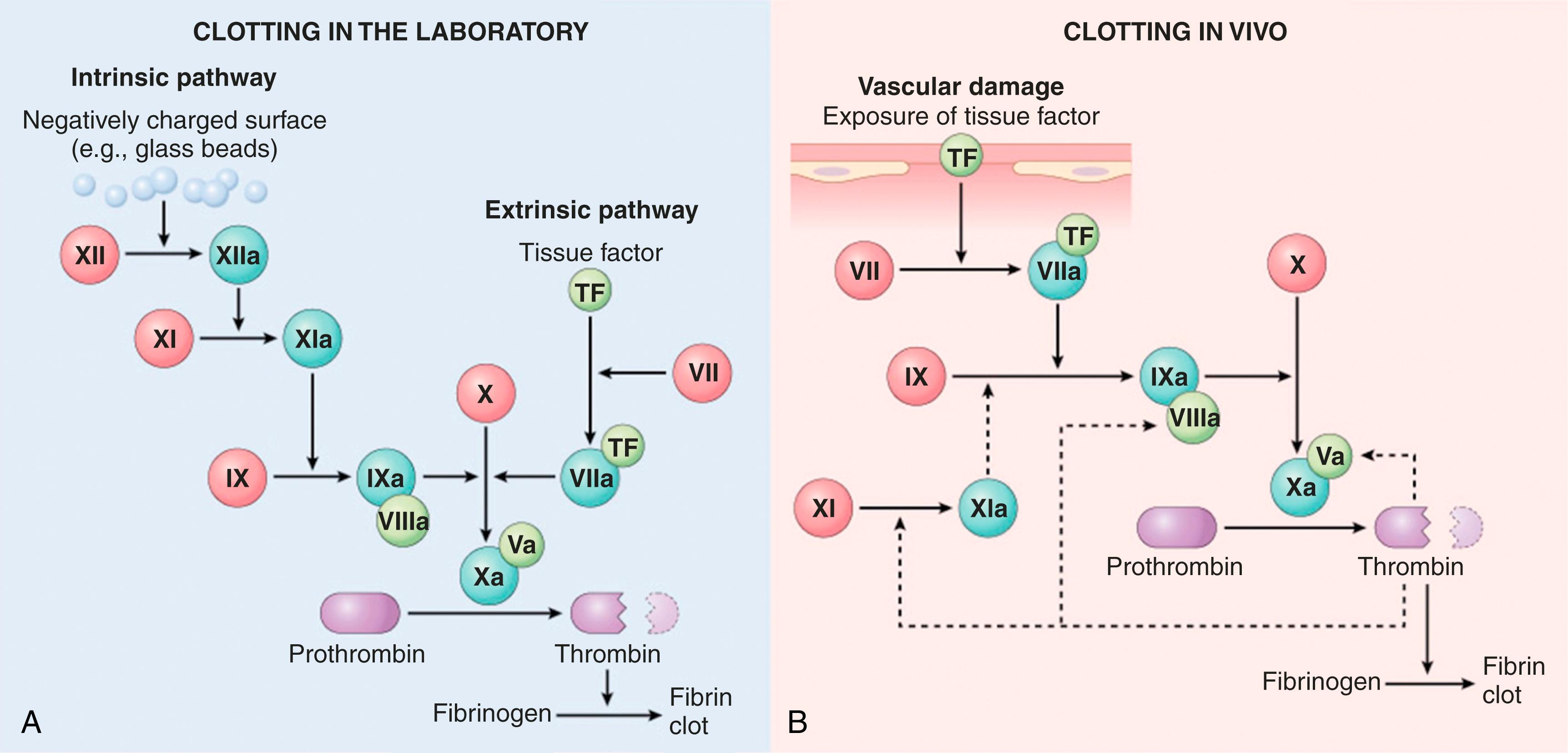
Coagulation factors participate in a series of amplifying enzymatic reactions that lead to the deposition of an insoluble fibrin clot. As discussed later, the dependency of clot formation on various factors differs in the laboratory test tube and in blood vessels in vivo ( Fig. 3.7 ). However, clotting in vitro and in vivo both follow the same general principles, as follows.
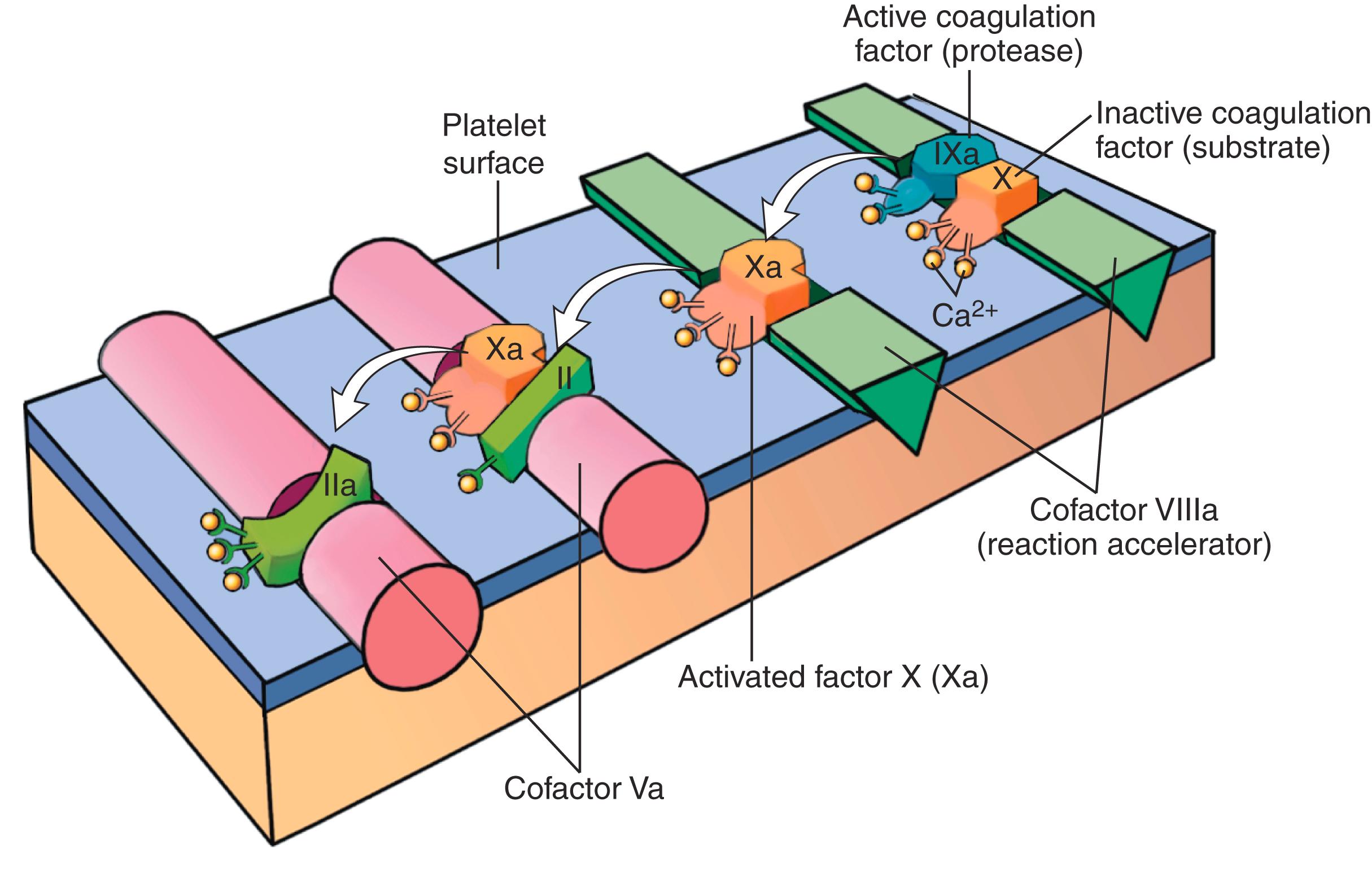
The cascade of reactions in the pathway can be likened to a “dance,” in which coagulation factors are passed from one partner to the next ( Fig. 3.8 ). Each reaction involves an enzyme (an activated coagulation factor), a substrate (an inactive proenzyme form of a coagulation factor), and a cofactor (a reaction accelerator). These components are assembled on the negatively charged phospholipid surface of activated platelets. Assembly of reaction complexes also depends on calcium, which binds to γ-carboxylated glutamic acid residues that are present in factors II, VII, IX, and X. The enzymatic reactions that produce γ-carboxylated glutamic acid require vitamin K and are antagonized by drugs such as warfarin, which interferes with vitamin K metabolism.
Based on assays performed in clinical laboratories, the coagulation cascade is divided into the extrinsic and intrinsic pathways (see Fig. 3.7A ).
The prothrombin time (PT) assay assesses the function of the proteins in the extrinsic pathway (factors X, VII, V, II [prothrombin], and fibrinogen). In brief, tissue factor, phospholipids, and calcium are added to plasma and the time for a fibrin clot to form is recorded.
The partial thromboplastin time (PTT) assay assesses the function of the proteins in the intrinsic pathway (factors XII, XI, X, IX, VIII, V, II, and fibrinogen). In this assay, clotting of plasma is initiated by the addition of negatively charged particles (e.g., ground glass) that activate factor XII together with phospholipids and calcium, and the time to fibrin clot formation is recorded.
Although the PT and PTT assays are of great utility in evaluating coagulation factor function in patients, they do not recapitulate the events that lead to coagulation in vivo. This point is most clearly made by considering the clinical effects of deficiencies of various coagulation factors. Deficiencies of factors V, VII, VIII, IX, and X are associated with moderate to severe bleeding, and prothrombin deficiency is incompatible with life. By contrast, factor XI deficiency only causes a mild bleeding disorder, and individuals with factor XII deficiency have no bleeding disorder at all. The physiologic role of factor XII is uncertain. Rare individuals with excessive factor XII activity are prone to angioedema, an inflammatory condition that may be triggered by the generation of bradykinin by factor XII through its ability to cleave high-molecular-weight kininogen.
Based on these observations, it is believed that, in vivo, the factor VIIa/tissue factor complex is the most important activator of factor IX and the factor IXa/factor VIIIa complex is the most important activator of factor X (see Fig. 3.7B ). The mild bleeding tendency seen in patients with factor XI deficiency is likely explained by the ability of thrombin to activate factor XI, a feedback mechanism that amplifies the coagulation cascade.
Among the coagulation factors, thrombin is the most important because it controls diverse aspects of hemostasis and links clotting to inflammation and repair. Among thrombin’s key activities are the following:
Conversion of fibrinogen into crosslinked fibrin. Thrombin converts soluble fibrinogen into fibrin monomers that polymerize into an insoluble fibril and amplifies the generation of fibrin by activating factors V, VIII, and XI. Thrombin also stabilizes fibrin clots by activating factor XIII, which covalently crosslinks fibrin.
Platelet activation. Thrombin is a potent inducer of platelet activation, aggregation, and contraction through its ability to activate PARs.
Effects on various cell types. PARs also are expressed on inflammatory cells, endothelium, and other cell types ( Fig. 3.9 ), and activation of these receptors by thrombin is believed to mediate effects that contribute to tissue repair.
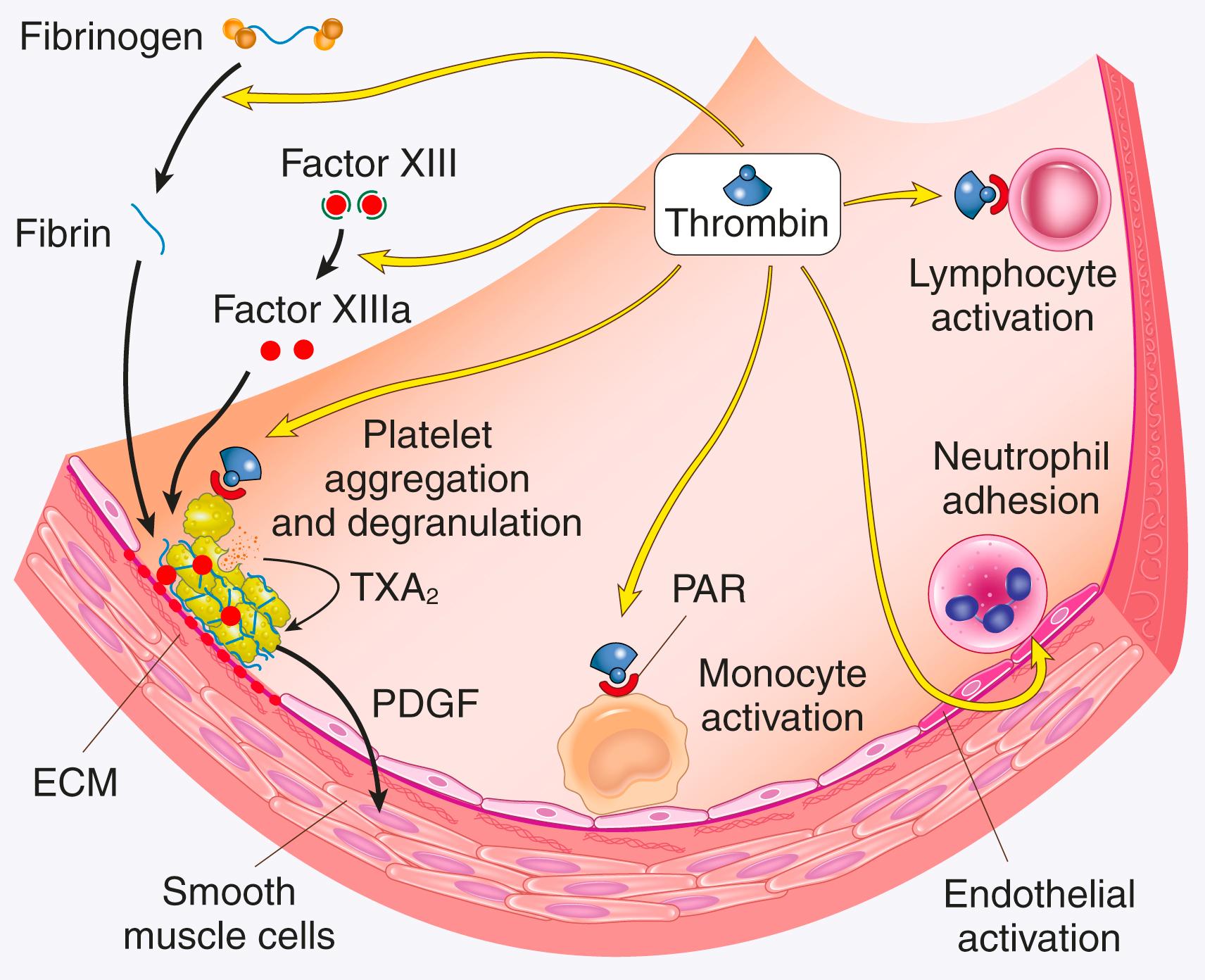
Anticoagulant effects. Remarkably, through mechanisms described later, on encountering normal endothelium, thrombin changes from a procoagulant to an anticoagulant; this reversal in function helps to prevent clots from extending beyond the site of the vascular injury.
Once initiated, coagulation must be restricted to the site of vascular injury to prevent deleterious consequences. One limiting factor is simple dilution: blood flowing past the site of injury washes out activated coagulation factors, which are rapidly removed by the liver. A second is the requirement for negatively charged phospholipids; these are provided mainly by activated platelets, which are not present away from the site of injury. However, the most important counterregulatory mechanisms involve factors that are expressed by intact endothelium adjacent to the site of injury (described later).
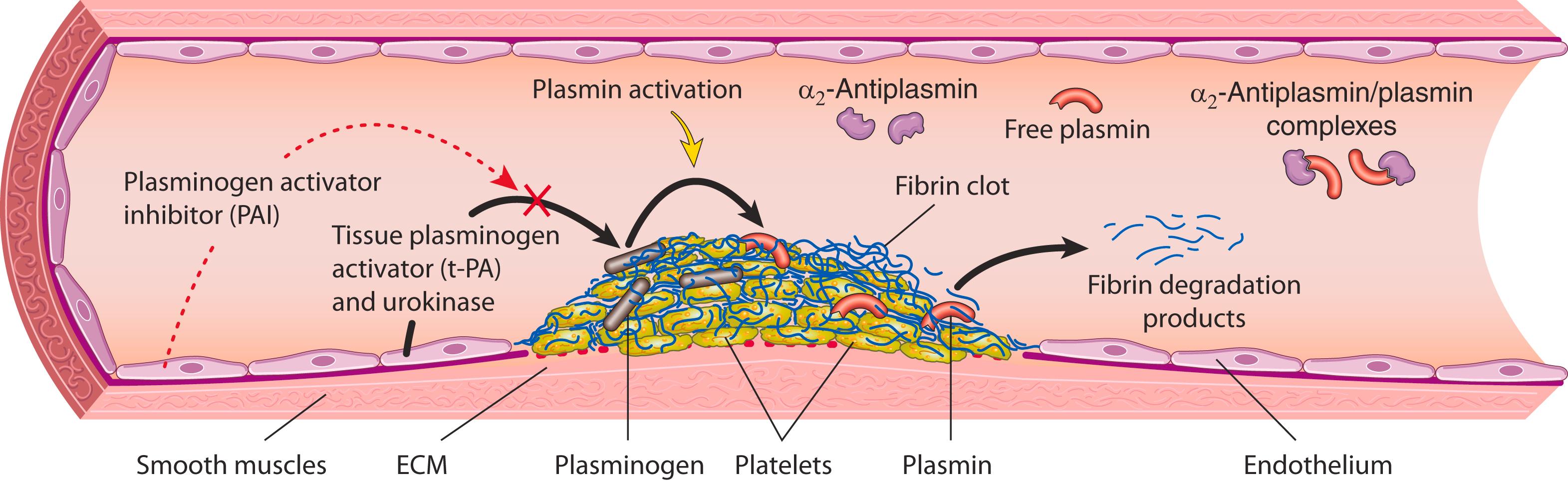
Activation of the coagulation cascade also sets into motion a fibrinolytic cascade that limits the size of the clot and contributes to its later dissolution ( Fig. 3.10 ). Fibrinolysis is accomplished largely through the enzymatic activity of plasmin, which breaks down fibrin and interferes with its polymerization. Elevated levels of breakdown products of fibrinogen (often called fibrin split products), most notably fibrin-derived D-dimers , are useful clinical markers of several thrombotic states (described later). Plasmin is generated from plasminogen, an inactive circulating precursor, by enzymatic cleavage. The most important plasminogen activator is tissue plasminogen activator (t-PA); it is synthesized principally by endothelium and is most active when bound to fibrin. This characteristic makes t-PA a useful therapeutic agent, since its fibrinolytic activity is largely confined to the site of a clot. Once activated, plasmin is in turn tightly controlled by factors such as α 2 -plasmin inhibitor, a plasma protein that binds and rapidly inhibits free plasmin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here