Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The health of cells and tissues depends on the circulation of blood, which delivers oxygen and nutrients and removes wastes generated by cellular metabolism. Under normal conditions, as blood passes through capillary beds, proteins in the plasma are retained within the vasculature, and there is little net movement of water and electrolytes into the tissues. This balance is often disturbed by pathologic conditions that alter endothelial function, increase vascular hydrostatic pressure, or decrease plasma protein content, all of which promote edema—the accumulation of fluid in tissues resulting from a net movement of water into extravascular spaces. Depending on its severity and location, edema may have minimal or profound effects. In the lower extremities, it may only make one's shoes feel snugger after a long sedentary day; in the lungs, however, edema fluid can fill alveoli, causing life-threatening hypoxia.
The structural integrity of blood vessels is frequently compromised by trauma. Hemostasis is the process of blood clotting that prevents excessive bleeding after blood-vessel damage. Inadequate hemostasis may result in hemorrhage, which can compromise regional tissue perfusion and, if massive and rapid, may lead to hypotension, shock, and death. Conversely, inappropriate clotting (thrombosis) or migration of clots (embolism) can obstruct blood vessels, potentially causing ischemic cell death (infarction) . Indeed, thromboembolism lies at the heart of three major causes of morbidity and death in high income countries: myocardial infarction, pulmonary embolism (PE), and cerebrovascular accident (stroke).
Herein, we focus on disorders of hemodynamics (edema, effusions, congestion, and shock), provide an overview of disorders of abnormal bleeding and clotting (thrombosis), and discuss the various forms of embolism.
Disorders that perturb cardiovascular, renal, or hepatic function are often marked by the accumulation of fluid in tissues (edema) or body cavities (effusions). Under normal circumstances, the tendency of vascular hydrostatic pressure to push water and salts out of capillaries into the interstitial space is nearly balanced by the tendency of plasma colloid osmotic pressure to pull water and salts back into vessels. There is usually a small net movement of fluid into the interstitium, but this drains into lymphatic vessels and ultimately returns to the bloodstream via the thoracic duct, keeping the tissues “dry” ( Fig. 4.1 ). Elevated hydrostatic pressure or diminished colloid osmotic pressure disrupts this balance and results in increased movement of fluid out of vessels. If the net rate of fluid movement exceeds the rate of lymphatic drainage, fluid accumulates. Within tissues the result is edema, and if a serosal surface is involved, fluid may accumulate within the adjacent body cavity as an effusion.
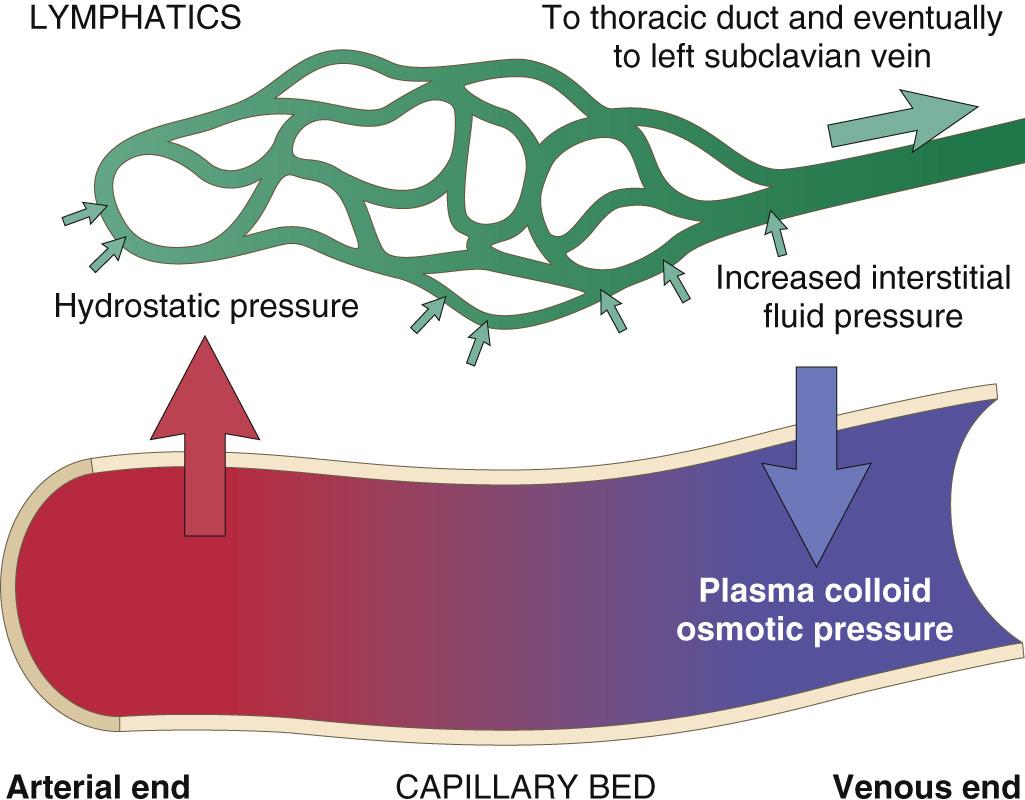
Edema fluids and effusions may be inflammatory or noninflammatory ( Table 4.1 ). Inflammation-related edema and effusions are discussed in detail in Chapter 3 . These protein-rich exudates accumulate due to increases in vascular permeability caused by inflammatory mediators. In contrast, noninflammatory edema and effusions are protein-poor fluids called transudates . Noninflammatory edema and effusions are common in many disorders, including heart failure, liver failure, renal disease, and malnutrition ( Fig. 4.2 ). We will now discuss the various causes of edema.
| Increased Hydrostatic Pressure |
| Impaired Venous Return |
|
| Arteriolar Dilation |
|
| Reduced Plasma Osmotic Pressure (Hypoproteinemia) |
|
| Lymphatic Obstruction |
|
| Sodium Retention |
|
| Inflammation |
|
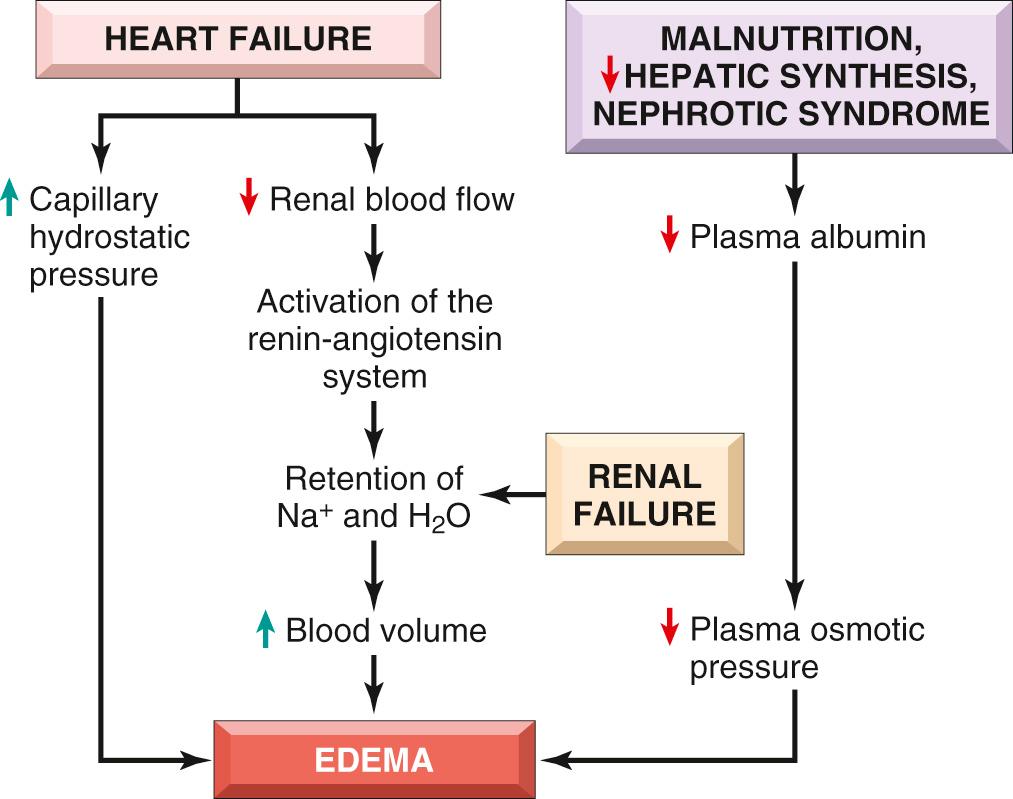
Increases in hydrostatic pressure are mainly caused by disorders that impair venous return. If the impairment is localized (e.g., a deep venous thrombosis [DVT] in a lower extremity), then the resulting edema is confined to the affected part. Conditions leading to systemic increases in venous pressure (e.g., congestive heart failure, Chapter 12 ) are understandably associated with more widespread edema.
Under normal circumstances albumin accounts for almost half of the total plasma protein; it follows that conditions leading to inadequate synthesis or increased loss of albumin from the circulation are common causes of reduced plasma oncotic pressure. Reduced albumin synthesis occurs mainly in severe liver diseases (e.g., end-stage cirrhosis, Chapter 18 ) and protein malnutrition ( Chapter 9 ). An important cause of albumin loss is the nephrotic syndrome ( Chapter 20 ), in which albumin leaks into the urine through abnormally permeable glomerular capillaries. Regardless of cause, reduced plasma osmotic pressure leads in a stepwise fashion to edema, reduced intravascular volume, renal hypoperfusion, and secondary hyperaldosteronism. Not only does the ensuing salt and water retention by the kidney fail to correct the plasma volume deficit, but it also exacerbates the edema, because the primary defect—a low plasma protein level—persists.
Increased salt retention—with obligate retention of associated water—causes both increased hydrostatic pressure (due to intravascular fluid volume expansion) and diminished vascular colloid osmotic pressure (due to dilution). Salt retention occurs whenever renal function is compromised, such as in primary kidney disorders and in cardiovascular disorders that decrease renal perfusion. One of the most important causes of renal hypoperfusion is congestive heart failure, which (like hypoproteinemia) results in the activation of the renin-angiotensin-aldosterone axis. In early heart failure, this response is beneficial, as the retention of sodium and water and other adaptations, including increased vascular tone and elevated levels of antidiuretic hormone, improve cardiac output and restore normal renal perfusion. However, as heart failure worsens and cardiac output diminishes, the retained fluid merely increases the hydrostatic pressure, leading to edema and effusions.
Trauma, fibrosis, invasive tumors, and infectious agents can all disrupt lymphatic vessels and impair the clearance of interstitial fluid, resulting in lymphedema in the affected part of the body. A dramatic example is seen in parasitic filariasis, in which the organism induces obstructive fibrosis of lymphatic channels and lymph nodes. This may result in edema of the external genitalia and lower limbs that is so massive as to earn the appellation elephantiasis . Severe edema of the upper extremity may also complicate surgical removal and/or irradiation of the breast and associated axillary lymph nodes in patients with breast cancer.
Edema is easily recognized grossly; microscopically, it is appreciated as clearing and separation of the extracellular matrix (ECM) and subtle cell swelling. Edema is most commonly seen in subcutaneous tissues, the lungs, and the brain. Subcutaneous edema can be diffuse or more conspicuous in regions with high hydrostatic pressures. Its distribution is often influenced by gravity (e.g., it appears in the legs when standing and the sacrum when recumbent), a feature termed dependent edema. Finger pressure over markedly edematous subcutaneous tissue displaces the interstitial fluid and leaves a depression, a sign called pitting edema.
Edema resulting from renal dysfunction often appears initially in parts of the body containing loose connective tissue, such as the eyelids; periorbital edema is thus a characteristic finding in severe renal disease. With pulmonary edema, the lungs are often two to three times their normal weight, and sectioning yields frothy, blood-tinged fluid—a mixture of air, edema, and extravasated red cells. Brain edema can be localized or generalized depending on the nature and extent of the pathologic process or injury. The swollen brain exhibits narrowed sulci and distended gyri, which are compressed by the unyielding skull ( Chapter 28 ).
Effusions involving the pleural cavity (hydrothorax), the pericardial cavity (hydropericardium), or the peritoneal cavity ( hydroperitoneum or ascites ) are common in a wide range of clinical settings. Transudative effusions are typically protein-poor, translucent, and straw colored; an exception are peritoneal effusions caused by lymphatic blockage (chylous effusion), which may be milky due to the presence of lipids absorbed from the gut. In contrast, exudative effusions are protein-rich and often cloudy due to the presence of white cells.
The consequences of edema range from merely annoying to rapidly fatal. Subcutaneous edema is important primarily because it signals potential underlying cardiac or renal disease; however, when significant, it can also impair wound healing and the clearance of infections. Pulmonary edema is a common clinical problem that is most frequently seen in the setting of left ventricular failure; it can also occur with renal failure, acute respiratory distress syndrome ( Chapter 15 ), and pulmonary inflammation or infection. Edema in the pulmonary interstitium and the alveolar spaces impedes gas exchange (leading to hypoxemia) and also creates a favorable environment for bacterial infection. Pulmonary edema is often exacerbated by pleural effusions, which may further compromise gas exchange by compressing the underlying pulmonary parenchyma. Peritoneal effusions (ascites) resulting most commonly from portal hypertension are prone to seeding by bacteria, leading to serious and sometimes fatal infections. Brain edema is life threatening; if severe, brain substance can herniate (extrude) through the foramen magnum, or the brain stem vascular supply can be compressed. Either condition can injure the medullary centers and cause death ( Chapter 28 ).
Edema is the result of the movement of fluid from the vasculature into the interstitial spaces; the fluid may be protein-poor (transudate) or protein-rich (exudate).
Edema may be caused by:
Increased hydrostatic pressure (e.g., heart failure)
Decreased colloid osmotic pressure caused by reduced plasma albumin, either due to decreased synthesis (e.g., liver disease, protein malnutrition) or to increased loss (e.g., nephrotic syndrome)
Increased vascular permeability (e.g., inflammation)
Lymphatic obstruction (e.g., infection or neoplasia)
Sodium and water retention (e.g., renal failure)
Hyperemia and congestion both stem from increased blood volumes within tissues, but have different underlying mechanisms and consequences. Hyperemia is an active process in which arteriolar dilation (e.g., at sites of inflammation or in skeletal muscle during exercise) leads to increased blood flow. Affected tissues turn red (erythema) because of increased delivery of oxygenated blood. Congestion is a passive process resulting from reduced venous outflow of blood from a tissue. It can be systemic, as in cardiac failure, or localized, as in isolated venous obstruction. Congested tissues have an abnormal blue-red color (cyanosis) that stems from the accumulation of deoxygenated hemoglobin in the affected area. In long-standing chronic passive congestion, the associated chronic hypoxia may result in ischemic tissue injury and scarring. In chronically congested tissues, capillary rupture can also produce small hemorrhagic foci; subsequent catabolism of extravasated red cells can leave residual telltale clusters of hemosiderin-laden macrophages. As a result of increased hydrostatic pressures, congestion commonly leads to edema.
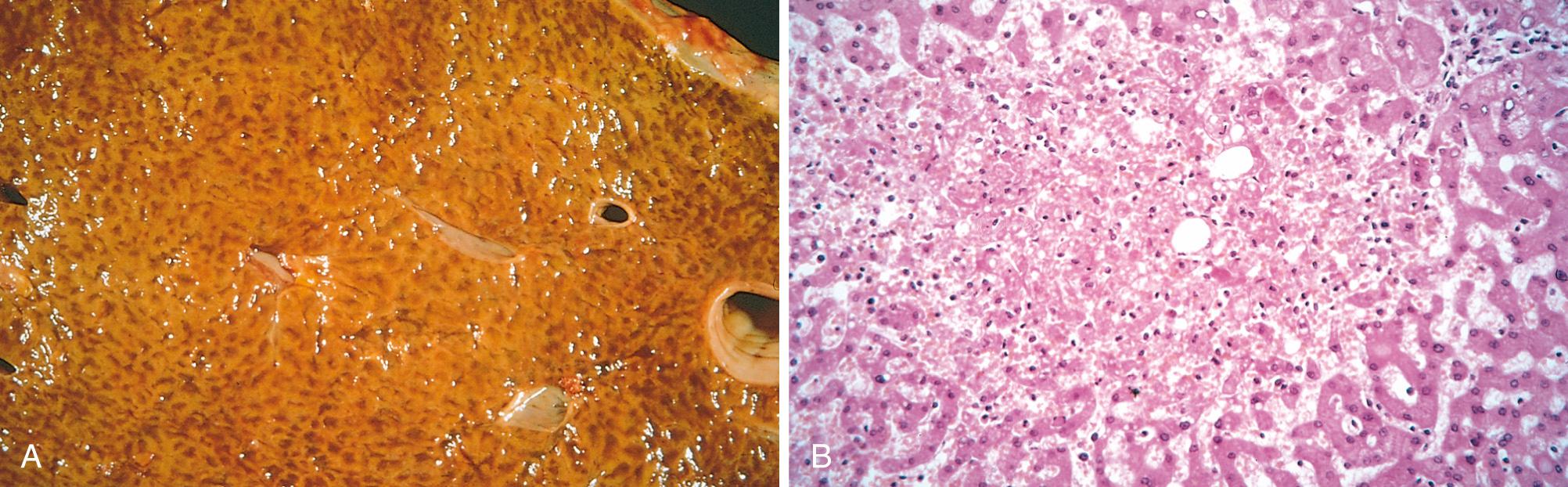
Congested tissues take on a dusky reddish-blue color (cyanosis) due to red cell stasis and the presence of deoxygenated hemoglobin. Microscopically, acute pulmonary congestion is marked by engorged alveolar capillaries, alveolar septal edema, and focal intra-alveolar hemorrhage. In chronic pulmonary congestion, which is often caused by congestive heart failure, the septa are thickened and fibrotic, and the alveoli often contain numerous macrophages laden with hemosiderin (heart failure cells) derived from phagocytosed red cells. In acute hepatic congestion, the central vein and sinusoids are distended. Because the centrilobular area is at the distal end of the hepatic blood supply, centrilobular hepatocytes may undergo ischemic necrosis, and the periportal hepatocytes—better oxygenated because of proximity to hepatic arterioles—may only develop fatty change. In chronic passive hepatic congestion, the centrilobular regions are grossly red-brown and slightly depressed (because of cell death) and are accentuated against the surrounding zones of uncongested tan liver (nutmeg liver) ( Fig. 4.3A ). Microscopically, there is centrilobular congestion and hemorrhage, hemosiderin-laden macrophages, and variable degrees of hepatocyte dropout and necrosis (see Fig. 4.3 B ).
Hemostasis can be defined simply as the process by which blood clots form at sites of vascular injury. Hemostasis is essential for life and is deranged to varying degrees in a broad range of disorders, which can be divided into two groups. In hemorrhagic disorders, characterized by excessive bleeding, hemostatic mechanisms are either blunted or insufficient to prevent blood loss. By contrast, in thrombotic disorders blood clots (often referred to as thrombi) form within intact blood vessels or within the chambers of the heart. As is discussed in Chapters 11 and 12 , thrombosis has a central role in the most common and clinically important forms of cardiovascular disease.
Although useful, it must be recognized that this division between bleeding and thrombotic disorders sometimes breaks down, in that generalized activation of clotting sometimes paradoxically produces bleeding due to the consumption of coagulation factors, as in disseminated intravascular coagulation (DIC) . To provide context for understanding disorders of bleeding and clotting, this discussion begins with normal hemostasis, focusing on the contribution of platelets, coagulation factors, and endothelium.
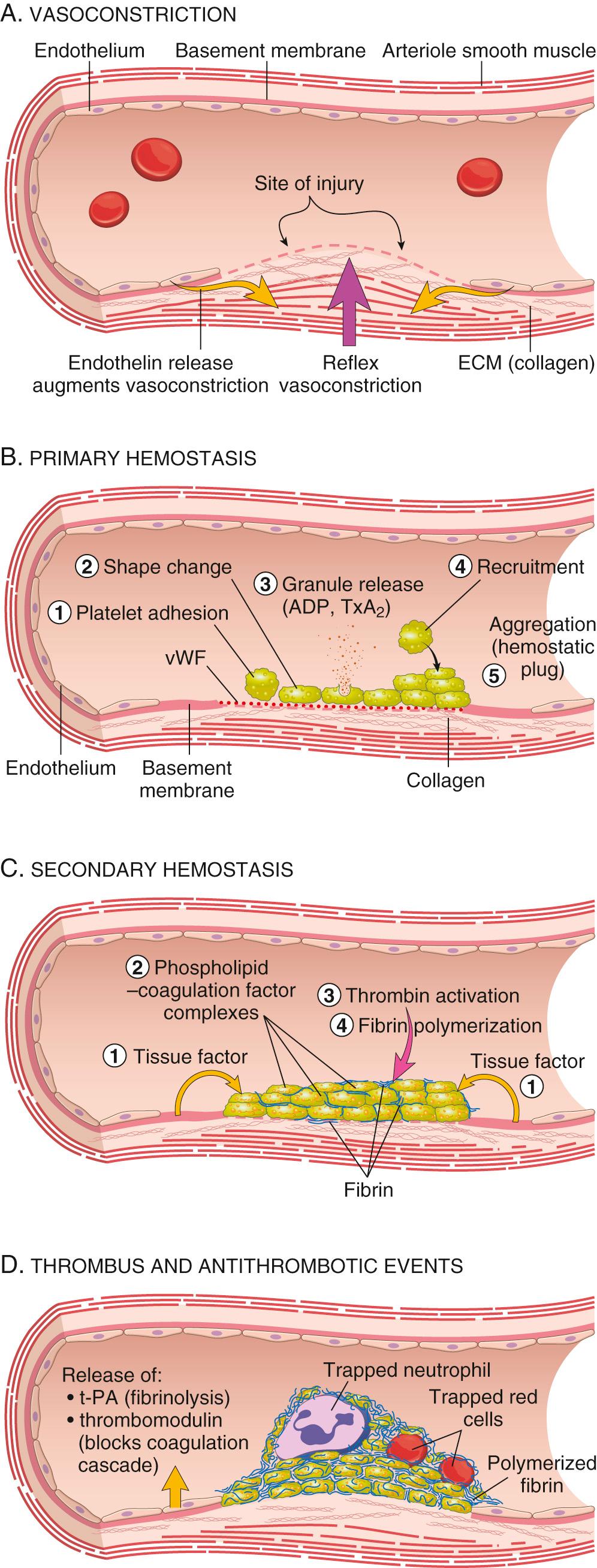
Hemostasis is a precisely orchestrated process involving platelets, clotting factors, and endothelium that occurs at the site of vascular injury and culminates in the formation of a blood clot, which serves to prevent or limit the extent of bleeding. The general sequence of events leading to hemostasis at a site of vascular injury is shown in Fig. 4.4 .
Arteriolar vasoconstriction occurs immediately and markedly reduces blood flow to the injured area (see Fig. 4.4A ). It is mediated by reflex neurogenic mechanisms and may be augmented by the local secretion of factors such as endothelin, a potent endothelium-derived vasoconstrictor. This effect is transient, however, and bleeding would resume if not for activation of platelets and coagulation factors.
Primary hemostasis: the formation of the platelet plug . Disruption of the endothelium exposes subendothelial von Willebrand factor (vWF) and collagen, which promote platelet adherence and activation. Activation of platelets results in a dramatic shape change (from small rounded discs to flat plates with spiky protrusions that markedly increase surface area), as well as the release of secretory granules. Within a few minutes, the secreted products recruit additional platelets that undergo aggregation to form a primary hemostatic plug (see Fig. 4.4B ).
Secondary hemostasis: deposition of fibrin. Vascular injury exposes tissue factor at the site of injury. Tissue factor is a membrane-bound procoagulant glycoprotein that is normally expressed by subendothelial cells in the vessel wall, such as smooth muscle cells and fibroblasts. Tissue factor binds and activates factor VII (see later), setting in motion a cascade of reactions that culminates in thrombin generation. Thrombin cleaves circulating fibrinogen into insoluble fibrin, creating a fibrin meshwork, and also is a potent activator of platelets, leading to additional platelet aggregation at the site of injury. This sequence, referred to as secondary hemostasis, consolidates the initial platelet plug (see Fig. 4.4C ).
Clot stabilization and resorption . Polymerized fibrin and platelet aggregates undergo contraction to form a solid, permanent plug that prevents further hemorrhage. At this stage, counterregulatory mechanisms (e.g., tissue plasminogen activator [t-PA] made by endothelial cells) are set into motion that limit clotting to the site of injury (see Fig. 4.4D ) and eventually lead to clot resorption and tissue repair.
It should be emphasized that endothelial cells are central regulators of hemostasis; the balance between the antithrombic and prothrombotic activities of endothelium determines whether thrombus formation, propagation, or dissolution occur. Normal endothelial cells express a variety of anticoagulant factors that inhibit platelet aggregation and coagulation and promote fibrinolysis; after injury or activation, however, this balance shifts, and endothelial cells acquire numerous procoagulant activities (activation of platelets and clotting factor, described earlier; see also Fig. 4.10 ). Besides trauma, endothelium can be activated by microbial pathogens, hemodynamic forces, and pro-inflammatory mediators.
We now describe roles of platelets, coagulation factors, and endothelium in hemostasis in greater detail, following the scheme illustrated in Fig. 4.4 .
Platelets play a critical role in hemostasis by forming the primary plug that initially seals vascular defects and by providing a surface that binds and concentrates activated coagulation factors. Platelets are disc-shaped anucleate cell fragments that are shed from megakaryocytes in the bone marrow into the bloodstream. Their function depends on several glycoprotein receptors, a contractile cytoskeleton, and two types of cytoplasmic granules. α -Granules have the adhesion molecule P-selectin on their membranes ( Chapter 3 ) and contain proteins involved in coagulation, such as fibrinogen, coagulation factor V, and vWF, as well as protein factors that may be involved in wound healing, such as fibronectin, platelet factor 4 (PF4, a heparin-binding chemokine), platelet-derived growth factor (PDGF), and transforming growth factor-β. Dense (or δ) granules contain adenosine diphosphate (ADP), adenosine triphosphate, ionized calcium, serotonin, and epinephrine.
After a traumatic vascular injury, platelets encounter constituents of the subendothelial connective tissue, such as vWF and collagen. On contact with these proteins, platelets undergo a sequence of reactions that culminate in the formation of a platelet plug (see Fig. 4.4B ).
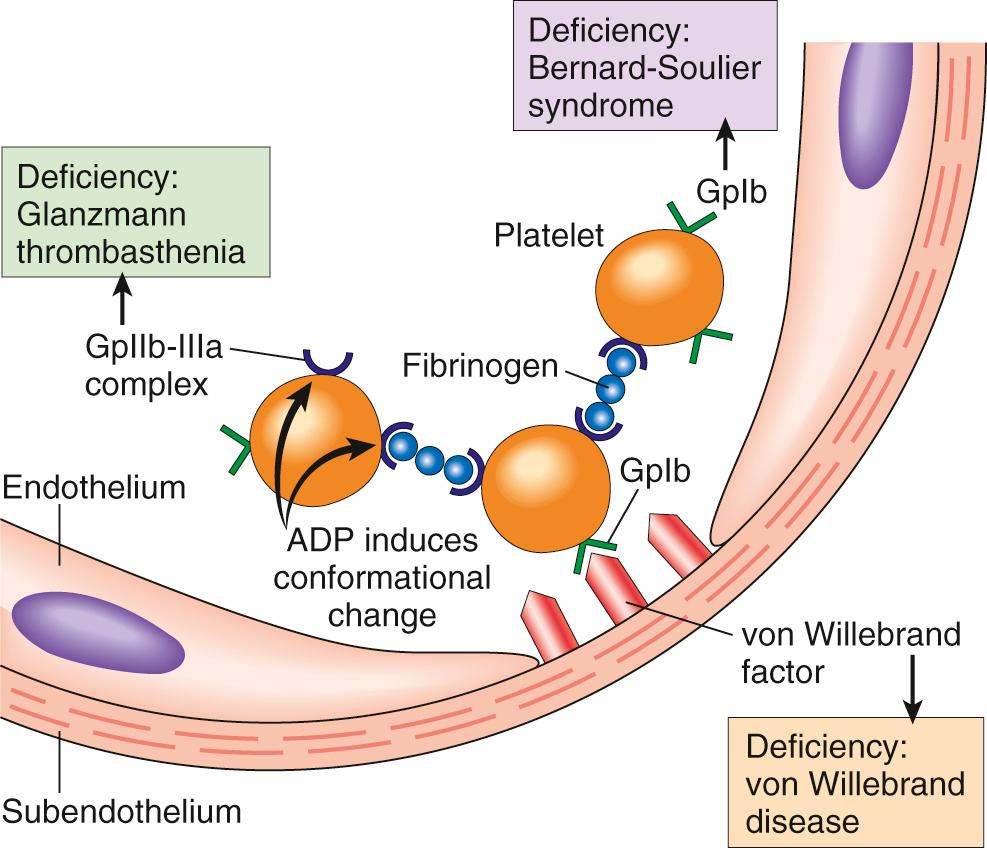
Platelet adhesion is mediated largely via interactions between the platelet surface receptor glycoprotein Ib (GpIb) and vWF in the subendothelial matrix ( Fig. 4.5 ). Platelets also adhere to exposed collagen via the platelet collagen receptor Gp1a/IIa. Notably, genetic deficiencies of vWF (von Willebrand disease, Chapter 14 ) or GpIb (Bernard-Soulier syndrome) result in bleeding disorders, attesting to the importance of these factors.
Platelets rapidly change shape following adhesion, being converted from smooth discs to spiky “sea urchins” with greatly increased surface area. This change is accompanied by conformational changes in cell surface glycoprotein IIb/IIIa that increase its affinity for fibrinogen and by the translocation of negatively charged phospholipids (particularly phosphatidylserine) to the platelet surface. These phospholipids bind calcium and serve as nucleation sites for the assembly of coagulation factor complexes.
Secretion (release reaction) of granule contents occurs along with changes in shape; these two events are often referred to together as platelet activation. Platelet activation is triggered by a number of factors, including the coagulation factor thrombin and ADP. Thrombin activates platelets through a special G-protein–coupled receptor referred to as protease-activated receptor-1 (PAR-1), which is switched on by a proteolytic cleavage carried out by thrombin. ADP is a component of dense-body granules; thus, platelet activation and ADP release begets additional rounds of platelet activation, a phenomenon referred to as recruitment . ADP acts by binding two G-protein–coupled receptors. P2Y 1 and P2Y 12 . Activated platelets also produce the prostaglandin thromboxane A 2 (TxA 2 ), a potent inducer of platelet aggregation. An understanding of the biochemical pathways involved in platelet activation has led to the development of drugs with antiplatelet activities. Aspirin , the oldest of all, inhibits platelet aggregation and produces a mild bleeding defect by inhibiting cyclooxygenase, a platelet enzyme that is required for TxA 2 synthesis. More recently, drugs that inhibit platelet function by antagonizing PAR-1 or P2Y 12 have been developed. All of these antiplatelet drugs are used in the treatment of coronary artery disease.
Platelet aggregation follows their activation. The conformational change in glycoprotein IIb/IIIa that occurs with platelet activation allows binding of fibrinogen, a large bivalent plasma polypeptide that forms bridges between adjacent platelets, leading to their aggregation. Predictably, inherited deficiency of GpIIb-IIIa results in a bleeding disorder called Glanzmann thrombasthenia . The initial wave of aggregation is reversible, but concurrent activation of thrombin stabilizes the platelet plug by causing further platelet activation and aggregation, and by promoting irreversible platelet contraction. Platelet contraction is dependent on the cytoskeleton and consolidates the aggregated platelets. In parallel, thrombin also converts fibrinogen into insoluble fibrin, cementing the platelets in place and creating the definitive secondary hemostatic plug . Entrapped red cells and leukocytes are also found in hemostatic plugs, in part due to adherence of leukocytes to P-selectin expressed on activated platelets.
Endothelial injury exposes the underlying basement membrane ECM; platelets adhere to the ECM primarily through the binding of platelet GpIb receptors to VWF.
Adhesion leads to platelet activation, an event associated with secretion of platelet granule contents, including calcium (a cofactor for several coagulation proteins) and ADP (a mediator of further platelet activation); dramatic changes in shape and membrane composition; and activation of GpIIb/IIIa receptors.
The GpIIb/IIIa receptors on activated platelets form bridging cross-links with fibrinogen, leading to platelet aggregation.
Concomitant activation of thrombin promotes fibrin deposition, cementing the platelet plug in place.
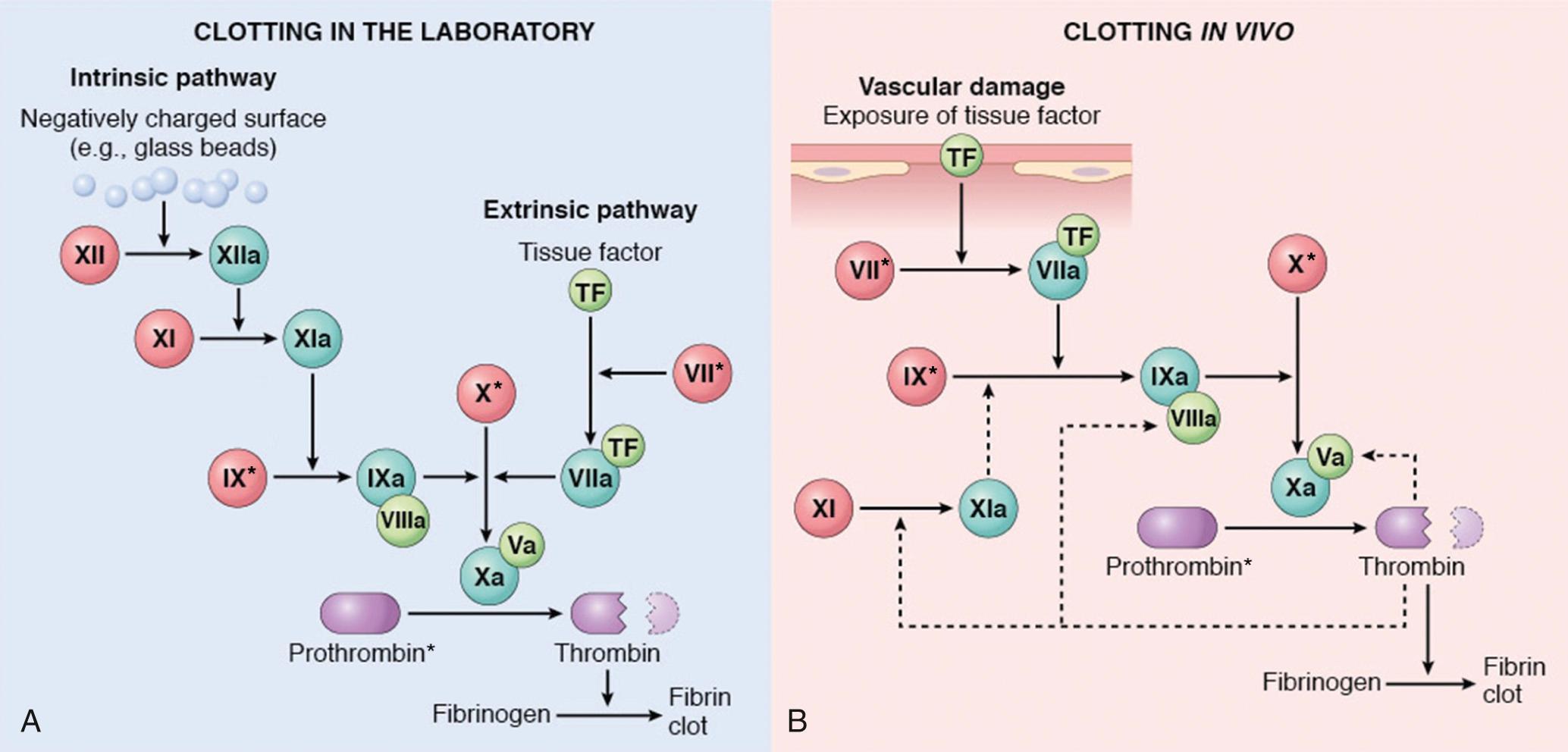
The coagulation cascade is a series of amplifying enzymatic reactions that lead to the deposition of an insoluble fibrin clot. As discussed later, the dependency of clot formation on various factors differs in the laboratory test tube and in blood vessels in vivo ( Fig. 4.6 ). However, clotting in vitro and in vivo both follow the same general principles, as follows.
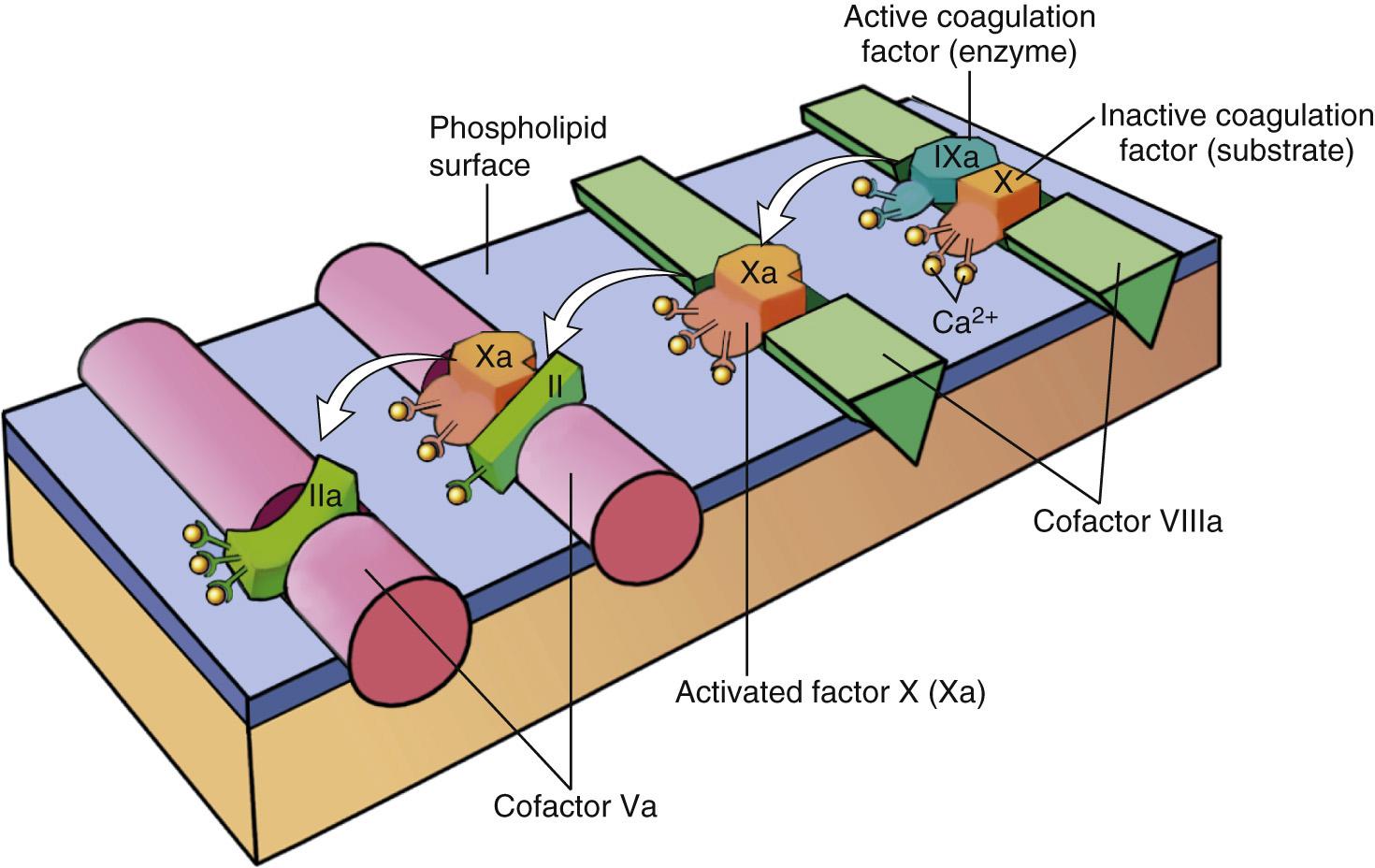
The cascade of reactions in the pathway can be likened to a “dance” in which coagulation factors are passed from one partner to the next ( Fig. 4.7 ). Each reaction step involves an enzyme (an activated coagulation factor), a substrate (an inactive proenzyme form of a coagulation factor), and a cofactor (a reaction accelerator). These components are assembled on a negatively charged phospholipid surface, which is provided by activated platelets. Assembly of reaction complexes also depends on calcium, which binds to γ-carboxylated glutamic acid residues that are present in factors II, VII, IX, and X. The enzymatic reactions that produce γ-carboxylated glutamic acid use vitamin K as a cofactor and are antagonized by drugs such as coumadin, used as an anticoagulant.
Based on assays performed in clinical laboratories, the coagulation cascade can be divided into the extrinsic and intrinsic pathways (see Fig. 4.6A ).
The prothrombin time (PT) assay assesses the function of the proteins in the extrinsic pathway (factors VII, X, V, II [prothrombin], and fibrinogen). In brief, tissue factor, phospholipids, and calcium are added to plasma, and the time for a fibrin clot to form is recorded.
The partial thromboplastin time (PTT) assay screens the function of the proteins in the intrinsic pathway (factors XII, XI, IX, VIII, X, V, II, and fibrinogen). In this assay, clotting of plasma is initiated by the addition of negatively charged particles (e.g., ground glass) that activate factor XII (Hageman factor) together with phospholipids and calcium, and the time to fibrin clot formation is recorded.
Although the PT and PTT assays are of great utility in evaluating coagulation factor function in patients, they do not recapitulate the events that lead to coagulation in vivo. This point is most clearly made by considering the clinical effects of deficiencies of various coagulation factors. Deficiencies of factors V, VII, VIII, IX, and X are associated with moderate to severe bleeding disorders, and prothrombin deficiency is likely incompatible with life. In contrast, factor XI deficiency is only associated with mild bleeding, and individuals with factor XII deficiency do not bleed and in fact may be susceptible to thrombosis. By contrast, there is evidence from experimental models suggesting that in some circumstances factor XII may contribute to thrombosis. These paradoxical findings may stem from involvement of factor XII in several pathways, including the pro-inflammatory bradykinin pathway as well as fibrinolysis (discussed later).
Based on the effects of various factor deficiencies in humans, it is believed that, in vivo, factor VIIa/tissue factor complex is the most important activator of factor IX and that factor IXa/factor VIIIa complex is the most important activator of factor X (see Fig. 4.6B ). The mild bleeding tendency seen in patients with factor XI deficiency is likely explained by the ability of thrombin to activate factor XI (as well as factors V and VIII), a feedback mechanism that amplifies the coagulation cascade.
Among the coagulation factors, thrombin is the most important, because its various enzymatic activities control diverse aspects of hemostasis and link clotting to inflammation and repair. Among thrombin's most important activities are the following:
Conversion of fibrinogen into cross-linked fibrin . Thrombin directly converts soluble fibrinogen into fibrin monomers that polymerize into an insoluble fibril, and also amplifies the coagulation process, not only by activating factor XI, but also by activating two critical cofactors: factors V and VIII. It also stabilizes the secondary hemostatic plug by activating factor XIII, which covalently cross-links fibrin.
Platelet activation . Thrombin is a potent inducer of platelet activation and aggregation through its ability to activate PAR-1, thereby linking platelet function to coagulation.
Pro-inflammatory effects . PARs also are expressed on inflammatory cells, endothelium, and other cell types ( Fig. 4.8 ), and activation of these receptors by thrombin is believed to mediate pro-inflammatory effects that contribute to tissue repair and angiogenesis.
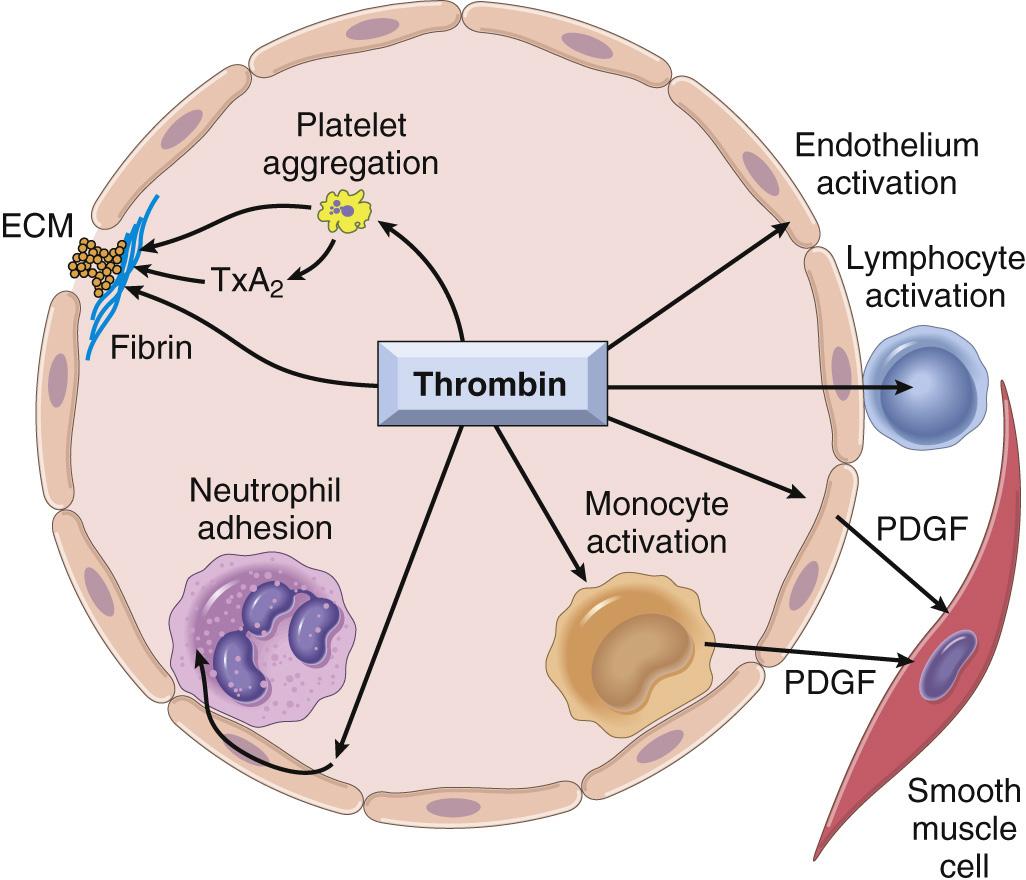
Anticoagulant effects. Remarkably, through mechanisms described later, on encountering normal endothelium, thrombin changes from a procoagulant to an anticoagulant; this reversal in function prevents clots from extending beyond the site of the vascular injury.
Once initiated, coagulation must be restricted to the site of vascular injury to prevent deleterious consequences. One limiting factor is simple dilution; blood flowing past the site of injury washes out activated coagulation factors, which are rapidly removed by the liver. A second is the requirement for negatively charged phospholipids, which, as mentioned, are mainly provided by platelets that have been activated by contact with subendothelial matrix at sites of vascular injury. However, the most important counterregulatory mechanisms involve factors that are expressed by intact endothelium adjacent to the site of injury (described later).
Activation of the coagulation cascade also sets into motion a fibrinolytic cascade that limits the size of the clot and contributes to its later dissolution ( Fig. 4.9 ). Fibrinolysis is largely accomplished through the enzymatic activity of plasmin, which breaks down fibrin and interferes with its polymerization. An elevated level of breakdown products of fibrinogen (often called fibrin split products), most notably fibrin-derived D-dimers, are a useful clinical marker of several thrombotic states (described later). Plasmin is generated by enzymatic catabolism of the inactive circulating precursor plasminogen , either by a factor XII–dependent pathway (possibly explaining the association of factor XII deficiency and thrombosis) or by plasminogen activators. The most important plasminogen activator is t-PA; it is synthesized principally by endothelium and is most active when bound to fibrin. This characteristic makes t-PA a useful therapeutic agent, since its fibrinolytic activity is largely confined to sites of recent thrombosis. Once activated, plasmin is in turn tightly controlled by counterregulatory factors such as α 2 -plasmin inhibitor, a plasma protein that binds and rapidly inhibits free plasmin.
Coagulation occurs via the sequential enzymatic conversion of a cascade of circulating and locally synthesized proteins.
Tissue factor elaborated at sites of injury is the most important initiator of the coagulation cascade in vivo.
At the final stage of coagulation, thrombin converts fibrinogen into insoluble fibrin that contributes to formation of the definitive hemostatic plug.
Coagulation normally is restricted to sites of vascular injury by:
Limiting enzymatic activation to phospholipid surfaces provided by activated platelets or endothelium
Circulating inhibitors of coagulation factors, such as antithrombin III, whose activity is augmented by heparin-like molecules expressed on endothelial cells
Expression of thrombomodulin on normal endothelial cells, which binds thrombin and converts it to an anticoagulant
Activation of fibrinolytic pathways (e.g., by association of t-PA with fibrin)
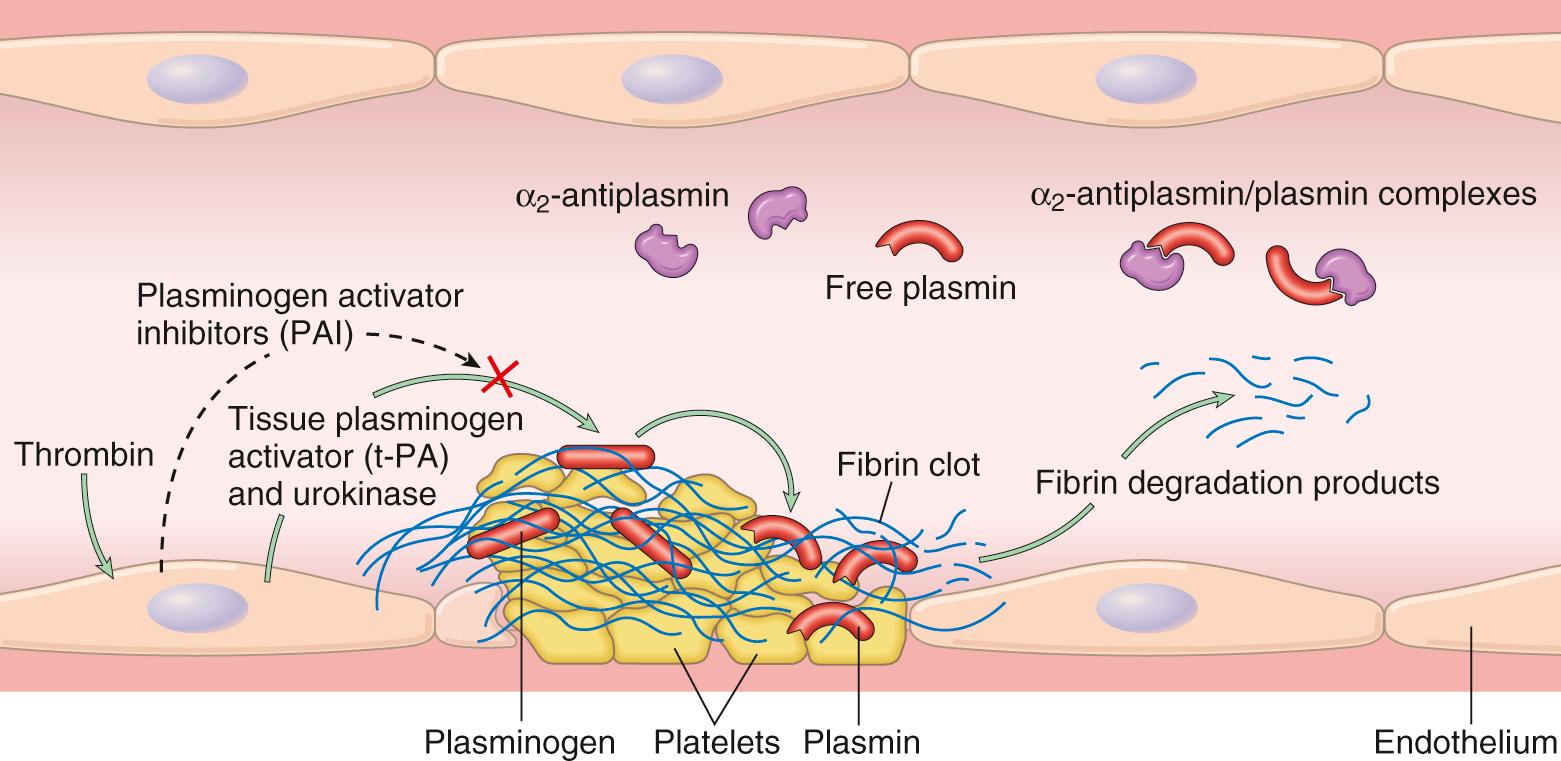
The balance between the anticoagulant and procoagulant activities of endothelium often determines whether clot formation, propagation, or dissolution occurs ( Fig. 4.10 ). Normal endothelial cells express a multitude of factors that inhibit the procoagulant activities of platelets and coagulation factors and that augment fibrinolysis. These factors act in concert to prevent thrombosis and to limit clotting to sites of vascular damage. However, if injured or exposed to pro-inflammatory factors, endothelial cells lose many of their antithrombotic properties. Here, we complete the discussion of hemostasis by focusing on the antithrombotic activities of normal endothelium; we return to the “dark side” of endothelial cells later when discussing thrombosis.
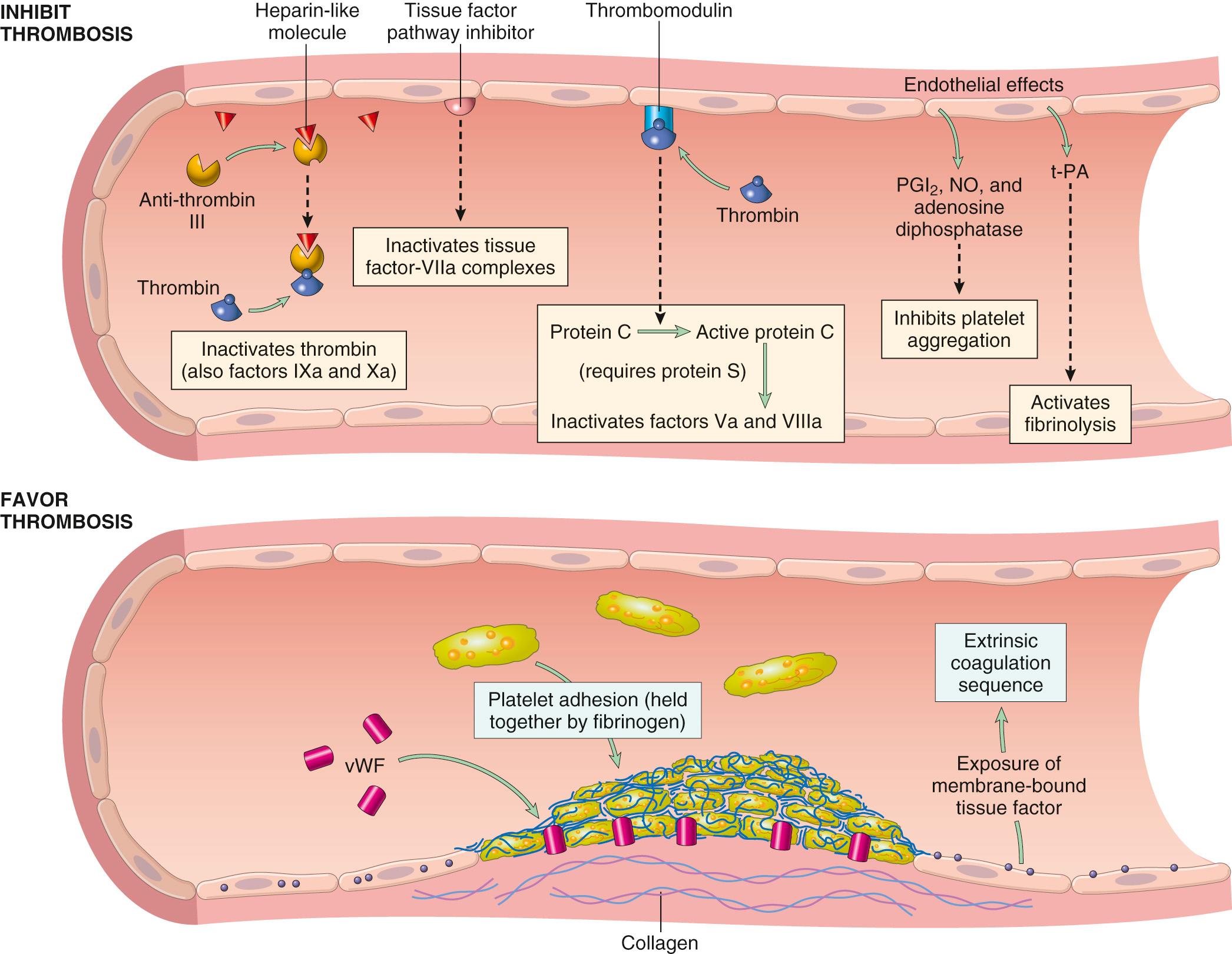
The antithrombotic properties of endothelium can be divided into activities directed at platelets, coagulation factors, and fibrinolysis.
Platelet inhibitory effects. An obvious effect of intact endothelium is to serve as a barrier that shields platelets from subendothelial vWF and collagen. However, normal endothelium also releases a number of factors that inhibit platelet activation and aggregation. Among the most important are prostacyclin (PGI 2 ), nitric oxide (NO), and adenosine diphosphatase ; the latter degrades ADP, already discussed as a potent activator of platelet aggregation. The major regulator of NO and PGI 2 production appears to be flow; precisely what senses flow is uncertain, though changes in cell shape and cytoskeleton are correlated. PGI 2 is produced by COX-1, which is expressed constitutively by “healthy” endothelium under normal flow conditions. NO is the product of endothelial nitric oxide synthase eNOS.
Anticoagulant effects. Normal endothelium shields coagulation factors from tissue factor in vessel walls and expresses multiple factors that actively oppose coagulation, most notably thrombomodulin, endothelial protein C receptor, heparin-like molecules, and tissue factor pathway inhibitor. Thrombomodulin and endothelial protein C receptor bind thrombin and protein C, respectively, in a complex on the endothelial cell surface. When bound in this complex, thrombin loses its ability to activate coagulation factors and platelets, and instead cleaves and activates protein C, a vitamin K–dependent protease that requires a cofactor, protein S. Activated protein C/protein S complex is a potent inhibitor of coagulation cofactors Va and VIIIa. Heparin-like molecules on the surface of endothelium bind and activate antithrombin III, which then inhibits thrombin and factors IXa, Xa, XIa, and XIIa. The clinical utility of heparin and related drugs is based on their ability to stimulate antithrombin III activity. Tissue factor pathway inhibitor (TFPI), like protein C, requires protein S as a cofactor and, as the name implies, binds and inhibits tissue factor/factor VIIa complexes.
Fibrinolytic effects. Normal endothelial cells synthesize t-PA, as already discussed, a key component of the fibrinolytic pathway.
Disorders associated with abnormal bleeding inevitably stem from primary or secondary defects in vessel walls, platelets, or coagulation factors, all of which must function properly to ensure hemostasis. The presentation of abnormal bleeding varies widely. At one end of the spectrum are massive bleeds associated with ruptures of large vessels such as the aorta or of the heart; these catastrophic events simply overwhelm hemostatic mechanisms and are often fatal. Diseases associated with sudden, massive hemorrhage include aortic dissection and aortic abdominal aneurysm ( Chapter 11 ) and myocardial infarction ( Chapter 12 ) complicated by rupture of the aorta or the heart. At the other end of the spectrum are subtle defects in clotting that only become evident under conditions of hemostatic stress, such as surgery, childbirth, dental procedures, menstruation, or trauma. Among the most common causes of mild bleeding tendencies are inherited defects in vWF ( Chapter 14 ), aspirin consumption, and uremia (renal failure); the latter alters platelet function through uncertain mechanisms. Between these extremes lie deficiencies of coagulation factors (the hemophilias, Chapter 14 ), which are usually inherited and lead to severe bleeding disorders if untreated.
Additional specific examples of disorders associated with abnormal bleeding are discussed throughout the book. The following are general principles related to abnormal bleeding and its consequences.
Defects of primary hemostasis (platelet defects or von Willebrand disease) often present with small bleeds in skin or mucosal membranes. These bleeds typically take the form of petechiae, minute 1- to 2-mm hemorrhages ( Fig. 4.11A ), or purpura, which are slightly larger (≥3 mm) than petechiae. It is believed that the capillaries of the mucosa and skin are particularly prone to rupture following minor trauma and that under normal circumstances platelets seal these defects virtually immediately. Mucosal bleeding associated with defects in primary hemostasis may also take the form of epistaxis (nosebleeds), gastrointestinal bleeding, or excessive menstruation (menorrhagia). A feared complication of very low platelet counts (thrombocytopenia) is intracerebral hemorrhage, which may be fatal.
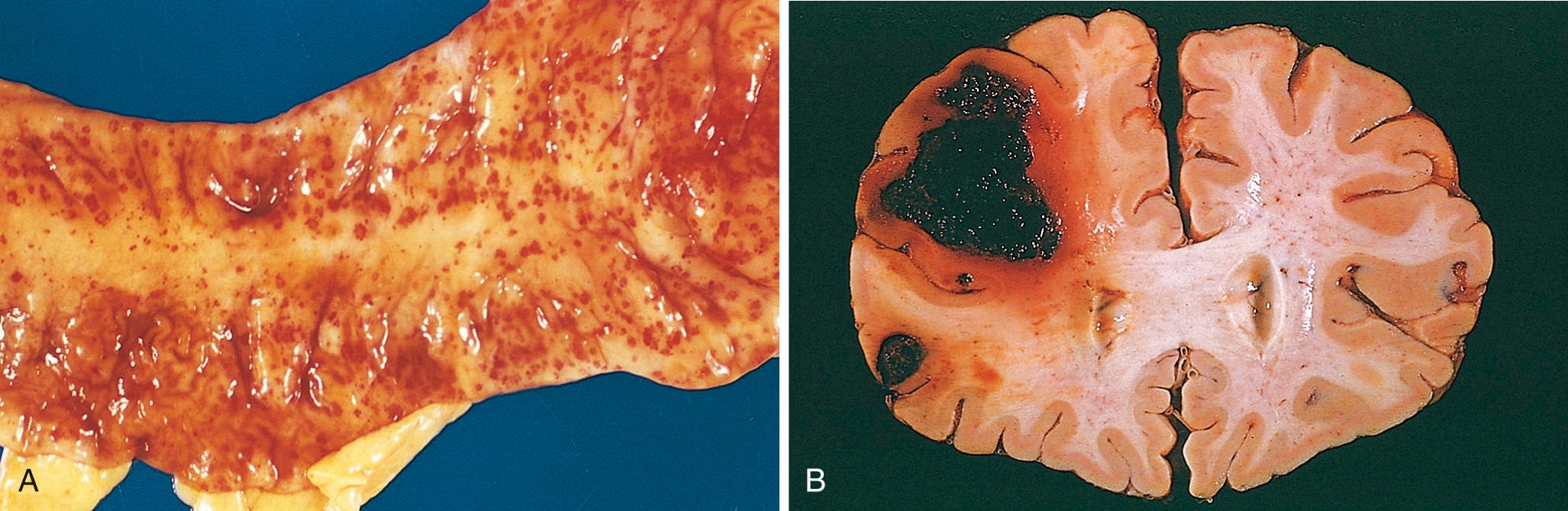
Defects of secondary hemostasis (coagulation factor defects) often present with bleeds into soft tissues (e.g., muscle) or joints. Bleeding into joints (hemarthrosis) following minor trauma is particularly characteristic of hemophilia ( Chapter 14 ). It is unknown why severe defects in secondary hemostasis present with this peculiar pattern of bleeding; as with severe platelet defects, intracranial hemorrhage, sometimes fatal, may also occur.
Generalized defects involving small vessels often present with “palpable purpura” and ecchymoses. Ecchymoses (sometimes simply called bruises ) are hemorrhages of 1 to 2 cm in size. In both purpura and ecchymoses, the volume of extravasated blood may be large enough to create a palpable mass of blood known as a hematoma . Purpura and ecchymoses are particularly characteristic of systemic disorders that disrupt small blood vessels (e.g., vasculitis, Chapter 11 ) or that lead to blood vessel fragility (e.g., amyloidosis, Chapter 6 ; scurvy, Chapter 9 ).
The clinical significance of hemorrhage depends on the volume of the bleed, the rate at which it occurs, and its location. Rapid loss of up to 20% of the blood volume may have little impact in healthy adults; greater losses, however, can cause hemorrhagic (hypovolemic) shock (discussed later). Bleeding that is relatively trivial in the subcutaneous tissues can cause death if located in the brain (see Fig. 4.11B ); because the skull is unyielding, intracranial hemorrhage may increase intracranial pressure to a level that compromises the blood supply or causes herniation of the brain stem ( Chapter 28 ). Finally, chronic or recurrent external blood loss (e.g., peptic ulcer or menstrual bleeding) causes iron loss and can lead to iron deficiency anemia. In contrast, when red cells are retained (e.g., hemorrhage into body cavities or tissues), iron is recovered and recycled for use in the synthesis of hemoglobin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here