Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter deals primarily with primary cutaneous B- and T-cell lymphomas, that is, lymphomas that initially or primarily affect the skin, and reactive lymphoid infiltrates, which may simulate a lymphoma (pseudolymphoma). Later sections of this chapter also address secondary cutaneous B- and T-cell lymphomas, that is, lymphomas that originate from an extracutaneous site and affect the skin, as well as leukemic infiltrates derived from “liquid tumors” that come to affect the skin.
Cutaneous lymphomas may behave in an indolent fashion and remain restricted to the skin in their disease manifestation. However, there are also aggressive types of cutaneous lymphomas that commonly progress to involve extracutaneous sites and that may be lethal. Each section of this chapter discusses the most commonly encountered hematopoietic neoplasms in the skin, distinguishing between those that fall into indolent or aggressive categories. The nomenclature used in this chapter adheres to the joint classification of the World Health Organization—European Organization for Research and Treatment of Cancer (WHO-EORTC). Because not all minor variants of these diseases are addressed here, the readers are referred to more specialized literature.
The majority (≈80%) of primary skin lymphomas are cutaneous T-cell lymphomas (CTCLs). Different types of CTCLs are recognized by distinct clinical and pathologic features. The most common type of CTCL is mycosis fungoides (MF).
This list of lymphomas follows the World Health Organization—European Organization for Research and Treatment of Cancer classification but does not include all of the entities described in that classification, such as primary cutaneous peripheral T-cell lymphoma, unspecified type, or angioimmunoblastic T-cell lymphoma. Only the entities discussed in this chapter are mentioned.
Mycosis fungoides (MF): patch, plaque, tumor stage, and variants of MF
Pagetoid reticulosis (localized disease)
Folliculotropic, syringotropic, granulomatous MF
Granulomatous slack skin
Spectrum of primary cutaneous CD30+ lymphoproliferative disorders
Anaplastic large cell lymphoma
Lymphomatoid papulosis
Subcutaneous panniculitis-like αβ T-cell lymphoma
Primary cutaneous CD4+ small/medium sized pleomorphic T-cell lymphoma (subtype of peripheral T-cell lymphoma)
Sézary syndrome
Adult T-cell leukemia/lymphoma
Extranodal NK/T-cell lymphoma, nasal type
Primary cutaneous aggressive epidermotropic CD8+ T-cell lymphoma (provisional)
Cutaneous γδ T-cell lymphoma (provisional)
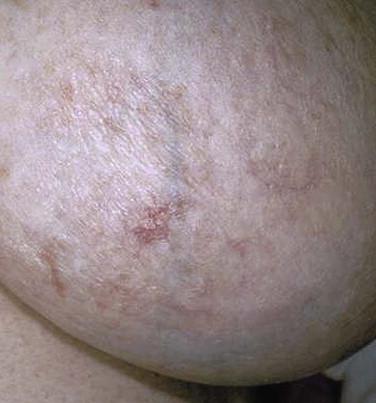
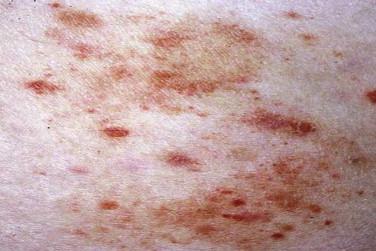
Mycosis fungoides accounts for approximately 70% of CTCL cases in the most recent Surveillance, Epidemiology, and End Results (SEER) data from 1973 to 2002. The annual age-adjusted incidence rate of MF has been reported to be approximately 6 per 1 million persons. MF is more commonly seen in men than women. It usually affects adults but may be seen in teens and rarely in children. The typical clinical presentation is that of persistent patches with a slight scale or a wrinkled surface. It is often accompanied by pruritus. The lesions may be pink to flesh-colored, hyperpigmented or hypopigmented, and have round to oval or annular morphology ( Figs. 14-1 to 14-4 ). Spontaneous resolution and expansion of areas may result in bizarre and arcuate to polycyclic shapes ( Fig. 14-5 ). Lesions may be atrophic ( Fig. 14-6 ) and associated with variegated color and telangiectasia (poikiloderma vasculare atrophicans–like appearance) or they may be purpuric ( Fig. 14-7 ). As the disease progresses, plaque and tumor lesions may develop. MF may also manifest as erythroderma.
Typically adult patients in fifth to sixth decades of life
Male-to-female ratio of 1.6 to 2 : 1
May occur anywhere but preferentially affects bathing trunk areas
Erythematous wrinkled, atrophic, or scaly patches
Plaques and tumors, which may be ulcerated
Pruritus common
Unusual variants: hypopigmented; palmoplantar; pigmented purpuric, poikilodermatous; bullous; guttate, verrucous, ichthyosiform
Stages I and II: localized skin involvement
T1: less than 10% of body surface area involved; no tumor lesions
Greater than 10% of body surface area involved; no tumor lesions
T3: presence of tumor lesion(s)
Stage III: generalized erythroderma (T4)
Stage IV: pathologically confirmed nodal or visceral involvement
Often protracted indolent course
Dependent on stage
Poor survival with extracutaneous involvement or large cell transformation
Dependent on stage
Topical steroids, PUVA, radiation for skin lesions
Chemotherapy or biologic response modifiers for stage IV disease
PUVA, Psoralen and ultraviolet A.
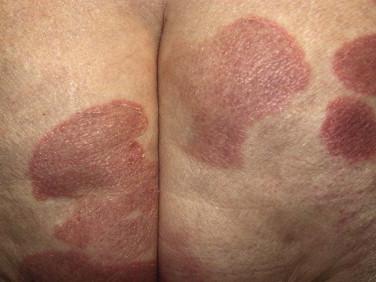
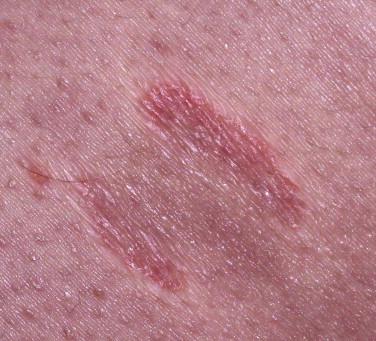
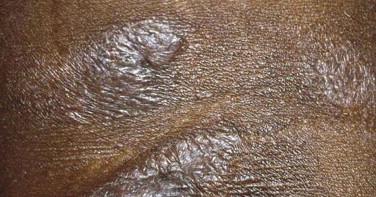

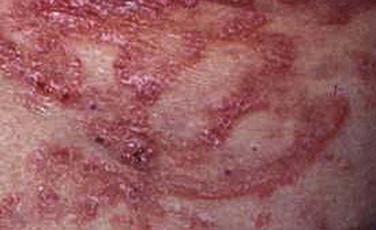
Patch and plaque lesions of MF tend to affect double-covered sites or follow a bathing trunk distribution (hip, buttock, groin, and breast). However, axillary or periaxillary and truncal lesions are also common. Some patients have persistent isolated patches only, but more commonly, the eruption progresses to a combination of generalized patches and plaques. A spectrum of different clinical phenotypes (patches, plaques, or tumors of variable size and shape) may be seen simultaneously. In advanced disease, lymph nodes, the peripheral blood vessels, and less frequently visceral organs may be involved by lymphoma.
Several unusual clinical variants of patch lesions of MF have been described. Hypopigmented MF (see Fig. 14-4 ) is typically seen in patients with darker skin tones but is also a common presentation in children and adolescents. It should be distinguished from pityriasis alba vitiligo and other causes of hypopigmentation. In MF palmaris et plantaris ( palmoplantar MF ), the lesions are hyperkeratotic; concentrated on acral sites, especially the palms and soles; and may simulate a keratoderma. The pigmented purpura variant of MF is characterized by cayenne pepper–colored or purpuric macules and patches (see Fig. 14-7 ). Such lesions may be indistinguishable from benign pigmented purpuric dermatosis. MF should be considered if there are multiple purpuric lesions on the buttock or sites other than the lower extremities. Smaller papulosquamous lesions simulating small plaque parapsoriasis or pityriasis lichenoides represent less common variants of MF. Rarely, bullous lesions of MF can be seen in a background of patch and plaque lesions. Bullous MF may arise with or without a background of classic MF lesions or may be targetoid in appearance, simulating erythema multiforme. Papuloerythroderma of Ofuji is a variant of MF characterized by erythematous, flat-topped, coalescent papules, with erythroderma-like aspects over the chest, abdomen, and limbs, sparing the skin folds of the trunk and pressure areas (deck-chair sign).
The presence of atypical small to intermediate-size lymphocytes, which tend to be located within or along the epidermis (a phenomenon commonly referred to as epidermotropism ) is characteristic of MF. In a classic patch or plaque lesion of MF, lymphocytes pepper the papillary dermis and epidermis and form small intraepidermal aggregates, so-called Pautrier microabscesses . Lymphocytes are typically distributed in a bandlike (lichenoid) pattern ( Fig. 14-8 ). The epidermis may show psoriasiform hyperplasia with hyperkeratosis and parakeratosis ( Fig. 14-9, A ). A common feature of epidermotropism is the presence of solitary lymphocytes aligned along the dermal-epidermal junction ( Figs. 14-9, B , and 14-10, A ). Lymphocyte atypia is characterized by a hyperconvoluted, hyperchromatic nucleus. Slits and crevices within the nucleus give it a cerebriform appearance ( Fig. 14-10, B ). Sometimes the lymphocytes are surrounded by perinuclear clearing or halo.
Variable epidermal changes: atrophy or psoriasiform hyperplasia
Bandlike lymphocytic infiltrate with epidermotropism
Lymphocyte atypia (hyperchromatism, cerebriform nuclei, perinuclear halos)
Wiry dermal collagen
Unusual histologic variants: pigmented purpuric, interstitial, granulomatous, poikilodermatous
Immunophenotype
Usually CD4-predominant T-cell infiltrate
May show loss of CD7
Clonal T-cell receptor (TCR) gene rearrangement common (may not be detectable in nearly half of early lesions)
Drug reaction (e.g., phenytoin-induced pseudo–mycosis fungoides)
Chronic eczema
Psoriasis
Lichenoid keratosis
Lichenoid pigmented purpuric dermatosis
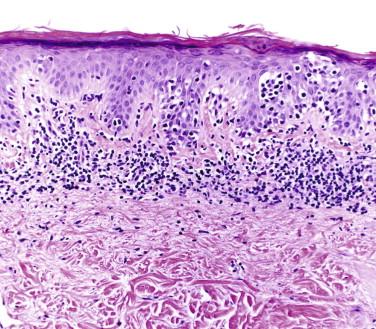
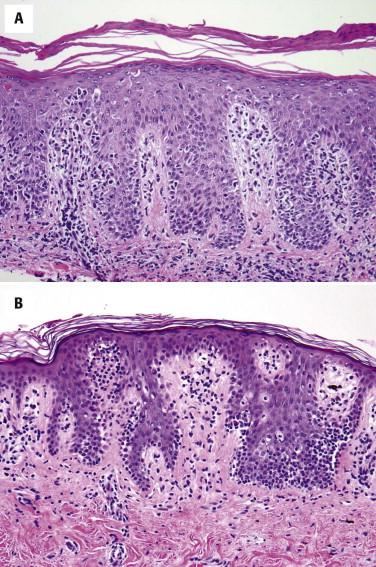
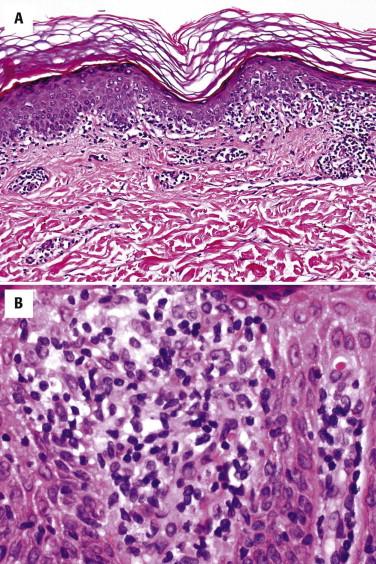
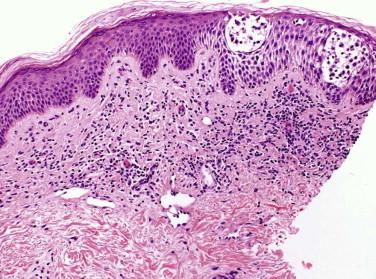
The lymphocytes tend to collect in a relatively “passive” epidermis without significant spongiosis or Civatte body formation. There are varying degrees of epidermal atrophy and rete hyperplasia. In early MF, there is a mild, irregular proliferation of rete ridges. The stratum corneum tends to be subtly altered, by compaction, with mild parakeratosis. Often the biopsy of an early patch lesion of MF lacks a number of classic features and reveals subtle or equivocal histologic findings. The papillary dermis may be peppered by atypical lymphocytes admixed with nonatypical lymphocytes. A helpful clue to the diagnosis of early MF is the presence of lymphocytes aggregating in dermal papillae. In more well-developed lesions, there is a more intense bandlike lymphocytic infiltrate, with fibroplasia of the papillary dermal collagen ( Fig. 14-11 ). The papillary dermal fibrosis has been referred to as wiry or fettuccine-like collagen bundle alteration. However, early, treated, or recurrent disease may lack this feature. Lymphocytes may also be distributed around superficial dermal vessels. An admixture of eosinophils, histiocytes, and (at times) plasma cells may be seen.
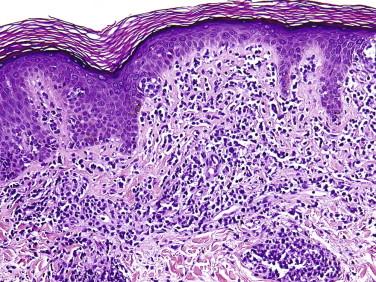
As plaque and tumor lesions develop, the dermal infiltrate becomes denser, and Pautrier's microabscesses may be found more frequently ( Fig. 14-12 ). In tumor lesions of MF, the infiltrate involves most of the reticular dermis ( Fig. 14-13 ) or may even extend into the subcutis. Tumor lesions tend to have more cells with intermediate to large nuclei ( Figs. 14-13 and 14-14 ). The number of large lymphocytes (greater than or equal to four times the size of a small lymphocyte) present is prognostically relevant. The term large cell transformation is applied when the percentage of large cells exceeds 25% of the lesional lymphocyte population or the large cells form discrete nodules. Large cell transformation portends a poor prognosis. It tends to occur more often in tumor lesions but can also be noted in patch or plaque lesions ( Fig. 14-12, B ). A subset of large cell tumors developing in lesions of MF has a CD30-positive immunophenotype (see Fig. 14-14 ).
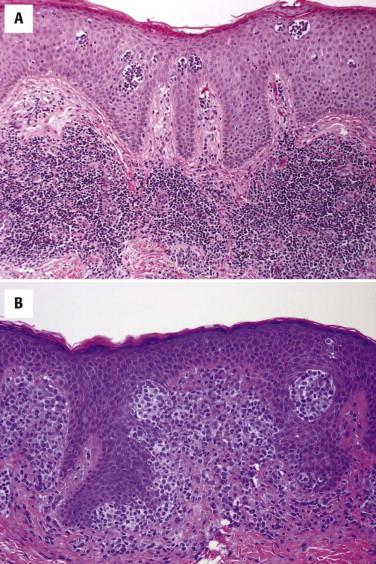
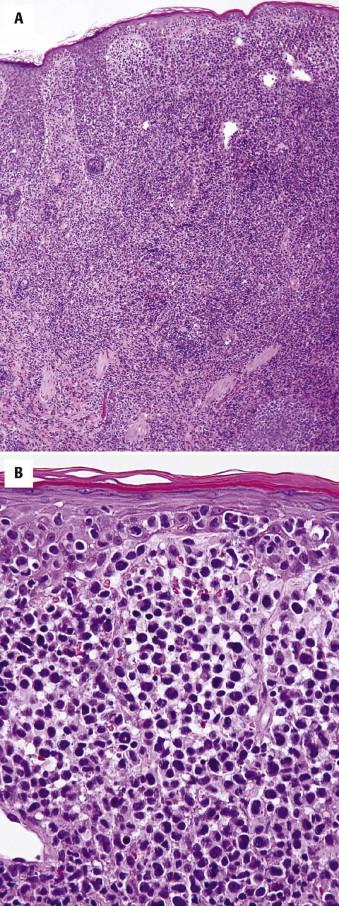

Hypopigmented MF has histologic features similar to patch lesions of classic MF, but there is diminished melanin pigment in lesional epidermis compared with adjacent clinically uninvolved skin. The lymphocytic infiltrate may be less intense, and the epidermis less acanthotic. Some variants of palmoplantar MF may show more prominent epidermotropism in addition to marked hyperkeratosis and epidermal hyperplasia. Pigmented purpura MF shows overlap features with lichenoid pigmented purpura, including erythrocyte extravasation, hemosiderin deposition, and often a subtle interface reaction ( Fig. 14-15, A ). MF with lichenoid features does not have a clinical presentation that is distinct from classic MF but histologically shows features of a lichenoid tissue reaction, including vacuolar and liquefactive basal keratinocyte alteration with dyskeratosis and colloid body formation. Interstitial MF clinically looks similar to patch stage MF but lacks a significant scale. Histologically, there are few, if any, lymphocytes within or near the epidermis. Atypical lymphocytes permeate the reticular dermis in an interstitial pattern ( Fig. 14-15, B ). Bullous MF may show either an intraepidermal blister resulting from confluence of Pautrier's microabscesses or a separation plane at the dermal-epidermal junction resulting from marked involvement by loosely cohesive neoplastic lymphocytes. The blister cavity contains atypical lymphocytes. There may be changes of MF at the edge of a blister. Spongiotic or eczematous variants of MF also exist ( Fig. 14-16 ).
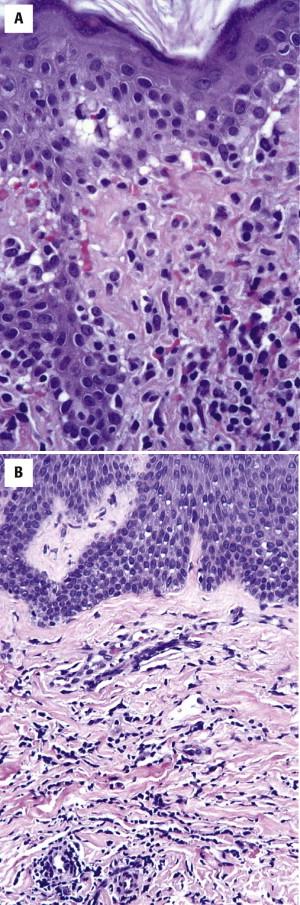
Mycosis fungoides, especially tumor lesions thereof, may be accompanied by a prominent granulomatous inflammatory reaction ( granulomatous MF ) and lead to possible diagnostic confusion with infection, sarcoidosis, or other non-neoplastic processes.
A diagnosis of MF is usually made on routine histology alone in conjunction with the clinical presentation. In diagnostically difficult, controversial, or histologically subtle cases, establishing the immunophenotype might help support the diagnosis.
Immunohistochemical stains permit assessment of the helper : suppressor ratio (CD4 : CD8) and detection of antigen loss. Results need be interpreted with caution because early disease or sparsely populated biopsies may not demonstrate classic immunophenotypic aberrations because of a population of suppressor host response.
Prototypical lesions of MF show an increase in the CD4 : CD8 ratio ( Fig. 14-17 ). However, there is no ratio number that proves the presence or absence of lymphoma. In early patch lesions of MF, the CD4 : CD8 ratio may be similar to reactive infiltrates, in which the ratio tends to range from 1 : 1 to 3 : 1. Although the immunophenotype of classic MF is CD3(+), CD2(+), CD4(+), TCR β(+), CD45 RO+, there are also cases that are CD8+/CD4–, CD8+/CD4+, or CD4–/CD8–.
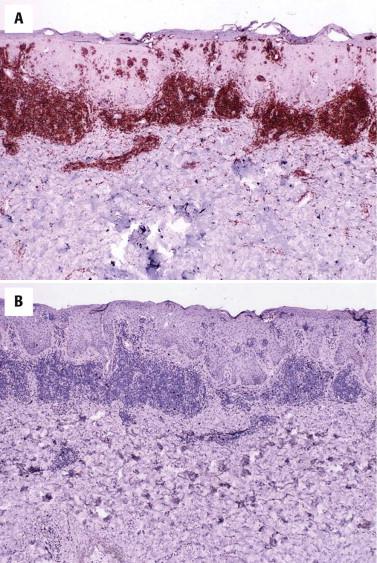
Although a reduction in staining for maturation T-cell markers such as CD7 or CD5 is commonly seen in MF, partial loss of CD7 can also occur in non-neoplastic inflammatory dermatoses. Even in bona fide MF, loss of CD7 staining may at times be seen only in late, histologically obvious lesions. Thus immunohistochemistry per se is of limited diagnostic value.
In many cases of MF, a monoclonal rearrangement of the T-cell receptor gene can be demonstrated by polymerase chain reaction (PCR) analysis, but results from clonality tests should be interpreted with caution. Although the detection of a T-cell clone may be used as additional evidence in support of MF, if the clinical and histologic findings favor lymphoma, it does not represent unequivocal proof thereof because clonal T-cell populations have also been demonstrated in non-neoplastic conditions, such as arthropod bites, eczema, or drug reactions. Likewise, the lack of detection of a T-cell clone in early or histologically subtle patch lesions of MF does not exclude the diagnosis because false-negative results occur in approximately 40% of early lesions of MF.
The differential diagnosis of MF depends on the clinical and histologic presentation. In early disease, all variants may show overlap with inflammatory dermatoses.
For patch lesions of MF, the most common problem is their distinction from chronic eczema, such as atopic, nummular, or allergic contact dermatitis. Spongiosis or the absence of epidermotropic atypical lymphocytes favors chronic eczema, but spongiosis may also occur in MF, and topical treatment with steroids may lead to temporary absence of epidermotropism. The pattern of papillary dermal fibrosis and cytology of lymphocytes are helpful features. The wiry collagen bundles often seen in MF are usually not found in eczema. Hyperchromatic cerebriform lymphocytes favor MF over eczema.
Mycosis fungoides may also be difficult to distinguish from psoriasis. Attention to subtle histologic clues (e.g., neutrophils in stratum corneum, hypervascular dermal papillae) and clinical context (associated other features of psoriasis, family history) lead to the correct diagnosis.
Histologic features identical to MF can also be seen in association with a drug reaction, in particular after the use of phenytoin. Clinical correlation (history of medication use) is essential to avoid a misdiagnosis.
Dermatophyte infections can be associated with histologic features that simulate various patterns that can be found in patch lesions of MF. Therefore, it is prudent practice to include a fungal stain in the workup of possible patch lesions of MF with only mild lymphoid atypia.
The pigmented purpura variant of MF can be difficult to differentiate from persistent lichenoid pigmented purpura, and patients may undergo several biopsies until a diagnosis of MF can be established. Cerebriform atypia in the epidermis and dermis, in conjunction with a clinical presentation of generalized involvement or lesions above the lower extremities, favors MF.
The lichenoid variant of MF shows overlap features with other lichenoid reactions such as lichen planus, lichen planus–like keratosis, lupus, lichenoid drug reaction, or pityriasis lichenoides chronica. Civatte bodies tend to be rare in lesions of MF but may be present. The presence of hyperchromatic cerebriform lymphocytes, lymphocytes with halos, or wiry papillary dermal collagen bundles favors lymphoma. Clinical correlation is essential.
Hypopigmented MF may have overlap with early vitiligo and often occurs in the same patient populations, that is, individuals with dark skin, making it difficult to distinguish these entities even with clinicopathologic correlation. Furthermore, both entities may have a CD8 immunophenotype. Epidermotropism, hydropic degeneration of basal cells, partial loss of pigment, preservation of some melanocytes, presence of lymphocytes within the papillary dermis, increased density of the dermal infiltrate, and wiry fibrosis of the papillary dermal collagen were shown to be detected with a significantly higher incidence in hypopigmented MF versus vitiligo. On the other hand, focal thickening of the basement membrane, complete loss of pigmentation, total absence of melanocytes, and absence or sparseness of lymphocytes in the dermal papillae were seen much more frequently in vitiligo.
With large cell transformation of MF, CD30-positive neoplasms may arise. Clinical history (of MF, concurrent patch or plaque lesions) and histologic context (adjacent features of classic MF) are needed for the distinction of lymphoma representing large cell transformation of MF from a primary cutaneous CD30-positive anaplastic large cell lymphoma (ALCL). This issue is important prognostically because patients with a primary cutaneous CD30-positive ALCL have longer survival times than those in whom the CD30-positive tumor represents MF with large cell transformation.
The majority of classic MF patients have an indolent clinical course over years to decades. The prognosis of the disease is defined by its stage. Patients with stage T1 disease (less than 10% of the body surface area involved by MF with no tumor lesions) have a survival time similar to that of an age-, sex-, and race-matched population. Poor prognostic factors include age older than 60 years, advanced stage (tumors, nodal, peripheral blood, or visceral involvement), and large cell transformation. When extracutaneous involvement or large cell transformation occurs, the expected survival period is usually less than 2 years.
Several treatment options are available, depending on the stage or extent of skin involvement. Topical therapies include nitrogen mustard, carmustine (BCNU), high-potency topical steroids, and topical retinoids such as tazarotene and imiquimod. Ultraviolet (UV) light, especially UVA in conjunction with psoralen (PUVA) and narrow-band UVB, is often used. Current Food and Drug Administration–approved systemic medications are bexarotene, tazarotene vorinostat, and denileukin diftitox. Other frequently used systemic medications include interferon, other oral retinoids (e.g., isotretinoin), methotrexate, and pralotrexate. Immune response modifiers and targeted therapies such as lenalidomide, programmed cell death 1 (PD1)-inhibitors, and C-C chemokinine receptor type 4 (CCR4) inhibitors are used for patients with advanced disease. Extracorporeal photopheresis may be used if there is significant peripheral blood involvement. Chlorambucil, liposomal doxorubicin, or gemcitabine may be used in advanced disease. Electron-beam therapy is frequently used in generally refractory skin disease, either alone or in conjunction with other therapies. A combination of these biologic therapies is often used if there is generalized or advanced disease. Combination chemotherapy is typically reserved for advanced disease or patients who no longer respond to biologic modifiers. Some patients may undergo bone marrow and allogeneic stem cell transplantation for recalcitrant advanced stage CTCL.
Folliculotropic mycosis fungoides (FMF) is a variant of MF in which the neoplastic T-cell infiltrate preferentially involves the hair follicles, at least in the early stages of the disease.
This may or may not be associated with mucinous degeneration of the follicular epithelium, which is termed follicular mucinosis (FM).
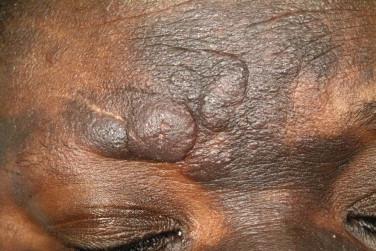
Folliculotropic mycosis fungoides is much less common than classic MF. Isolated lesions of benign FMF may occur in children as well as in adults. FMF with or without mucinosis typically occurs in adults, with a male predominance. In contrast with classic MF, in which the “double-covered” sites are more frequently involved, in FMF, the lesions are concentrated in the head and neck region ( Fig. 14-18 ). Very early lesions may show minimal changes that include follicular-distributed mild erythema or flesh-colored papules or patches of alopecia with follicular prominence. Other clinical presentations include various acneiform sequelae of follicular alteration, such as comedones, fine white follicular spines (see Fig. 14-18 ), milia or small infundibular cysts, and foreign body reactions to follicular rupture. In advanced disease, mucinorrhea, tumid plaques, and nodules may be seen, and when associated with loss of eyebrow hair, a leonine facies may ensue ( Fig. 14-19 ). Lesions of FMF typically arise as a pink solitary or oligolesional indurated or somewhat translucent-appearing erythematous plaque on the head and neck with alopecia. Features of FMF may coincide or collide with classic lesions of MF.
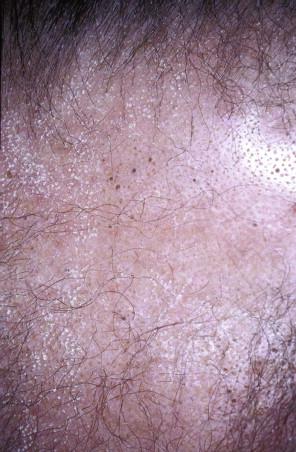
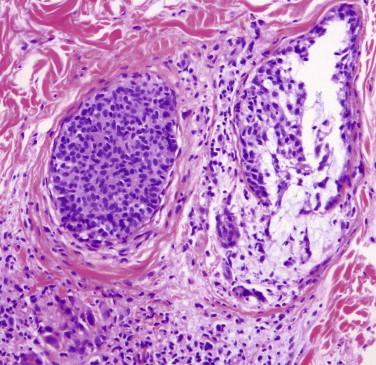
Follicular mucinosis refers to the presence of mucinous edema within the follicular epithelium ( Fig. 14-20 ). There may be a mild lymphocytic infiltrate without atypia. In well-developed lesions of FMF, hyperchromatic cerebriform lymphocytes are dispersed within the follicular epithelium and along the follicular dermal junction, with variable degrees of mucinosis or spongiosis. In pure cases of FMF, there is no or minimal epidermal involvement (“sparing of the interfollicular epidermis”) ( Fig. 14-21 ). However, commonly, the interfollicular dermis and epidermis may also be involved. There may be alteration of the follicle, resulting in comedonal changes, keratin plugging, or follicular rupture (see Fig. 14-21 ).
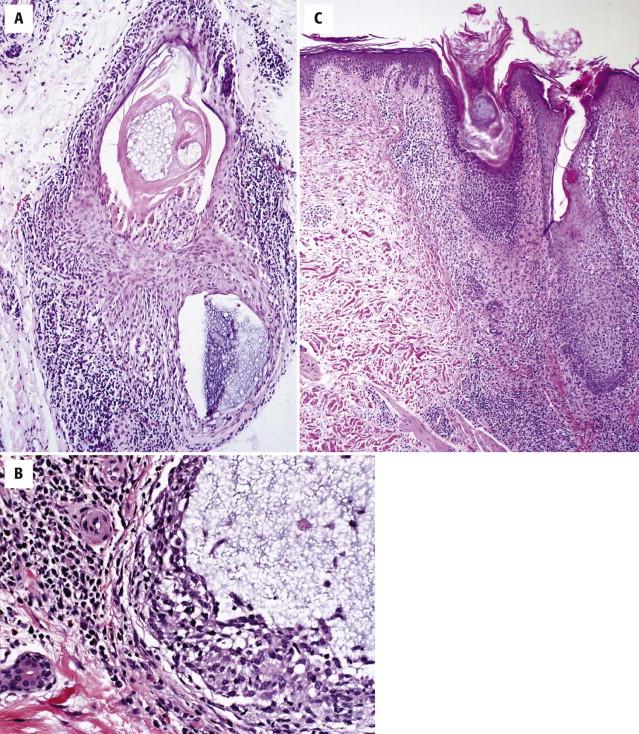
In advanced lesions, there is a dense infiltrate of atypical lymphocytes involving the follicles as well as the epidermis and dermis. Eosinophils are often abundant and may be associated with eosinophilic spongiosis. Follicular distortion and rupture result in a granulomatous component, potentially simulating a reactive infiltrate.
The immunophenotype is similar to that of classic MF. Clonal TCR gene rearrangement is often detected but may be negative in early disease. Ancillary studies cannot reliably distinguish benign FM from folliculotropic lymphoma.
Folliculotropic MF can be difficult to diagnose. In the absence of cerebriform nuclear atypia, it may be impossible to distinguish FMF from benign FM. Clinically, solitary or few lesions limited to the head and neck area in a younger patient population have been reported to occur more often in patients with limited benign FM.
Early lesions of FMF may also be confused with lymphocyte-rich follicular inflammatory processes, including, lupus erythematosus (LE), and rosacea. Because spongiosis may be present in FMF, it may be confused with an eczematous dermatitis. Even in advanced disease, the presence of eosinophils and secondary changes such as spongiosis or granulomatous reactions to follicular rupture may simulate cutaneous lymphoid hyperplasia.
The prognosis is thought to be worse for the follicular variant of MF than for classic MF in patch or plaque stage. Patients with FMF have been found to have a 5-year survival rate that is similar to patients with tumor stage MF. Treatment is similar to that of classic MF, requiring multimodality therapy, often in conjunction with PUVA.
This variant of MF is characterized by epitheliotropism of the eccrine apparatus. It can arise alone or in conjunction with classic or folliculotropic MF.
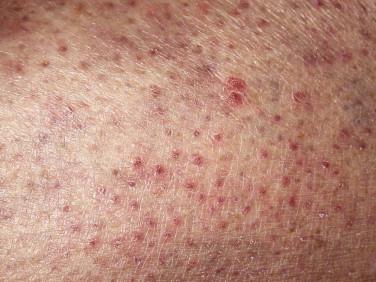
Clinically, there are initially discrete red-brown macules with minimal scale, concentrated on the extremities, palms, and soles ( Fig. 14-22 ). There may be anhidrosis. As the lesions advance, these eccrine-distributed lesions may merge into scaly patches and plaques ( Fig. 14-23 ). Alopecia and follicular accentuation may be present. Often the lesions are solitary.

There is a syringotropic atypical lymphocytic infiltrate ( Fig. 14-24 ). This may arise alone or in conjunction with patch or plaque lesions of MF. The diagnostic features may not be apparent if an eccrine duct is not present in the sections, and one must be sure to specifically assess for this possibility by evaluating the deep eccrine coils. The eccrine ducts and glands are infiltrated and surrounded by atypical T lymphocytes. Eccrine gland hyperplasia may give rise to complex epithelial structures with a cribriform pattern of ductal hyperplasia or squamatization (“syringometaplasia”).
The differential diagnosis of syringotropic MF includes a lymphoid drug eruption, lupus, or lichen striatus, all of which may involve the eccrine duct. If an eccrine gland is not present in the sections, the lymphocytic infiltrate may be too sparse to render a diagnosis of lymphoma, simulating “normal skin” or a nonspecific perivascular dermal hypersensitivity reaction. If there is a prominent dermal component and one does not recognize the syringotropism, the differential diagnosis includes a peripheral T-cell lymphoma or atypical cutaneous lymphoid hyperplasia.
Currently, there is no strong evidence that the presence of syringotropism has an impact on prognosis or treatment.
Pagetoid reticulosis (PR) is a variant of MF that derives its name from the histologic feature of prominent intraepidermal (pagetoid) distribution of atypical lymphocytes.
Oligolesional or solitary PR (Woringer-Kolopp disease) is characterized by one or few, usually indolent, scaly to keratotic lesions, often occurring on the extremities. Historically, a disseminated variant of PR has been referred to as Ketron-Goodman disease . The latter is associated with a more aggressive clinical course and is now considered as a different subtype of CTCL, termed primary cutaneous aggressive CD8 epidermotropic CTCL .
Pagetoid reticulosis is characterized by a lymphocytic infiltrate that predominantly localizes to within the epidermis and the dermal-epidermal junction ( Fig. 14-25, A ). Lymphocytes may be hyperchromatic cerebriform or show perinuclear halos. Typically, many atypical lymphocytes are distributed in a “buckshot” scattered pattern at all layers of the epidermis. However, not all unilesional forms of MF show prominent pagetoid epidermotropism. Some lesions display a bandlike pattern with involvement of both epidermis and superficial dermis ( Fig. 14-25, B ) and only limited pagetoid invasion of the lower spinous cell layer.
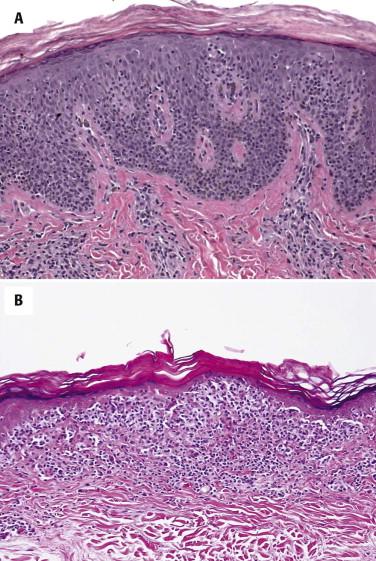
The infiltrate is immunopositive for CD3 and CD5. In contrast with classic MF, many of the cases show a predominance of CD8-positive cells within the epidermis. CD7 and CD2 expression may be lost.
Histologically, it is not possible to distinguish PR from the aggressive CD8 cytotoxic CTCL variants, although the latter may show a more pleomorphic infiltrate. Lesions of clinically more classic MF or palmoplantar MF may also show histologic features of PR. Clinical correlation is essential to distinguish unilesional or oligolesional MF from classic MF.
Solitary or oligolesional variants typically have an indolent course. Simple surgical excision may suffice. Local radiation therapy or topical steroids may also be used.
Granulomatous slack skin (GSS) is a recognized clinical-pathologic variant of MF, and it is distinct from “granulomatous” MF. The latter refers to a microscopic variant, in which there is a granulomatous or sarcoidal component to the infiltrate in a patient with otherwise classic MF or Sézary syndrome.
Granulomatous slack skin is very rare. It generally affects patients at a younger age than classic MF and may manifest in the teens to young adulthood. The characteristic manifestation is that of lax skin or atrophic or pendulous plaques in the axillary and inguinal folds. Initially, lesions are indurated plaques, which slowly become wrinkled.
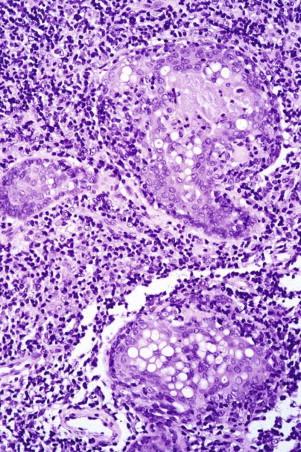
There is a pandermal and subcutaneous infiltrate of atypical lymphocytes admixed with evenly distributed epithelioid and multinucleated giant cells, many of which contain numerous nuclei ( Fig. 14-26 ). The multinucleated giant cells demonstrate prominent elastophagia and lymphophagocytosis (emperipolesis). Eosinophils and plasma cells are often present. Epidermotropism is usually minimal to absent.
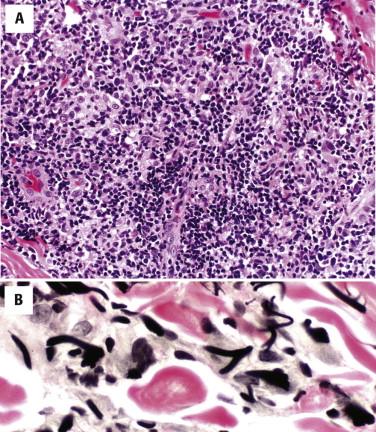
Elastic tissue stains demonstrate loss of elastic fibers. Immunohistochemistry shows a CD4-predominant T-cell infiltrate. There may be loss of CD5 and CD7. These tumors typically show monoclonal rearrangements of the TCR.
Multinucleated giant cells with numerous nuclei, emperipolesis, elastophagia, and lymphocyte atypia are a distinct constellation of features that help differentiate GSS from other granulomatous and lymphoid infiltrates, such as granulomatous infiltrates in MF, infectious causes of granulomatous infiltrates, granulomatous hypersensitivity reactions, and cutaneous lymphoid hyperplasia with anetoderma. The latter can be excluded by immunophenotyping, demonstrating a mixed T- and B-cell infiltrate. GSS does not tend to show an admixture of neutrophils, epithelioid histiocytes, and necrosis, which may be seen in infectious granulomata.
Patients with GSS tend to have a protracted clinical course. Various topical and systemic agents listed above for MF may be tried, although the lesions may be difficult to completely eradicate. If the lesions are localized, radiation therapy or surgical excision may be helpful. The main concern is the development of a secondary extracutaneous lymphoma, which is reported to occur with this subgroup. Treatment is similar to that of MF.
Sézary syndrome represents the leukemic form of CTCL characterized by erythroderma, lymphadenopathy, and neoplastic T cells (Sézary cells) in the skin, lymph nodes, and blood.
Patients have erythroderma ( Fig. 14-27, A ), often associated with edema, induration, and scaling of the skin. Palmar or plantar keratoderma is often present. Lower eyelid ectropion, alopecia, and nail dystrophy may also be seen.
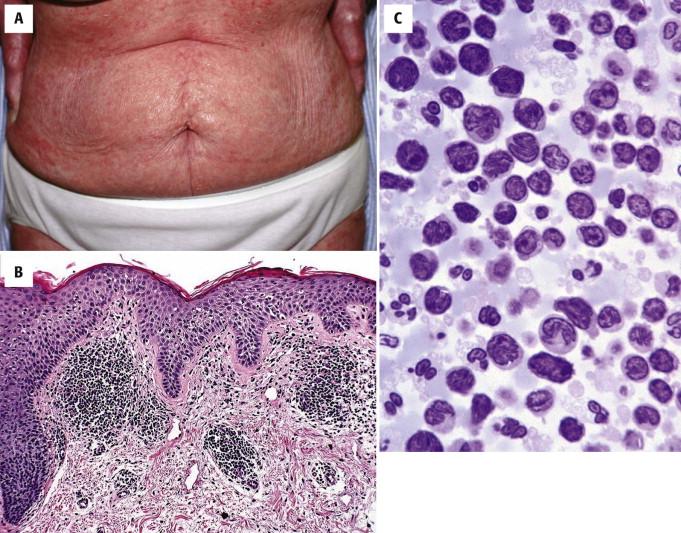
The histopathology of Sézary syndrome is often overall subtler than in classic MF. Often there is a perivascular lymphocytic infiltrate in the upper dermis, with mild acanthosis ( Fig. 14-27, B ). The lymphocytes in Sézary syndrome display cytologic atypia, with a spectrum of larger cerebriform cells (Sézary cells) and smaller hyperchromatic lymphocytes with cerebriform nuclear contours (Lutzner cells). Eosinophils and plasma cells may accompany the lymphocytic infiltrate. Epidermotropism and cerebriform lymphoid atypia may be prominent but are often subtle. Frequently, the epidermis shows foci of mild spongiosis, making a distinction from erythrodermic eczema difficult without correlation with assessment for peripheral blood involvement and clinical context (history and laboratory data).
The diagnosis of Sézary syndrome requires documentation of neoplastic T cells in the peripheral blood in the context of erythroderma. This is typically done by flow cytometry, but morphologic assessment by peripheral blood smear or buffy coat analysis may also support the diagnosis ( Fig. 14-27, C ). The number of hyperconvoluted or Sézary cells required for a diagnosis of Sézary syndrome depends on the presence of other diagnostic features. Current criteria (by the International Society for Cutaneous Lymphoma) for the diagnosis of Sézary syndrome include an absolute Sézary cell count of at least 1000 cells/mm 3 ; immunophenotypical abnormalities, including a CD4/CD8 ratio of 10 or higher (measured by flow cytometry) and loss of T-cell antigen (CD2, CD3, CD4, CD5) expression; or the documentation of a T-cell clone in the blood by PCR test for TCR gene rearrangement or cytogenetic studies.
The differential diagnosis includes erythroderma because of other causes, such as atopic dermatitis, psoriasis, pityriasis rubra pilaris, mastocytosis, and drug reactions. It is not always possible to distinguish between lymphoma and non–lymphoma-related erythroderma on histologic grounds alone if there is only mild lymphoid atypia. Examination of the peripheral blood and clinical correlation are critical for a final diagnosis. Adult T-cell leukemia/lymphoma (ATLL) (see the following) may show histology indistinguishable from Sézary syndrome. It is best distinguished by documenting human T-lymphotropic virus 1 (HTLV–1) lymphocyte infection and multilobated lymphocytes in the peripheral blood. Evidence of prior infection per se is not sufficient for the diagnosis because only a small percentage of patients infected with HTLV-1 will develop ATLL.
Patients with erythrodermic CTCL or Sézary syndrome have a poorer prognosis than patients with early patch or plaque disease, with a 5-year survival rate of approximately 11%, but this may be higher in some institutions. Treatment usually entails multimodality therapy using a combination of regimens outlined for skin-restricted CTCL, often in conjunction with extracorporeal photopheresis and systemic therapy (bexarotene, interferon, or chemotherapy).
The CD30-positive lymphoproliferative disorder spectrum includes lymphomatoid papulosis (LyP), ALCL, and “borderline” lesions.
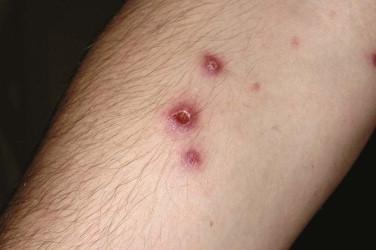
Lymphomatoid papulosis presents as a chronic, recurrent, self-healing eruption of papules that progress to papulonecrotic lesions before resolving ( Fig. 14-28 ). People of all ages can be affected, including children, but LyP has a peak incidence in the fifth decade of life. Typically, the lesions occur in crops and are found in different stages of evolution at clinical examination. Erythematous papules with scale crust may become ulcerated and necrotic. The eruption of LyP may recur over many years to decades. The most commonly affected sites include the trunk and proximal extremities; however, lesions may be seen on the face, palms, soles, and scalp. The lesions may be restricted to one region of the body or limb. Oral mucosal involvement is rare. Papules (less than 1 cm in size) are the most characteristic presentation. However, the presence of nodules measuring 1 to 2 cm is not uncommon. If nodules are present, this raises concerns for progression to or coexistence of ALCL or may lead to a designation of a borderline lesion if no definitive distinction between LyP and ALCL can be made. Lesions of LyP may heal with postinflammatory dyschromia or an atrophic scar. Resolution tends to occur within 3 to 6 weeks, but they may not completely regress for several months. At least 5% to 20% of patients have a concurrent or preceding diagnosis of Hodgkin's or non-Hodgkin's lymphoma (often MF or ALCL) or may develop such in the future.
May occur at any age but most often in mid adult life
No site predilection; often on the trunk and extremities
Small papules and papulonecrotic lesions that appear in crops
May be asymptomatic or pruritic
Lesions tend to resolve spontaneously between 2 and 6 weeks
Waxing and waning of this process may last for decades
Often chronic indolent disease
No impact on life expectancy
May be associated with lymphoma (mycosis fungoides, anaplastic large cell lymphoma, Hodgkin's disease) in 5% to 15% of affected individuals
Topical steroids, especially for pruritic lesions
Phototherapy
Therapy is not necessary in many cases
Low-dose methotrexate
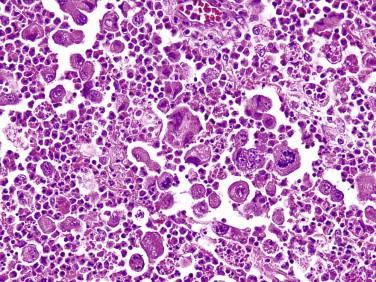
The histology of LyP varies, depending in part on the evolutionary stage of the lesion. Different lesional morphologies are often seen in the same patient at different biopsy sites. The most typical architectural pattern of the infiltrate is a wedge shape with the broad base of the wedge aligning with the epidermis ( Fig. 14-29 ). Associated epidermal hyperplasia, ulceration, spongiosis, and focal epidermal necrosis are common. Hair follicles may be involved and may be dystrophic. The lymphocytic infiltrate usually involves both the epidermis and dermis. It is typically composed of a mixture of large cells, small to intermediate-sized atypical lymphocytes, and a polymorphous background of neutrophils and eosinophils in variable proportions. Anaplastic binucleate cells with prominent nucleoli, similar to Reed-Sternberg cells of Hodgkin's disease, may be seen.
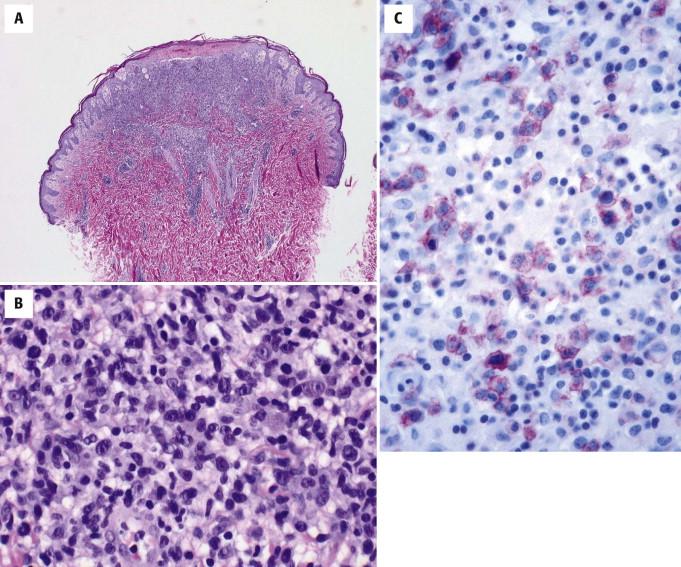
Subtypes A, B, C, and recently D and E of LyP have been described, referring to variations in the architectural, cytomorphologic, and immunohistochemical composition of the lymphoid infiltrate. In type A, the infiltrate is polymorphous, being composed of lymphocytes, neutrophils, and eosinophils admixed with large histiocyte-like CD30-positive lymphocytes. CD30 is expressed in a crisp membranous and Golgi pattern and generally represents less than 50% of the infiltrate. Type B LyP is similar (or identical in appearance) to papular MF because the infiltrate is composed predominantly of smaller cerebriform lymphocytes (Lutzner cells) aligned along the dermo-epidermal junction, often demonstrating prominent epidermotropism. In the past, these cells were said to be negative for CD30; however, with recent improvements in immunohistochemical techniques and reagents, many type B LyP cases are known to be CD30 positive. Type C LyP is composed of a more exuberant population of large atypical CD30-positive cells in clusters and sheets, often histologically indistinguishable from ALCL on small biopsies ( Fig. 14-30 ). Type D LyP is a lichenoid and epidermotropic form of LyP characterized by CD8-positive, βF1-positive, cytotoxic marker–positive T cells, histologically indistinguishable from a primary cutaneous aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma. Recently, LyP type E (angioinvasive LyP) has been described as an oligolesional, characteristically ulcerated, large necrotic eschar-like but eventually self-healing lesion with prominent angioinvasion and angiodestruction by small to medium-sized atypical CD8-positive, CD30-positive T cells.
Variable; often wedge-shaped infiltrate with associated epidermal hyperplasia and ulcer
Type A
Most common variant (≈75% of all lesions of lymphomatoid papulosis)
Scattered large (25 to 40 µm) CD30-positive mononuclear cells intermingled with mixed inflammatory cell infiltrate (small lymphocytes, neutrophils, eosinophils)
Type B
Dermal infiltrate of small (8 to 15 µm) atypical hyperchromatic lymphocytes with epidermotropism
CD30-positive cells usually absent
Type C
Sheets of large atypical (anaplastic) CD30-positive lymphocytes
Associated mixed inflammation
Type D
Lichenoid epidermotropic
CD8 positive
Type E
Angioinvasive
Immunophenotype
The large cells are positive for CD30; usually also positive for CD4
May coexpress CD56 (10% of cases)
Type B lymphomatoid papulosis is usually negative for CD30
Clonal T-cell receptor gene rearrangement—variable (present or absent)
t(2;5) translocation is absent
Anaplastic large cell lymphoma (types A and C)
Hypersensitivity reactions with CD30-positive cells
Pityriasis lichenoides et varioliformis acuta
Papular mycosis fungoides (type B)
Aggressive epidermotropic CD8-positive cytotoxic T-cell lymphoma (type D)
Angiocentric T-cell lymphoma(s)
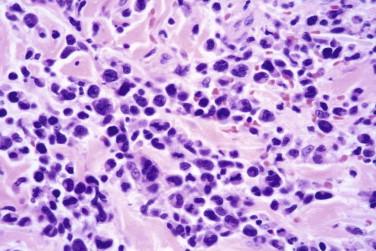
Immunoreactivity for CD30, typically in a membranous pattern with occasional focal cytoplasmic staining, is characteristic ( Fig. 14-31 ). The small to intermediate-sized lymphocytes are often predominantly composed of CD4-positive cells, but there is considerable variation in the CD4 : CD8 ratio. Antigen deletion (CD7, CD2, CD3, CD5) may be seen. Type B LyP may be negative for CD30. Some lesions (particularly in type D LyP) may express CD56 or cytotoxic markers, such as TIA-1 or granzyme B. PCR analysis may or may not demonstrate the presence of a T-cell clone.
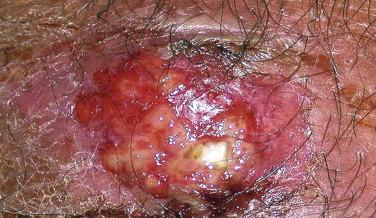
As described earlier, the differential diagnosis of individual lesions of LyP depends on the morphology of the biopsied lesion; examples such as the need to distinguish type B LyP versus MF (especially MF of the papular variant), type C LyP versus ALCL, or type D LyP versus cytotoxic CD8+ T-cell lymphoma by clinical features have been briefly discussed. Ultimately for such cases, correlation with the clinical presentation and course is imperative for the correct diagnosis. If multiple small papular lesions evolve and spontaneously regress, a diagnosis of LyP can be made confidently, irrespective of the proportion of CD30-positive large cells, cytotoxic CD8-positive epidermotropic cells, or MF-like CD30-negative cells. Diagnostic problems usually arise when only a few lesions are present that are intermediate in size (1 to 3 cm), which may persist longer but eventually involute weeks or months after biopsy. If such a lesion rich in CD30-positive large cells eventually clinically regresses and involutes, one should still consider LyP. Because ALCL may also self-involute, a provisional category of a “borderline lesion” of CD30-positive lymphoproliferative disease has been proposed for diagnostically difficult lesions.
A special problem is the presence of LyP-like lesions that have developed within a patch or plaque of MF. If large CD30-positive cells are present, the differential diagnosis is that of secondary LyP in MF versus large cell transformation of MF. Knowledge of the clinical context and evolution of the lesion(s) is necessary for the correct diagnosis. If a patient has a history of LyP or has multiple other lesions of LyP, a small CD30-positive cluster within a patch or plaque of MF should not be too quickly interpreted as large cell lymphoma and should be considered to be LyP unless the lesion persists and grows as would be expected for large cell transformation.
Clinically and histopathologically, LyP may show overlap with pityriasis lichenoides et varioliformis acuta (PLEVA). PLEVA tends to have a higher number of lesions and a shorter overall course of the disease. Although classic PLEVA differs from LyP, some patients with PLEVA may have lesions with histology that is difficult to distinguish from LyP. Furthermore, PLEVA may exhibit CD30 positivity. A clear separation between LyP and PLEVA is not always possible. LyP and PLEVA may also coexist.
When considering LyP and reviewing immunostains for CD30, one should bear in mind that CD30-positive cells can be seen in many non-neoplastic lymphocyte-mediated disorders such as hypersensitivity reactions from an arthropod bite or a drug. The large CD30-positive cells tend to be fewer in these lesions than in LyP, but the clinical history is the key element to making the distinction in appearance to papular MF because the infiltrate is composed predominantly of smaller cerebriform lymphocytes, which are frequently negative for CD30 and demonstrate prominent epidermotropism. Type C LyP is composed of a more prominent population of atypical CD30-positive cells that may be in clusters and sheets, simulating ALCL (see Fig. 14-30 ). Other less frequent features include lymphocytic vasculitis, marked epidermal hyperplasia, and prominent involvement of follicular epithelium. A syringotropic variant, a bandlike presentation similar to MF, and associated keratoacanthoma have also been described.
Lymphomatoid papulosis is a chronic condition that may last decades. Patients with LyP will not die from this condition but may have morbidity related to an associated lymphoma. Treatment of LyP is not always necessary. LyP responds to PUVA, interferon, methotrexate, and systemic retinoids, but because none of these modalities or chemotherapy has been proved to alter the natural history, the condition recurs after discontinuing therapy. Associated cutaneous CD30-positive lymphoma, MF, and systemic Hodgkin's or non-Hodgkin's lymphoma is seen in 5% to 20% of cases. These neoplasms may occur concurrent with, before, or after a diagnosis of LyP.
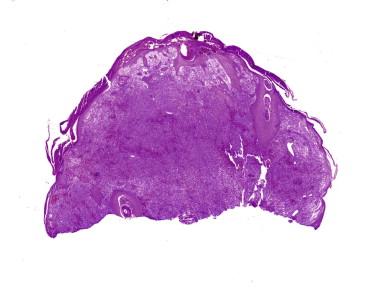
Anaplastic large cell lymphoma, previously referred to as Ki-1 lymphoma or CD30-positive lymphoma, is a neoplasm composed of CD30-positive lymphocytes. Primary cutaneous ALCL is restricted to the skin, but similar tumors may occur in the skin secondarily from systemic ALCL. Primary ALCL may affect any age but is rare in children. After MF, it is the second most common form of primary CTCL. Lesions are usually solitary nodules (greater than 1 to 2 cm) (see Fig. 14-31 ), tumors, or plaques that frequently ulcerate or become necrotic. The extremities are frequently involved as several grouped nodules or papules confined to a region or in multiple anatomic sites. Spontaneous regression may be seen in approximately 40%. Such tumors may arise in a patient with LyP or MF before or after diagnosis of LyP or MF. Tumors with the appearance of an ALCL arising in the context of MF are by convention regarded as large cell transformation, an interpretation that is often supported by shared clonal TCR gene rearrangements.
Primary cutaneous anaplastic large cell lymphoma (ALCL) tends to affect middle-aged or older individuals
May rarely be seen in children
Men are more often affected than women
Systemic ALCL tends to affect young adults as well as elderly people
Head, extremities, and buttock
Usually solitary rapidly growing nodule with ulceration
Nodules are reddish brown and indurated
Favorable prognosis with a 5-year survival rate of 90%
May spontaneously regress
Radiation therapy
Simple excision for localized solitary skin lesion
Biologic therapy such as bexarotene for multifocal disease
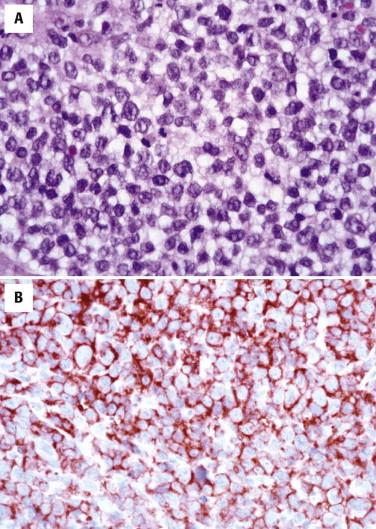
The histology of ALCL can be similar to that described for LyP. However, the lesions tend to be larger and extend more deeply ( Fig. 14-32 ). There are often dense cohesive sheets of CD30-positive cells present comprising the majority of the lesional cell population ( Fig. 14-33 ). Tumor nodules most often appear to be situated in the dermis; however, clusters of the cells may involve intratumoral vessels, or large portions of the tumor may be present intravascularly. Most often the atypical lymphocytes are medium to large in size, with classic intranuclear protrusions, horseshoe- or kidney bean–shaped nuclei, and multinucleation comprising the “hallmark cells” seen in all ALCL ( Fig. 14-34 ). However, the infiltrates may alternatively be composed of small pleomorphic cells. Admixed eosinophils or neutrophils may be numerous. There may be less involvement of the epidermis than in LyP, but acanthosis and secondary epidermal changes with exocytosis or ulceration are also common with ALCL. The pseudoepitheliomatous epidermal hyperplasia overlying lesions of ALCL can occasionally be so exuberant as to simulate squamous cell carcinoma.
Dense nodular or diffuse infiltrate by large atypical mononuclear cells
Epidermotropism common
Secondary surface changes common: ulceration, epidermal hyperplasia
Variable associated mixed inflammatory cell infiltrate of lymphocytes, histiocytes, neutrophils, and eosinophils
Immunophenotype
Large cells are positive for CD30
Variable expression of T-cell markers
Negative for ALK
Clonal TCR gene rearrangement present in most cases (>90%)
Secondary involvement of the skin by systemic anaplastic large cell lymphoma
Leukemia with immunoreactivity for CD30
Nonlymphoid tumors: melanoma, poorly differentiated carcinoma
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here