Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The hematopoietic and lymphoid systems are affected by a wide spectrum of diseases. One useful way to organize these disorders is based on whether they primarily affect red cells, white cells, or the coagulation system, which includes platelets and clotting factors. The most common red cell disorders are those that lead to anemia, a state of red cell deficiency. Clinically significant white cell disorders, by contrast, are most often associated with excessive proliferation resulting from malignant transformation. Derangements in blood coagulation may result in hemorrhagic diatheses (bleeding disorders). Blood products are frequently lifesaving when given to patients but may also produce serious complications, which are reviewed in brief. Finally, splenomegaly, a feature of numerous diseases, is discussed at the end of the chapter, as are tumors of the thymus.
Although these divisions are useful, in reality the production, function, and destruction of red cells, white cells, and components of the hemostatic system are closely linked, and derangements primarily affecting one cell type or component of the system often lead to alterations in others. Other levels of interplay and complexity stem from the anatomically dispersed nature of the hematolymphoid system and the capacity of both normal and malignant white cells to “traffic” between various compartments. Hence, a patient who is diagnosed with lymphoma by lymph node biopsy may also be found to have neoplastic lymphoid cells in the bone marrow and blood. The malignant clone of lymphoid cells in the marrow may suppress hematopoiesis, giving rise to low blood cell counts (cytopenias), and the dissemination of tumor cells to the liver and spleen may lead to organomegaly. Thus, in both benign and malignant hematolymphoid disorders, a single underlying abnormality can result in diverse systemic manifestations. Keeping these complexities in mind, we will use the time-honored classification of hematolymphoid disorders based on predominant involvement of red cells, white cells, and the hemostatic system.
Disorders of red cells can result in anemia or, less commonly, polycythemia (an increase in red cells, also known as erythrocytosis ). Anemia is defined as a decrease in the red cell mass to subnormal levels and results in a reduction of the oxygen-transporting capacity of blood.
Anemia can stem from bleeding, increased red cell destruction (hemolysis), or decreased red cell production. These mechanisms serve as one basis for classifying anemia ( Table 10.1 ). In some entities both mechanisms may occur; for example, in thalassemia, both reduced red cell production and increased destruction contribute to anemia. With the exception of anemia caused by chronic renal failure or chronic inflammation (described later), the decrease in tissue oxygen tension that accompanies anemia triggers increased production of the growth factor erythropoietin from specialized cells in the kidney. Erythropoietin in turn drives a compensatory hyperplasia of erythroid precursors in the bone marrow and, in severe anemia, the induction of extramedullary hematopoiesis within the secondary hematopoietic organs (the liver, spleen, and lymph nodes). In well-nourished persons who become anemic because of acute bleeding or increased red cell destruction (hemolysis), the compensatory response can increase the production of red cells 5- to 8-fold. The marrow response is signaled by the appearance of increased numbers of newly formed red cells (reticulocytes) in the peripheral blood. By contrast, anemia caused by decreased red cell production (aregenerative anemia) is associated with subnormal reticulocyte counts (reticulocytopenia).
| Blood Loss |
|
| Increased Destruction (Hemolytic Anemias) |
| Intrinsic (Intracorpuscular) Abnormalities |
|
| Extrinsic (Extracorpuscular) Abnormalities |
|
| Impaired Red Cell Production |
|
Anemia can also be classified on the basis of red cell morphology, which often points to particular causes. Features that provide etiologic clues include the size, color, and shape of the red cells. These are judged subjectively by visual inspection of peripheral smears and also are expressed quantitatively using the following indices:
Mean cell volume (MCV): the average volume of each red cell, expressed in femtoliters (cubic microns)
Mean corpuscular hemoglobin (MCH): the average mass of hemoglobin per red cell, expressed in picograms
Mean corpuscular hemoglobin concentration (MCHC): the average concentration of hemoglobin in a given volume of packed red cells, expressed in grams per deciliter
Red cell distribution width (RDW): the coefficient of variation of red cell volume
Red cell indices are quantified by specialized instruments in clinical laboratories. The same instruments also determine the reticulocyte count, a simple measure that distinguishes between hemolytic anemia and anemia due to reduced production (see later). Adult reference ranges for these tests are shown in Table 10.2 . Depending on the differential diagnosis, a number of other blood tests may also be performed to evaluate anemia, including (1) serum iron indices (iron levels, iron-binding capacity, transferrin saturation, and ferritin concentrations), which help distinguish among microcytic anemia caused by iron deficiency, chronic inflammation, or thalassemia; (2) plasma unconjugated bilirubin, haptoglobin, and lactate dehydrogenase levels, which are abnormal in hemolytic anemia; (3) serum and red cell folate and vitamin B 12 concentrations, which are low in megaloblastic anemia; (4) hemoglobin electrophoresis, which is used to detect abnormal hemoglobins; and (5) the Coombs test, which is used to detect antibodies or complement bound to red cells in suspected cases of antibody-mediated hemolytic anemia. In isolated anemia, tests performed on the peripheral blood usually suffice to establish the cause. By contrast, when anemia occurs along with thrombocytopenia and/or granulocytopenia, it is much more likely to be associated with marrow aplasia or infiltration; in such instances a marrow examination is usually warranted.
| Units | Men | Women | |
|---|---|---|---|
| Hemoglobin (Hb) | g/dL | 13.2–16.6 | 11.6–15.0 |
| Hematocrit (Hct) | % | 38–49 | 35–45 |
| Red cell count | ×10 6 /μL | 4.4–5.6 | 3.9–5.1 |
| Reticulocyte count | % | 0.6–2.7 | 0.6–2.7 |
| Mean cell volume (MCV) | fL | 78–98 | 78–98 |
| Mean cell Hb (MCH) | pg | 26–34 | 26–34 |
| Mean cell Hb concentration (MCHC) | g/dL | 32–36 | 31–36 |
| Red cell distribution width (RDW) | 11.8–14.5 | 12.2–16.1 |
a Reference ranges vary among laboratories. The reference ranges for the laboratory providing the result should always be used in interpreting a laboratory test. Reference values from https://mayocliniclabs.com/ by permission of Mayo Foundation for Medical Education and Research. All rights reserved.
As discussed later, the clinical consequences of anemia are determined by its severity, rapidity of onset, and underlying pathogenic mechanism. If the onset is slow, the deficit in O 2 -carrying capacity is compensated for by increases in cardiac output, respiratory rate, and red cell 2,3-diphosphoglycerate (DPG), a glycolytic pathway intermediate that enhances the release of O 2 from hemoglobin. These adaptive changes mitigate the effects of mild to moderate anemia in otherwise healthy persons but are less effective in those with compromised pulmonary or cardiac function. Pallor, fatigue, and lassitude are common to all forms of anemia. Features that are specific to various subtypes are discussed in the following sections.
Anemia of blood loss can be divided into anemia caused by acute bleeding (hemorrhage) and anemia caused by chronic blood loss (described later). The effects of acute bleeding are mainly due to the loss of intravascular volume, which if greater than 20%, can lead to cardiovascular collapse, shock, and death. If the patient survives and is resuscitated with oral or intravenous fluids, hemodilution begins at once and achieves its full effect within 2 to 3 days; only then is the full extent of the red cell loss revealed. The anemia is normocytic and normochromic. Recovery from blood loss is enhanced by a compensatory rise in erythropoietin, which stimulates increased red cell production and reticulocytosis following a lag of 5 to 7 days.
With chronic blood loss, iron stores are gradually depleted. Iron is essential for hemoglobin synthesis and erythropoiesis, and its deficiency leads to a chronic anemia of underproduction. Iron deficiency anemia can occur in other clinical settings as well; it is described later along with other forms of anemia caused by decreased red cell production.
Hemolytic anemias are a diverse group of disorders that have as a common feature accelerated red cell destruction. The red cell life span is shortened to less than its normal 120 days, often markedly so. The resulting anemia and low tissue O 2 levels stimulate erythropoietin release from the kidney, leading to increased production of reticulocytes by the bone marrow. Thus, marrow erythroid hyperplasia and peripheral blood reticulocytosis are hallmarks of hemolytic anemias. In severe hemolytic anemias, the erythropoietic drive may be so pronounced that extramedullary hematopoiesis appears in the liver, spleen, and lymph nodes.
There are several ways to organize hemolytic anemias. One approach groups them according to whether the pathogenic red cell defect is intrinsic (intracorpuscular) or extrinsic (extracorpuscular) (see Table 10.1 ). A second more clinically useful approach groups hemolytic anemias according to whether hemolysis is primarily extravascular or intravascular. Extravascular hemolysis is caused by defects that increase the destruction of red cells by phagocytes, particularly in the spleen. The spleen contains large numbers of macrophages, the principal cells responsible for the removal of damaged or antibody-coated red cells from the circulation. Because marked alterations of shape are necessary for red cells to navigate the splenic sinusoids, any reduction in red cell deformability makes this passage difficult, and red cells that become “stuck” are phagocytosed by resident splenic macrophages. As described later in the chapter, diminished deformability is a major cause of red cell destruction in several hemolytic anemias. Findings that are relatively specific for extravascular hemolysis (as compared to intravascular hemolysis) include the following:
Hyperbilirubinemia and jaundice, stemming from degradation of hemoglobin in macrophages
Varying degrees of splenomegaly due to “work hyperplasia” of phagocytes in the spleen
If long-standing, increased risk of cholelithiasis with formation of bilirubin-rich gallstones (pigment stones)
Intravascular hemolysis , by contrast, is characterized by injuries so severe that red cells burst within the circulation. Intravascular hemolysis may result from mechanical forces (e.g., turbulence created by a defective heart valve) or biochemical or physical agents that severely damage the red cell membrane (e.g., fixation of complement or exposure to clostridial toxins or heat). Findings that distinguish intravascular hemolysis from extravascular hemolysis include the following:
Hemoglobinemia, hemoglobinuria, and hemosiderinuria. Hemoglobin released into the circulation is small enough to pass into the urinary space. Here, it is partially resorbed by renal tubular cells and processed into hemosiderin, which is then lost in the urine when renal tubular cells are sloughed.
Loss of iron, which may lead to iron deficiency if hemolysis is persistent. By contrast, iron recycling by phagocytes is very efficient, and so iron deficiency is not a feature of extravascular hemolytic anemias.
A feature of both intravascular and extravascular hemolysis is decreased serum levels of haptoglobin, a plasma protein that binds free hemoglobin and is then removed from the circulation. Apparently, macrophages “regurgitate” sufficient hemoglobin during consumption of red cells to cause haptoglobin levels to fall, even when hemolysis is entirely extravascular.
We now turn to some of the relatively common hemolytic anemias.
This disorder stems from inherited (intrinsic) defects in the red cell membrane that lead to the formation of spherocytes, nondeformable cells that are highly vulnerable to sequestration and destruction in the spleen. Hereditary spherocytosis is usually transmitted as an autosomal dominant trait; a more severe, autosomal recessive form of the disease affects a small minority of patients.
Hereditary spherocytosis is caused by inherited defects in the membrane skeleton, a network of proteins that stabilizes the lipid bilayer of the red cell ( Fig. 10.1 ). The major membrane skeleton protein is spectrin, a long, flexible heterodimer that self-associates at one end and binds short actin filaments at its other end. These contacts create a two-dimensional meshwork that is connected to the intrinsic membrane proteins band 3 and glycophorin via linker proteins like ankyrin and band 4.1. The common feature of the mutations that cause hereditary spherocytosis is that they weaken interactions between the membrane skeleton and intrinsic red cell membrane proteins. This results in the destabilization of the lipid bilayer of red cells, which shed membrane vesicles into the circulation as they age. Little cytoplasm is lost in the process and as a result the surface area-to-volume ratio decreases progressively with time until the cells become spherical ( Fig. 10.1 ).
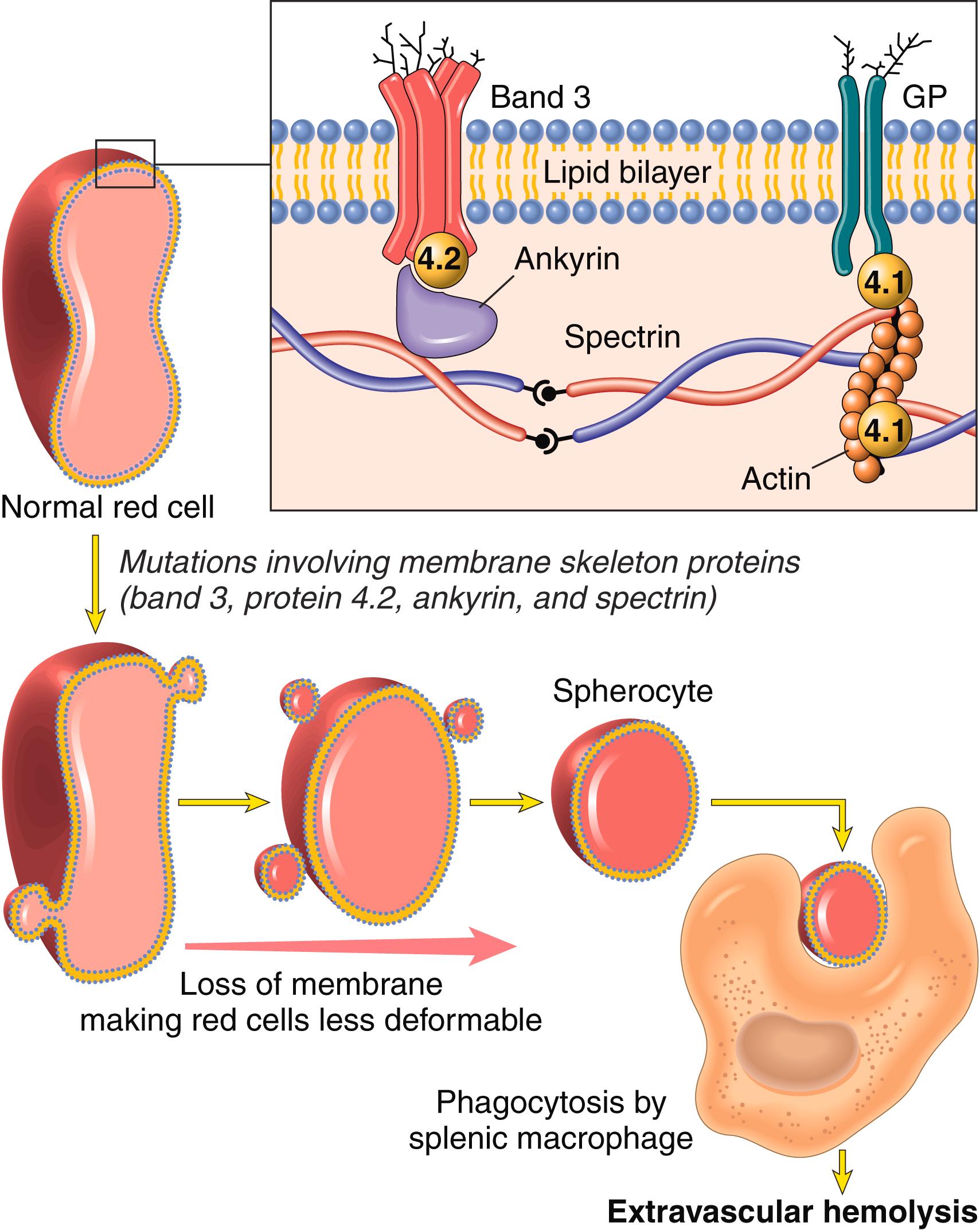
The floppy discoid shape of normal red cells allows considerable latitude for shape changes. By contrast, spherocytes have limited deformability and are sequestered in the splenic cords, where they are destroyed by the plentiful resident macrophages ( Fig. 10.1 ). The critical role of the spleen in hereditary spherocytosis is illustrated by the beneficial effect of splenectomy; although the red cell defect and spherocytes persist, the anemia is corrected.
On peripheral blood smears, spherocytes are dark red and lack central pallor ( Fig. 10.2 ). The excessive red cell destruction and resultant anemia lead to a compensatory hyperplasia of red cell progenitors in the marrow and an increase in red cell production marked by reticulocytosis. Splenomegaly is more common and prominent in hereditary spherocytosis than in other forms of hemolytic anemia. The splenic weight is usually between 500 g and 1000 g (normal, 150 g to 200 g). The enlargement results from marked congestion of the splenic cords and increased numbers of macrophages. Phagocytosed red cells are seen within macrophages lining the sinusoids and within the cords. Other general features of hemolytic anemia may also be seen, including cholelithiasis, which occurs in 40% to 50% of patients with hereditary spherocytosis.
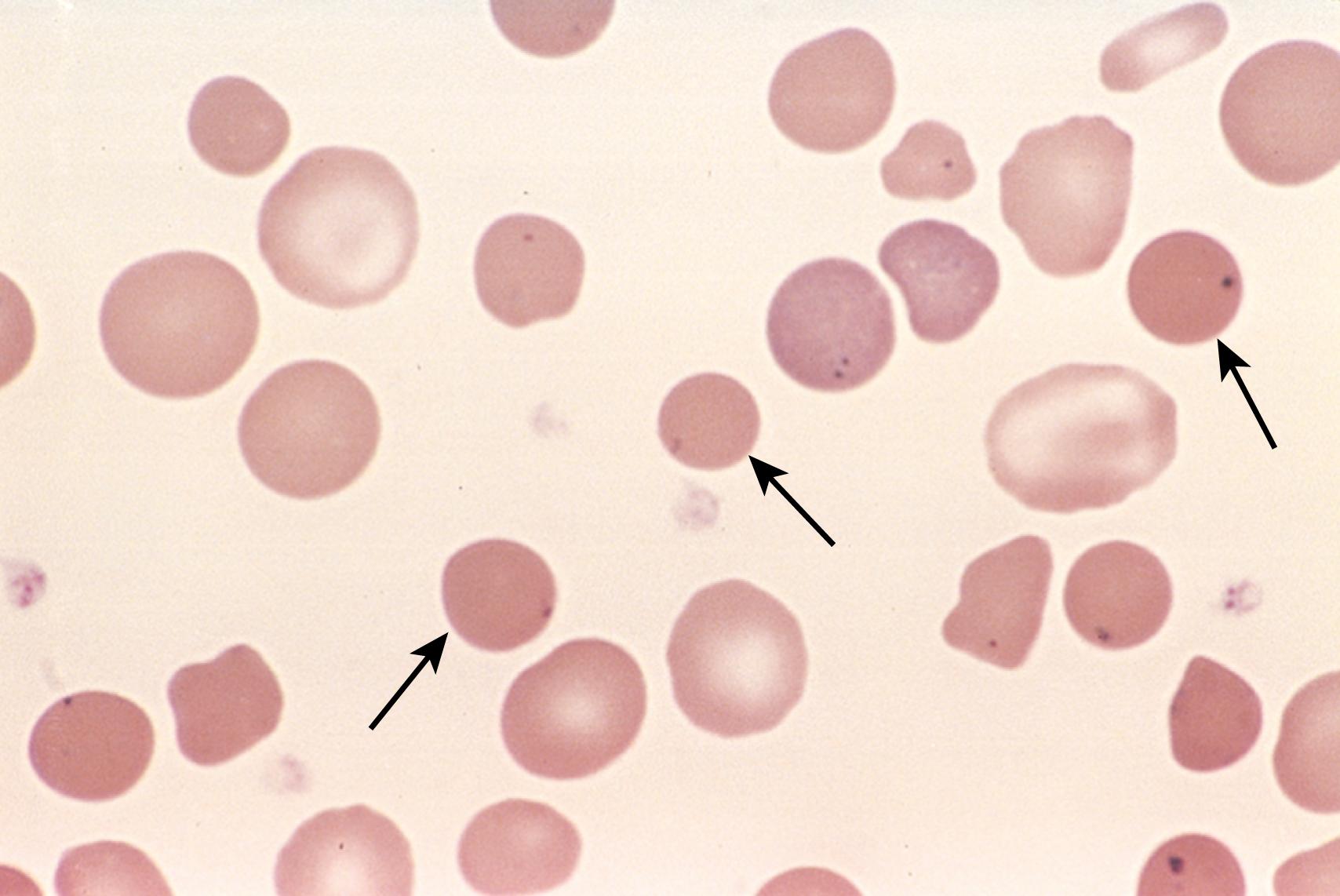
The characteristic features are anemia, splenomegaly, and jaundice. The anemia is variable in severity, ranging from subclinical to profound; most commonly it is moderate in degree. Because of their spherical shape, red cells in hereditary spherocytosis show increased osmotic fragility when placed in hypotonic salt solutions, a characteristic that can help establish the diagnosis.
The course is generally stable but may be punctuated by aplastic crises, the most severe of which are triggered by parvovirus B19 infection . This virus has a marked tropism for erythroblasts, which undergo apoptosis during viral replication. Until the immune response controls the infection (usually in 10 to 14 days), the marrow may be virtually devoid of red cell progenitors. Because of the shortened life span of red cells in hereditary spherocytosis, a lack of red cell production, even for a few days, results in rapid worsening of the anemia. Blood transfusions may be needed to support patients until the infection is cleared.
There is no specific treatment. Splenectomy improves the anemia by removing the major site of red cell destruction. The benefits of splenectomy must be weighed against the increased risk of serious infections by encapsulated bacteria, particularly in children. Partial splenectomy is gaining favor in young children because this approach produces hematologic improvement while maintaining protection against sepsis. The downside is that because the partially resected spleen eventually regains its size, many patients will need a second resection. The hope is that this can be delayed until later in childhood, when the risk of serious infection is lower.
Hemoglobinopathies are a group of hereditary disorders caused by inherited mutations that lead to structural abnormalities in hemoglobin. Sickle cell anemia, the prototypic hemoglobinopathy, is caused by a mutation in β-globin that creates sickle hemoglobin (HbS). Numerous other hemoglobinopathies have been described, but these are less common and beyond the scope of this discussion.
Sickle cell anemia is the most common familial hemolytic anemia. The presence of HbS is protective against falciparum malaria and because of this selective pressure the HbS allele is prevalent in areas where malaria was (and, in some instances, still is) endemic, including equatorial Africa and parts of India, southern Europe, and the Middle East. In the United States, approximately 8% of people of African descent are heterozygous HbS carriers, and about 1 in 600 have sickle cell anemia.
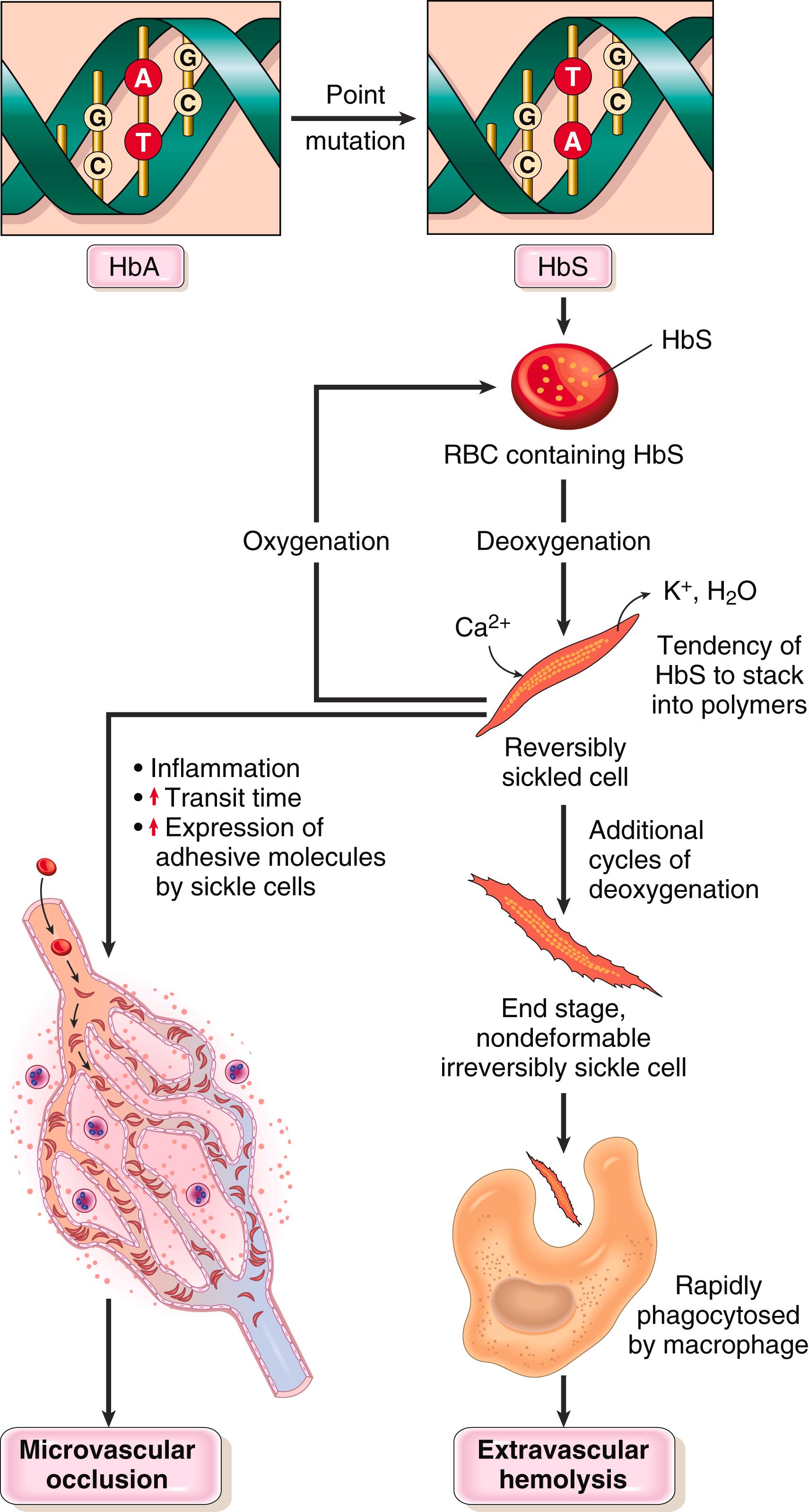
Sickle cell anemia is caused by a single amino acid substitution in β-globin that results in a tendency for deoxygenated HbS to self-associate into polymers. Normal hemoglobins are tetramers composed of two pairs of similar chains. On average, the normal adult red cell contains 96% HbA (α 2 β 2 ), 3% HbA2 (α 2 δ 2 ), and 1% fetal Hb (HbF, α 2 γ 2 ). In patients with sickle cell anemia, HbA is completely replaced by HbS, whereas in heterozygous carriers, only about half is replaced. HbS differs from HbA by having a valine residue instead of a glutamate residue at the 6th amino acid position in β-globin. On deoxygenation HbS molecules undergo a conformational change that allows polymers to form via intermolecular contacts involving the abnormal valine residue. These polymers distort the red cell, which assumes an elongated crescentic or sickle shape ( Fig. 10.3 ).
The sickling of red cells is initially reversible on reoxygenation. However, membrane distortion produced by each sickling episode leads to an influx of calcium, which causes the loss of potassium and water and also damages the membrane skeleton. With time, this cumulative damage creates irreversibly sickled cells that are prone to hemolysis.
Three factors are particularly important in determining whether clinically significant polymerization of HbS occurs in patients:
The intracellular levels of hemoglobins other than HbS. In heterozygotes approximately 40% of Hb is HbS and the remainder is HbA, which interacts only weakly with deoxygenated HbS. Because HbA greatly retards HbS polymerization, the red cells of HbS heterozygotes have little tendency to sickle in vivo. Such persons are said to have sickle cell trait. Similarly, because fetal hemoglobin (HbF) interacts weakly with HbS, newborns with sickle cell anemia do not manifest the disease until HbF falls to adult levels, generally around the age of 5 to 6 months. Hemoglobin C (HbC), another mutant β-globin, has a lysine residue instead of the normal glutamic acid residue at position 6. Like HbS, HbC confers protection to falciparum malaria and is prevalent in similar populations (sub-Saharan Africa, parts of India, etc.). Due to equatorial African ancestry, about 2.3% of Americans of African descent are heterozygous carriers of HbC, and about 1 in 1250 are compound HbC/HbS heterozygotes.
The intracellular concentration of HbS. The polymerization of deoxygenated HbS is strongly concentration dependent. Thus, red cell dehydration, which increases the Hb concentration, facilitates sickling. Conversely, the coexistence of α-thalassemia (described later), which decreases the Hb concentration, reduces sickling.
The time required for red cells to pass through the microvasculature. The normal transit times of red cells through capillary beds are too short for significant polymerization of deoxygenated HbS to occur. Hence, the tissues that are most susceptible to obstruction by sickle cells are those in which blood flow is sluggish, such as the spleen and the bone marrow. However, sickling may occur in other microvascular beds in the presence of factors that retard the passage of red cells, particularly inflammation. Recall that inflammation slows blood flow by increasing the adhesion of leukocytes and red cells to endothelium and by inducing the exudation of fluid through leaky vessels ( Chapter 2 ). In addition, sickle red cells have a greater tendency than normal red cells to adhere to endothelial cells, as repeated bouts of sickling cause membrane damage that makes the red cells abnormally “sticky.” These factors conspire to prolong the transit times of sickle red cells, increasing the probability of clinically significant vascular obstruction.
The sickling of red cells has two major pathologic consequences: chronic moderately severe hemolytic anemia, due to red cell membrane damage; and vascular obstructions, which result in ischemic tissue damage and pain crises ( Fig. 10.4 ). The mean life span of red cells in sickle cell anemia averages only 20 days (one-sixth of normal) and the severity of the hemolysis correlates with the fraction of irreversibly sickled cells that are present in the blood. Vasoocclusion, by contrast, is not related to the number of irreversibly sickled cells and instead appears to be triggered by superimposed factors such as infection, inflammation, dehydration, and acidosis, all of which enhance the tendency of red cells to arrest and sickle within the microvasculature.
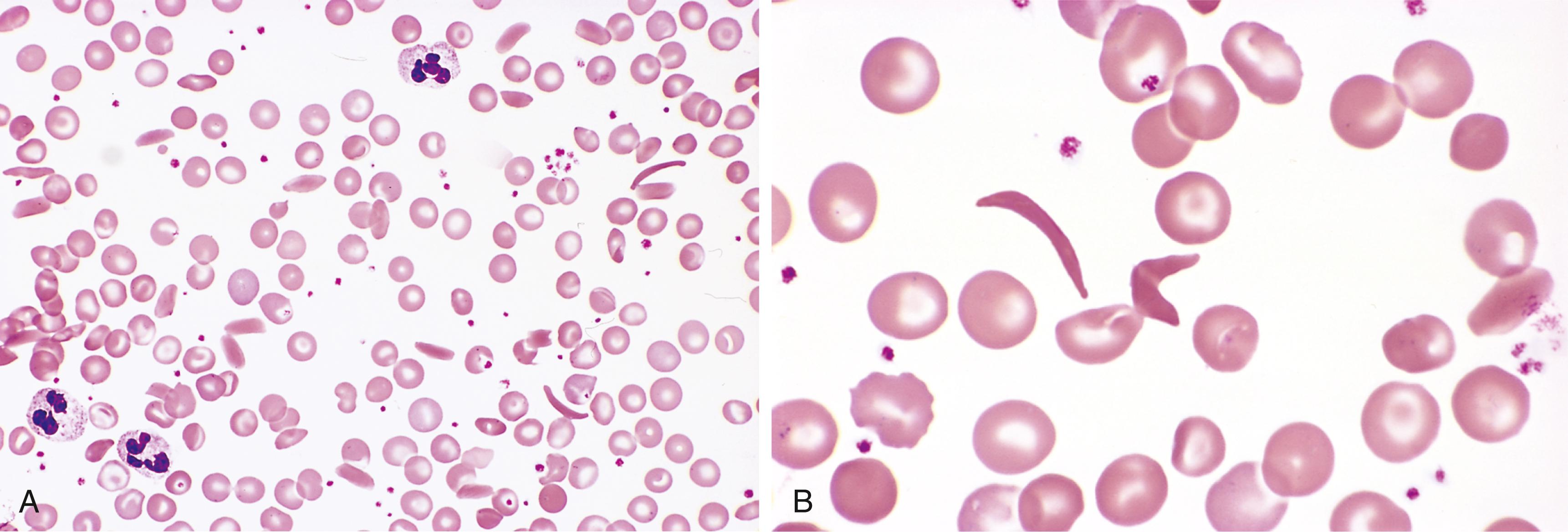
The abnormalities in sickle cell anemia stem from (1) chronic hemolytic anemia, (2) increased breakdown of heme to bilirubin, and (3) microvascular obstructions, which provoke tissue ischemia and infarction. In peripheral smears, elongated, spindled, or boat-shaped irreversibly sickled red cells are evident (see Fig. 10.3 ). Both the anemia and the vascular stasis produce hypoxia-induced fatty changes in the heart, liver, and renal tubules. There is a compensatory hyperplasia of erythroid progenitors in the marrow. The cellular proliferation in the marrow often causes bone resorption and secondary new bone formation, resulting in prominent cheekbones and changes in the skull resembling a “crewcut” in radiographs. Extramedullary hematopoiesis may appear in the liver and spleen.
In children there is moderate splenomegaly (splenic weight up to 500 g) due to red pulp congestion caused by entrapped sickled red cells. However, chronic splenic red cell stasis produces hypoxic damage and infarcts, which with time reduce the spleen to a useless nubbin of fibrous tissue. This process, referred to as autosplenectomy, is complete by adulthood.
Vascular congestion, thrombosis, and infarction can affect any organ, including the bones, liver, kidney, retina, brain, lung, and skin. The bone marrow is particularly prone to ischemia because of its sluggish blood flow and high rate of metabolism. Priapism, another frequent problem, can lead to penile fibrosis and erectile dysfunction. As with the other hemolytic anemias, pigment gallstones are common.
Sickle cell disease is marked by chronic hemolytic anemia and superimposed vasoocclusive crises. It is usually asymptomatic until 6 months of age when the shift from HbF to HbS is complete. The anemia is moderate to severe; most patients have hematocrits of 18% to 30% (normal range, 38% to 48%). The chronic hemolysis is associated with hyperbilirubinemia and compensatory reticulocytosis.
Much more serious are vasoocclusive crises, which are characteristically associated with pain and often lead to tissue damage and significant morbidity and mortality. Among the most common and serious of these crises are the following:
Hand-foot syndrome, resulting from infarction of bones in the hands and feet, is the most common presenting symptom in young children.
Acute chest syndrome, in which sluggish blood flow in an inflamed lung (e.g., an area of pneumonia) leads to sickling within hypoxemic pulmonary beds. This exacerbates pulmonary dysfunction, creating a vicious circle of worsening pulmonary and systemic hypoxemia, sickling, and vasoocclusion. Acute chest syndrome may also be triggered by fat emboli emanating from infarcted bone.
Stroke, which sometimes occurs in the setting of the acute chest syndrome. Stroke and the acute chest syndrome are the two leading causes of ischemia-related death.
Proliferative retinopathy, a consequence of vasoocclusions in the eye that can lead to loss of visual acuity and blindness.
Another acute event, aplastic crisis, is caused by a sudden decrease in red cell production. As in hereditary spherocytosis, this is usually triggered by infection of erythroblasts by parvovirus B19 and, although severe, is self-limited.
In addition to these crises, patients with sickle cell disease are prone to infections. Both children and adults with sickle cell disease are functionally asplenic, making them susceptible to infections caused by encapsulated bacteria, such as pneumococci. In adults the basis for “hyposplenism” is autoinfarction. In the earlier childhood phase of splenic enlargement, congestion caused by trapped sickled red cells apparently interferes with bacterial sequestration and killing; hence, even children with enlarged spleens are at risk for developing fatal septicemia. Patients with sickle cell disease are also predisposed to bacterial osteomyelitis, which may result from bacterial seeding of infarcted bone. The most common causative organisms are encapsulated bacterial species and gram-negative organisms, particularly E. coli and Salmonella .
In homozygous sickle cell disease, irreversibly sickled red cells are seen in routine peripheral blood smears. In sickle cell trait, sickling can be induced in vitro by exposing cells to hypoxia. In the United States, newborn screening for sickle cell disease is now mandated; HbS and other hemoglobins are identified by methods such as gel electrophoresis in blood obtained by heel-stick. Prenatal diagnosis of sickle cell anemia is performed by analyzing fetal DNA obtained by amniocentesis or biopsy of chorionic villi.
The clinical course of sickle cell disease is highly variable. As a result of improvements in supportive care, approximately 50% of patients now survive beyond the fifth decade. Of particular importance is vaccination and prophylactic treatment with penicillin to prevent pneumococcal infections, especially in children younger than age 5. A mainstay of therapy is hydroxyurea, a “gentle” inhibitor of DNA synthesis. Hydroxyurea reduces pain crises and lessens the anemia through several effects, including (1) an increase in levels of HbF; (2) an anti-inflammatory effect because of the inhibition of white cell production; (3) an increase in red cell size, which lowers the intracellular hemoglobin concentration; and (4) its metabolism to NO, a potent vasodilator and inhibitor of platelet aggregation. More recently, encouraging results have been obtained with allogeneic bone marrow transplantation and corrective gene therapy, both of which are potentially curative.
Thalassemias are inherited disorders caused by mutations in globin genes that decrease the synthesis of α- or β-globin. Decreased synthesis of one globin chain results not only in a deficiency of Hb but also in the formation of intracellular precipitates from the excess of unpaired normal globin chain that cause red cell damage and hemolysis. The mutations that cause thalassemia are particularly common in Mediterranean, African, and Asian regions in which malaria is endemic. As with HbS, it is hypothesized that globin mutations associated with thalassemia protect against falciparum malaria.
A diverse collection of α-globin and β-globin mutations underlie the thalassemias, which are autosomal codominant conditions. As described previously, adult hemoglobin, or HbA, is a tetramer composed of two α chains and two β chains. The α chains are encoded by two α-globin genes lying in tandem on chromosome 16, whereas the β chains are encoded by a single β-globin gene located on chromosome 11. The clinical features vary widely depending on the specific combination of mutated alleles that are inherited by the patient ( Table 10.3 ).
| Clinical Syndrome | Genotype | Clinical Features | Molecular Genetics |
|---|---|---|---|
| β -Thalassemias | Mainly point mutations that lead to defects in the transcription, splicing, or translation of β-globin mRNA | ||
|
Homozygous β-thalassemia (β 0 /β 0 , β + /β + , β 0 /β + ) | Severe anemia; regular blood transfusions required | |
|
Variable (β 0 /β + , β + /β + , β 0 /β, β + /β) | Moderately severe anemia; regular blood transfusions not required | |
|
Heterozygous β-thalassemia (β 0 /β, β + /β) | Asymptomatic with mild or absent anemia; red cell abnormalities seen | |
| α -Thalassemias | Mainly gene deletions | ||
|
−/α, α/α | Asymptomatic; no red cell abnormality | |
|
−/−, α/α (Asian) −/α, −/α (black African, Asian) |
Asymptomatic, resembles β-thalassemia minor | |
|
−/−, −/α | Moderately severe; resembles β-thalassemia intermedia | |
|
−/−, −/− | Lethal in utero without transfusions |
Mutations associated with β-thalassemia fall into two categories: (1) β 0 , in which no β-globin chains are produced; and (2) β + , in which there is reduced (but detectable) β-globin synthesis. Sequencing of β-thalassemia genes has shown more than 100 different causative mutations, a majority consisting of single-base changes. Persons inheriting one abnormal allele have β -thalassemia minor (also known as β -thalassemia trait ), which is asymptomatic or mildly symptomatic. Most people inheriting any two β 0 and β + alleles have β-thalassemia major ; occasionally, persons inheriting at least one β + allele have a milder disease termed β-thalassemia intermedia . In contrast with α-thalassemia (described later), gene deletions rarely underlie β-thalassemia ( Table 10.3 ).
The mutations responsible for β-thalassemia are diverse and disrupt β-globin synthesis in several ways. The most common lead to abnormal RNA splicing, whereas others lie in the β-globin gene promoter (leading to decreased transcription) or in coding regions (leading to decreased translation). The specific nature of the mutation determines whether the outcome is a β + or β 0 allele.
Defective synthesis of β-globin in β-thalassemia contributes to anemia through two mechanisms: (1) inadequate HbA formation, resulting in small (microcytic), poorly hemoglobinized (hypochromic) red cells; and (2) accumulation of unpaired α-globin chains, which form toxic precipitates that severely damage the membranes of red cells and erythroid precursors. A large fraction of erythroid precursors is so badly damaged that they die by apoptosis in the bone marrow ( Fig. 10.5 ), a phenomenon termed ineffective erythropoiesis, and the few red cells that are produced have a shortened life span. Ineffective hematopoiesis is also associated with an inappropriate increase in the absorption of dietary iron, which without medical intervention inevitably leads to iron overload . The increased iron absorption is caused by low plasma levels of hepcidin, a critical negative regulator of iron absorption that is discussed later.
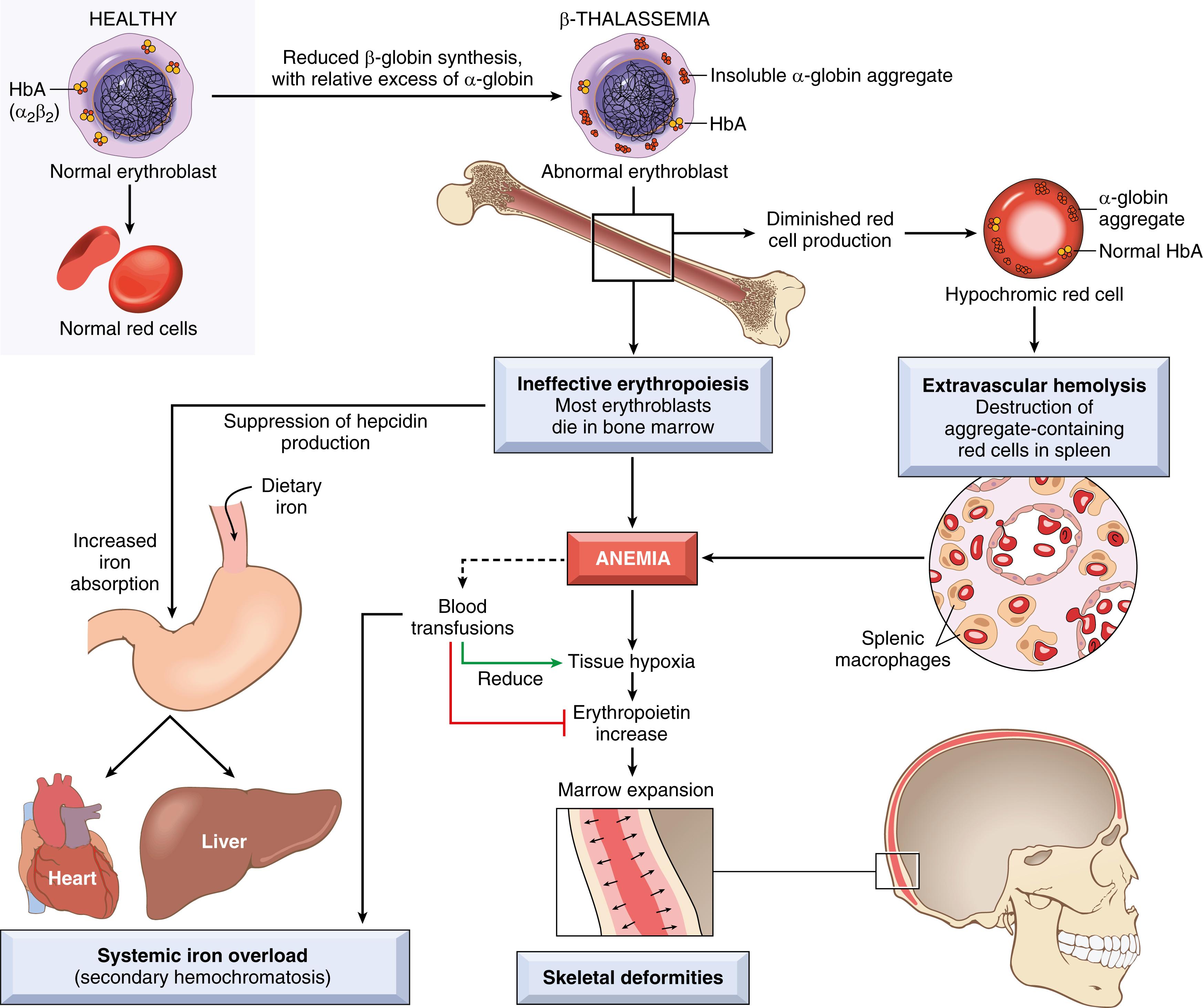
Unlike β-thalassemia, α-thalassemia is caused mainly by deletions involving one or more of the α-globin genes. The severity of the disease is proportional to the number of α-globin genes that are deleted (see Table 10.3 ). For example, loss of a single α-globin gene produces a silent-carrier state, whereas deletion of all four α-globin genes is lethal in utero because the red cells have virtually no oxygen-delivering capacity. With loss of three α-globin genes there is a relative excess of β-globin or (early in life) γ-globin chains. Excess β-globin and γ-globin chains form relatively stable β4 and γ4 tetramers known as HbH and Hb Bart, respectively, which cause less membrane damage than the free α-globin chains that are found in β-thalassemia; as a result, ineffective erythropoiesis is less pronounced in α-thalassemia. Unfortunately, both HbH and Hb Bart have an abnormally high affinity for oxygen, which prevents release of oxygen in tissues and thus renders them ineffective at delivering oxygen.
A range of morphologies is seen, depending on the specific underlying molecular lesion. On one end of the spectrum is β-thalassemia minor and α-thalassemia trait, in which abnormalities are confined to the peripheral blood. In smears, the red cells are small (microcytic) and pale (hypochromic), but regular in shape. Often seen are target cells, cells with an increased surface area-to-volume ratio that allows the cytoplasm to collect in a central dark red “puddle.” On the other end of the spectrum, in β-thalassemia major peripheral blood smears show marked microcytosis, hypochromia, poikilocytosis (variation in cell shape), and anisocytosis (variation in cell size). Nucleated red cells (normoblasts) are also seen that reflect the underlying erythropoietic drive. β-Thalassemia intermedia and HbH disease are associated with peripheral smear findings that lie between these two extremes.
The anatomic changes in β-thalassemia major are similar to those seen in other hemolytic anemias but profound in degree. Ineffective erythropoiesis and hemolysis result in a striking hyperplasia of erythroid progenitors, with a shift toward early forms. The expanded erythropoietic marrow may completely fill the intramedullary space of the skeleton, invade the bony cortex, impair bone growth, and produce skeletal deformities. Extramedullary hematopoiesis and hyperplasia of mononuclear phagocytes result in prominent splenomegaly, hepatomegaly, and lymphadenopathy. The ineffective erythropoietic precursors consume nutrients and produce growth retardation and a degree of cachexia reminiscent of that seen in cancer patients. Unless steps are taken to prevent iron overload, during the span of years severe hemosiderosis develops (see Fig. 10.5 ). HbH disease and β-thalassemia intermedia are also associated with splenomegaly, erythroid hyperplasia, and growth retardation related to anemia, but these are less severe than in β-thalassemia major.
β-Thalassemia trait and α-thalassemia trait are typically asymptomatic. There is usually only a mild microcytic hypochromic anemia; these patients have a normal life expectancy. Iron deficiency anemia is associated with a similar red cell appearance and must be excluded by appropriate laboratory tests (described later).
β-Thalassemia major manifests postnatally as HbF synthesis diminishes. Affected children have growth retardation that commences in infancy. They are sustained by blood transfusions, which improve the anemia and reduce the skeletal deformities associated with excessive erythropoiesis. With transfusions alone, survival into the second or third decade is possible, but systemic iron overload gradually develops owing to inappropriate uptake of iron from the gut and the iron load in transfused red cells. The excessive uptake of iron from the gut occurs because the expanded pool of erythroblasts secrete a hormone called erythroferrone, which circulates to the liver and suppresses the release of hepcidin (described later). Unless patients are treated aggressively with iron chelators, cardiac dysfunction from secondary hemochromatosis inevitably develops and often is fatal in the second or third decade of life. When feasible, hematopoietic stem cell transplantation at an early age is the treatment of choice.
HbH disease and β-thalassemia intermedia are not as severe as β-thalassemia major because the imbalance in globin chain synthesis is not as profound and hematopoiesis is more effective. Anemia is of moderate severity, patients usually do not require transfusions, and iron overload is rarely seen.
The diagnosis of β-thalassemia major can be strongly suspected on clinical grounds. Hb electrophoresis shows a profound reduction or absence of HbA and increased levels of HbF. The HbA 2 level may be normal or increased. Similar but less severe changes are noted in patients affected by β-thalassemia intermedia. Prenatal diagnosis of β-thalassemia is challenging but can be made in specialized centers by DNA analysis. In fact, thalassemia was the first disease diagnosed by DNA-based tests, opening the way for the field of molecular diagnostics. The diagnosis of β-thalassemia minor is made by Hb electrophoresis, which typically shows a reduced level of HbA (α 2 β 2 ) and an increased level of HbA 2 (α 2 δ 2 ). HbH disease can be diagnosed by detection of β4 tetramers by electrophoresis.
Red cells are constantly exposed to both endogenous and exogenous oxidants, which are normally inactivated by reduced glutathione (GSH). Abnormalities affecting enzymes responsible for the synthesis of GSH leave red cells vulnerable to oxidative injury and hemolysis. By far the most common of these conditions is glucose-6-phosphate dehydrogenase (G6PD) deficiency. The G6PD gene is on the X chromosome. More than 400 G6PD variants have been identified, but only a few are associated with disease.
G6PD deficiency is typically associated with transient episodes of intravascular hemolysis caused by exposure to an environmental factor (usually infectious agents or drugs) that produces oxidant stress. Incriminated drugs include antimalarials (e.g., primaquine), sulfonamides, nitrofurantoin, phenacetin, aspirin (in large doses), and vitamin K derivatives. Consumption of certain foods, such as fava beans, may also lead to hemolysis. More commonly, episodes of hemolysis are triggered by infection, which induce phagocytes to generate oxidants as part of the host response. These oxidants, such as hydrogen peroxide, are normally sopped up by GSH, which is converted to oxidized GSH in the process. Because regeneration of GSH is impaired in G6PD-deficient cells, oxidants are free to “attack” other red cell components, including globin chains. Oxidized hemoglobin denatures and precipitates, forming intracellular inclusions called Heinz bodies, which can damage the red cell membrane so severely that intravascular hemolysis results. Other cells with less damage lose their deformability and suffer further injury when splenic phagocytes attempt to “pluck out” the Heinz bodies, creating bite cells ( Fig. 10.6 ). Such cells become trapped on recirculation to the spleen and are destroyed by phagocytes.
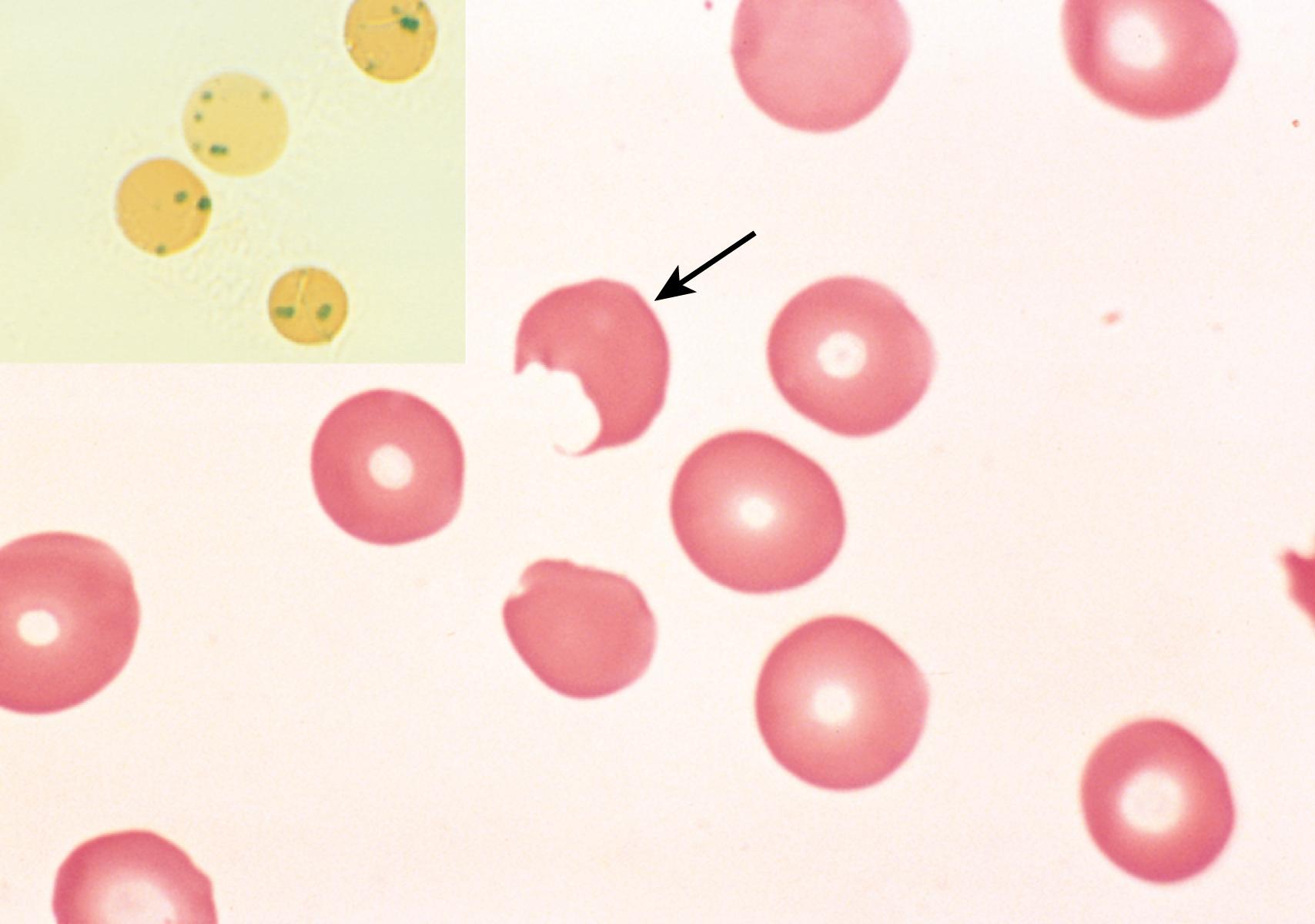
Hemolysis typically develops 2 or 3 days after drug exposure and is of variable severity. Because G6PD is X-linked, the red cells of affected males are uniformly deficient and vulnerable to oxidant injury. By contrast, random inactivation of one X chromosome in heterozygous females ( Chapter 4 ) creates two populations of red cells, one normal and the other G6PD deficient. Most carrier females are unaffected except for those with a large proportion of deficient red cells (a chance situation known as unfavorable lyonization). Susceptibility to hemolysis among different mutated forms of G6PD varies according to the degree of G6PD deficiency. In the case of the G6PD A - variant, which is common in areas of Africa where malaria is endemic, the half-life of the variant is only modestly decreased. As a result, only older red cells are susceptible to lysis. Because the marrow compensates for the anemia by increasing its production of new red cells with adequate levels of G6PD, the hemolysis abates even if the drug exposure continues. Other variants such as G6PD Mediterranean, found mainly in the Middle East, produce more marked enzyme deficiency and as a result the hemolysis that occurs on exposure to oxidants is more severe.
Paroxysmal nocturnal hemoglobinuria (PNH) is a hemolytic anemia that stems from acquired mutations in PIGA , a gene required for the synthesis of phosphatidylinositol glycan (PIG), which serves as a membrane anchor for many proteins. Because PIGA is X-linked, normal cells have only one active PIGA gene, mutation of which is sufficient to cause PIGA deficiency. The pathogenic mutations in PNH occur in an early hematopoietic progenitor that is capable of giving rise to red cells, leukocytes, and platelets. Progeny of the PIGA -mutated clone lack the ability to make “PIG-tailed” proteins, including several that limit the activity of complement; as a result, red cells derived from PIGA -deficient precursors are inordinately sensitive to lysis by the complement C5b-C9 membrane attack complex. Leukocytes share the same deficiency but are less sensitive to complement than are red cells, and so red cells take the brunt of the attack. The nocturnal hemolysis that gives PNH its name occurs because complement fixation is enhanced by the decrease in blood pH that accompanies sleep (owing to CO 2 retention). However, most patients present less dramatically with anemia and iron deficiency resulting from chronic intravascular hemolysis. Interestingly, PNH is sometimes associated with aplastic anemia, which may precede or follow the onset of PNH. The basis for this association is uncertain.
The most feared complication of PNH is thrombosis, which often occurs within abdominal vessels such as the portal vein and the hepatic vein. The prothrombotic state also is somehow related to excessive complement activity, as eculizumab, a therapeutic antibody that binds C5 and inhibits the assembly of the C5b–C9 membrane attack complex, greatly lessens the incidence of thrombosis as well as the degree of intravascular hemolysis. Eculizumab has no effect on early stages of complement fixation, and treated patients continue to have varying degrees of extravascular hemolysis because of the deposition of C3b on red cell surfaces. Loss of C5b-C9 activity in patients receiving eculizumab poses a risk for Neisseria infections, particularly meningococcal sepsis; thus, all treated patients must be vaccinated against N. meningococcus .
Immunohemolytic anemia is caused by antibodies that bind to antigens on red cell membranes. These antibodies may arise spontaneously or be induced by exogenous agents such as drugs or chemicals. Immunohemolytic anemia is uncommon and is classified based on (1) the nature of the antibody and (2) the presence of predisposing conditions, summarized in Table 10.4 .
| Warm Antibody Type |
|
| Cold Antibody Type |
|
The diagnosis depends on the detection of antibodies and/or complement on red cells. This is done with the direct Coombs test, in which the patient’s red cells are incubated with antibodies against human immunoglobulin or complement. These antibodies cause the patient’s red cells to clump (agglutinate), indicating that the patient’s red cells are coated with immunoglobulin and/or complement. The indirect Coombs test, which assesses the ability of the patient’s serum to agglutinate test red cells bearing defined surface determinants, can then be used to characterize the target of the antibody.
In this entity, hemolysis results from the binding of high-affinity autoantibodies to red cells, which are then removed from the circulation by phagocytes in the spleen and elsewhere. In addition to erythrophagocytosis, incomplete consumption (“nibbling”) of antibody-coated red cells by macrophages removes membrane and transforms red cells into spherocytes, which are rapidly destroyed in the spleen, just as in hereditary spherocytosis (described earlier). Warm antibody immunohemolytic anemia is caused by immunoglobulin G (IgG) or (rarely) IgA antibodies that are active at 37°C. More than 60% of cases are idiopathic (primary), while 25% occur in the setting of an immunologic disorder (e.g., systemic lupus erythematosus) or are induced by drugs. The clinical severity is variable, but most patients have chronic mild anemia and moderate splenomegaly and require no treatment.
The mechanisms of hemolysis induced by drugs are varied and, in some instances, poorly understood. Drugs such as α-methyldopa induce autoantibodies against intrinsic red cell constituents, in particular Rh blood group antigens. Presumably, the drug somehow alters the immunogenicity of native epitopes and thereby circumvents T-cell tolerance ( Chapter 5 ). Other drugs, such as penicillin, induce an antibody response by binding covalently to red cell membrane proteins and thereby produce neoantigens. Sometimes antibodies recognize a drug in the circulation and form immune complexes that are deposited on red cells. Here they may fix complement or act as opsonins, either of which can lead to hemolysis.
Cold antibody immunohemolytic anemia is usually caused by low-affinity IgM antibodies that bind to red cell membranes only at temperatures below 30°C, such as may occur in distal parts of the body (e.g., ears, hands, and toes) in cold weather. Cold agglutinins sometimes appear transiently during recovery from pneumonia caused by Mycoplasma spp. and infectious mononucleosis, producing a mild anemia of little clinical importance. More significant chronic forms of cold agglutinin hemolytic anemia occur in association with certain B-cell neoplasms or as an idiopathic condition.
IgM binding to red cells initiates the fixation of complement, but later steps of the complement cascade occur inefficiently at temperatures lower than 37°C. As a result, red cells with bound IgM are coated with complement C3 fragments C3b and C3d but are not lysed intravascularly. When these cells travel to warmer areas, the weakly bound IgM is released, but the coating of C3b and C3d fragments remains. Because C3b and C3d fragments are opsonins ( Chapter 2 ), the cells are phagocytosed by macrophages, mainly in the spleen and liver; hence, in most cases the hemolysis is mainly extravascular. Because IgM is pentavalent, each molecule can bind to more than one red cell, crosslinking red cells and causing them to clump (agglutinate). Sludging of blood in capillaries because of agglutination of red cells often produces Raynaud phenomenon in the extremities of affected individuals.
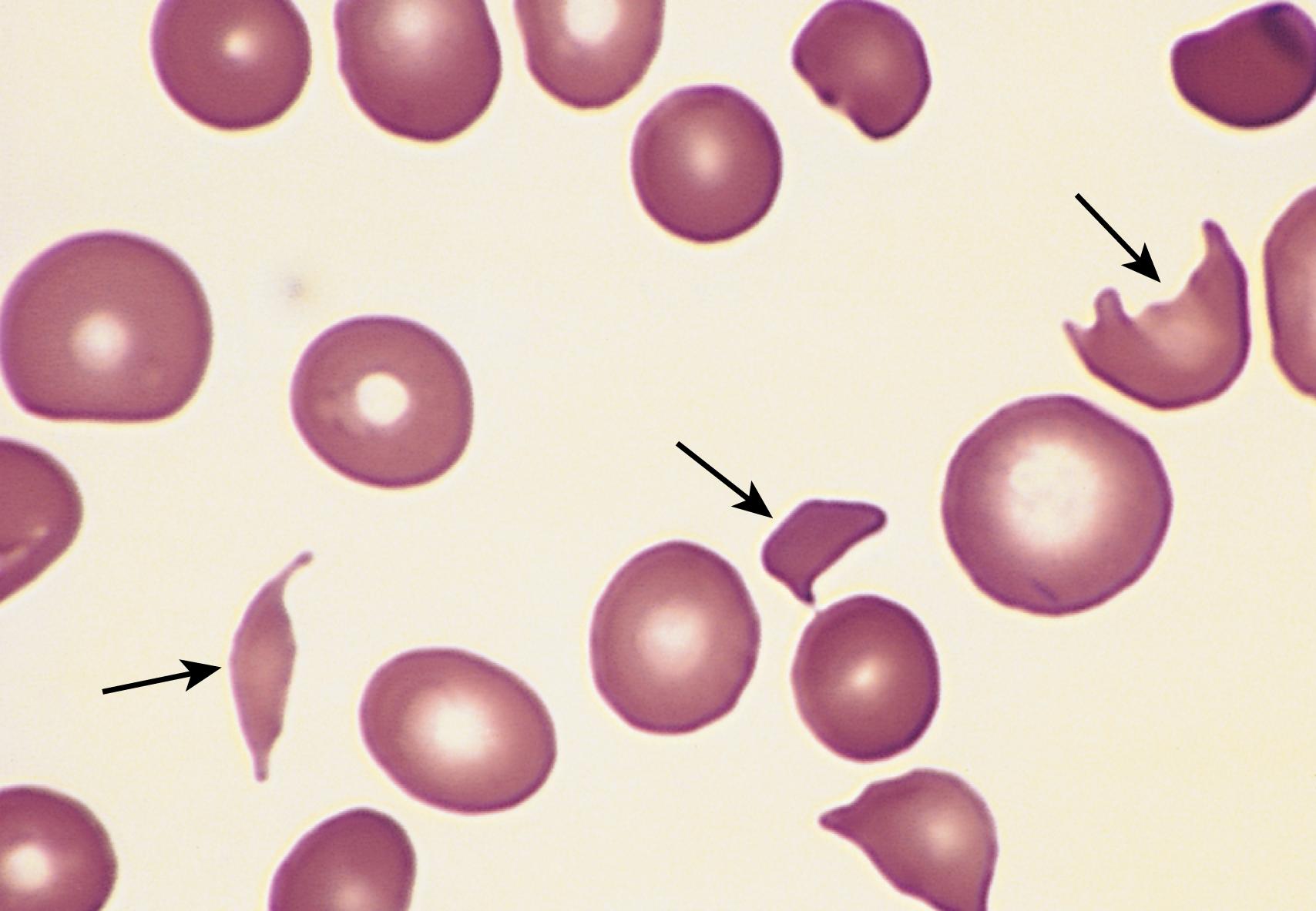
Hemolysis of red cells due to their exposure to abnormal mechanical forces occurs in two major settings. Clinically significant traumatic hemolysis is sometimes produced by dysfunctional cardiac valve prostheses, which may create sufficient turbulence to shear red cells (the blender effect). More commonly, traumatic hemolysis occurs incidentally during an activity producing repeated physical pounding of one or more body parts (e.g., marathon racing, karate chopping, bongo drumming). Microangiopathic hemolytic anemia is observed in pathologic states in which small vessels become partially obstructed or narrowed by lesions that predispose passing red cells to mechanical damage. The most frequent of these conditions is disseminated intravascular coagulation (DIC) (see later), in which vessels are narrowed by the intravascular deposition of fibrin. Other causes of microangiopathic hemolytic anemia include severe hypertension, thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), and disseminated intravascular cancer, in which tumor cells occlude small vessels. Mechanical fragmentation of red cells (schistocytosis) leads to the appearance of characteristic “burr cells,” “helmet cells,” and “triangle cells” in peripheral blood smears ( Fig. 10.7 ). Although microangiopathic hemolysis is not usually a major clinical problem in and of itself, it often points to a serious underlying condition. TTP and HUS are discussed later in this chapter.
In 2020 the World Health Organization estimated that there were more than 200 million cases of malaria worldwide that resulted in over 600,000 deaths, making it one of the most serious afflictions of humans. Malaria is endemic in Asia and Africa, but with widespread jet travel, cases are now seen all over the world. It is caused by five species of plasmodia. Of these, the most important is Plasmodium falciparum, which is responsible for tertian malaria (falciparum malaria), a disorder with a high fatality rate. The other four species of Plasmodium that infect humans— Plasmodium malariae, Plasmodium vivax, Plasmodium knowlesi, and Plasmodium ovale —cause relatively mild disease. All forms are transmitted by the bite of female Anopheles mosquitoes and humans are the only natural reservoir.
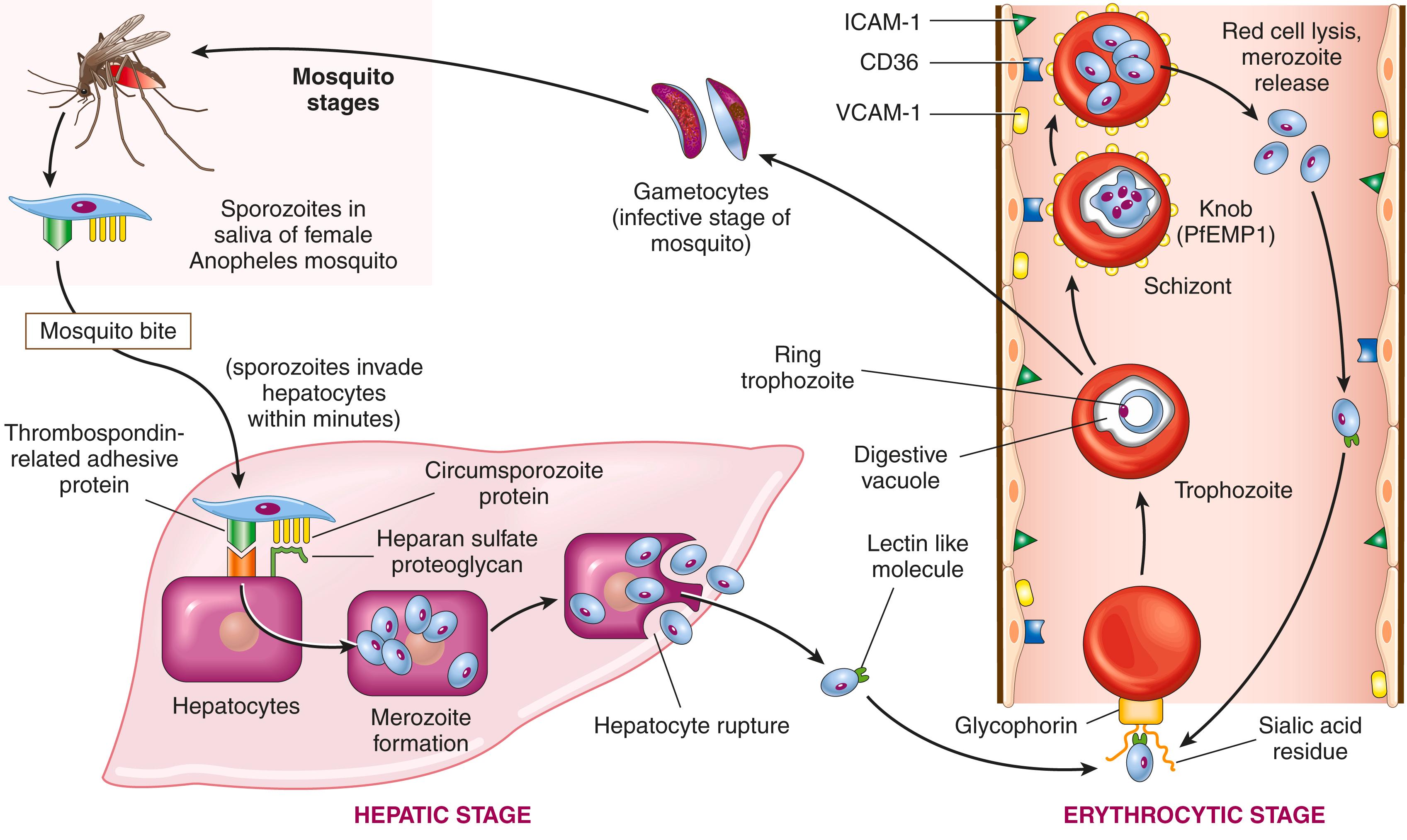
The life cycle of plasmodia is complex and varies among species; Fig. 10.8 shows the life cycle of P. falciparum . As Anopheles mosquitoes feed on human blood, sporozoites are introduced into the circulation and travel through the blood to the liver, where two sporozoite surface proteins, thrombospondin-related adhesive protein and circumsporozoite protein, bind to factors such as proteoglycans on the surface of hepatocytes. The sporozoites then enter the liver and differentiate into merozoites . After an incubation period of 1 to 4 weeks, the infected hepatocytes rupture and release the merozoites. Next, a lectinlike molecule on the surface of the merozoite binds to sialidated glycophorin, a red cell transmembrane protein, allowing the merozoite to invaginate into red cells within a “digestive” vacuole. Intraerythrocytic organisms then differentiate into trophozoites, which follow two paths. Some trophozoites differentiate into gametocytes, which restart the life cycle in mosquitoes when the infected human is again bitten by another Anopheles mosquito. Most trophozoites differentiate into schizonts, which express an adhesion molecule called PfEMP1 ( Plasmodium falciparum erythrocyte membrane protein 1) that concentrates in knoblike extensions on the red cell surface. Normally, red cells bear negatively charged surfaces that interact poorly with endothelial cells, but PfEMP1 binds to adhesion molecules on the surface of endothelium such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and CD36, causing the parasitized red cells to arrest in capillary beds. After a period of several days, schizonts differentiate into merozoites, leading to lysis of the infected red cell and another cycle of red cell infection.
Fatal falciparum malaria often involves the small vessels of the brain, a complication known as cerebral malaria. In an unfortunate minority of patients, mainly children, this process involves cerebral vessels, which become engorged and occluded.
Red cell trophozoites from each Plasmodium species have a somewhat distinctive appearance, allowing expert observers to determine which species is responsible for an infection from examination of appropriately stained thick smears of peripheral blood. The destruction of red cells leads to hemolytic anemia, with its attendant features and laboratory findings. A characteristic brown malarial pigment derived from hemoglobin called hematin is released from the ruptured red cells and produces discoloration of the spleen, liver, lymph nodes, and bone marrow. Activation of defense mechanisms in the host leads to a marked hyperplasia of mononuclear phagocytes, producing massive splenomegaly and occasional hepatomegaly.
Malaria is associated with episodic shaking chills and fever that coincide with the release of “showers” of merozoites from infected red cells at intervals of approximately 24 hours for P. knowlesi; 48 hours for P. vivax, P. ovale, and P. falciparum; and 72 hours for P. malariae. Hemolytic anemia of varying severity is a constant feature. Cerebral malaria associated with P. falciparum is rapidly progressive; convulsions, coma, and death usually occur within days to weeks. Falciparum malaria more often pursues a chronic course that may be punctuated by blackwater fever , a poorly understood complication marked by massive intravascular hemolysis, hemoglobinemia, hemoglobinuria, jaundice, and renal failure.
With appropriate drug therapy, the prognosis for patients with most forms of malaria is good; however, falciparum malaria is becoming more difficult to treat due to the emergence of drug-resistant strains. Because of the potentially serious consequences of the disease, early diagnosis and treatment are important. An encouraging advance is the recent development of a vaccine containing sporozoite antigens; although the protection afforded by the vaccine is partial, when fully deployed it is expected to prevent thousands of cases of fatal cerebral malaria in children. Currently, the best method of preventing malaria is by public health measures such as removal of stagnant bodies of water (mosquitoes breeding sites) close to living areas, use of insecticide-treated bed nets, and prophylactic intake of antimalarial drugs.
Anemias of diminished erythropoiesis include those caused by an inadequate dietary supply of nutrients, particularly iron, folic acid, and vitamin B 12 . Other anemias of this type are associated with bone marrow failure (aplastic anemia), systemic inflammation (anemia of chronic inflammation), or bone marrow infiltration by tumor or inflammatory cells (myelophthisic anemia). In this section, some common examples of anemias of these types are discussed individually.
Iron deficiency is the most common nutritional deficiency in the world and results in clinical signs and symptoms that are mostly related to anemia. About 10% of people living in higher-resource countries and 25% to 50% of those in lower-resource countries are anemic, and in both settings the most frequent cause is iron deficiency. The factors responsible for iron deficiency differ in various populations and are best understood in the context of iron metabolism.
The normal total body iron mass is about 2.5 g for women and 3.5 g for men. Approximately 80% of this iron is present in hemoglobin, myoglobin, and iron-containing enzymes (e.g., catalase, cytochromes). The remaining 15% to 20% of body iron is in a storage pool consisting of hemosiderin and ferritin-bound iron, which is mainly found in macrophages in the liver, spleen, and bone marrow and in skeletal muscle cells. Because serum ferritin is largely derived from the storage pool, the serum ferritin level is usually a good surrogate measure of iron stores. Bone marrow aspiration to assess iron stores is another reliable but more invasive method for estimating iron stores. Iron is transported in the plasma bound to the protein transferrin. Normally, transferrin is about 33% saturated with iron, yielding serum iron levels that average 120 μg/dL in men and 100 μg/dL in women. Thus, the normal total iron-binding capacity of serum is 300 to 350 μg/dL.
In keeping with the high prevalence of iron deficiency, evolutionary pressures have produced metabolic pathways that are strongly biased toward iron retention. Iron is lost at a rate of 1 to 2 mg/day through the shedding of mucosal and skin epithelial cells. This loss must be balanced by the absorption of dietary iron, which is tightly regulated (described later). The normal daily Western diet contains 10 to 20 mg of iron. Most is found in heme within meat and poultry, with the remainder present as inorganic iron in vegetables. About 20% of heme and 1% to 2% of nonheme iron are absorbable; hence, the average Western diet contains sufficient iron to balance fixed daily losses.
Regulation of iron absorption occurs within the duodenum ( Fig. 10.9 ). After reduction by duodenal cytochrome B, a ferric reductase, ferrous iron (Fe 2+ ) is transported across the apical membrane of enterocytes by divalent metal transporter-1 (DMT1). A second transporter, ferroportin, then moves iron from the cytoplasm to the plasma across the basolateral membrane. The newly absorbed iron is oxidized by hephaestin and ceruloplasmin to ferric iron (Fe 3+ ), the form that binds to transferrin. Both DMT1 and ferroportin are widely distributed in the body and are involved in iron transport in many tissues. As depicted in Fig. 10.9 (middle panel), part of the iron that enters enterocytes is delivered to transferrin by ferroportin, whereas the remainder is incorporated into cytoplasmic ferritin and is lost through the exfoliation of epithelial cells.
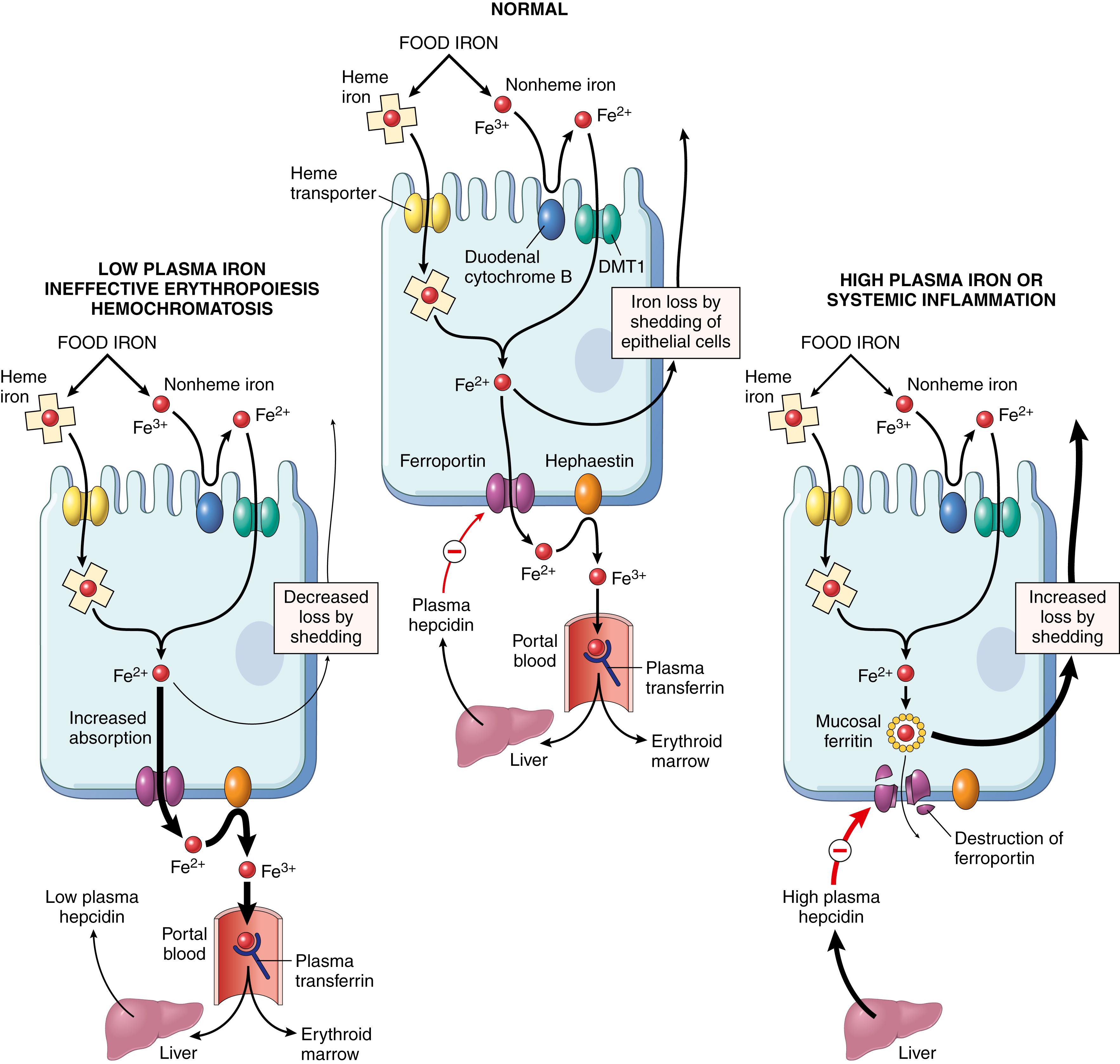
The fraction of dietary iron that is absorbed from the duodenum is determined by the plasma level of hepcidin, a small peptide secreted by the liver in an iron-dependent fashion that negatively regulates ferroportin. Plasma iron levels are “sensed” by a protein called HFE that is expressed on the surface of hepatocytes, and as iron levels rise, HFE and associated proteins send signals that upregulate hepcidin production, creating a feedback loop that maintains iron stores within a physiologic range. In addition, hepatic hepcidin production is positively regulated by inflammatory mediators (e.g., IL-6) and negatively regulated by erythroferrone, which is secreted from erythroblasts in the bone marrow.
Alterations in hepcidin production are a common feature of disorders of iron metabolism. Thus, disorders associated with sustained high levels of erythroferrone (e.g., β-thalassemia major) or inherited defects in HFE (hereditary hemochromatosis, Chapter 14 ) suppress hepcidin production ( Fig. 10.9 , left panel ), leading to iron overload, whereas chronic inflammation stimulates hepcidin production ( Fig. 10.9 , right panel ), leading to the anemia of chronic inflammation (discussed later).
Iron deficiency arises in a variety of settings:
Chronic blood loss is the most important cause of iron deficiency anemia in higher-resource countries. The most common sources of bleeding are the gastrointestinal tract (e.g., peptic ulcers, colon cancer, hemorrhoids) and the female genital tract (e.g., menorrhagia, metrorrhagia, endometrial cancer).
In lower-resource countries, low intake and poor bioavailability of iron because of predominantly vegetarian diets are the most common causes of iron deficiency. In the United States, low dietary intake is infrequent but is sometimes seen in infants fed exclusively milk, in individuals with food insecurity, and the elderly.
Increased iron demands not met by normal dietary intake occur worldwide during pregnancy and infancy.
Malabsorption of iron may occur with celiac disease, various forms of gastritis, or after gastrectomy ( Chapter 13 ).
Regardless of cause, iron deficiency develops insidiously. Iron stores are depleted first, marked by a decline in serum ferritin and the absence of stainable iron in bone marrow macrophages. These changes are followed by a decrease in serum iron and a rise in serum transferrin. Ultimately, the capacity to synthesize hemoglobin, myoglobin, and other iron-containing proteins is diminished, leading to microcytic anemia, impaired work and cognitive performance, and even reduced immunocompetence.
In most instances iron deficiency anemia is mild and asymptomatic. Nonspecific manifestations, such as weakness, listlessness, and pallor, may be present in severe cases. With long-standing anemia, abnormalities of the fingernails, including thinning, flattening, and “spooning,” may appear. A curious but characteristic neurobehavioral complication is pica, the compunction to consume nonfoodstuffs such as dirt or clay.
In peripheral smears, red cells are microcytic and hypochromic ( Fig. 10.10 ). Characteristic findings include decreased hematocrit; hypochromic, microcytic red cell indices; low serum ferritin and iron levels; low transferrin saturation; increased total iron-binding capacity; and, ultimately, response to iron therapy. For unclear reasons, the platelet count is often high. Erythropoietin levels are elevated, but the marrow response is blunted by the iron deficiency; thus, marrow cellularity is usually only slightly increased.
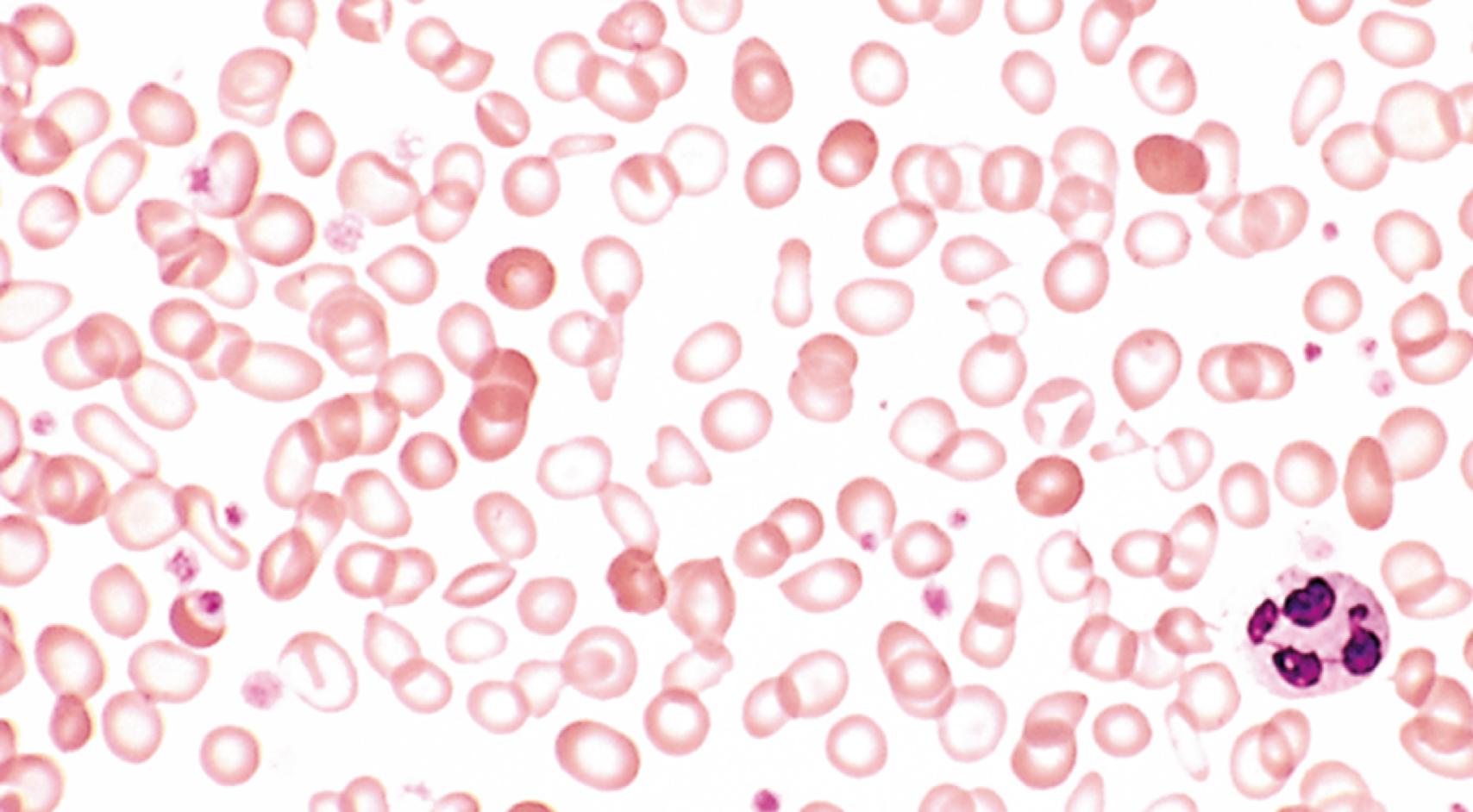
Persons often die with iron deficiency anemia but virtually never of it. An important point to remember is that in well-nourished persons, microcytic hypochromic anemia is not a disease but rather a symptom of another underlying disorder (e.g., colon cancer leading to chronic blood loss).
Often referred to as the anemia of chronic disease, anemia associated with chronic inflammation is the most common form of anemia in hospitalized patients. It superficially resembles the anemia of iron deficiency but arises instead from the suppression of erythropoiesis by systemic inflammation. It occurs in a variety of disorders associated with sustained inflammation, including the following:
Chronic microbial infections such as osteomyelitis, bacterial endocarditis, and lung abscess
Chronic immune disorders such as rheumatoid arthritis and Crohn disease
Cancers such as Hodgkin lymphoma and carcinomas of the lung and breast
The anemia of chronic inflammation stems from high levels of plasma hepcidin, which blocks the transfer of iron to erythroid precursors by downregulating ferroportin in marrow macrophages. The elevated hepcidin levels are caused by pro-inflammatory cytokines such as IL-6, which increase hepatic hepcidin synthesis. In addition, chronic inflammation blunts erythropoietin synthesis by the kidney, lowering red cell production by the marrow. The functional advantages of these adaptations in the face of systemic inflammation are unclear; they may serve to inhibit the growth of iron-dependent microorganisms.
Serum iron levels usually are low in the anemia of chronic disease, and the red cells may be slightly hypochromic and microcytic. In contrast to iron deficiency anemia, however, storage iron in the bone marrow and serum ferritin are increased and the total iron-binding capacity is reduced. Administration of erythropoietin and iron can improve the anemia but only effective treatment of the underlying condition is curative.
The two principal causes of megaloblastic anemia are folate deficiency and vitamin B 12 deficiency. Both vitamins are required for DNA synthesis and the effects of their deficiency on hematopoiesis are identical. However, the causes and consequences of folate and vitamin B 12 deficiency differ in important ways. We will first review some of the common features and then touch on those that are specific to folate and vitamin B 12 deficiency.
Megaloblastic anemia stems from metabolic defects that lead to inadequate biosynthesis of thymidine, one of the building blocks of DNA. Folate and vitamin B 12 are both essential for thymidine synthesis, which is required for DNA replication. Thymidine deficiency causes abnormalities in rapidly dividing cells throughout the body, but the hematopoietic marrow is most severely affected. Because the synthesis of RNA and cytoplasmic elements proceeds in parallel at a relatively normal rate and thus outpaces that of DNA, the hematopoietic precursors show nuclear-cytoplasmic asynchrony (described later). This maturational derangement contributes to the anemia in several ways. DNA synthesis is so defective in many red cell progenitors that a DNA damage response is triggered, leading to apoptosis (ineffective hematopoiesis) . Other progenitors mature into red cells but do so after fewer cell divisions, further diminishing the output of red cells. Granulocyte and platelet precursors are also affected (although not as severely), and most patients present with pancytopenia (anemia, thrombocytopenia, and granulocytopenia).
Certain morphologic features are common to all megaloblastic anemias. The bone marrow is markedly hypercellular and contains numerous megaloblastic erythroid progenitors. Megaloblasts are larger than normal erythroid progenitors (normoblasts) and have delicate, finely reticulated nuclear chromatin (indicative of nuclear immaturity). As megaloblasts differentiate and acquire hemoglobin, the nucleus retains its finely distributed chromatin and fails to undergo the chromatin clumping typical of normoblasts, a classic example of nuclear-cytoplasmic asynchrony. Granulocytic precursors also demonstrate nuclear-cytoplasmic asynchrony, yielding giant metamyelocytes. Megakaryocytes may also be abnormally large and contain bizarre multilobed nuclei.
In the peripheral blood the earliest change is the appearance of hypersegmented neutrophils ( Fig. 10.11 ), which appear before the onset of anemia. Normal neutrophils have three or four nuclear lobes, but in megaloblastic anemias they often have five or more. The red cells typically include large egg-shaped macroovalocytes; the MCV is often greater than 110 fL (normal, 78–98 fL). Although macroovalocytes appear hyperchromic, in reality their hemoglobin concentration is normal. Large, misshapen platelets also may be seen. Morphologic changes in other systems, especially the gastrointestinal tract, also occur, giving rise to some of the clinical manifestations.
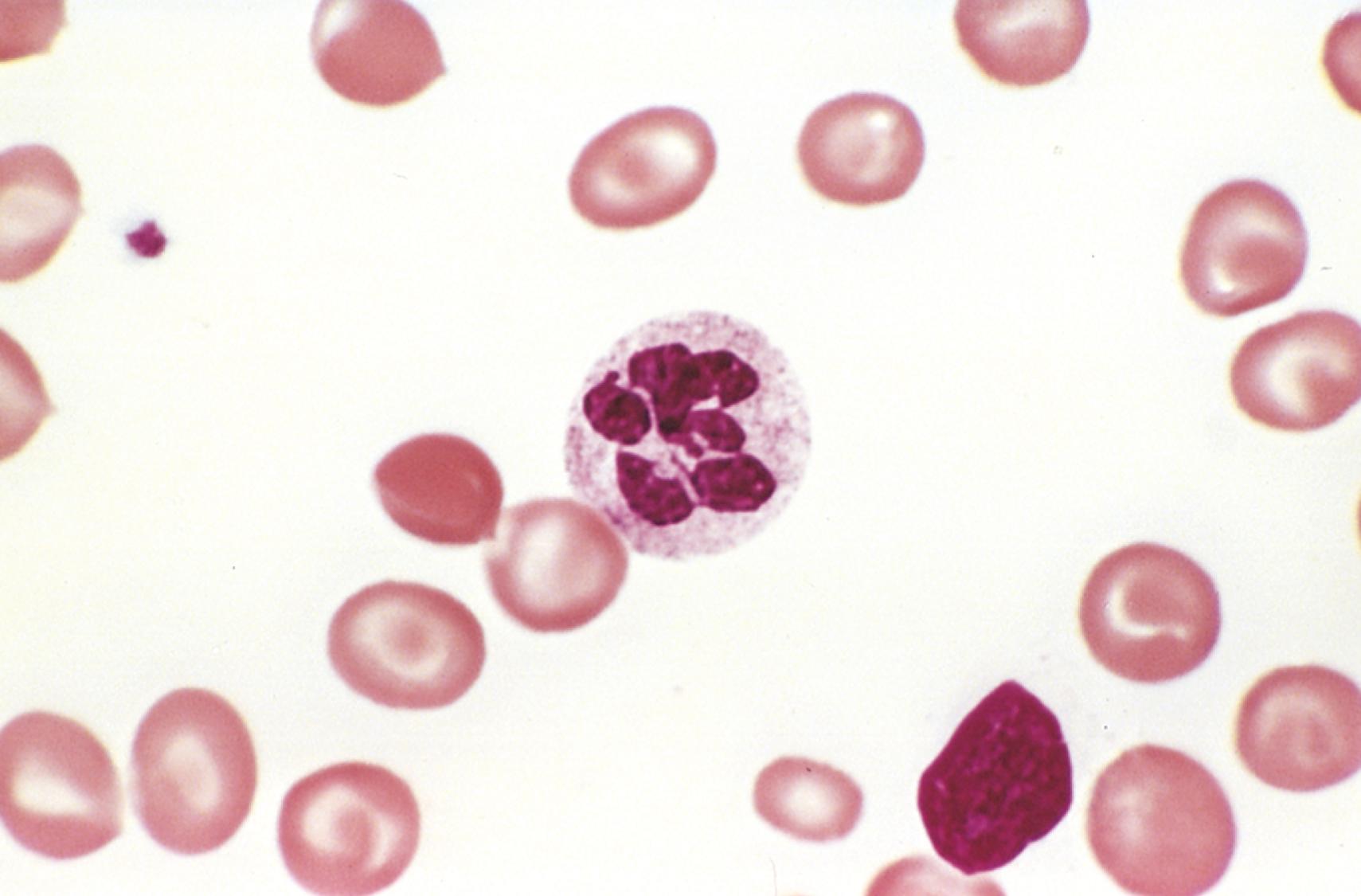
Folate deficiency is usually the result of inadequate dietary intake, sometimes complicated by increased metabolic demands. Folate is present in nearly all foods but is destroyed by 10 to 15 minutes of cooking; as a result, folate stores are marginal in a surprising number of healthy persons. The risk of folate deficiency is highest in those with a poor diet (individuals with food insecurity and the elderly) or those with increased metabolic needs (pregnant women and patients with chronic hemolytic anemias such as sickle cell disease). Deficiency may also stem from problems with absorption or metabolism. Food folates are predominantly in polyglutamate form and must be split into monoglutamates for absorption, a conversion that is inhibited by acidic foods and substances found in beans and other legumes. Some drugs such as phenytoin also interfere with folate absorption, while others such as methotrexate inhibit folate metabolism. Malabsorptive disorders such as celiac disease and environmental enteropathy ( Chapter 13 ) that affect the upper third of the small intestine, where folate is absorbed, may also impair folate uptake.
Tetrahydrofolate acts as an acceptor and donor of one-carbon units in several reactions that are required for the synthesis of deoxythymidine monophosphate (dTMP), which is used in DNA synthesis. Although the metabolism and functions of folate are complex, here it is sufficient to note that its conversion within cells from dihydrofolate to tetrahydrofolate by dihydrofolate reductase (the target of the drug methotrexate) is particularly important for dTMP synthesis. If intracellular stores of folate fall, insufficient dTMP is synthesized and DNA replication is blocked, leading to megaloblastic anemia.
The onset of the anemia of folate deficiency is insidious, associated with nonspecific symptoms such as weakness and easy fatigability. The clinical picture may be complicated by coexistent deficiency of other vitamins, especially in those with alcohol use disorder. Because the cells lining the gastrointestinal tract, like the hematopoietic system, turn over rapidly, symptoms referable to the alimentary tract, such as sore tongue, are common. Unlike in vitamin B 12 deficiency, neurologic abnormalities do not occur.
The diagnosis of a megaloblastic anemia is readily made from the examination of smears of peripheral blood and bone marrow. The anemia of folate deficiency is best distinguished from that of vitamin B 12 deficiency by measuring serum and red cell folate and vitamin B 12 levels.
Vitamin B 12 is widely present in foods, is resistant to cooking and boiling, and is even synthesized by gut flora. Thus, unlike folate, vitamin B 12 deficiency is virtually never caused by inadequate intake except in vegetarians who scrupulously avoid milk and eggs. Instead, deficiencies typically arise from some abnormality that interferes with vitamin B 12 absorption. Absorption of vitamin B 12 requires intrinsic factor, which is secreted by the parietal cells of the fundic mucosa ( Fig. 10.12 ). Vitamin B 12 is freed from binding proteins in food through the action of pepsin in the stomach and it then binds to a salivary protein called haptocorrin . In the duodenum, bound vitamin B 12 is released from haptocorrin by the action of pancreatic proteases and associates with intrinsic factor. This complex is transported to the ileum, where it is endocytosed by ileal enterocytes that express a receptor for intrinsic factor called cubilin on their surfaces. Within ileal cells, vitamin B 12 associates with transcobalamin II and is secreted into the plasma. Transcobalamin II in turn delivers vitamin B 12 to the liver and other cells of the body, including rapidly proliferating cells in the bone marrow and the gastrointestinal tract.
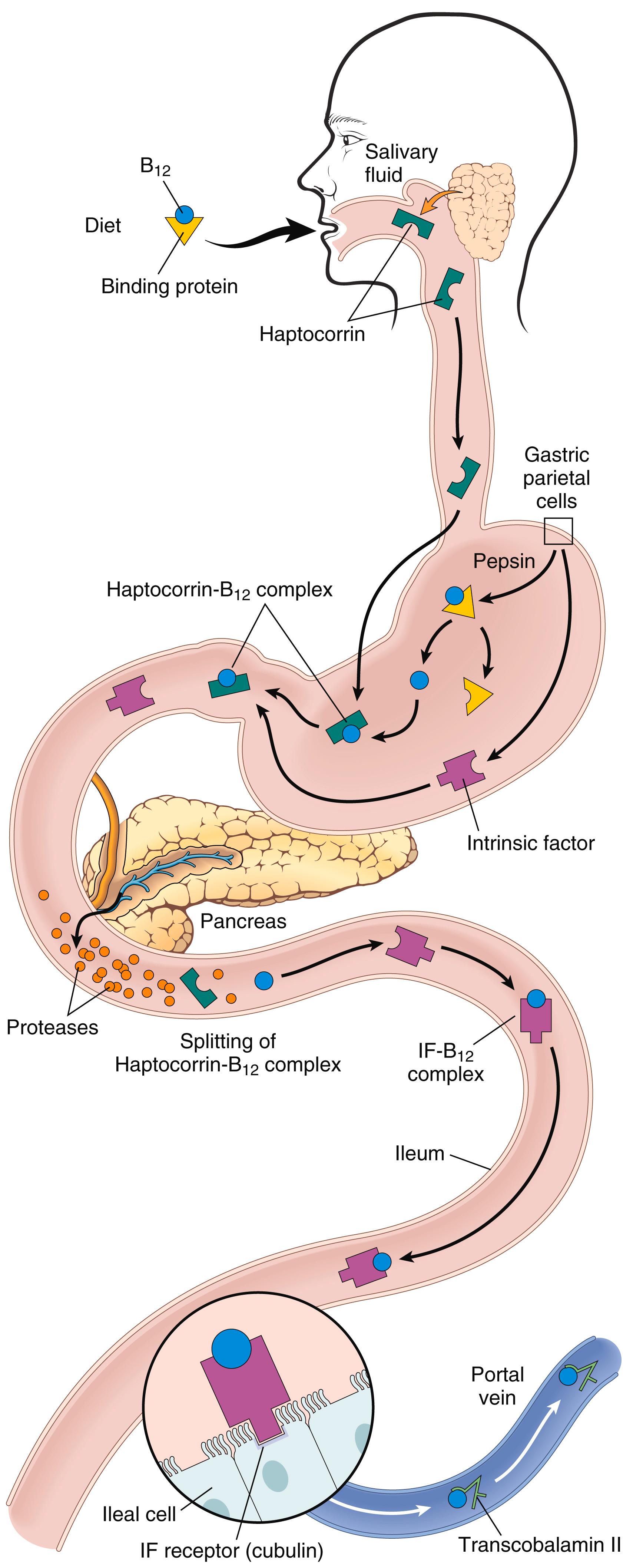
After absorption, the body handles vitamin B 12 efficiently. It is stored in the liver, which normally contains reserves sufficient to support bodily needs for 5 to 20 years. Because of the large storage capacity of the liver, clinical presentations of vitamin B 12 deficiency typically follow years of unrecognized malabsorption.
The most frequent cause vitamin B 12 deficiency is pernicious anemia, which results from an autoimmune attack on the gastric mucosa that suppresses the production of intrinsic factor ( Chapter 13 ). Histologically, there is a chronic atrophic gastritis marked by a loss of parietal cells, a prominent infiltrate of lymphocytes and plasma cells, and megaloblastic changes in mucosal cells similar to those found in erythroid precursors. The serum of most affected patients contains several types of autoantibodies that block the binding of vitamin B 12 to intrinsic factor or prevent binding of the intrinsic factor–vitamin B 12 complex to cubilin. Autoantibodies are of diagnostic use, but they are not thought to be the primary cause of the gastric pathology; rather, it seems that an autoreactive T-cell response initiates gastric mucosal injury and triggers the formation of autoantibodies. When the mass of intrinsic factor–secreting cells falls below a threshold (and reserves of stored vitamin B 12 are depleted), anemia develops.
Other causes of vitamin B 12 malabsorption include gastrectomy (leading to loss of intrinsic factor–producing cells), ileal resection (resulting in loss of intrinsic factor–B 12 complex–absorbing cells), and disorders that disrupt the function of the distal ileum (such as Crohn disease, environmental enteropathy, and Whipple disease). Particularly in older persons, gastric atrophy and achlorhydria may interfere with the production of acid and pepsin, which are needed to release vitamin B 12 from its bound form in food.
The metabolic defects responsible for the anemia of vitamin B 12 deficiency are intertwined with folate metabolism. Vitamin B 12 is required for recycling tetrahydrofolate, the form of folate needed for DNA synthesis. In keeping with this relationship, the anemia of vitamin B 12 deficiency is reversed by administration of folate. Importantly, however, folate administration does not prevent and may worsen neurologic symptoms that are specific to vitamin B 12 deficiency. The main neurologic lesions associated with vitamin B 12 deficiency are demyelination of the posterior and lateral columns of the spinal cord, sometimes beginning in the peripheral nerves. In time, axonal degeneration may supervene. The severity of the neurologic manifestations is not related to the degree of anemia and neurologic disease may even occur in the absence of overt megaloblastic anemia.
The manifestations of vitamin B 12 deficiency are nonspecific. As with all anemias, findings include pallor, easy fatigability, and (in severe cases) dyspnea and congestive heart failure. The increased destruction of erythroid progenitors because of ineffective erythropoiesis may give rise to mild jaundice. Megaloblastic changes in the oropharyngeal epithelium may produce a beefy red tongue. The spinal cord disease (subacute combined degeneration) begins with symmetric numbness, tingling, and burning in the feet or hands, followed by unsteadiness of gait and loss of position sense, particularly in the toes. Although the anemia responds dramatically to parenteral vitamin B 12 , the neurologic manifestations often fail to resolve. As discussed in Chapter 13 , patients with pernicious anemia have an increased risk for the development of gastric carcinoma.
Findings supporting the diagnosis of vitamin B 12 deficiency are (1) low serum vitamin B 12 levels, (2) normal or elevated serum folate levels, (3) moderate to severe macrocytic anemia, (4) leukopenia with hypersegmented granulocytes, and (5) a dramatic increase in reticulocytes 2 to 3 days after treatment with vitamin B 12 . Pernicious anemia is associated with all of these findings plus the presence of serum antibodies to intrinsic factor.
Aplastic anemia is a disorder in which multipotent myeloid stem cells are suppressed, leading to bone marrow failure and pancytopenia. The marrow is often virtually devoid of recognizable hematopoietic elements. It must be distinguished from pure red cell aplasia, in which only erythroid progenitors are affected and anemia is the only manifestation.
The pathogenesis of aplastic anemia is not fully understood, but two major etiologies have been invoked: an extrinsic, immune-mediated suppression of marrow progenitors and an intrinsic abnormality of stem cells. Experimental studies have focused on a model in which activated T cells suppress hematopoietic stem cells. It is hypothesized that stem cells are first antigenically altered by exposure to drugs, infectious agents, or other unidentified environmental insults. This provokes a cellular immune response, during which activated Th1 cells produce cytokines that suppress hematopoietic progenitors. In keeping with this scenario, immunosuppressive therapy directed against T cells restores hematopoiesis in 60% to 70% of patients.
Alternatively, the notion that aplastic anemia results from an intrinsic stem cell abnormality is supported by observations showing that from 5% to 10% of patients have inherited defects in telomerase, which is needed for the maintenance and stability of chromosomes. It is hypothesized that telomerase defects lead to premature senescence of hematopoietic stem cells. Of further interest, the bone marrow cells in an additional 50% of cases have unusually short telomeres, possibly stemming from as-yet undiscovered defects in telomerase or excessive replication of hematopoietic stem cells. These two mechanisms are not mutually exclusive, because genetically altered stem cells (e.g., those with abnormal telomeres) also might express “neoantigens” that serve as targets for a T-cell attack.
Aplastic anemia affects persons of all ages and both sexes. The slowly progressive anemia causes the insidious development of weakness, pallor, and dyspnea. Thrombocytopenia often manifests with petechiae and ecchymoses. Neutropenia may set the stage for serious infections.
It is important to separate aplastic anemia from anemia caused by marrow infiltration (myelophthisic anemia), “aleukemic leukemia,” and granulomatous diseases, which may have similar clinical presentations but are easily distinguished by examination of the bone marrow. Aplastic anemia does not cause splenomegaly; if present, another diagnosis should be sought.
The prognosis is unpredictable. Withdrawal of an inciting drug sometimes leads to recovery, but this is the exception. Hematopoietic stem cell transplantation is often curative, particularly in patients younger than 40 years of age. Transfusion necessary to correct anemia sensitizes patients to alloantigens, producing a high rate of engraftment failure; thus, it must be minimized in persons eligible for transplantation. Successful transplantation requires “conditioning” with immunosuppressive radiation or chemotherapy, reinforcing the notion that autoimmunity has an important role in the disease. As mentioned earlier, patients who are not transplant candidates often benefit from immunosuppressive therapy.
Myelophthisic anemia is caused by extensive infiltration of the marrow by tumors or other lesions. It is most commonly associated with metastatic breast, lung, or prostate cancer. Other tumors, advanced tuberculosis, lipid storage disorders, and osteosclerosis may produce a similar clinical picture. The principal manifestations include anemia and thrombocytopenia; in general, the white cell series is less affected. Characteristic misshapen red cells, some resembling teardrops, are seen in the peripheral blood. Immature granulocytic and erythrocytic precursors may also be present (leukoerythroblastosis) along with mild leukocytosis. Treatment is directed at the underlying condition.
Polycythemia (erythrocytosis) denotes an increase in red cells per unit volume of peripheral blood. It may be absolute (defined as an increase in total red cell mass) or relative. Relative polycythemia results from dehydration, such as occurs with water deprivation, prolonged vomiting, diarrhea, or excessive use of diuretics. Absolute polycythemia is described as primary when the increased red cell mass results from an autonomous proliferation of erythroid progenitors, and secondary when the excessive proliferation stems from elevated levels of erythropoietin. Primary polycythemia is usually caused by a myeloid neoplasm, polycythemia vera, considered later in this chapter. The increases in erythropoietin that lead to secondary forms of absolute polycythemia stem from diverse causes ( Table 10.5 ).
| Relative |
|
| Absolute |
| Primary |
|
| Secondary |
|
Disorders of white cells include deficiencies (leukopenias) and proliferations, which may be reactive or neoplastic. Reactive proliferation in response to diseases such as microbial infections is common. Neoplastic disorders, although less common, are more ominous. They cause approximately 9% of all cancer deaths in adults and approximately 30% in people younger than 20 years of age.
Presented next are brief descriptions of some nonneoplastic conditions, followed by more detailed considerations of the malignant proliferations of white cells.
Leukopenia results most commonly from a decrease in granulocytes, the most numerous circulating white cells. Lymphopenia is less common; it is associated with rare congenital immunodeficiency diseases, advanced human immunodeficiency virus (HIV) infection, and treatment with high doses of corticosteroids. Only the more common leukopenias of granulocytes are discussed here.
A reduction in the number of granulocytes in blood is known as neutropenia or, when severe, agranulocytosis. People who are neutropenic are susceptible to severe, potentially fatal bacterial and fungal infections. The risk of infection rises sharply as the neutrophil count falls below 500 cells/μL.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here