Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Parasitic diseases are not common in medical, let alone hematologic, practice in North America or Europe. However, much of the world’s population is infected by and becomes symptomatic as a result of a plethora of parasites, and many of these infections represent global public health problems.
Although, some significant parasitic diseases are transmitted in temperate climates, the majority of parasites of significance to human health are endemic in the tropical world. This reflects not only socioeconomic circumstances but also the origin of our species in tropical Africa, where the human host, parasites, and also vectors have established complex relationships over evolutionary timescales. Notwithstanding such geographic variation in the incidence of parasitic disease, both travelers and recent immigrants now present to hematology clinics and laboratories all over the world with increasing frequency. Even in those circumstances, there are marked variations in practice in North America as well as in Europe, where the United Kingdom reports more cases of imported malaria than the United States and indeed has a 10-fold greater incidence of malaria per capita, reflecting the increased frequency of travel to and from endemic areas compared with North American populations.
Patients with malaria, leishmaniasis, trypanosomiasis, and babesiosis may present directly or indirectly to hematologists. This chapter is concentrated on the biologic, clinical, and hematologic features of these infections and the hematologic aspects or complications of their treatment. Comprehensive accounts of the general medical aspects of these diseases are provided in many other recent textbooks.
Malaria is a major public health problem in tropical areas, and it is estimated that it is responsible for approximately 400,000 deaths annually and 150 to 300 million infections. The vast majority of morbidity and mortality caused by malaria is caused by infection with Plasmodium falciparum , although Plasmodium vivax, Plasmodium ovale , and Plasmodium malariae are also responsible for human infections. A fifth species, Plasmodium knowlesi , has been shown to cause human infection in some parts of Southeast Asia (for review, see the article by Jeyaprakasam et al. ). In endemic areas, a significant proportion of the mortality and morbidity is from anemia. In Europe and North America, malaria is not infrequently a clinical problem in travelers or recent arrivals from malaria-endemic areas, and hematologists may be involved in the diagnosis and management of the disease. Moreover, in nonendemic areas, malaria may cause a fatal transfusion-transmitted infection, and detection of blood donors who may be carrying the disease represents a major challenge for blood services.
Approximately 1 billion people live in areas of endemic or epidemic malaria. The global mortality and morbidity were revised to 350 million cases and 1 million deaths per year, respectively, following an evaluation of the prevalence of infection in Southeast Asia in 2005 ( Fig. 154.1 ). There is, however, substantial evidence that the incidence of severe disease has fallen considerably across the world in the last 15 years, following the widespread introduction of artemisinin combination treatment, impregnated bed nets, and residual spraying because the resources available for malaria control have increased substantially through the Global Fund, the World Health Organization’s Global Malaria Programme, and the Roll Back Malaria Partnership to End Malaria ( https://endmalaria.org/ ). The current estimated annual death total from malaria in Africa has fallen from 700,000 in 2004 to 386,000 in 2019.
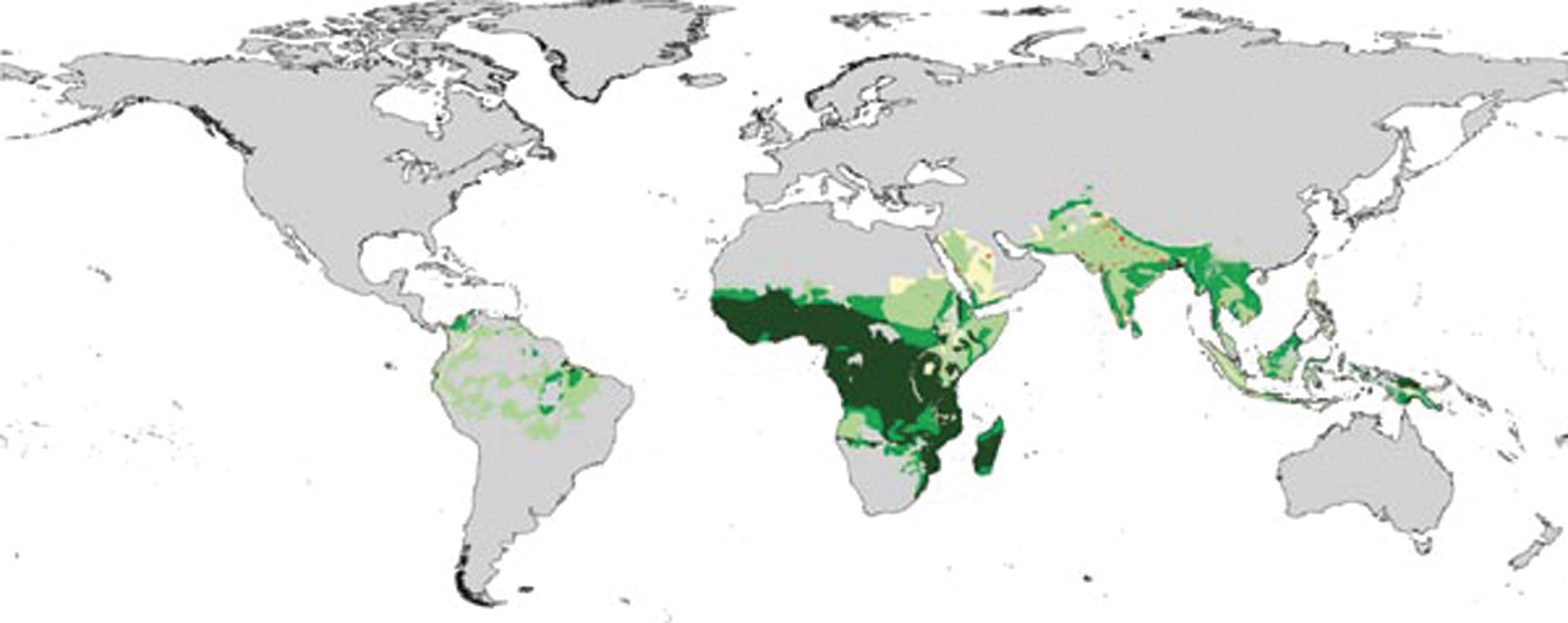
The distribution of malaria is determined by features of host, vector, and parasite. In summary, the global distribution of autochthonous or endogenous malaria is limited by the lower temperature limits for development of the parasite in the mosquito (sporogony) of 20°C for P. falciparum and 15°C for other human malarias. Within these limits, transmission does not occur above 1500 m in arid regions or in the Central and South Pacific (because of the absence of suitable vectors). In addition, P. vivax malaria is rare in Africa, where the population frequency of the blood group Duffy negative (Fya − Fyb − ) is high. P. ovale requires a lengthy period of sporogony and is confined to areas of Africa and Southeast Asia with a high density of susceptible Anopheles spp. P. knowlesi is transmitted from macaque monkeys in forest areas of Borneo, Malaysia, Thailand, and Vietnam.
In some malarious areas the seasonal pattern of clinical malaria is determined by the increase in vector density after rainfall, leading to an increase in new infections as transmission increases. In naive individuals, parasites can cause chronic infection lasting many months.
The intensity of transmission determines the distribution of clinical symptoms in different age groups. In general, in areas of high transmission, younger children experience severe disease. Where transmission is less intense, older children experience severe disease. Finally, if the rate of transmission is very low, few cases of malaria are seen in any age group, and such populations would have little natural immunity. In such areas, a sudden increase in vectorial capacity (through the accidental introduction of efficient vectors or higher density, biting, or survival of the resident vectors), more rapid parasite sporogony, or migration of infected or nonimmune populations can result in epidemics where large numbers fall ill in all age groups. The transition from high to low transmission has been classified by holoendemicity, hyperendemicity, mesoendemicity, and hypoendemicity. These categories can be related epidemiologically to age-specific rates of parasite prevalence or splenomegaly and theoretically to the reproductive ratio of malarial infection.
Malaria exerts a substantial selection for human traits that protect from infection. About a third of the variability in the risk of severe and complicated malaria is now explained by additive host genetic effects. Substantial protection from malaria is derived from sickle cell trait and thalassemia traits which are truly polymorphic characteristics in many parts of the world. Other red cell traits conferring resistance to severe disease include the ABO blood groups and polymorphisms of ATPas4 (the major calcium pump on red cells) and polymorphisms of the glycophorins, including the Dantu blood group antigen. Understanding genetic epidemiology has provided the foundation of population genetics and has provided classic examples of principles of genetic selection in vivo—for example, balancing selection for sickle cell trait and negative epistasis for sickle cell trait and α-thalassemia. The homozygous forms of these characteristics cause significant clinical disease, such as sickle cell disease, β-thalassemia, and glucose-6-phosphate dehydrogenase (G6PDH) deficiency. In endemic areas these genetic diseases represent major public health problems (for review, see Williams and Luzzatto et al. ).
In P. falciparum (see later for a discussion of the other human parasites) the infective sporozoite forms are inoculated into the bloodstream from the salivary glands of a female Anopheles mosquito ( Fig. 154.2 ). These thin, needle-shaped cells, 10 to 12 μm in length, circulate briefly with a half-life of approximately 30 minutes before traversing macrophages and several hepatocytes and ultimately residing in a single hepatocyte. Here rapid multiplication takes place over 5 to 8 days to produce a liver schizont, 80 μm in diameter and containing 30,000 ± 10,000 merozoites that are released into the bloodstream, where they infect erythrocytes. When ready to leave hepatocytes, the parasite induces cell death in the hepatocytes and causes the release of merozoites in membrane-enclosed structures or merosomes that are extruded from the infected cell, thereby avoiding host cell defense mechanisms.
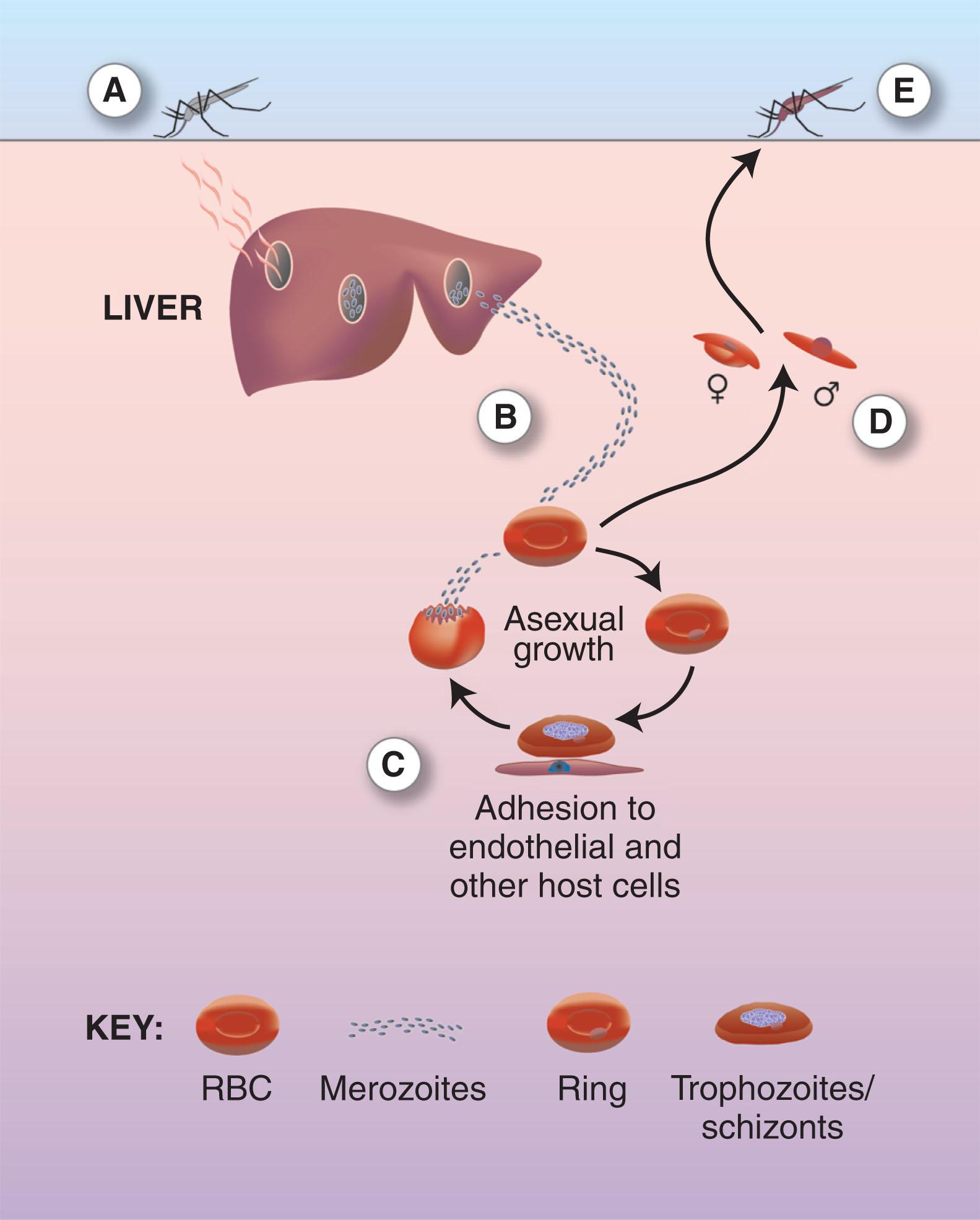
The merozoites bind and then invade red blood cells (RBCs; for review of RBC and merozoite interactions, see Satchwell ). The host plasma membrane is invaginated to form the parasitophorous vacuole. For the first 10 hours the developing parasites appear as fine “ring forms.” Between 10 and 15 hours the cytoplasm thickens, and 16 hours after invasion, granules of the black pigment hematin, the end product of hemoglobin (Hb) digestion, begin to appear. Ligands are expressed at the surface of the infected RBC that mediate adhesion to host receptors on venular endothelium. These trophozoites no longer circulate throughout the body but are sequestered in the peripheral circulation. Nuclear division begins, at approximately 30 hours, to form schizonts containing up to 32 merozoites. At 48 hours the RBC is ruptured to release the merozoites into the circulation to continue further cycles of asexual multiplication.
The erythrocytic cycle of schizogony may achieve a 10-fold increase in parasitemia in vivo and a patent or microscopically detectable infection 6 days after the liver stage is completed. After two or more cycles the infection becomes clinically apparent by the paroxysms of fever that accompany the release of merozoites. Cycles of schizogony continue until the rate of parasite multiplication is reduced by chemotherapy, specific or nonspecific defense mechanisms, or occasionally the demise of the host.
Some merozoites do not multiply but become committed during the previous erythrocytic cycle to form male or female gametocytes. Gametocytes are distinguished by dispersed pigment in a single nucleus, in a fully grown parasite, and are sequestered for the first 5 days of their development in the peripheral circulation. Thus 8 to 10 days after the start of clinical infection, mature, crescent-shaped gametocytes appear in the blood.
The sexual phase (or sporogony) of the parasite life cycle begins after a male and female gametocyte are ingested by a feeding female Anopheles mosquito. In the midgut of the mosquito the gametocytes shed the RBC membrane. This change is apparently precipitated by the drop in temperature. A female gametocyte forms a single macro-gamete, but male gametocytes undergo several rounds of nuclear division to produce flagellated microgametes. These microgametes are motile and migrate to fertilize a macrogamete. The resulting zygote enlarges to form a mobile ookinete and migrates through the epithelial wall of the mosquito midgut to rest finally on the external surface. The oocyst divides repeatedly to form up to 10,000 sporozoites, which travel up through the hemolymph to enter the acinar cells of the salivary glands. Once there, they are infective when injected into the host.
Major differences exist in the life cycles of other human Plasmodium spp. First, in P. vivax and P. ovale infections, some sporozoites entering the liver form dormant hypnozoites that begin to divide only after a variable period of some months to cause further blood-stage infections or relapses. Second, the cycle of erythrocytic development in P. malariae takes 72 hours and thus causes quartan fever (i.e., on days 1 and 4).
Malaria gives ample reasons for both increased RBC destruction and reduced RBC production (see Fig. 154.3 for overview).
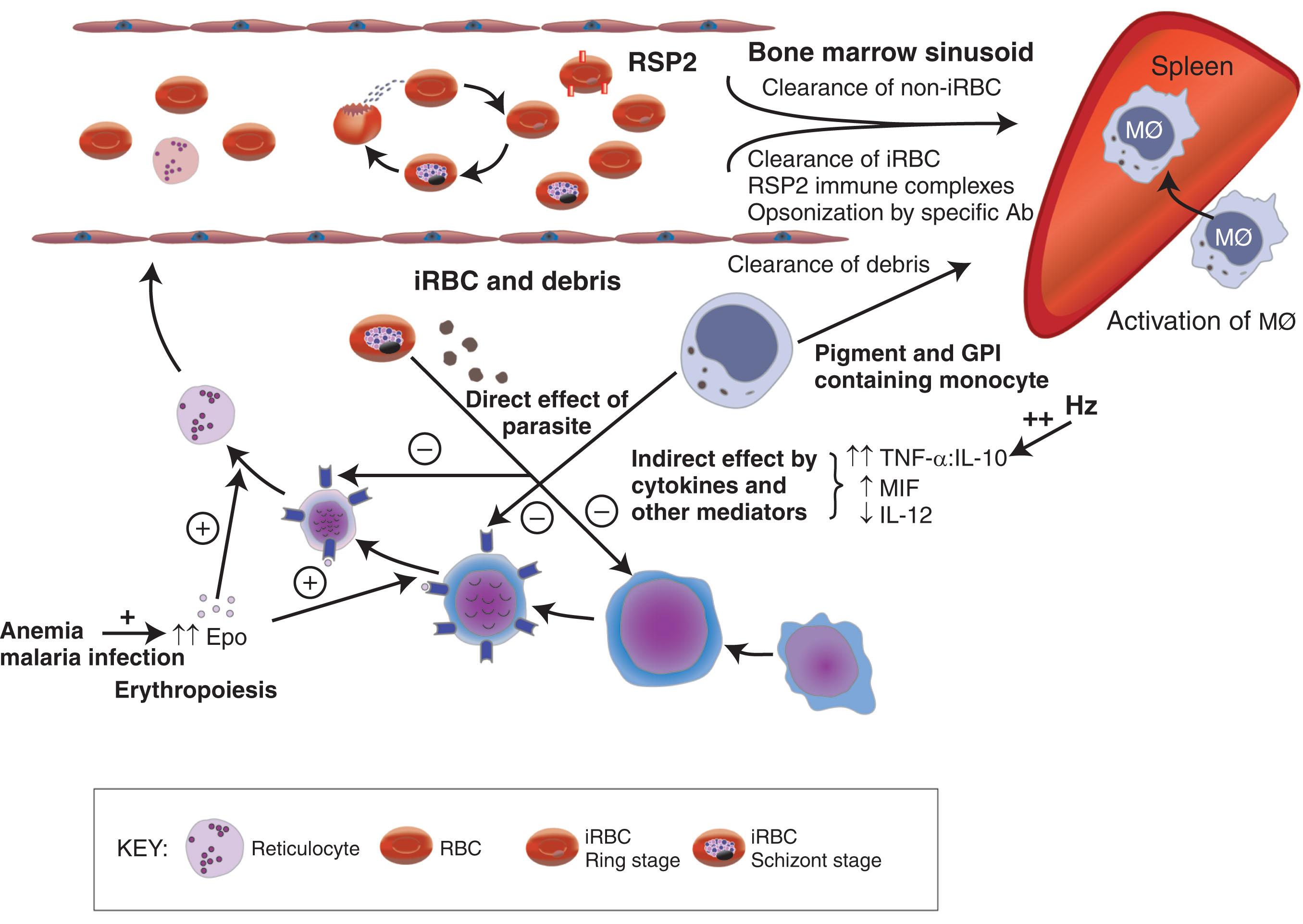
Destruction of RBCs is inevitable as parasites complete their 48-hour growth cycle and lyse their temporary host cell. Some parasites may be removed from erythrocytes as immature ring forms by phagocytic cells, leaving the RBCs with residual parasite antigens to continue to circulate, albeit with reduced survival. Infected erythrocytes may also be phagocytosed by macrophages following opsonization by immunoglobulins and/or complement components. Other signals for recognition of infected erythrocytes by macrophages include abnormally rigid membranes and exposure of phosphatidylserine and other altered host antigens and, in animal models of malaria, antiphosphatidylserine antibodies.
The activity and the number of macrophages are increased in malarial infection, and increased removal of uninfected cells may occur. Moreover, the signals for recognition of uninfected erythrocytes for removal by macrophages are enhanced. Uninfected erythrocytes bind increased amounts of immunoglobulin and/or complement as detected in the direct antiglobulin test (DAT or Coombs test). These antibodies do not have a particular specificity but are more likely to represent immune complexes absorbed onto the surface of RBCs. Furthermore, hemozoin may activate complement directly and cause deposition of C3b on uninfected RBCs. However, an association between positive DAT results and severe anemia has not been established. More recent studies have shown not only that RBCs from patients with malaria were more susceptible to phagocytosis but also that RBCs from children with acute malaria had increased surface immunoglobulin G (IgG) and low levels of RBC CR1 and CD55 compared with control subjects.
Some degree of splenomegaly is a normal feature of malarial infection, and the prevalence of splenomegaly in regions of malarial transmission is used as a major indicator of the level of malarial endemicity. The importance of the spleen in host defense against malaria has been demonstrated in experimental systems, and individuals whose spleens have been surgically removed are thought to be more susceptible to severe infection. Indeed, the phenomenon of parasitic sequestration is thought to have evolved primarily as an immune evasion strategy so the mature parasite can avoid passing through the spleen.
Active erythrophagocytosis is a conspicuous feature within the bone marrow during P. vivax and P. falciparum malaria, and it is highly probable that this also occurs within the spleen. Cytokines may be responsible for activating macrophages during malarial infection. Children with acute P. falciparum malaria have high circulating levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), a synergistic combination of cytokines that activates macrophages.
Researchers in several studies have attempted to define the pathophysiologic changes in the spleen during acute malaria. In animal models, malaria is accompanied by increased intravascular clearance of infected or rigid, heat-treated cells by the spleen, as well as alterations in the splenic microcirculation. In studies of human malaria, it has been found that increased splenic clearance of heated RBCs occurs during acute attacks. Ex vivo models of splenic function suggest that the spleen removes not only mature infected RBCs but also uninfected cells marked for clearance by low deformability, aggregated band 3, complement deposition, or phosphatidylserine exposure. Moreover, “pitting” of ring-stage infected cells may remove the parasite and leave the RBC marked with parasite antigen to return to the circulation (reviewed by White ).
Changes to the uninfected RBCs during infection also contribute to their own enhanced clearance by phagocytes. Uninfected RBCs in children and adults with severe disease are less deformable, and this is a significant predictor of the severity of anemia and indeed outcome, consistent with the notion that these cells are being removed by the spleen. It has also been found that IgG-sensitized RBCs are rapidly removed from the circulation by the spleen and that unusually rapid clearance persists well into the convalescent phase.
Thus, all the available evidence points to increased reticuloendothelial clearance in P. falciparum malaria, persisting long after recovery. These changes are presumably a host defense mechanism, maximizing the clearance of parasitized erythrocytes. Nevertheless, the contribution of clearance of uninfected erythrocytes to anemia during acute malaria is substantial and it has been estimated that loss of unparasitized erythrocytes accounts for approximately 90% of the drop in Hb resulting from a single infection. The severity and duration of anemia is determined not only by red cell clearance but also by dyserythropoiesis and reticulocytopenia.
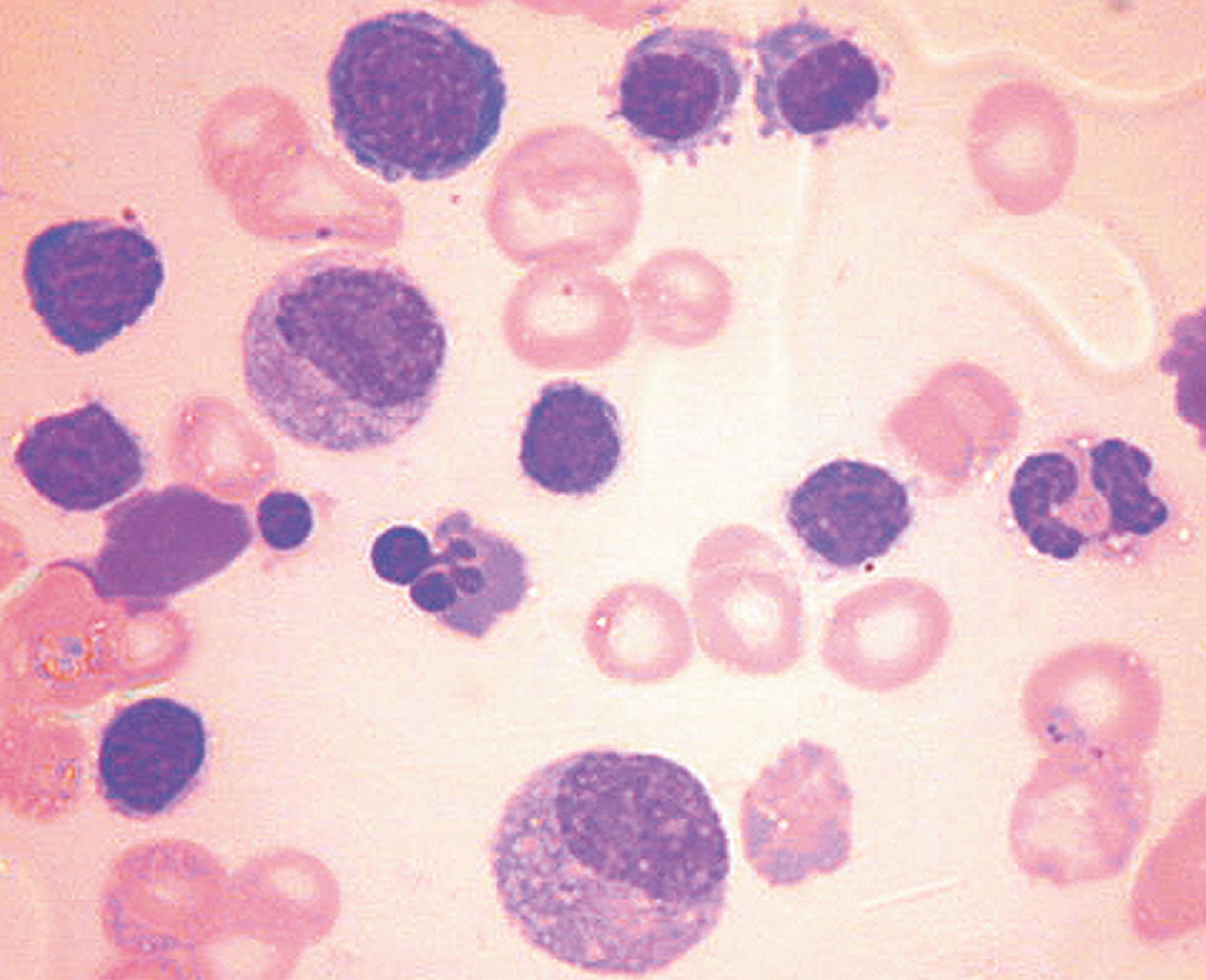
Reticulocytopenia has been confirmed in numerous clinical studies of malarial anemia. The histopathologic study of the bone marrow of children with malarial anemia shows erythroid hyperplasia with increased numbers of erythroid precursors ( Fig. 154.4 ). However, the maturation is abnormal by light and electron microscopy. Abdalla et al. described the hallmark characteristics of such abnormal maturation, namely cytoplasmic and nuclear bridging and irregular nuclear outline. They later confirmed that the distribution of the erythroid progenitors through the cell cycle is abnormal in malarial anemia, with an increased proportion of cells in the G 2 phase compared with healthy control subjects. Dörmer et al. confirmed these findings using the same criteria as Abdalla et al.; they studied six patients with falciparum malaria before and after treatment.
Bone marrow dyserythropoiesis persists for many weeks following the start of malaria treatment in acute malaria and reticulocyte counts are therefore low when patients are symptomatic. In such settings, Hb concentration in acute falciparum malaria is lowest around 1 week after presentation with fever, while in acute vivax malaria the nadir in Hb is a few days after presentation. In acute uncomplicated malaria, anemia is worse in younger children and those with protracted infections. In high transmission areas, where previous infections may confer some protection from the effects of infection, Hb concentrations often increase soon after treatment.
Given the importance of erythropoietin (Epo) to erythropoiesis, attention has been focused on the levels of this crucial cytokine in malarial infection. Serum Epo was appropriately raised in studies of African children with malarial anemia, and high levels of this cytokine have been associated with better outcomes of disease. African children with severe malaria, particularly young children, were shown to have supraphysiologic levels of Epo compared with age-matched community control subjects with nonmalarial anemia. Evidence from some observational clinical studies suggested that these high levels of Epo in children with malarial anemia were associated with a cytoprotective effect and so reduce the risk for neurologic sequelae or death in children with cerebral malaria. Other studies in children over 18 months old with cerebral malaria, showed that high Epo levels were associated with prolonged coma duration and increased mortality. Moreover, clinical studies using Epo as an adjuvant in other neurological diseases have shown that although Epo was associated with some neurological benefits, treatment with Epo increased mortality from other causes. There have been no large-scale trials of recombinant Epo in malaria.
The prime candidates for the host factors mediating dyserythropoiesis are imbalances of TNF-α, IFN-γ, and interleukin-10 (IL-10). The concentrations of TNF-α and IFN-γ have been correlated with the severity of the disease. Whereas low concentrations of TNF-α (<1 ng/mL) stimulate erythropoiesis, higher levels of TNF-α have been shown to suppress erythropoiesis. Furthermore, it is possible that high levels of these inflammatory cytokines may contribute to reduced and abnormal production of erythrocytes and also to increased erythrophagocytosis.
Recent evidence has suggested that the release of hepcidin is associated with malarial infection and that high levels of hepcidin could contribute to the sequestration of iron and impair erythropoiesis. The stimulus for hepcidin secretion may be proinflammatory cytokines such as IL-6, but malaria-infected erythrocytes may also enhance hepcidin production by peripheral blood mononuclear cells. Intriguingly, hepcidin released during blood-stage parasitemia may be a key regulator of P. falciparum liver-stage development. These data now support suggestions from large-scale studies of iron supplementation that iron may be unhelpful and possibly even harmful when given during acute malarial infection. Indeed, there are both clinical and experimental evidence that iron deficiency may confer protection from severe malaria. Malaria parasites show reduced growth in RBCs from subjects with iron deficiency, and infection is augmented during the phase of iron supplementation after severe iron deficiency.
High levels of the T-helper cell type 2 (Th2)-type cytokine, IL-10, might prevent the development of severe malarial anemia. Low levels of IL-10 have been described in African children with severe malarial anemia. Similarly, defective IL-12 production has been shown experimentally to be associated with increased severity of malaria in a rodent model, and low IL-12 levels have been associated with severe malaria in African children. The role of IL-12 in clinical malaria appears complex, but IL-12 and other cytokines may activate adaptive natural killer (NK) cells to reduce parasitemia by antibody-dependent cytotoxicity.
There is also substantial evidence that lysate of infected erythrocytes may directly modulate the function of host cells. During its blood stage, the malaria parasite proteolyzes host Hb in an acidic vacuole to obtain amino acids, releasing heme as a byproduct, which is auto-oxidized to potentially toxic hematin (aquaferriprotoporphyrin IX [H 2 O−Fe III PPIX]). β-Hematin forms as a crystalline cyclic dimer of Fe III PPIX and is complexed with protein and lipid products as malarial pigment or hemozoin. Schwarzer et al. showed that the function of monocytes and of monocyte-derived macrophages is severely inhibited after ingestion of malaria pigment or hemozoin.
The hemozoin polymer of heme moieties may be complexed with biologically active compounds. The oxidation of membrane lipids catalyzed by the ferric heme produces the lipoperoxides. There is accumulating evidence that 4-hydroxynonenal (HNE) and other lipoperoxides, including 15-hydroxyarachidonic acid [15-( R , S )-HETE], may play a role in the pathophysiology of malaria. It has been shown that HNE and HETE are generated in parasitized erythrocytes and that HNE and endoperoxides produced in pigment-containing monocytes or macrophages may cause cell-cycle arrest and impair erythroid growth. Hemozoin may also directly inhibit erythroid development in vitro and cause apoptosis of erythroid precursors. Furthermore, increased levels of plasma hemozoin and pigment in monocytes have been associated with anemia.
In children presenting with acute malaria, bacteremia is associated not only with anemia but also with excess mortality. Malaria infection is particularly associated with non-typhi salmonella bacteriemia, due at least in part to impaired neutrophil function.
Parvovirus B19 may cause a transient reticulocytopenia and thus severe and sudden anemia in those with a hemolytic anemia or fetal anemia and intrauterine death. Most children in Africa have serologic evidence of infection early in life. However, acute infection with this virus is uncommon in those presenting with severe malarial anemia, and a recent parvovirus B19 infection and falciparum malaria appear to be independent risk factors for anemia in children in Africa.
In endemic areas the etiology of anemia is complex. Acute or chronic malarial infection is a major precipitating factor in children with severe anemia causing admission to hospital.
Longitudinal studies from The Gambia have shown that whereas the mean Hb levels in children vary significantly through the year, anemia is much more common in the rainy season, when malaria transmission is at its highest. However, the rains are also associated with an increase in waterborne diarrheal disease and poor food supplies. So the seasonal increase in anemia in malaria-endemic areas arises on a background of low or frankly deficient stores of hematinics and/or other micronutrients. Iron deficiency, which affects at least 20% of the world's population, is a major factor in the seasonal surge of anemia in the tropical rainy season. Low iron stores at birth and dietary iron deficiency may be exacerbated by hookworm or schistosomal infection. It is now also clear that malarial infection is associated with reduced uptake of available iron and reduced incorporation of iron into developing erythroid cells. Folate deficiencies and/or increased requirements may occur in many populations, although frank folate deficiency is uncommon.
A low baseline Hb level at the start of the season for malaria transmission is a major risk factor for developing severe malarial anemia and has encouraged studies aimed at preventing the development of anemia by hematinic supplementation with or without antimalarial prophylaxis or surveillance.
Quantifying the contribution of these individual factors to anemia reliably requires intervention studies. In Tanzania, Menendez et al. and Schellenberg et al. have shown that iron supplementation and antimalarial prophylaxis prevented 30% and 60%, respectively, of all cases of moderate anemia presenting during the malaria transmission season in children and in infants.
Translating the results of these studies from well-defined and carefully controlled study areas has not been easy. Considerable concerns about iron supplementation programs for children have been raised in sub-Saharan Africa and in areas of high malaria endemicity. One large trial of iron supplementation was stopped because of increased hospital admission and death in the group receiving iron. However, meta-analysis of iron supplementation in malaria-endemic areas has shown that iron alone or with antimalarial treatment does not increase the risk for clinical malaria or death when regular malaria surveillance and treatment services are provided. It is increasingly clear that iron supplementation in these areas must be based on either defining iron-deficient children or combining iron administration with effective infection control and treatment strategies.
The signs and symptoms of malarial infection in humans are caused by the asexual blood stage of the parasite. Infection with blood-stage parasites may result in a wide range of outcomes and pathologic conditions. Indeed, the spectrum of severity ranges from asymptomatic infection to rapidly progressive, fatal illness. The clinical presentation of malarial infection is also wide and influenced by age, immune status, and pregnancy, as well as by the species, genotype, and perhaps the geographic origin of the parasite. In endemic areas, many infections present as an uncomplicated febrile illness. In more severe forms of the disease, children may present with prostration or inability to take oral fluids, or in younger children an inability to suckle. Alternatively, these children may exhibit a number of syndromes of severe disease, including anemia, coma, respiratory distress, and hypoglycemia and may also have a high rate of bacteremia.
In most age groups, anemia is frequently accompanied by more than one syndrome of severe disease, and the already substantial case fatality rate of 15% to 20% for severe malaria in African children rises significantly when multiple syndromes of severe disease are present. The age distribution of anemia and other syndromes of severe disease is a consistent but puzzling feature of the epidemiology of clinical malaria. Children born in endemic areas are protected from severe malaria in the first 6 months of life by the passive transfer of maternal immunoglobulins and by fetal Hb. Beyond infancy, the most common form of presentation of severe disease changes from anemia in children aged 1 to 3 years old, in areas of high transmission, to cerebral malaria in older children, in areas of lower transmission. As transmission intensity declines further, severe malaria is most frequently found in older age groups.
The blood stage of falciparum malaria may cause life-threatening anemia; an Hb level of less than 5 g/dL is considered to represent severe disease. Children with anemia may present with malaise, fatigue, and dyspnea or respiratory distress, which usually represents metabolic acidosis, but in an ill child acute respiratory infection must be carefully excluded.
Acidosis is due largely to excessive lactic acid and other anions. Salicylate toxicity and dehydration may also play a role. However, the majority of children presenting with respiratory distress are severely anemic, have a metabolic acidosis secondary to reduced oxygen-carrying capacity, and respond to rapid transfusion of fresh blood.
A minority of those with respiratory distress do not respond to appropriate resuscitation. They probably represent a heterogeneous clinical group and may have renal failure, systemic bacterial infection, or a more profound syndrome of systemic disturbance caused by malarial parasites.
Resuscitation of children with anemia and/or severe metabolic problems must be conducted carefully. Fluid boluses given to African children with shock and life-threatening infections showed somewhat surprisingly that rapid infusion of crystalloids or albumin (20 to 40 mL/kg) significantly increased 48-hour mortality in these critically ill children with impaired perfusion. The TRACT trial has recently provided additional evidence for management of severe anemia and shown that conservative management of uncomplicated severe anemia (Hb 40 to 60 g/L) is safe, if children are reviewed regularly. The trial showed that severely anemic children without fever can be safely transfused with 30 mL/kg of blood but a blood infusion of 20 mL/kg is needed for safe transfusion of febrile children with severe anemia (see box on Transfusion Management of Severe Anemia in African Children .). Fluid resuscitation and blood transfusion must be carefully supervised, and it may be relevant that after transfusion in children with acute malarial anemia, BNP levels are high in children with severe malarial anemia suggesting that cardiac function may be compromised and should be carefully monitored during fluid and blood therapy for acute malaria.
The Phase III Transfusion and Treatment of severe anemia in African Children Trial (TRACT) showed:
conservative management of uncomplicated severe anemia [hemoglobin (Hb) 40–60 g/L] was safe
transfusion volume (20 vs. 30 mL/kg whole blood equivalent) for children with severe anemia (Hb <60 g/L) had strong but opposing effects on mortality, depending on fever status (>37.5°C).
In 2020 a stakeholder meeting of pediatric and blood transfusion groups from Africa reviewed the results and additional analyses and concluded:
the definition of severe anemia can be standardized as Hb <60 g/L
children with uncomplicated severe anemia do not require immediate transfusion, but do require monitoring because ~50% will develop severe and life-threatening anemia requiring subsequent transfusion
clinical and Hb monitoring reviews are needed in a child with severe anemia (both uncomplicated and complicated) to identify those needing transfusion
the most effective and safest volume of blood for transfusion depends on the axillary temperature when a blood transfusion is ordered
if temperature is equal to or <37.5°C then transfuse 30 mL/kg whole blood or 15 mL/kg red cell concentrate
if temperature is >37.5°C then transfuse 20 mL/kg whole blood or 10 mL/kg red cell concentrate
there is no need for separate recommendations for children with malaria (even those with high parasite burdens)
optimal transfusion of SCD patients or of children with poor nutritional status may need further specific studies.
The sudden appearance of Hb in the urine, indicating severe intravascular hemolysis leading to hemoglobinemia and hemoglobinuria or blackwater fever, received particular attention in early studies of anemia in expatriates living in endemic areas. There was an association between blackwater fever and the irregular use of quinine for chemoprophylaxis. This drug can act as a hapten and stimulate production of a drug-dependent, complement-fixing antibody. Recent studies of sudden intravascular hemolysis have shown that it is rare in Africa but more common in Southeast Asia and Papua New Guinea, where some cases are associated with G6PD deficiency and treatment with a variety of drugs, including quinine, mefloquine, and artesunate. Treatment with artesunate frequently induces anemia without induction of antibodies. Post-artesunate delayed hemolysis (PADH) occurs in 40% of returning travelers with severe malaria treated with artesunate, occurring more commonly in patients of European than of African origin. Hemolysis is largely, but not entirely, due to clearance by splenic macrophages of once-infected cells that may circulate for many days after removal of the ring-stage parasite from red cells. The once-infected cells retain several parasite proteins, including histidine-rich protein-2. Post-artesunate hemolysis is associated with increased levels of this protein, which can be detected by testing at 1:500 dilution of blood with the rapid diagnostic dipsticks for HRP-2 antigen.
The anemia of falciparum malaria is typically normocytic and normochromic, with a notable absence of reticulocytes, although microcytosis and hypochromia may be present because of the very high frequency of α- and β-thalassemia traits and/or iron deficiency in many endemic areas.
The anemia of malaria may be accompanied by changes in the white cell and platelet counts and in clotting parameters, but these changes are not in themselves diagnostic, nor do they guide management. Malaria is accompanied by a modest leukocytosis, although leukopenia may also occur. Occasionally, leukemoid reactions have been observed. Leukocytosis has been associated with severe disease. A high neutrophil count may also suggest intercurrent bacterial infection. Monocytosis and increased numbers of circulating lymphocytes are also seen in acute infection, although the significance of these changes is not established. However, malarial pigment is often seen in neutrophils and in monocytes and has been associated with severe disease and unfavorable outcome.
Thrombocytopenia is almost invariable in malaria and so may be helpful as a sensitive but nonspecific marker of active infection. However, severe thrombocytopenia (platelet count <50 × 10 9 /L) is rare. Increased removal of platelets may follow absorption of immune complexes or platelet activation, but there is no evidence for platelet-specific alloantibodies. Thrombocytopenia has not generally been associated with disease severity, although platelets have been shown to contribute to disease pathology in both animal and human malaria. Moreover, in human infections, platelets may form clumps with infected erythrocytes. One explanation of inconsistent relationships reported between the severity of malaria and the thrombocytopenia may be that findings of low levels of platelets might not only be a marker of parasite burden but also be protective from severe disease as parasite growth is inhibited by physiological concentrations of platelets. Platelet factor 4 may bind to and enter malaria-infected RBCs and induce cell death of intra-erythrocytic parasites.
Disordered coagulation and clinical evidence of bleeding are not infrequent in nonimmune adults contracting malaria and presenting with severe disease. Patients may present with bleeding at injection sites, gums, or epistaxis. Abnormal results of laboratory tests for hemostasis, suggesting activation of the coagulation cascade, occur in acute infection. However, histologic evidence of intravascular fibrin deposition is notably absent in adults dying as a result of severe malaria. Factor XIII, normally responsible for cross-linking fibrin, is inactivated during malarial infection, and these data may explain low levels of fibrin deposition in the face of increased procoagulant activity.
During acute disease the levels of a disintegrin and metalloproteinase with thrombospondin motif 13 (ADAMTS13) protease are moderately reduced, and the concentration of high-molecular-weight von Willebrand multimers is increased, but the role of this adhesive pathway in the etiology of severe and cerebral malaria has not been established. Infected RBCs from different isolates or clones may variously bind to a series of endothelial receptors including CD36 and ICAM-1. Adhesion of infected RBCs to the endothelial receptor for protein C is associated with cerebral malaria and may induce endothelial activation and brain swelling.
The bone marrow is typically hypercellular. The most striking findings are of grossly abnormal development of erythroid precursors or dyserythropoiesis. The developing erythroid cells typically demonstrate cytoplasmic and nuclear bridging and irregular nuclear outline. These changes are probably central to the pathophysiology of malarial anemia and are discussed in detail later. The proportion of abnormal erythroid precursors and the degree of dyserythropoiesis are markedly greater in chronic compared with acute infection, suggesting that the inhibition and abnormal maturation of erythroid precursors may have somewhat differing etiologies in acute and chronic infection.
The role of hematinic deficiency in children presenting with malaria and anemia may be difficult to assess. The relative importance of absolute and functional iron deficiency have not been defined. Iron will not be absorbed during acute infection, because hepcidin levels are raised. Nevertheless, many hospitals give a course of iron supplementation after an episode of acute malaria, although no general guidelines have been established. Chronic hemolysis may increase folate requirements, but frank deficiency is uncommon in children presenting with acute malaria, at least in East Africa. Folate deficiency may be more common in West Africa, and protocols for folate supplementation after malaria must reflect local experience.
Pregnancy is accompanied by a series of physiologic changes that predispose not only to anemia but also to malaria. Hemodilution causes a physiologic decrease in Hb. Moreover, the demand for both iron and folate increases as the fetus grows and often precipitates frank folate or iron deficiency, particularly in multigravid women.
Occult malarial infection, often without fever, may cause anemia and placental dysfunction. This effect is greatest in primigravidas and has been attributed to the adhesion of parasitized erythrocytes to chondroitin sulfate A and hyaluronic acid in the placenta (for review, see Rogerson et al. ). Fetal growth is impaired, and babies born to women with placental malaria are, on average, 100 g lighter than those born to women without malaria. The subsequent contribution of malaria to infant mortality is substantial. Furthermore, the increase in hematopoiesis demanded by hemolysis during malarial infection may precipitate frank folate deficiency. Finally, women who are not immune to malaria are more likely to develop hypoglycemia and pulmonary edema during pregnancy. The increase in maternal and fetal morbidity and mortality secondary to malaria may be prevented by routine hematinic supplementation, implementation of insecticide-impregnated bed-nets, and by intermittent treatment with antimalarials during the second and third trimesters with sulfadoxine-pyrimethamine (Fansidar) where parasites remain sensitive to this combination, dihydroartemisinin-piperaquine in Africa and areas of drug resistance to sulphadoxine-primethamine.
Although splenomegaly secondary to malarial infection usually regresses as immunity is acquired, some people living in endemic areas develop progressive, massive splenomegaly. The pathophysiology of such hyperreactive malarial splenomegaly (HMS) is poorly understood but certainly results in B-cell hyperplasia with high levels of polyclonal IgM, reaching a level greater than two standard deviations (SDs) above the local reference mean. The specific antimalarial antibody titer is high, although only a small proportion of the polyclonal IgM response is directed at malarial antigens. B-cell hyperplasia may provoke proliferation of both T cells and macrophages, and IgM levels may cause cryoglobulinemia and stimulate erythrophagocytosis.
Patients may present typically between 20 and 40 years old, with massive splenomegaly without lymphadenopathy, anemia that may be severe, neutropenia, and thrombocytopenia. The anemia may cause life-threatening hemolytic crises and/or neutropenia associated with severe bacterial infection. Thrombocytopenia is rarely symptomatic. The bone marrow shows hypercellularity, with a lymphocytosis in marrow and peripheral blood. The high IgM levels distinguish HMS from chronic lymphocytic leukemia or other lymphoproliferative disorders. However, the polyclonal proliferation of B cells may transform to a true clonal lymphoproliferative disorder. This appears to be derived from a naive B cell, although the exact classification of such lymphoproliferative disorders is poorly defined ; however, there is a single report of a high incidence of B prolymphocytic leukemia in one population.
Treatment for HMS is lifelong antimalarial prophylaxis. Signs and symptoms usually subside over 1 to 2 years, but relapse may occur if antimalarial therapy ceases. Splenectomy is contraindicated because it is technically difficult, with considerable intra-operative mortality, and it may predispose to severe infection (for review see Leoni et al. ).
In P. vivax and P. ovale malaria, high parasitemias are rare because invasion of erythrocytes is limited to reticulocytes. However, there is an emerging consensus that P. vivax monoinfection may cause severe disease with cerebral malaria and/or anemia (for review, see Price et al. ). The vivax-infected RBC can adhere to host cells, but sequestration in the peripheral circulation and organ-specific syndromes of disease are much less common than in falciparum malaria.
P. vivax malaria has been associated with anemia during pregnancy and with low birth weight of children of affected mothers. Here cytokines or other inflammatory mediators appear to cause placental dysfunction. P. malariae infection is also rarely fatal but is distinguished by the persistence of blood-stage parasites for up to 40 years. It can, however, cause a progressive and fatal nephrotic syndrome.
The diagnosis of malaria is based on the identification of circulating blood-stage parasites. The standard methods of preparing thick and thin films are straightforward and allow a simple method to diagnose infection ( Table 154.1 ). However, malaria diagnosis poses particular problems for inexperienced staff. The main biologic problem is that the level of circulating parasites is only weakly associated with the overall parasite burden because falciparum-infected erythrocytes are sequestered in capillary venules; thus the parasitemia is not a reliable guide to the severity of disease. Second, missing or delaying the diagnosis of falciparum malaria may result in serious morbidity and mortality. Finally, the most sensitive methods for diagnosis of infection, namely microscopy, require both skill and time.
|
In endemic areas, laboratory staff are skilled at the examination of thick films and routinely are able to detect 1 parasite in 100 high-power fields of a thick film, which corresponds to a sensitivity of approximately 5 to 50 parasites/μL. Thin films are used for determining the species of the parasites, and the circulating asexual forms of the four main malaria species can be readily identified, whereas the sexual forms (gametocytes) of the species require some skill and regular practice ( Figs. 154.5 and 154.6 ).
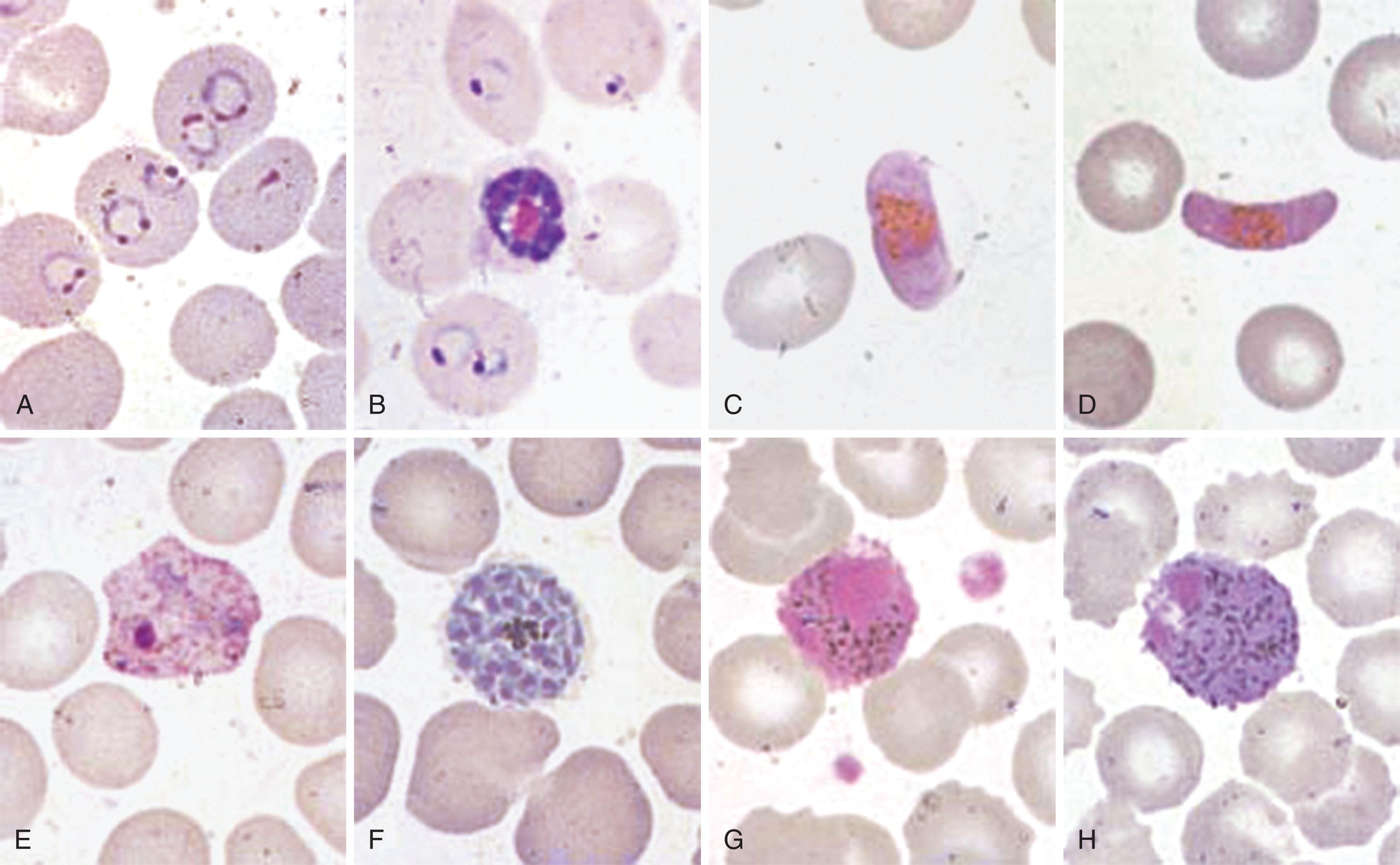
Nevertheless, diagnosis of malaria by microscopy in nonendemic countries has proven problematic. Routine laboratories may only achieve sensitivities of the order of 500 parasites/μL using thick films. Quality assurance schemes show that performance of routine hematology laboratories in the recognition of and species determination of malaria parasites is poor.
There has therefore been a strong drive to use nonmicroscopic methods for malaria diagnosis. Until recently, it has been the received wisdom that these methods are not sufficiently sensitive for clinical diagnosis but may have a role in detecting parasites of more than 500 parasites/μL when experienced staff are not available and/or as part of an out-of-hours service. However, advances in rapid antigen tests and in loop-mediated isothermal amplification (LAMP) amplification of deoxyribonucleic acid (DNA) have brought modern technology closer to an established role in the laboratory service and have enabled a significant change in practice where resources are limited. Operationally, this means that hematology laboratories need to make the diagnosis of malaria and must maintain the skills needed for reliable examination of thick films.
A number of methods based on the fluorescent staining of parasite DNA and/or ribonucleic acid (RNA) and the concentration of parasites have been devised. However attractive these methods may appear, their sensitivity is limited by background staining of cellular debris, and the limit of their sensitivity is approximately 100 parasites/μL The time taken in preparing samples and the specialized equipment and skills needed to use these methods limit their effectiveness in routine practice (for review see Amir ).
Detection of circulating malarial antigens by rapid diagnostic tests (RDTs) is another potentially attractive alternative to the laborious method of screening blood films. There are over 200 different RDT brands that utilize three antigens, namely, Plasmodium histidine-rich protein 2 (PfHRP-2), Plasmodium lactate dehydrogenase (pLDH), and Plasmodium aldolase (pALDO). pfHRP-2 is produced exclusively by P. falciparum and the assay is very Pf sensitive, but an alternative antigen(s) is needed in areas where P. vivax is more common. The sensitivity of these immunochromatographic tests decreases with low parasitemia (<100 parasites/μL), genetic variability of the antigen(s) including deletion of PfHRP-2, and prozone effect when the parasitemia is over 4%. The formulation of the tests using dipstick antigens allows rapid testing to be performed by laboratory staff. However, the sensitivity is variable and may range from 100 to 1000 parasites/μL, and this is comparable to the sensitivity achieved by inexperienced microscopists and may approach that achieved by experienced microscopists.
In lower- and middle-income countries, RDTs have allowed change in strategy from empiric treatment of a febrile child for malaria to parasitological diagnosis before therapy, with guidance to confirm RDT diagnoses with microscopy. WHO malaria treatment guidelines now recommend against routine presumptive treatment unless strong clinical suspicion of a severe disease without the opportunity for a timely laboratory diagnosis. The current recommendations for malaria diagnosis in the United Kingdom emphasize that the optimum diagnostic procedure is examination of thick and thin blood films by an expert to detect and speciate the malarial parasites. The sensitivity of RDTs is highest for P. falciparum and P. vivax malaria. However, RDTs for other Plasmodium species are not as reliable.
Amplification of circulating parasite DNA using the repeated ribosomal RNA (rRNA) genes is an extremely sensitive method of malaria diagnosis. The sensitivity may be as low as 0.005 parasites/μL or 5 parasites/mL. Undoubtedly, this is a useful tool for reference laboratories and epidemiologic surveys. LAMP amplification of parasite DNA is more sensitive than RDTs but less sensitive than conventional polymerase chain reaction (PCR) at low-level parasitemia. LAMP tests require DNA extraction and some basic molecular techniques and so are unlikely to replace RDTs and microscopy in the point-of-care diagnosis for suspected cases of malaria but may be suitable for epidemiological surveys of asymptomatic infection.
Malaria requires urgent effective chemotherapy to prevent progression of disease and may be the most crucial public health intervention to reduce global mortality from malaria. The drug treatment of malaria must take account of the expected pattern of drug resistance in the area where infection was contracted, the severity of clinical disease, and the species of parasite. The spread of drug-resistant parasites and the optimal use of affordable, effective drugs are of continual concern, and these have recently been reviewed ( Table 154.2 ).
| Oral Dose | Parenteral Dose | Side Effects | |
|---|---|---|---|
| Sulfadoxine-pyrimethamine |
|
For IM injection, doses as for oral |
|
| Artemether |
|
|
Reticulocytopenia |
| Artesunate | 4 mg/kg once daily for 7 days |
|
Reticulocytopenia |
| Artemether/lumefantrine |
|
||
| Quinine | 10 mg/kg of salt (maximum 600 mg) every 8 h for 7 days, together with or followed by either doxycycline 200 mg once daily for 7 days or clindamycin 450 mg every 8 h for 7 days [unlicensed indication] | 20 mg/kg in 10 mL/kg isotonic fluid over 4 h, then 10 mg/kg over 2 h given every 12 h in children and every 8 h in adults, together with or followed by either doxycycline 200 mg once daily for 7 days or clindamycin 450 mg every 8 h for 7 days [unlicensed indication] | Thrombocytopenia, intravascular hemolysis (blackwater fever) when used prophylactically, nausea, tinnitus, deafness |
Artemisinin-based combination treatments have been the mainstay of treatment for falciparum malaria in Southeast Asia for more than 15 years and are now recommended as first-line treatment throughout the rest of the world. However, resistance to these drugs is emerging, and very young children (particularly those underweight for age), patients with high parasitemias, and patients in very low transmission intensity areas with emerging parasite resistance are at risk for treatment failure and should be monitored closely. The hematologic side effects of antimalarial drugs in use today are few. Amodiaquine is associated with neutropenia and agranulocytosis. Artemisinin-based treatments may cause reticulocytopenia and hemolysis in approximately 10% to 15% of patients following intravenous artesunate treatment (see above). High levels of circulating PfHRP-2 predict late hemolysis and memoglobin concentrations should be checked approximately 14 days following treatment in those treated with intravenous artemisinins.
In severely ill patients, good nursing care is vital. Monitoring and treatment of fits and hypoglycemia are essential, and antipyretics should be given. Certainly, blood transfusion is required for the treatment of severe malarial anemia,
A consensus has emerged following the TRACT trial (see box on Transfusion Management of Severe Anemia in African Children ). Children with uncomplicated severe anemia do not require immediate transfusion and should be monitored as half these children will develop severe and life-threatening anemia requiring subsequent transfusion. The most effective and safest volume of blood for transfusion for afebrile children is 30 mL/kg whole blood and for febrile children is 20 mL/kg whole blood. Longer-storage RBC units are not inferior to shorter-storage RBC units for tissue oxygenation as measured by reduction in blood lactate levels and improvement in cerebral tissue oxygen saturation among children with severe anemia.
Whatever clinical guidelines emerge, in reality blood transfusion in the heartland of malaria-endemic areas is beset by many practical and theoretic problems. First, the absence of well-characterized donor panels (and thus systematic blood collection) frequently jeopardizes the supply of blood. Second, even when standard screening for human immunodeficiency virus (HIV) is in place, the residual risk for viral transmission in the serologic window of infectivity varies from country to country but recent estimates are that the risks remain for HIV at 1 in 3000 to 1 in 16,000, for hepatitis C 1:200 to 1:5000, and hepatitis B 1:400 to 1:2000. At a practical level, positive indirect antiglobulin test results in the setting of acute infection may make the exclusion of alloantibodies difficult. Depending on the clinical urgency and transfusion history, the least serologically incompatible blood may have to be given.
One therapeutic option available in North America and in Europe for the urgent treatment of nonimmune patients with severe disease would be an exchange blood transfusion. This procedure removes non-sequestered, infected erythrocytes but there are no randomized trials supporting the use of exchange transfusion in malaria. Some experts have suggested that this treatment could be given for hyperparasitemia (>20%) in severely ill nonimmune patients, but there is doubt whether this rationale is still relevant when artesunate kills circulating, non-sequestered ring-stage infected erythrocytes. The salient features that make this clinical problem a major public health concern are the very large numbers of children affected and the difficulty of satisfactory treatment by blood transfusion outside specialist centers.
Malaria is undoubtedly the most common transfusion-transmitted infection in the world. In endemic areas a large proportion of adult donors will be parasitemic, perhaps 20% to 80%, depending on the rate of transmission. Here donor deferral is impractical, and treatment of recipients with a course of effective antimalarials is the most practical alternative. In nonendemic areas, transmission of malaria is an occasional but potentially devastating complication of blood transfusion, and considerable thought and resources are required to combat the problem effectively.
The first case of transfusion-transmitted malaria (TTM) was in 1911. Between 1911 and the mid-1970s the incidence of TTM rose to more than 140 cases per year, with P. vivax the most common species causing infection, although the proportion of cases from P. falciparum has steadily increased, perhaps reflecting the speed and destination of international travel. It is striking that the background problem, namely malaria in returned travelers, is much more common in the United Kingdom than in the United States, with the per capita incidence differing by nearly a factor of 10 and a higher proportion of cases from P. falciparum in Europe and the United Kingdom compared with the United States.
Recent experience in the United Kingdom and the United States has emphasized the seriousness of TTM. Two of the last five cases of malaria owing to blood transfusion in the United Kingdom were fatal. In the United States, 14 cases of TTM were reported between 1990 and 1999, but only 5 cases were reported between 2000 and 2009. Detecting these cases after transfusion is frequently delayed because malarial infection acquired in nonendemic countries rarely figures in immediate differential diagnosis and requires careful examination of the blood film.
The mainstay of preventing TTM in the United Kingdom is donor deferral backed up by detection of circulating antibodies to malaria antigens. The guidelines for donor deferral have recently been revised ( Table 154.3 ). These criteria recognize that malaria in the nonimmune patient is likely to present within four months of return from an endemic area and that significant immunity to falciparum malaria may be acquired by residence after 6 months in a malarious area.
| Donor Risk Category | Guidelines |
|---|---|
| Resident |
|
| History of malaria | Permanent deferral unless malarial antibody test results are negative at least 3 years after cessation of treatment or last negative test results |
| Undiagnosed febrile illness |
|
| All other risks | Deferral for 12 months or 4 months if malarial antibody test results are negative |
The criteria also require that residents, as well as those having had malaria or an undiagnosed febrile illness, may be reinstated, after four months without malaria or a fever that could have been due to malaria or three years after treatment for an episode of malaria, if antimalarial antibodies cannot be detected. The importance of antimalarial antibody testing rests on the fact that it is a very sensitive method to detect chronic infection, whereas the identification of circulating malarial antigens or nucleic acids or microscopy would fail to detect a level of 1 parasite/mL, which would still give a highly infectious dose in a unit of blood.
The assays for antimalarial antibodies previously used indirect immunofluorescence antibody tests (IFATs) to detect reactivity to a crude parasite lysate as a target antigen. However, several enzyme-linked immunosorbent assays (ELISAs) using recombinant malarial antigens have proved to be a more practical if slightly less sensitive alternative to IFATs. These tests detect antimalarial antibodies in less than 2% of donors who have visited endemic areas. It has been calculated that the return of 90% of malarial antibody–positive visitors to the donor pool releases an extra 50,000 units per year in the United Kingdom, and this is a highly cost-effective process to reduce the attrition of eligible blood donors. In the United States, over 200,000 donors per year are deferred after travel to malaria-endemic areas.
Donor deferral is based on the potential of a donor to carry malaria and is therefore based on the area of travel, length of stay or residence, elapsed time since leaving the endemic area, and history of malaria. It has been repeatedly shown that application of even the most thorough donor questionnaires allows some of those carrying malaria to give blood because guidelines are frequently incorrectly applied or questions are answered inaccurately in routine practice.
In Canada donors reporting diagnosis or treatment of malaria defer permanently, and in the United States donors are deferred for 3 years after treatment. These criteria and the respective regulations of other major international blood services will inevitably cause unnecessary deferral of those who never actually had malaria but also may permit some individuals with low-level chronic infection to donate because malaria may very occasionally present more than 3 years after travelers return from endemic areas. The last case of malaria transmitted in the United Kingdom was by someone who had left a malarious area 8 years previously, and the longest recorded case of recrudescence of malarial infection is 44 years for P. malariae .
Permanent deferral of all those visiting malaria-endemic areas is unlikely to be a viable strategy to prevent TTM because donor bases are declining. Preventing malaria transmission through blood transfusion requires comprehensive, regularly reviewed, and effectively implemented guidelines for donor deferral and laboratory testing. Even the best strategy is a compromise, and medical laboratory staff should be aware of the rare but potentially serious possibility of fever after transfusion that could be caused by malaria.
Leishmaniasis is a generic term for infection by 30 or so species of the obligate intracellular parasites from the genus Leishmania , and different species may cause systemic or visceral, mucocutaneous or cutaneous infection. Visceral leishmaniasis (VL), or kala-azar, presents with a wide spectrum of systemic and hematologic features.
VL occurs in all countries bordering the Mediterranean Sea and across the Middle East, including Saudi Arabia and Yemen. Indian VL occurs in the eastern regions of India (particularly Assam, Bengal, Bihar, Uttar Pradesh, Tamil Nadu, and Sikkim) and in Nepal and Bangladesh. African kala-azar is endemic in Kenya, Ethiopia, and the Sudan and sporadically elsewhere in tropical Africa. In the Americas, VL occurs in foci across Mexico, Central America, Colombia, Venezuela, Guyana, Brazil, Bolivia, and northern Argentina ( Fig. 154.7 ).
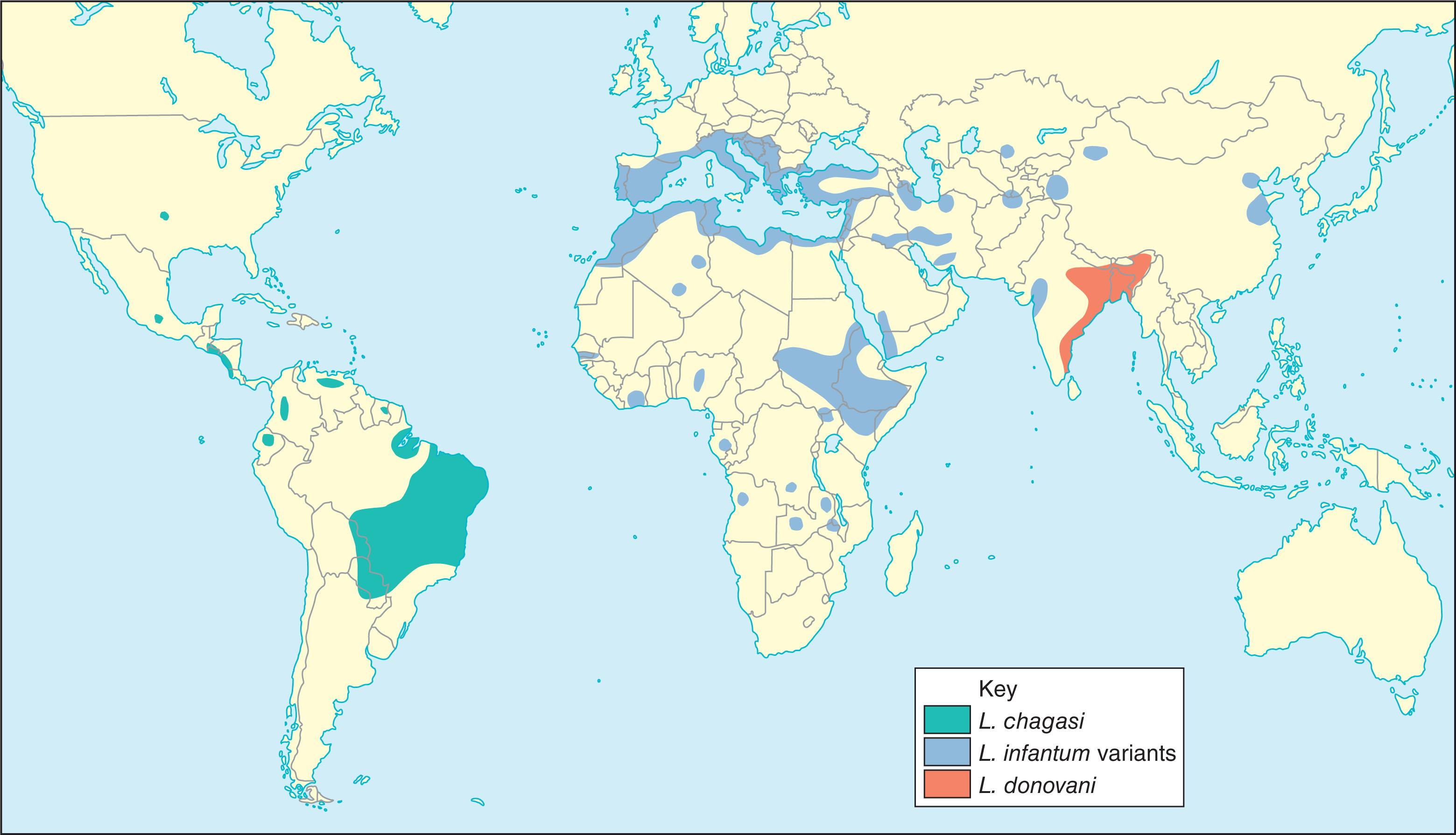
The total burden of disease is difficult to estimate but significant. Leishmaniasis burden is endemic in 88 countries, with 15,000 to 40,000 new cases of VL per year, with the vast majority occurring in India, Bangladesh, Nepal, Northern Sudan, and northeastern Brazil. VL is associated with poverty and undernutrition in endemic areas, particularly in the hyperendemic foci of the southern part of the Sudan and the Ganges river basin. In the Mediterranean area, it is increasingly seen in association with HIV infection, where infection rates in HIV-infected people may be as high as 10%.
VL is caused by a number of species of the Leishmania donovani complex. In the Mediterranean region and areas in the Middle East and Central Asia through to China, Leishmania infantum predominates, whereas L. donovani is more prevalent in India. Leishmania tropica is a less common cause of VL in these areas. Throughout their range in the Old World, parasites are transmitted by the female sandfly of the Phlebotomus genus. Leishmaniasis is caused by different parasites and vectors in the New World, where Leishmania chagasi and Leishmania amazonensis are transmitted by the Lutzomyia genus of sandfly.
Leishmania organisms are present in blood, and so the disease can be transmitted by blood transfusion, as a sexually transmitted disease, as a congenital infection, by needle sharing for intravenous drug abuse, or within a laboratory by intradermal inoculation of L. donovani promastigotes. Very few cases of leishmaniasis have occurred as transfusion-transmitted infections in Europe or North America, with under 10 cases in infants or immunocompromised patients reported over the past 50 years. In endemic areas, this problem represents a much greater but unquantifie risk.
Leishmania amastigotes live and multiply within macrophages by binary fission. They are round or ovoid bodies, approximately 2 to 3 μm in diameter. Occasional rupture of cells allows invasion of uninfected monocytes and macrophages by free forms. Sandflies ingest amastigotes within macrophages from blood or skin. In the insect’s stomach, free amastigotes multiply and divide asexually, becoming elongated and developing flagella as metacyclic promastigotes. Within 2 weeks, such infective forms migrate through the lining of the stomach and enter the proboscis of the sandfly, allowing them to be inoculated in the human host while the sandfly takes a blood meal. Promastigotes are taken up by macrophages, where they become amastigotes by simple fission.
The large, elongated, fusiform promastigote measures 15 to 20 μm in length and 0.5 to 3.5 μm in width. Cultured promastigotes may also demonstrate rounded forms 4 to 5 μm in diameter. In Giemsa or other Romanowsky stains, a large nucleus and smaller, distinct rodlike kinetoplast are obvious. The organisms must be distinguished from Histoplasma capsulatum .
Parasites spread within macrophages to local lymph nodes and then to the liver, spleen, and bone marrow. They are also present more widely, in particular in the gastrointestinal tract and epidermis. In the subclinical cases, a cellularly mediated immune response causes a granulomatous lesion and resolution of the infection. However, in clinical VL little if any inflammatory response to the rapidly extensive and expanding parasite-laden macrophages is seen. Where it shows, a granuloma develops at the site of the initial inoculation but may not be apparent at the time of presentation.
The spectrum of clinical disease is wide, ranging from asymptomatic infection to acute or chronic illness. Perhaps only 1% to 3% of all infections are symptomatic, with an incubation period of 10 days to 10 years but typically between 3 and 6 months.
The high number of seropositive individuals in relation to clinical cases suggests that spontaneous cure without symptoms or with mild systemic symptoms and hepatosplenomegaly occurs in the majority of individuals. In a large series of children with VL in Brazil, the overall case fatality rate was 10%, and mucosal bleeding, jaundice, dyspnea, bacterial infections, and low neutrophil count of less than 500/mm or low platelet count of less than 50,000/mm 3 were associated with a poor outcome.
In endemic areas, a significant number of seropositive children, who do not develop classic VL, have a subclinical form of disease with malaise, fever, poor weight gain, intermittent cough, diarrhea, hepatomegaly, and variable splenomegaly. In these cases, leishmania were neither cultured nor seen in bone marrow aspirates. There are also reports of VL presenting as mild, nonspecific illness with fever, fatigue, cough, and abdominal pain in army personnel returning from the Middle East. In these cases, L. tropica was isolated from bone marrow or lymph nodes.
Patients with typical chronic VL often present with malaise and considerable fatigue and the slow onset of low-grade fever, anorexia, and weight loss. They usually have anemia, progressive and occasionally massive splenomegaly, hepatomegaly, lymphadenopathy, and hypergammaglobulinemia, with increasing skin pigmentation (hence the name kala-azar from the Hindi for “black sickness”). Patients may have jaundice, petechia, or purpura; heart murmurs; and edema. The size of the spleen is related to the length of infection and severity of the pancytopenia. In Africa, patients may have diffuse polymorphic papular lesions at presentation. Oral, nasal, nasopharyngeal, and laryngeal ulcers may occur during active disease or after treatment. The large spleen, often extending to the right iliac fossa, may be painful. Other features of acute disease include cough, epistaxis, and in some severe cases concurrent infection of the respiratory or gastrointestinal tracts and/or tuberculosis.
The typical features of VL may be preceded by bacteremia, bacterial infection, acute hepatitis, or Guillain-Barré syndrome. VL may be associated with hepatic necrosis, cholecystitis, or neuropathy. Occasionally, lymphadenopathy may occur without systemic features of the disease. Here histology shows noncaseating granulomas and amastigotes within macrophages. A more acute presentation in recent immigrants and visitors to endemic areas may include a high periodic fever similar to classic malaria, with malnutrition, bleeding, hepatitis, and/or acute renal failure (for review see Torres-Guerrero et al. ).
Coinfection with HIV and VL is now a well-recognized clinical entity in the Mediterranean region and undoubtedly occurs more widely (for review, see van Griensven et al. and Lindoso et al. ). VL occurs in the setting of late-stage acquired immunodeficiency syndrome (AIDS) with CD4 + lymphocyte counts of less than 200 × 10 6 /L. Patients present with fever, splenomegaly, and pancytopenia with frequent and sometimes atypical involvement of the gastrointestinal and respiratory systems and skin. The typical hematologic features of VL are sometimes absent, and serologic tests are often negative, although antibodies to 14- and 16-kDa Leishmania antigens may be detected by Western blot analysis. However, organisms are plentiful in bone marrow and even in buffy coat preparations. Some patients may have few symptoms, and the diagnosis of VL in immunocompromised patients requires a high index of suspicion. Treatment is difficult, with poor responses to pentavalent antimony. Liposomal amphotericin is the drug of choice. However, relapses are common, and maintenance treatment is required.
VL may also occur after transplant and after immunosuppressive treatments, including chemotherapy, rituximab, or other immunodeficiency states. Although patients present with a typical combination of fever, pancytopenia, and splenomegaly, the diagnosis may be missed if not considered.
VL causes a moderate normocytic, normochromic anemia. The pathogenesis of anemia is multifactorial and includes hemodilution, shortened RBC survival, and reduced erythropoiesis. The plasma iron is low, with plentiful stored iron typical of the anemia of chronic disease. One report has suggested that Epo levels are reduced compared with what would be expected for the degree of anemia. Occasionally, folate deficiency secondary to malabsorption and/or increased cell turnover or coexistent iron deficiency are associated with macrocytic or microcytic anemia, respectively.
The direct Coombs test results are usually positive for C3 components, and IgG and anti-I agglutinins may be present, but the presence and strength of the test is not correlated with the severity of the anemia. The positive direct Coombs test results appear to be caused by absorbed immune complexes onto erythrocytes. The neutrophil survival is reduced, and the differential white blood cell count shows neutropenia, relative lymphocytosis, and low eosinophil levels, with a white blood cell count typically in the range 2 to 4 × 10 9 L.
Neutrophil function may be impaired. Pancytopenia is more severe in those with concurrent HIV infection. A leukemoid reaction to infection has been reported.
Typically, the platelet count is reduced to 50 to 200 × 10 9 /L as platelet survival is reduced. Mucosal bleeding may occur, but extensive purpura is uncommon.
The prothrombin time is usually mildly prolonged to 2 to 4 seconds longer than in control subjects, secondary to impaired liver function. Fibrinolytic activity is increased, and in advanced cases, fibrinogen levels may be reduced. Disseminated intravascular coagulation or vasculitis may occur. Full coagulation screening is advisable if a splenic aspirate or bone marrow biopsy is planned.
The bone marrow is usually hypercellular, with increased erythroid, myeloid, and platelet precursors. Lymphocytes may be increased, and macrophages contain Leishman-Donovan bodies ( Fig. 154.8 ). If weight loss and malabsorption are extensive, the marrow may undergo gelatinous transformation.
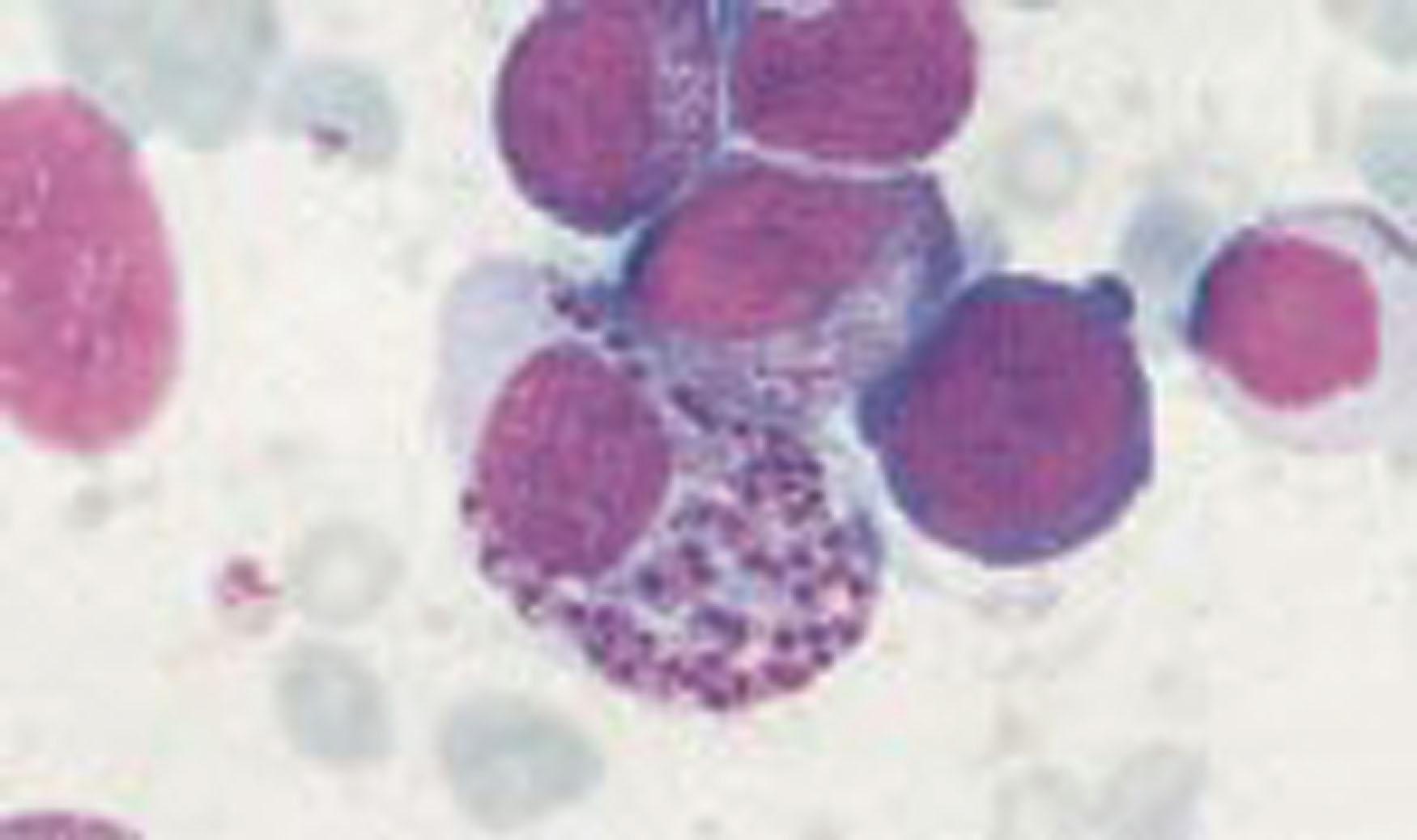
The mechanisms of leukopenia and thrombocytopenia are multifactorial. There is evidence for reduced bone marrow production of granulocytes and platelets and also for their autoimmune destruction and/or increased splenic clearance. After treatment, hematologic recovery is slow, and full recovery may take many months.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here