Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
bone morphogenetic protein
dispatched
days post coitum
fibroblast growth factor
fused
Hedgehog
Hedgehog interacting protein
hepatic stellate cell
intraflagellar transport
pancreatic intraepithelial neoplasia
pancreatic ductal adenocarcinomas
patched
smoothened
suppressor of fused
transforming growth factor β
We are built from hundreds of specialized cell types that make up our organs. The development and postnatal maintenance of these cell lineages are tightly controlled and incredibly stable in time. Cells are capable to go through major shifts in protein expression in time in response to physiological and environmental cues without changing cellular phenotype. Each cell type is defined by the expression of a unique combination of proteins transcribed and posttranscriptionally modified from the templates of our 20,000–25,000 genes. One of the central questions in biology is how the information contained in these genes can translate into a spatially complex multicellular organism. The idea that such complexity is possible because cellular phenotype is coupled to cellular position is at least 100 years old. We have learned much about the proteins involved in the specification of cellular position from developmental biologists that have used models such as the regulation of segmentation of the Drosophila larval epidermis, development of the vertebrate limb bud, neurogenesis, and somite formation.
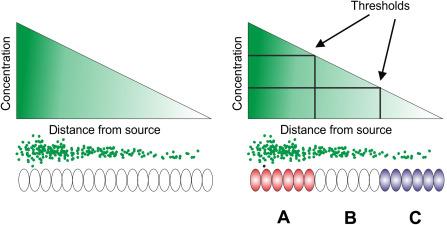
Most of the evolutionary conserved signaling pathways that act to couple cellular position to cellular phenotype have been first identified in genetic screens in Drosophila most notably the systematic screens performed by Nusslein-Volhard and Wieschaus. The type of molecules that have emerged from these genetic screens can be classified in two broad categories, intrinsic and extrinsic factors. Intrinsic signals such as kinases, phosphatases, and transcription factors function within the cell and thus act in a cell-autonomous manner. Extrinsic signals are soluble or transmembrane factors that act among cells and regulate cell-nonautonomous processes. Extrinsic information is critical for a complex tissue composed of multiple different cell types to organize appropriately. These tissues contain gradients of soluble factors that act in a concentration-dependent manner to control cell fate. Such factors have been termed morphogens. This concept was most clearly voiced by Wolpert in the 1960s, who developed the French Flag model ( Fig. 4.1 ). A morphogen receiving cell has one or more concentration thresholds that result in the expression of a distinct set of target genes. Thus, morphogen concentration gradients confer positional information by specifying distinct cellular phenotypes in a field of receiving cells depending on their distance from the source of the signal. Signaling molecules from the hedgehog (Hh)-pathway, the Wnt-pathway, the transforming growth factor (TGF) β-superfamily, and receptor tyrosine kinase pathways have been shown to act as morphogens in several models of development and are used in the regulation of cell fate throughout evolution and in most developing tissues. This chapter will summarize our current understanding of the Hh-pathway and its role in the development and postnatal maintenance of the GI tract.
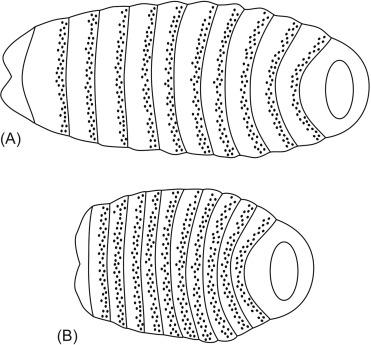
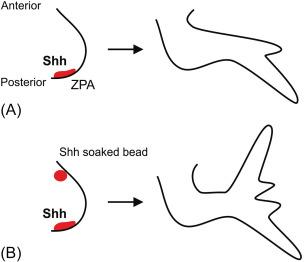
The Hh gene was identified in the first systematic screen for patterning genes in Drosophila . In the larvae of wild-type Drosophila , a band of bristles called denticles is present across the anterior half of each segment, whereas the posterior half is smooth (the so-called naked cuticle). In screening for mutations that affect the segmental pattern of the Drosophila larva, Nüsslein-Volhard and Wieschaus identified a group of mutants that affected the patterning within the segments but at the same time left the number of segments unaltered. In one of these segment polarity mutants, the posterior half of each segment failed to develop, resulting in a larva that is entirely covered by denticles ( Fig. 4.2 ). This phenotype gave the larva the aspect of a Hedgehog ( Hemiechinus ), hence the name. After much effort, the nucleotide sequence of the Hh gene was finally obtained, and the mammalian Hh homologues were identified. Echelard et al. identified three Hh genes in the mouse. They were named Sonic Hedgehog ( Shh ), after a popular Sega computer game character, Desert Hedgehog ( Dhh ), after an Egyptian species of Hedgehog ( Hemiechinus auritus ), and Indian Hedgehog ( Ihh ), a Hedgehog species endemic in Pakistan ( Hemiechinus micropus ). Riddle et al. found that Shh protein mediates the action of the zone of polarizing activity (ZPA) in the chick limb bud ( Fig. 4.3 ). The ZPA is a region at the posterior margin of the chick limb bud responsible for normal anteroposterior patterning. Shh was shown to colocalize with this previously described region and to alter limb patterning in a way similar to ZPA grafting. At the same time, a Hh homologue was identified in zebra fish ( Danio rerio ) and shown to be expressed in tissues with organizing activity. In 1995, a new Hh homolog was found in zebra fish and was named Tiggy-Winkle Hh, after a character in a children’s book. Most phyla of the eukaryotic kingdom have now been shown to contain Hh homologues, and the expression of vertebrate Hh genes has been detected in a variety of tissues during development. Shh , Dhh , and Ihh are highly conserved between mouse and humans. Unfortunately, little is known about factors that control the transcription of Hh genes, particularly in the gut.
In order for the Hh protein to act as a potent morphogen, it needs to be processed in different ways ( Fig. 4.4 A). After removal of the signal sequence, approximately 45 kDa large Hh proteins are cleaved auto-catalytically yielding an 19 kDa N-terminal fragment that contains all the signaling functions, and a 26 kDa C-terminal fragment that has no other function than to catalyze the cleavage. The unusual posttranslational processing of Hh proteins is not limited to this autocatalytic processing of the primary translated product. In the course of the cleavage, the Shh protein is covalently palmitoylated. Palmitoylation in the secretory pathway is atypical as palmitoylation is usually restricted to the cytoplasm and thus only affects proteins without a signal peptide and not proteins entering the secretory vesicular pathway. Palmitoylation is essential to both activity of the Hh protein and the signaling range. Palmitoylated recombinant Shh has an approximately 30-fold higher biological activity than unpalmitoylatable mutants suggesting that this acylation is important for enhancing biological activity. Even more atypical than the palmitoylation is the covalent attachment of a cholesterol moiety to the C-terminal part of Hh-N, making Hh proteins the only established example of protein sterolation. Conflicting reports have been published on the role of the cholesterol moiety in Hh signaling. The lipid-modified (processed) Hh signaling domain (Hh-Np) is hydrophobic and thus able to bind to the cell membrane where it mediates local signals. Dispatched (Disp), a 12-pass transmembrane protein with a sterol-sensing domain, is required for the release of Hh-Np from the cell membrane. Disp −/− mutant mice present with a similar phenotype as mice that lack the signaling receptor Smoothened (Smo), suggesting Disp is required for all Hh signaling.
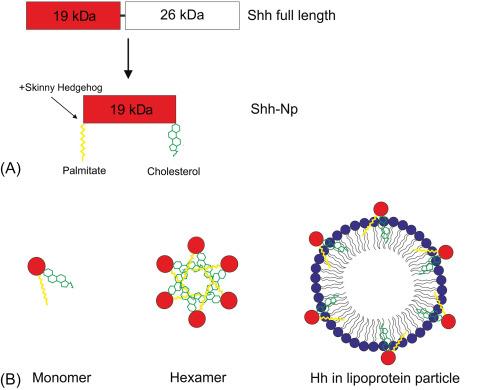
Despite the fact that lipid-modified Hh is not soluble, it is known to form a concentration gradient through a tissue and is able to activate long-range target gene expression over at least 8–10 cell diameters. Different models have been proposed to explain how the lipophilic Hh can spread through an aqueous tissue ( Fig. 4.4 B). Several laboratories have shown that both cholesterol and palmitate modifications are required to mediate multimerization complexes of NH 2 -terminal Hh proteins. The lipid moieties are thought to be embedded in the core of these complexes, in analogy to micelles. A second model proposes that the lipid moieties of Hh-Np associate with lipoprotein particles. The role of lipoproteins as vehicles for long-range Hh targets has been demonstrated by several groups who showed that depletion of circulating lipophorin particles, the Drosophila equivalent of lipoproteins, reduces the range of Hh signaling. Distribution of the lipid-modified Hh protein is further facilitated by interactions with proteoglycans, heparin, and chondroitin sulfates. Besides being the receptor for the Hh ligand, Ptch has been shown to function as a lipophorin receptor. Furthermore, Callejo et al. demonstrated that Ptch modulates intracellular lipid homeostasis, independently of its role in the regulation of Hh signaling. A link between Hh signaling and lipid metabolism may have important implications for vertebrate Hh signaling in diseases as atherosclerosis and adipogenesis-related diseases, such as osteoporosis, lipodystrophy, diabetes, and obesity.
Hh signal transduction is highly unusual, containing many features unique to this signaling system. The Hh-binding receptor Patched (Ptch) is a 12-transmembrane protein with two large hydrophilic extracellular loops that mediate Hh binding. However, Ptch does not convey the Hh signal to the intracellular components of the pathway itself like a conventional receptor. Rather, binding of Hh to Ptch alleviates the inhibitory effect of Ptch on another membrane receptor, the 7-transmembrane protein Smoothened (Smo). Two Ptch genes exist in vertebrates, Ptch1 and Ptch2 , but Ptch1 mediates most, if not all, effects of Hh signaling. In the absence of Hh, Ptch inhibits signaling by Smo. Upon binding of Hh to Ptch, this inhibition is alleviated and Smo will activate Hh target gene transcription ( Fig. 4.5 ). Ptch1 is a transcriptional target of Hh signaling and acts in a negative- feedback loop to restrict the range of Hh signaling in a tissue. In addition to Ptch, several other proteins have been identified that bind Hh and modulate signaling. Hh-interacting protein ( Hhip ) is a transcriptional target that acts as a negative regulator of Hh signaling by binding Hh on receiving cells. An additional class of Hh-binding proteins has been identified in Drosophila , interference Hedgehog (Ihog), and Brother of IHog (Boi), which interact directly with Ptch and have been shown to be essential for Hh transduction and target gene expression. Two vertebrate homologues are known, Cdo and Boc. These Hh-binding proteins facilitate binding and positively regulate signaling.
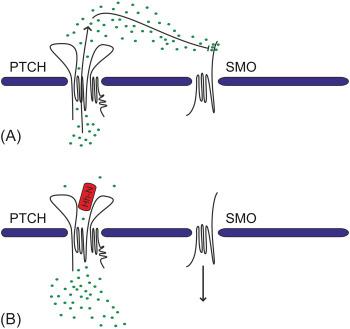
The mechanism of the inhibitory action of Ptch on Smo is only partially understood. Ptch belongs to the resistance-nodulation-division (RND) family of small molecule pumps and is closely related to the Niemann-Pick C1 (NPC1) protein, a transport protein involved in cholesterol homeostasis, which can act as a proton driven pump to flux small lipophilic molecules across the bilayer. A plausible hypothesis is that Ptch functions as a pump that regulates the availability of a compound that mediates the function of Smo. Mammalian Smo activity can be modulated by binding to lipophilic molecules structurally related to sterols, and it is thought that a structurally related endogenous ligand for Smo might exist. There is increasing evidence indicating that sterols may act as endogenous activators of Smo. Sterols are required for Hh signaling because sterol depletion or genetic defects in cholesterol biosynthesis results in inadequate signaling. Furthermore, some oxysterols, generated by cholesterol oxidation, are able to activate Smo by binding to the extracellular, cysteine-rich domain (CRD) of Smo. Recently, cholesterol itself has been proposed as an endogenous molecule to bind and activate Smo by causing a conformational change of the CRD. However, if cholesterol is also a substrate for the predicted transporter activity of Ptch1 at the side of the primary cilium is still a matter of active investigation. For a complete review of the Hh signaling molecules see Ref. .
The regulation of Smo activation and downstream Hh signaling have been only partly revealed. Most studies were performed in Drosophila ; however, this part of the pathway seems to have diverged considerably during evolution from Drosophila to mammals. For example, Drosophila do not have cilia, whereas in vertebrates, the Hh-pathway is dependent on the primary cilium. In Drosophila , fused (Fu) is essential for Hh signaling, whereas Fu is dispensable for Hh signaling in mammals. Therefore, we will focus on experiments performed in vertebrates where possible. In Drosophila , the Hh-pathway acts through a single transcription factor cubitus interruptus (Ci). In vertebrates, three Ci homologues have been identified termed Gli-1 to Gli-3. Gli2 acts mainly as a transcriptional activator, whereas Gli3 can also be processed into a transcriptional repressor. Gli1 is a transcription factor as well as a target of Hh signaling and functions as an amplifier of activated Hh signaling. Primary cilia have been shown to play a major role in Hh signaling in vertebrate systems. They are specialized subcellular signaling centers that are required for the response to developmental signals ( Fig. 4.6 ). Evidence that Hh signaling depends on cilia first came from a phenotype-based mutation screen, in which genes encoding components of the intraflagellar transport (IFT) machinery were disrupted. IFT proteins are responsible for maintenance of the cilia and for transport of particles along the axoneme, a microtubule-based cytoskeletal structure in the inner core of the cilium. Mutations in IFT proteins have been shown to lead to similar phenotypes as mutations in the Hh signaling pathway. IFT proteins act downstream of membrane proteins Ptch and Smo and upstream of the Gli transcription factors, for a review see Ref. . The importance of cilia in vertebrate Hh signaling was further demonstrated by the fact that all key components of the Hh-pathway are enriched in cilia. Both Ptch1 and Smo show dynamic, Hh-dependent trafficking in cilia. In the absence of Hh ligand, Ptch1 is localized at the cell surface of the ciliary base where it waits to bind extracellular Hh ligands. Although Smo circulates in and out of the cilium, Smo is mainly localized outside of the cilium at the cell membrane and intracellular vesicles. Upon binding of Hh to Ptch1, the receptor-ligand complex is internalized in endosomal vesicles, Smo accumulates at the ciliary membrane, and Hh target genes are activated by Gli proteins.
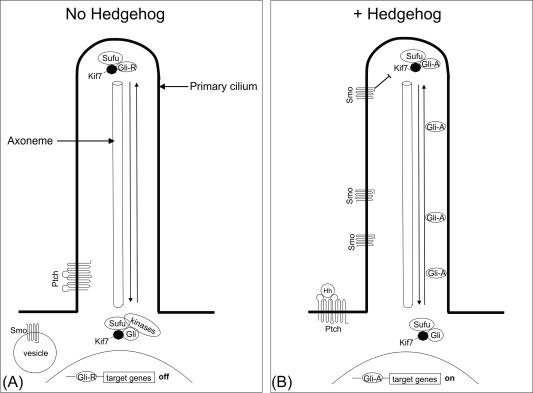
Gli2 and Gli3 are the major Glis to transduce the Hh signal in the gut. Gli2, which functions predominantly as activator of Hh signaling, shifts from a primarily cytoplasmic localization to the distal cilium tip and the nucleus, where it activates gene expression. In the absence of Hh, Gli3 is restrained to the cytoplasm by Suppressor of fused (Sufu) and proteolyzed into a truncated transcriptional repressor. Gli2 can also function as a repressor of Hh target expression; however, processing of Gli3 is much more efficient than that of Gli2. Sufu-Gli3 interaction is therefore a major control point in the Hh-pathway. Genetic inactivation of Sufu leads to constitutive activation of Hh signaling and in humans Sufu is known as a tumor suppressor gene.
Gli3 is converted into a transcriptional repressor by a complex of protein kinases. PKA initiates a partial proteolytic processing reaction in which PKA and subsequently CK1 and GSK3β phosphorylate Gli3. This phosphorylation can result in ubiquitination and partial degradation that generates a truncated version of Gli3 that acts as a dominant negative Gli. In the presence of Hh, Gli3, and Sufu dissociate, and an active form of Gli3 enters the nucleus to activate target gene expression. It is unknown by which mechanism the Hh signal counteracts Sufu’s repression of Gli activity, but some studies suggest that Smo activation can reduce PKA activity by activation of G protein Gα1 functions, leading to dephosphorylation and degradation of Sufu and subsequent dissociation of the Gli3-Sufu complex. Another protein that plays a role as a regulator of Hh signaling is Kif7, the homolog of Drosophila Cos2. Activity of Kif7 is dependent on the presence of the primary cilium. Upon Hh signal activity it traffics along the ciliary axoneme and localizes to the distal tip of the cilium where it controls cilium architecture creating an optimal environment for Sufu and Gli3 to interact. Kif7 behaves similarly to Drosophila Cos2 as a predominantly negative regulator of Hh target genes and probably serves as a scaffold for the production of Gli repressors. For a comprehensive review on the Hh signaling pathway see Ref. .
Following the release of transcriptional repression, Hh target genes will be transcribed. Regulation of target genes differs in time and between organs. Ptch1, Gli1, and Hhip are the most widely used as readouts for Hh-pathway activity, but many more Hh targets have been described. Ptch1 and Hhip are not only targets of Hh signaling but also part of a negative feedback loop and diminish the Hh response. On the other hand, Gli1 functions as transcriptional target that further enhances signaling and thus is part of a positive feedback loop.
The GI tract develops from a simple tubular structure composed of cells derived from all three germ layers. Cells from the endoderm will form the epithelial layer; mesodermal cells differentiate into mesenchymal cell types such as myocytes and fibroblasts whereas neural cells originate from the ectoderm and innervate the GI tract. The gut tube acquires regional specialization along the longitudinal axis of the developing embryo. The fate of cells that form this tube is restricted to become part of one of the roughly four specialized tissue units of the esophagus, stomach, small intestine, and colon. Other small groups of cells bud from the original tube to form the thyroid, thymus, lungs, liver, and pancreas. A second axis of specialization is the cross sectional or vertical axis. The constant renewal of epithelial cells in the adult occurs along this vertical axis. Most patterning mechanisms along these axes involve cross talk of morphogens between cells of the different germ layers.
Hh signaling plays a role in the specification of cellular fate along both the longitudinal and the vertical axis and its regulation is involved in budding of gut tube-derived organs. The endodermal cell layer expresses Hh family members Shh and Ihh. We will discuss our current understanding of the role of Hh signaling in GI development in three sections that will cover the three segments of the gut and its derivatives: foregut (esophagus, trachea, lungs, thymus, stomach, liver, and pancreas), the midgut (small intestine), and hindgut (colon and anus). For a detailed description of gastrulation, which takes place early in embryological development, and axis formation see Ref. .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here