Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cancer is a disease of genetic instability. Although only a few specific alterations seem to be required for generation of the malignant phenotype, at least in colon carcinoma there are approximately 10,000 estimated mutations at time of diagnosis. The genetic plasticity of cancer cells allows them to frequently escape the precise molecular targeting of a single signaling node or pathway, making them ultimately nonresponsive to molecularly targeted therapeutics. Even Gleevec (Novartis Pharmaceuticals Corp.), a well-recognized, clinically active Bcr-Abl tyrosine kinase inhibitor, can eventually lose its effectiveness under intense, drug-dependent selective pressure, because of either mutation of the drug interaction site or expansion of a previously existing resistant clone. Most solid tumors at the time of detection are already sufficiently genetically diverse to resist single-agent molecularly targeted therapy. However, such an enhanced rate of mutation suggests that cancer cells should be highly dependent on efficient chaperoning and degradation of terminally misfolded proteins, because unchecked accumulation of misfolded proteins can initiate apoptosis. Further, a multipronged attack on tumor cell signaling is likely to prove more efficacious than is targeting individual molecular pathways. Thus, pharmacologic agents that inhibit a cancer cell’s ability to dispose of misfolded protein, allow for simultaneous attack of multiple signaling nodes, or possess both properties simultaneously should be of benefit. Hsp90 inhibitors and proteasome inhibitors display these characteristics and are actively being developed as novel anticancer agents.
A number of signaling proteins that are Hsp90 clients (see the website maintained by D. Picard, http://www.picard.ch/ as well as several excellent reviews ) also mediate acquisition of the eight hallmarks of cancer and promote cancer cell survival in the face of environmental stress ( Figure 56-1 ). Therefore, it is not surprising that inhibition of Hsp90 may collapse or significantly weaken a cancer cell’s “safety net.” Several excellent reviews provide an in-depth description of the many signaling nodes regulated by Hsp90.
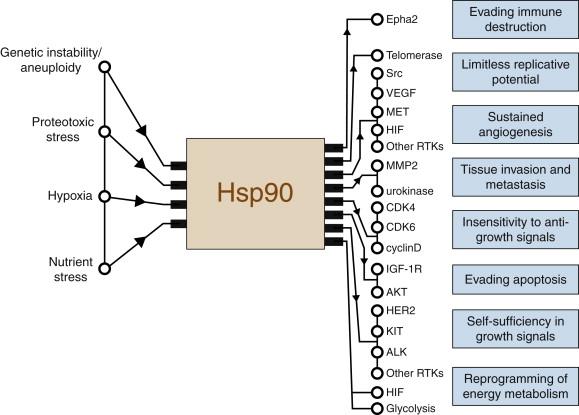
Cancer cells are subjected to extreme environmental stress, such as hypoxia and acidosis, as well as the exogenously applied environmental stresses of chemotherapy or radiation. These stresses tend to generate free radicals that can cause significant physical damage to cellular proteins. Given the combined protective role of molecular chaperones toward damaged proteins and the dependence of multiple signal transduction pathways on Hsp90, it is therefore not surprising that molecular chaperones in general, and Hsp90 in particular, are highly expressed in most tumor cells. Hsp90 may provide a unique molecular target in cancer cells for an additional reason. In Drosophila and Arabidopsis model systems, Hsp90 has been suggested to be a buffer of genetic mutation. Extrapolating this function to genetically unstable cancer cells, it is tempting to speculate that Hsp90 may be critical to a cancer’s ability to survive in the presence of a constitutively high mutation rate. Indeed, a recent study identified the Hsp90 inhibitor tanespimycin (17-AAG) in an unbiased in vitro screen for drugs active against aneuploid cancer cell lines.
The benzoquinoid ansamycin antibiotics, first isolated from the actinomycete Streptomyces hygroscopicus var. geldanus var. nova, include geldanamycin (GA) and its semisynthetic derivatives, 17-allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) and the more water-soluble 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG, alvespimycin) and 18,21-didehydro-17-demethoxy-18,21-dideoxo-18,21-dihydroxy-17-(2-propenylamino) geldanamycin (IPI-504, retaspimycin) ( Figure 56-2 ). These small molecules were the first identified inhibitors of Hsp90, and they (and second-generation Hsp90 inhibitors) have shown activity in cancer clinical trials. In particular, RECIST responses have been documented in patients with HER2 + breast cancer previously treated with trastuzumab (Herceptin), and in EML4-ALK + non–small-cell lung cancer. Activity has also been seen in bortezomib-naïve and refractory multiple myeloma.
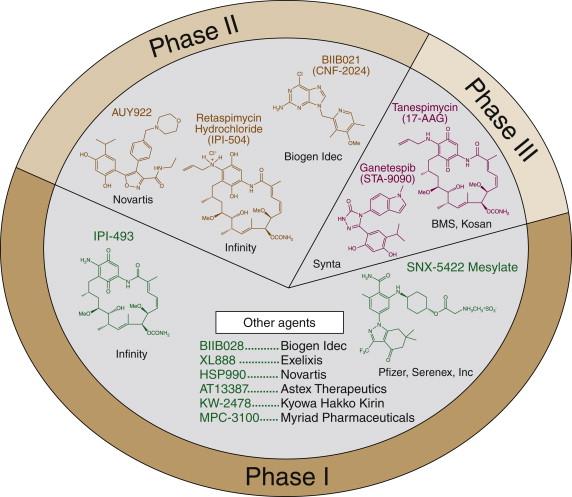
Several recent, excellently detailed reviews of the mechanics of Hsp90 function are in the scientific literature. Hsp90 is a conformationally flexible ATPase that associates with a distinct set of co-chaperones depending on ATP binding to an amino-terminal purine binding pocket. Identification of this pocket as the GA binding site led Chiosis and colleagues to design a series of highly potent purine scaffold Hsp90 inhibitors with markedly improved drug-like properties. Workman and colleagues used a high-throughput screen based on inhibition of Hsp90 ATPase activity to identify 3,4-diarylpyrazoles as a novel class of Hsp90 inhibitors. Other groups have developed and validated a number of structurally distinct inhibitors of this purine pocket. As of early 2012, more than 10 Hsp90 inhibitors had reached clinical trial (see Figure 56-2 ).
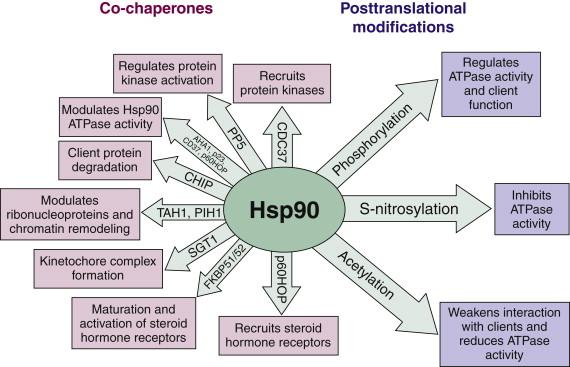
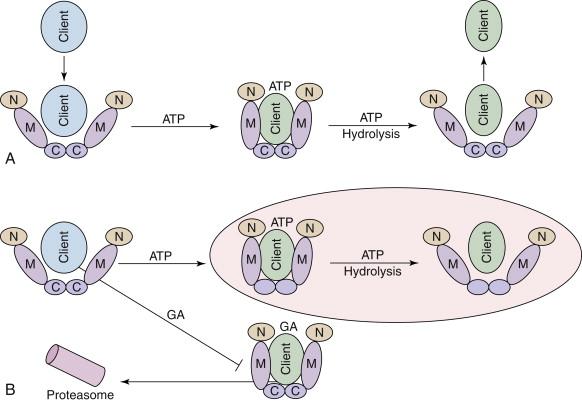
A model of Hsp90 function has emerged in which ATP binding to the amino-terminal pocket initiates a series of conformational changes that endow Hsp90 with ATPase activity. This process involves participation of a number of co-chaperones that interact with Hsp90 to form a “super-chaperone machine.” Certain co-chaperones play specific roles in this dynamic process ( Figure 56-3 ). ATP hydrolysis completes the chaperone cycle, at which point the process (which is frequently iterative) can begin again. Many Hsp90 client proteins first associate with an Hsp70/Hsp40 chaperone complex. This assemblage associates with Hsp90 via p60Hop, an Hsp90/Hsp70 interacting protein. At this point, when the client protein is being loaded on Hsp90, the chaperone is thought to be in an apo (nucleotide-free) conformation. ATP binding alters Hsp90 conformation, causing it to release p60Hop and the Hsp70/Hsp40 complex, and to recruit another set of co-chaperones, including p23 and certain immunophilins. In the case of kinase client proteins, the co-chaperone p50Cdc37 usually delivers the client to Hsp90. Although ATP hydrolysis is essential for chaperone activity, the ATPase activity of Hsp90 is very weak. Association of the co-chaperone Aha1 is necessary to increase Hsp90 ATPase activity sufficiently to drive the chaperone cycle forward. In higher eukaryotes, the ordered association/dissociation of client proteins and co-chaperones is also affected by a series of sequential phosphorylation events. Other posttranslational modifications also affect Hsp90 chaperone activity or client binding (see Figure 56-3 ).
A highly orchestrated and tightly regulated process drives the ATP-dependent Hsp90 super-chaperone machine. Hsp90 inhibitors block the chaperone cycle by displacing ATP from the amino-terminal purine pocket, inducing client protein ubiquitination and proteasome-mediated degradation ( Figure 56-4 ). Although the mechanics of this process are not well understood, they appear to involve a “handing-off” of the client from inhibitor-bound Hsp90 to an Hsp70-ubiquitin ligase complex. E3 ubiquitin ligases that have been implicated in mediating Hsp90 inhibitor-induced client protein ubiquitination in mammals include CHIP and Cullin 5. Importantly, even if the proteasome is inhibited, client proteins are not rescued from Hsp90 inhibition, but instead accumulate in a misfolded, inactive state in detergent-insoluble subcellular complexes. A selection of several oncogenic Hsp90 clients (in addition to HER2 and EML4-ALK, mentioned earlier) is shown in Table 56-1 . The reader is directed to the references therein for more detailed information.
| Cancer | Hsp90 Client | References |
|---|---|---|
| Anaplastic large cell lymphomas | NMP-ALK | |
| Acute myeloid leukemia | FLT3 | |
| Chronic myelogenous leukemia | BCR-ABL | |
| Human mastocytosis Gastrointestinal stromal tumors |
KIT | |
| Melanomas | B-RAF EGFR |
, |
| Prostate cancer | Androgen receptor | |
| Clear cell renal cell carcinoma (ccRCC) Nonhereditary sporadic ccRCC |
HIF1-α | |
| Glioblastoma | VEGF | |
| Leukemia | BCL2 APAF |
|
| Small-cell lung cancer | MET | |
| Multiple endocrine neoplasia type 2 (MEN2A, MEN2B) Familial medullary thyroid carcinoma (FMTC) Papillary carcinoma of thyroid |
RET |
Hsp90 itself is also subject to ubiquitination and degradation. Wee1-mediated phosphorylation of a conserved tyrosine residue (Y38 in human Hsp90α) determines the ubiquitination and degradation of a nuclear pool of the chaperone, with consequences for Hsp90 function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here