Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The temporal bone is divided into five portions:
Mastoid (posterolateral part that includes the mastoid process: attachment for sternocleidomastoid and other muscles)
Petrous portion (pyramidal-shaped medial part; inner ear structures, internal auditory canal [IAC], petrous apex)
Squamous portion (anterolateral part; forms lateral wall of middle cranial fossa)
Tympanic portion (forms bulk of external auditory canal [EAC] and middle ear space)
Styloid portion
Cartilaginous portion
Bony portion
Composed of air-filled spaces that contain the ossicles and are divided into:
Epitympanum (attic; above imaginary line between scutum tip and tympanic facial nerve)
Mesotympanum (middle ear proper between epitympanum and hypotympanum)
Hypotympanum (shallow space below imaginary line between tympanic annulus and cochlear promontory)
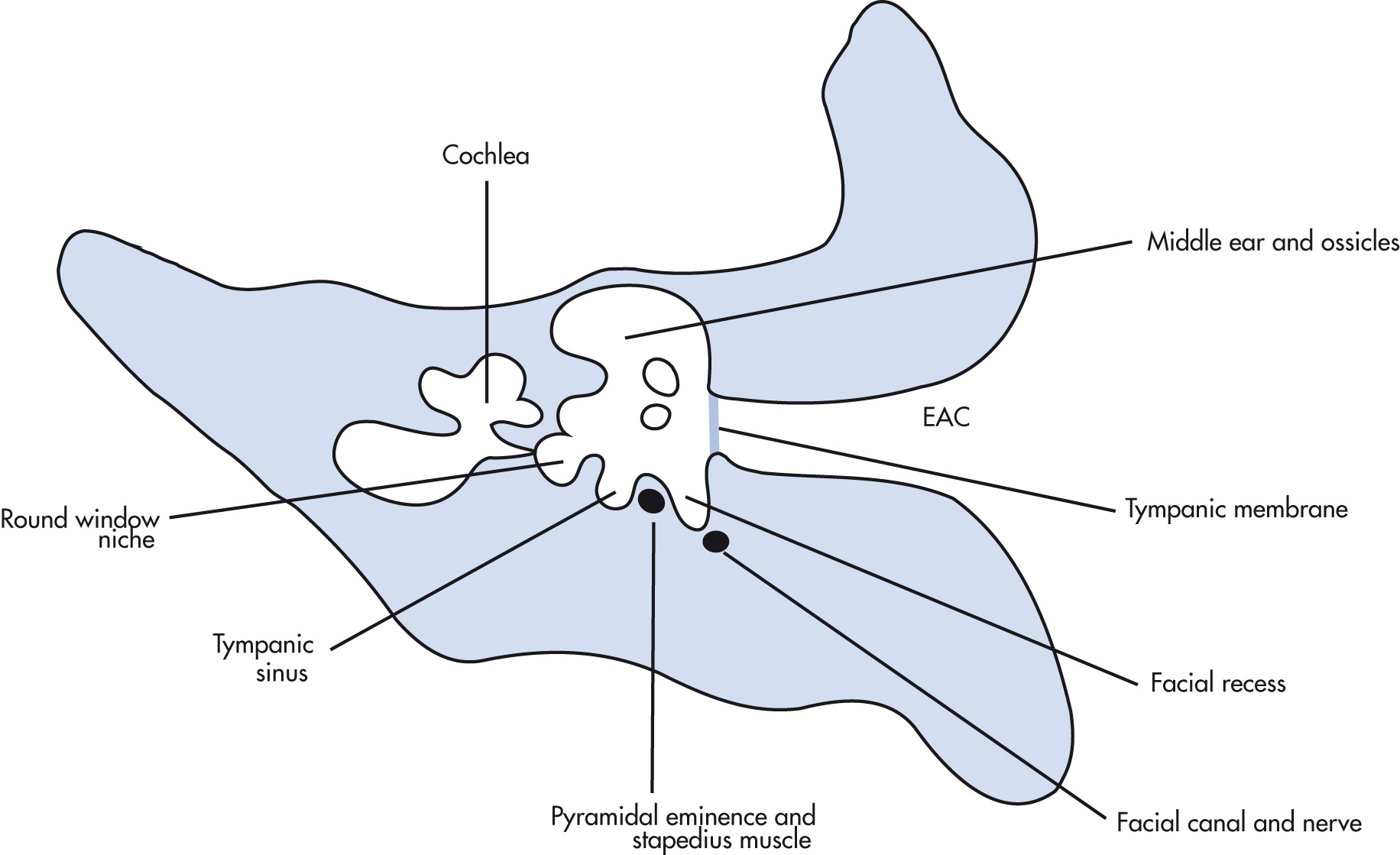
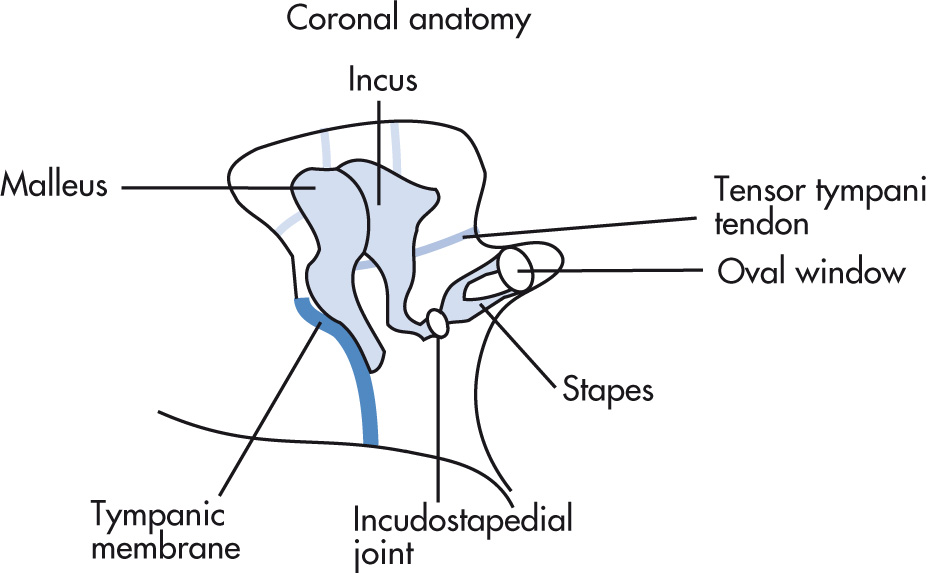
Boundaries of the middle ear are tympanic membrane (TM) laterally, the tegmen superiorly, and the inner ear medially. Posteriorly, there are three important structures (facial nerve recess, pyramidal eminence, and sinus tympani). The eustachian tube (pressure equalization) connects the middle ear with the nasopharynx. The lateral epitympanic recess (Prussak space) is a common location for retraction pockets and cholesteatoma. Three ossicles transmit sound waves from the TM to the oval window in the vestibule:
Malleus
Incus
Stapes (two crura, one footplate)
The inner ear (labyrinth) consists of:
Three semicircular canals, which connect to vestibule
Vestibule (utricle and saccule) between semicircular canals and cochlea
Cochlea (sensorineural hearing), which connects to:
Stapes → oval window
Round window (allows for counterpulsation of fluid)
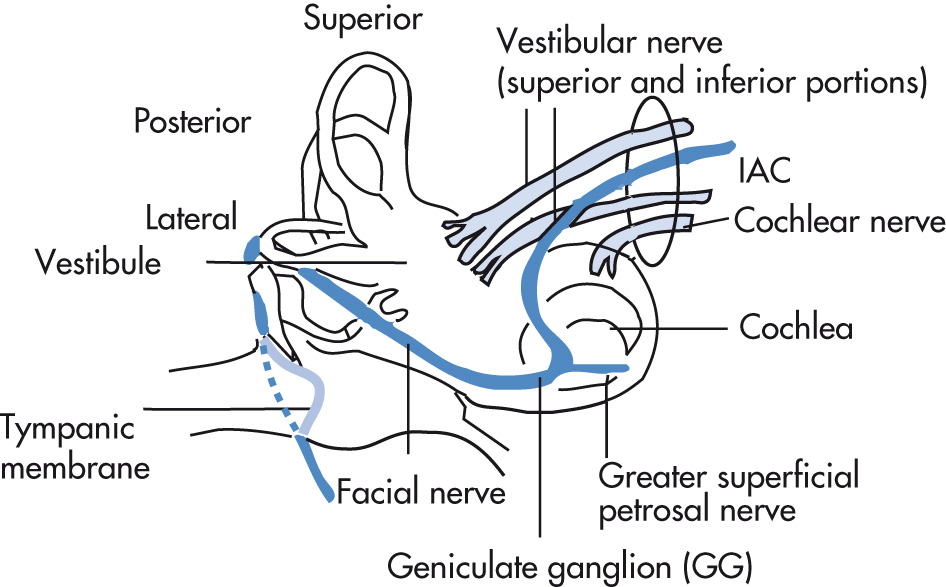
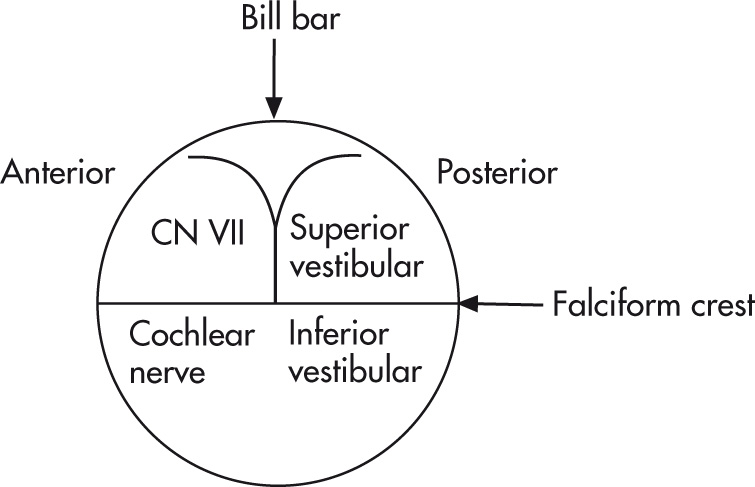
Left and right IAC should not differ >2 mm in diameter. Contents of IAC are divided by the falciform crest and Bill bar:
Facial nerve (anterior superior): cranial nerve (CN) VII (“7 up”)
Cochlear part (anterior inferior): CN VIII (“Coke down”)
Superior vestibular nerve (posterior): CN VIII
Inferior vestibular nerve (posterior): CN VIII
Four main parts of the facial nerve:
Intraaxial segment
Cisternal segment
Intratemporal segment:
Meatal (IAC) segment
Labyrinthine segment
Tympanic (horizontal) segment
Mastoid (vertical) segment
Extracranial segment:
Exits the skull through the stylomastoid foramen
Immediately gives off the posterior auricular branch/nerve
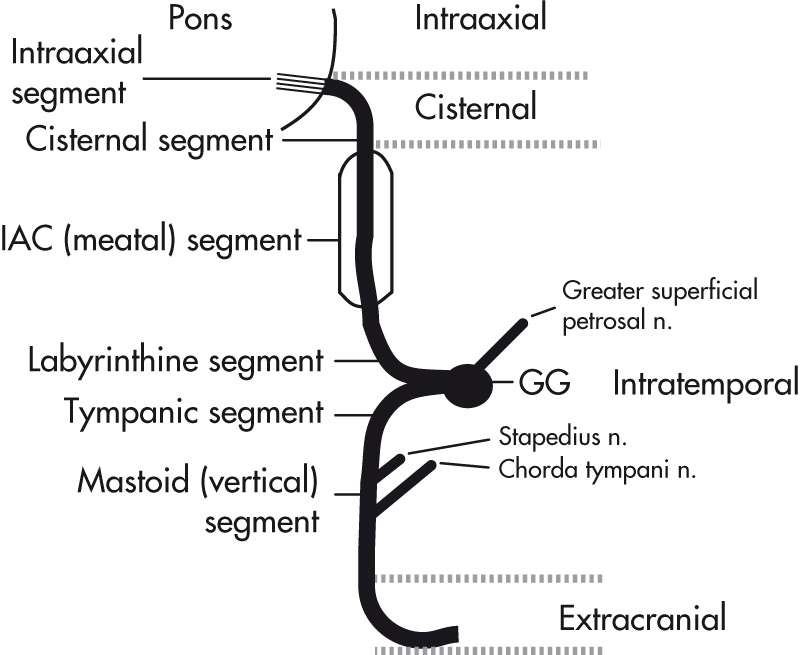
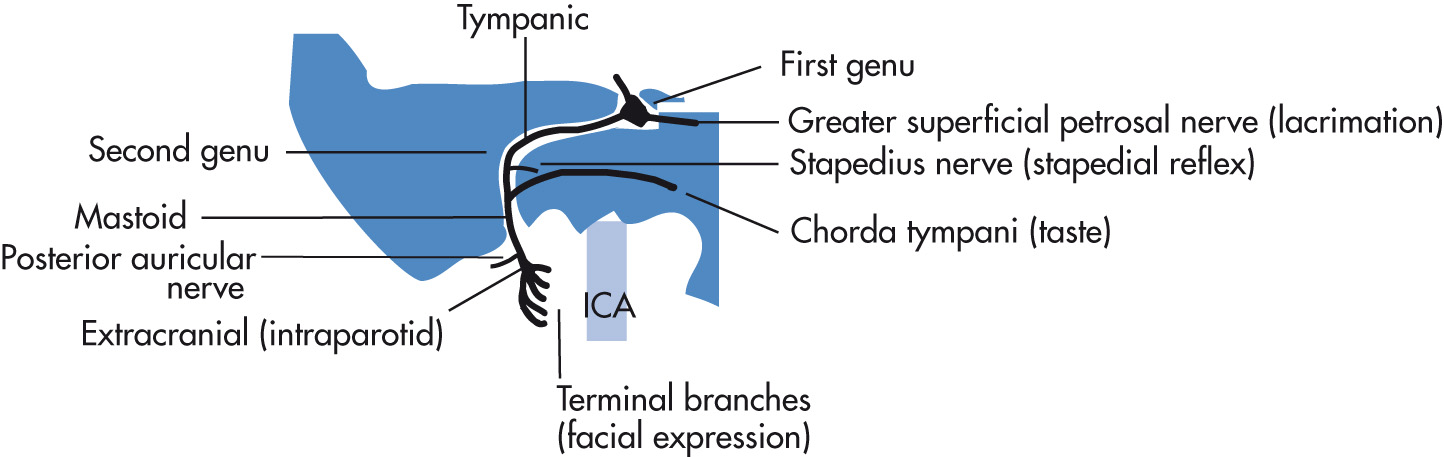
Main trunk courses through the parotid gland and gives rise to five terminal branches. The intratemporal segment of the facial nerve gives rise to three branches (see Fig. 7.6 ):
Greater superficial petrosal nerve
Stapedius nerve
Chorda tympani nerve
Conductive
Disruption of sound conduction that may be secondary to abnormalities in the EAC, TM, ossicles, or oval window
Common underlying causes: otitis, cholesteatoma, otosclerosis, trauma
Sensorineural
Evaluate inner ear, IAC
Common underlying causes: idiopathic, hereditary, vestibular Schwannoma, enlargement of vestibular aqueduct, labyrinthitis ossificans, otosclerosis (cochlear otosclerosis), trauma
Normal vascular variants
Aberrant ICA
Jugular bulb anomalies (high or dehiscent jugular bulb, diverticulum)
Persistent stapedial artery
Vascular tumors
Glomus jugulare paraganglioma
Glomus tympanicum
Vascular abnormalities
Skull base dural arteriovenous fistula/arteriovenous malformation (AVM)
Atherosclerotic carotid artery stenosis
Carotid-cavernous fistula (CCF)
ICA dissection or aneurysm at the petrous apex
Fibromuscular dysplasia
Dural sinus stenosis
Transverse-sigmoid sinus diverticulum
Other causes of tinnitus
Idiopathic intracranial hypertension
Paget disease
Otosclerosis
Ménière disease
Periauricular swelling and ecchymosis (Battle sign)
Bleeding into EAC or hemotympanum
Hearing loss
Tinnitus
Vertigo
Cerebrospinal fluid (CSF) leak
Facial paresis
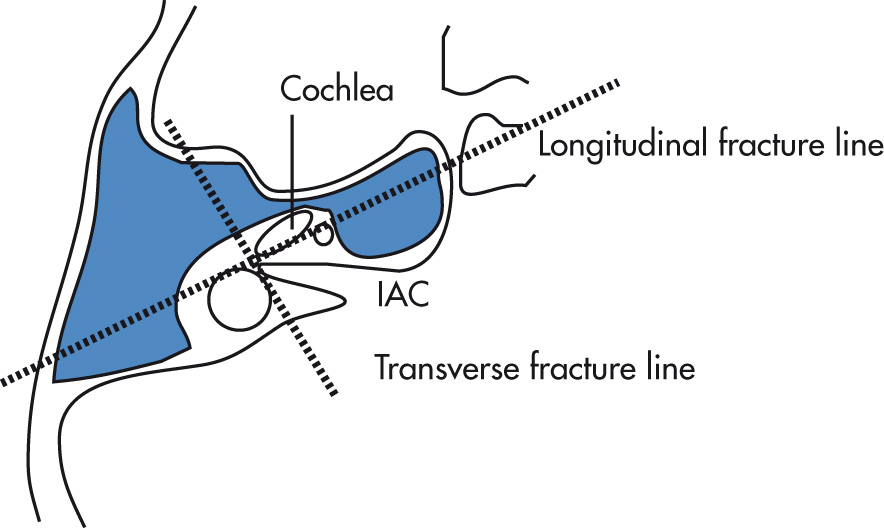
| Parameter | Longitudinal Fractures | Transverse Fractures |
|---|---|---|
| Frequency | 80% | 20% |
| Fracture line | Parallel to long axis | Perpendicular to long axis |
| Typically involves | Middle ear | Inner ear |
| Labyrinth | Typically spared | Commonly involved |
| Ossicles | Involved: conductive hearing loss | Commonly spared |
| Tympanic membrane | Involved | Frequently spared |
| Facial paralysis | Approx. 20% | Approx. 50% |
a Fractures may also be characterized as oblique: mixed features of longitudinal and transverse fractures, cross the petrotympanic fissure.
Ossicular fractures or dislocations
TM perforation
Hemotympanum
CN VII paralysis
CSF otorrhea
Meningitis, abscess
Sinus thrombosis, rare
Labyrinthitis ossificans (late)
Also assess for associated cervical spine fracture or fracture extension to carotid canal.
Ossicular fracture or dislocation: varied, conservative or surgery
Facial nerve injury: frequently conservative, but occasionally requires surgical decompression
Labyrinthine fistulae: surgery if persistent
CSF leaks: surgery if persistent
Otitis media (fluid in the middle ear cavity): acute, subacute, chronic forms
Children: common
Adults: less common; exclude nasopharyngeal carcinoma (NPC), which can cause eustachian tube obstruction and serous otitis media.
Mastoiditis: fluid-filled mastoid cells are also often present but not specific for infection.
Coalescent mastoiditis: occurs if medical treatment of acute otomastoiditis fails. Enzymatic resorption of mastoid septa and development of an intramastoid empyema seen as erosive changes on computed tomography (CT); comparison with the opposite side can be helpful in subtle cases.
Labyrinthitis
Meningitis
Subdural empyema or epidural abscess
Dural venous sinus thrombosis
Petrositis (infection of petrous air cells)
Gradenigo syndrome (rare complication of petrositis: triad of otomastoiditis, sixth nerve palsy, and pain in the distribution of the fifth nerve)
Subperiosteal or Bezold (via defect at the mastoid tip) abscess
Focal encephalitis, brain abscess, otitic hydrocephalus
Acute onset peripheral facial nerve palsy secondary to herpes simplex virus (HSV) that the majority of time resolves within 2 to 3 months. Imaging typically performed for atypical or persistent palsy. By magnetic resonance imaging (MRI), there is enhancement along the facial nerve involving intracanalicular (“fundal tuft”) and labyrinthine segments that normally do not enhance. Note that mild enhancement of the tympanic and mastoid segments of the facial nerve can be seen as a normal finding due to vascular supply. Facial nerve enhancement is nonspecific and can occur in other inflammatory and neoplastic conditions (especially if the nerve is enlarged, consider secondary to perineural spread of a tumor).
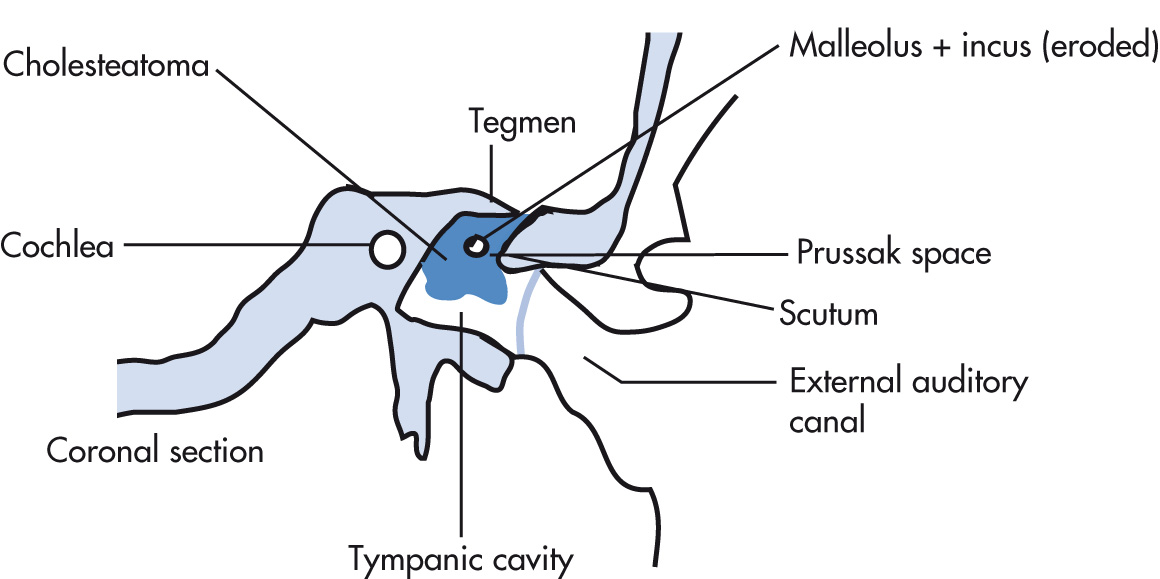
Mass of stratified squamous epithelium and keratin debris. Cause: epithelial cells accumulate in the middle ear via a perforation of the TM. The most accepted theory is that they arise from TM retraction pockets resulting from decreased intratympanic pressure. Types:
Acquired (98%): chronic middle ear infection (common). Arise in attic (Prussak space; pars flaccida cholesteatoma), or from pars tensa (sinus tympani).
Congenital (epidermoid; 2%): cholesteatoma arises from epithelial nests in middle ear, mastoid, or petrous bone, including the labyrinth (uncommon).
High resolution temporal bone CT first line, excellent for detailed bone evaluation; MRI can be helpful for complex cases and cholesteatoma distinction.
Soft tissue mass in middle ear
Borders may be well- or ill-defined.
Erosion of incus and otic spur (scutum) common
Bone resorption (collagenase) is typical and occurs most commonly in:
Ossicles
Lateral semicircular canal (fistula)
Tegmen tympani
Facial canal
Mastoid air cells may be underpneumatized and sclerotic secondary to chronic infection.
Labyrinthine fistula formation in lateral semicircular canal is less common.
It is often impossible to distinguish chronic middle ear infection from cholesteatoma in cases with little bone destruction on CT. However, MRI can be useful for distinction of cholesteatoma (restricted diffusion, no enhancement) from inflammatory changes or granulation tissue (enhances).
| Parameter | Attic Cholesteatoma | Sinus Cholesteatoma |
|---|---|---|
| TM perforation | Pars flaccida | Pars tensa |
| Location | Prussak's space | Sinus tympani |
| Ossicles displaced | Medially | Laterally |
| Bone erosion | Lateral tympanic wall (erosion of scutum is an early finding) | Initially subtle |
| Ossicle erosion | Head of malleus and long process of incus | Short process of incus and stapes |
Labyrinthine fistula (dehiscence of semicircular canals—most frequently lateral canal)
Facial nerve paralysis (involvement of facial nerve canal)
Invasion of tegmen tympani, petrous apex, or sigmoid plate
Automastoidectomy
Constitute approximately 2% of temporal bone cholesteatomas and may affect middle ear, EAC, mastoid, or petrous bone, including the labyrinth (uncommon). The most common location is the anterosuperior portion of the middle ear near the eustachian tube or stapes. The typical presentation is in a young patient with otherwise normal mastoid pneumatization and without history of chronic ear infections. Histologically, the cholesteatoma is made up of squamous cell lining, keratin debris, and cholesterol.
Mix of hemorrhage, cholesterol crystals, and granulation tissue that may be present in temporal bone, including the petrous apex or rarely the middle ear. Depending on the location of the expansile erosive lesion and involvement of critical structures, they can be associated with hearing loss, tinnitus, and CN palsies.
Expansile erosive osseous lesion of the petrous apex
Smooth margins
CT: isodense relative to brain, no enhancement, no calcification
MRI: hyperintense relative to brain on T1-weighted (T1W) images because of methemoglobin and cholesterol crystals
Cholesterol granulomas retain their high signal on fat suppressed T1W images, distinguishing them from fatty lesions.
| Epidermoid (Congenital Cholesteatoma) | Cholesterol Granuloma (Cholesterol Cyst) | Mucocele a | |
|---|---|---|---|
| CT | ≤ | Isodense, no calcium, no enhancement | < or occasionally > |
| T1W | ≤ (lamination) | > (cholesterol) | ≤ or occasionally > |
| T2W | > | > (with peripheral low signal hemosiderin ring) | > or occasionally < |
a Density and MRI signal characteristics of mucoceles can vary depending on protein content.
Severe, life-threatening Pseudomonas aeruginosa infection in older adults with diabetes. Aggressive infection of EAC, adjacent soft tissues, and skull base that spreads via cartilaginous fissures of Santorini and can extend into:
Middle ear and mastoid air cells
Variable involvement of skull base, including osteomyelitis
Phlegmon and abscesses may spread in any direction:
Parapharyngeal, parotid, or masticator space
Intracranially
Anteriorly to temporomandibular joint (TMJ)
Early findings may manifest as thickened mucosa of EAC and soft tissue swelling; more advanced disease can have bone erosion associated with the opacification.
Mastoiditis
Skull base osteomyelitis
Sinus phlebitis and thrombosis
Phlegmon or abscess in nearby soft tissues and spaces
Ossification of the membranous labyrinth as a sequela of previous infectious, inflammatory, traumatic, or surgical injury to the inner ear. Seen as ossification within the membranous labyrinth on high resolution computed tomography (HRCT) and foci of low signal on T2-weighted (T2W) MRI within the otherwise high signal fluid of the membranous labyrinth.
Glomus tumors (chemodectomas) arise from chemoreceptor cells in multiple sites in the head and neck. The majority are benign and approximately 10% are multiple; thus it is important to check other common locations in the head and neck during imaging (glomus jugulare, vagale, and carotid body tumor). Glomus tympanicum represents the most common middle ear tumor.
Glomus jugulare: origin at jugular bulb; more common
Glomus tympanicum: arises from paraganglia along inferior tympanic nerve (Jacobson nerve), frequently on the cochlear promontory
Glomus vagale
Carotid body tumor
Pulsatile tinnitus (most common)
Conductive hearing loss
Rarely, facial nerve paralysis
Other focal neurologic symptoms depending on location as well as systemic symptoms such as sudden blood pressure (BP) fluctuations if the tumor secretes catecholamines
Glomus tympanicum typically presents as a small enhancing soft tissue mass centered over the cochlear promontory. Enhancement distinguishes tumors from obstructive secretions.
Glomus jugulare (glomus jugulotympanicum) is centered in the region of the jugular foramen. Most common path of spread is to the middle ear. It is accompanied by permeative bone changes in the jugular foramen. Characteristic findings by MRI are multiple low signal intensity areas that represent flow voids in the tumor. This has a salt-and-pepper appearance.
Glomus vagale paraganglioma: arises from paraganglia in the nodose ganglion of the vagus nerve in the nasopharyngeal carotid (or poststyloid parapharyngeal) space; displaces carotid anteromedially, jugular vein posterolaterally, and does not splay the carotid bifurcation.
Carotid body paraganglioma: arises from carotid glomus bodies (paraganglia); splays the external carotid artery (ECA) and internal carotid artery (ICA).
Intense contrast enhancement by CT, MRI, angiography
Large tumors erode bone.
Meningioma
Facial schwannoma may arise anywhere along the course of CN VII. In the IAC, the tumor may be indistinguishable from a vestibular schwannoma unless it can be seen to arise from the facial nerve.
Osteoma
Adenoma (ceruminoma), benign but locally aggressive, rare
Endolymphatic sac tumor, benign but locally aggressive; permeative destructive lesion with spiculated calcified tumor matrix
Epidermoid (primary cholesteatoma): not a true neoplasm
Carcinoma (CA) (most common malignant tumors)
Squamous cell carcinoma (SCC) arising from the EAC
Adenocarcinoma
Lymphoma
Metastases: breast, lung, renal, prostate; neuroblastoma and leukemia in children
Chondrosarcoma, other primary bone tumors
Rhabdomyosarcoma in children and late teens
The osseous labyrinth (otic capsule) is the normal dense bone of the inner ear surrounding the cochlea, vestibule, and semicircular canals. In otosclerosis, the capsule is replaced by vascular, irregular bony trabeculae (lucent on CT) and later by new bone within the lucent areas in the late, chronic healing phase. Unknown cause, sporadic or autosomal dominant (AD) gene transmission, frequently bilateral. Patients (female > male) present with progressive conductive or mixed hearing loss.
Fenestral: sclerosis or spongiosis around oval window, including fixation of stapes. Diagnosis is usually made from clinical and audiometric findings (conductive hearing loss). Begins as radiolucent focus at the anterior margin of the oval window. Early disease may not be seen on imaging or may be very subtle.
Cochlear (retrofenestral) otosclerosis: involves pericochlear bony labyrinth. CT findings:
Deossification around cochlea (lucent halo)
Sclerosis occurs later in disease (mixed lucencies and densities)
Disease begins at fissula ante fenestram (fenestral); fenestral alone most common (85%, conductive hearing loss); approximately 15% progress to cochlear (mixed hearing loss).
CT shows a small defect or dehiscence in the bony wall of the superior or less commonly posterior semicircular canal. Seen as ≥2 mm dehiscence of roof of superior semicircular canal on coronal or ideally transverse oblique (Poschl) CT reformats for superior semicircular canal dehiscence. Clinically presents with dizziness and vertigo +/– nystagmus with loud sound or pressure (Tullio phenomenon). Clinical syndrome + positive CT = semicircular canal dehiscence syndrome. Can also be associated with inner ear conductive hearing loss. The cause is unknown, likely developmental, possibly also secondary to trauma.
Paget disease
Fibrous dysplasia
Osteogenesis imperfecta
Skull base osteopetrosis
Craniometaphyseal dysplasia (Pyle disease)
Craniodiaphyseal dysplasia and Camurati-Engelmann disease (progressive diaphyseal dysplasia)
Cleidocranial dysostosis
Cochlear incomplete partition type I: spectrum of abnormalities ranging from lack of internal structure of the cochlea to severe cystic cochleovestibular malformation
Cochlear incomplete partition type II: incomplete partition with deficient interscalar septum between middle and apical turns (Mondini anomaly: historic terminology – incomplete partition type II + large vestibular aqueduct)
Cochlear hypoplasia: small, underdeveloped cochlea, typically <2 turns
Cochlear aplasia: absent cochlea with variable anomalies of other inner ear structures
Labyrinthine aplasia: aplasia or hypoplasia of otic capsule, petrous apex hypoplasia, and other associated temporal bone abnormalities (Michel aplasia: historic terminology for complete labyrinthine aplasia)
Common cavity malformation: undifferentiated cochlea and vestibule represented by a common cavity of variable size.
Large vestibular aqueduct: ≥1 mm diameter at midpoint and/or ≥2 mm at operculum perpendicular to long axis of vestibular aqueduct; rule of thumb—abnormal if larger than the posterior semicircular canal. High incidence of associated cochlear abnormalities (also see incomplete partition II above).
Small IAC
The presence of a fistula in the petrous portion of the temporal bone may lead to otorrhea, pneumocephalus, meningitis, or abscess; rare.
Acquired lesion of the petrous apex can potentially have similar complications depending on extent and violation of adjacent structures.
| Syndrome/Disease | Inner Ear | Middle Ear | Outer Ear | Other Abnormalities |
|---|---|---|---|---|
| Otocraniofacial | ||||
| Treacher-Collins syndrome | ++ | ++ | ++ | Coloboma, mandibular hypoplasia (micrognathia), microtia |
| Otocervical | ||||
| Klippel-Feil syndrome | ++ | ++ | ++ | Cervical fusion, short neck, low posterior hairline, limited cervical motion |
| Cleidocranial dysplasia (dysostosis) | + | + | ++ | Hypoplastic, malformed, or absent clavicles; large head, underdeveloped facial bones |
| Otoskeletal | ||||
| Osteogenesis imperfecta | ++ | + | − | Fractures, blue sclera in some subtypes |
| Osteopetrosis | + | ++ | ++ | Autosomal recessive: macrocephaly, deafness and blindness; autosomal dominant much less severe, may even be asymptomatic |
| Other | ||||
| Hemifacial macrosomia (Goldenhar syndrome, oculoauriculovertebral spectrum) | + | ++ | ++ | Facial asymmetry, microtia, variable ocular abnormalities, cervical spine deformity |
| CHARGE | ++ | ++ | ++ | Coloboma, heart anomaly, choanal atresia, mental retardation, genital hypoplasia |
| Pendred syndrome | ++ | − | − | Thyroid organification defect with goiter |
| Branchio-oto-renal syndrome | ++ | ++ | ++ | Branchial and renal anomalies |
| Thalidomide | ++ | ++ | ++ | Short limbs, cardiac and GI abnormalities |
a There can be variations in the type and extent of otologic abnormalities associated with each syndrome.
Anterior skull base: broadly consists of floor of anterior cranial fossa and the roof of the nose, ethmoid air cells, and orbits.
Central skull base: broadly consists of floor of middle cranial fossa, roof of sphenoid sinus, and greater wing of sphenoid.
Anterior and central skull base boundary:
Medially: posterior margin of planum sphenoidale (tuberculum sellae)
Laterally: lesser wing of sphenoid/sphenoid
| Opening | Cranial Nerve | Artery | Vein |
|---|---|---|---|
| Optic canal | II | Ophthalmic artery | |
| Superior orbital fissure | III, IV, V1, VI | Orbital branches of middle meningeal artery (occasionally), recurrent meningeal branches of lacrimal artery | Ophthalmic veins |
| Inferior orbital fissure | Infraorbital nerve (V2) | Infraorbital artery | Ophthalmic vein (inferior division) |
| Carotid canal | Sympathetic plexus | ICA | |
| Foramen rotundum | V2 | Artery of foramen rotundum | Emissary veins |
| Foramen ovale | V3 | Accessory meningeal artery | Emissary veins |
| Foramen spinosum | Meningeal (recurrent) branch of mandibular nerve, lesser petrosal nerve (occasionally) | Middle meningeal artery | Middle meningeal vein |
| Foramen lacerum (not a true foramen) | Nerve of the pterygoid canal (formed by greater petrosal and deep petrosal nerves) | Terminal (meningeal) branches of ascending pharyngeal artery | Emissary veins |
| Vidian (pterygoid) canal | Vidian nerve | Vidian artery | Vidian vein |
| Stylomastoid foramen | VII | Stylomastoid artery | |
| Jugular foramen pars nervosa | IX, Jacobson nerve | Inferior petrosal sinus | |
| Jugular foramen pars vascularis | X, XI, Arnold nerve | Posterior meningeal artery | Jugular bulb |
| Hypoglossal canal | XII |
Important space and potential route of spread of disease in the deep face
Contents:
Pterygopalatine ganglion
Maxillary nerve (V2) via foramen rotundum
Distal internal maxillary artery via pterygomaxillary fissure
Relations and boundaries:
Anterior: posterior maxillary sinus wall
Posterior: pterygoid plates and inferior part of lesser wing of sphenoid
Superior: inferior orbital fissure
Inferior: tapers into the greater palatine canal
Medial wall: perpendicular plate of palatine bone, sphenopalatine foramen superiorly
Lateral wall: pterygomaxillary fissure (vertical communication with infratemporal fossa)
Lesion affecting the anterior skull base can be centered in the bone, arise in the anterior cranial fossa above, or arise from sinonasal cavities below; localization of lesion center can help with the differential.
Meningioma: classic locations olfactory groove and planum sphenoidale
Olfactory neuroblastoma (esthesioneuroblastoma): malignant tumor arising from olfactory mucosa in superior nasal cavity
Heterogeneously enhancing mass in upper nasal cavity
Larger tumors extend to the floor of anterior cranial fossa with erosive bone changes.
May have “waist” at level of cribriform plate
Peripheral tumor cysts at intracranial tumor-brain margin are highly suggestive of diagnosis.
Osteoma (sinonasal): well-defined nonaggressive bony mass, most common in frontal and ethmoid sinuses.
Metastasis: bone destruction, may involve dura or brain parenchyma
Sinonasal SCC: most common primary sinonasal malignancy, ethmoid sinus and nasal cavity primaries may extend to the anterior skull base.
Aggressive lesion with bone destruction
Sinonasal undifferentiated CA: rare, highly aggressive sinonasal malignancy
Sinonasal lymphoma: cephalad extension from nasal cavity through skull base
Typically, homogeneous lesions with relatively high density on unenhanced CT
Septal destruction, occasional bone remodeling
On MRI, relatively low signal on T2W images, homogeneously enhancing
Sinonasal melanoma: highly malignant, nasal cavity often site of origin
Bone destruction more frequent than remodeling
On MRI lesions may have intrinsically high signal on T1W and low signal on T2W images (paramagnetic properties of melanin)
Avid enhancement
Hemangiopericytoma: hypervascular lesion with aggressive growth pattern
Dural-based mass that may have internal flow voids
Intensely enhancing
Sinonasal nerve sheath tumor: benign neoplasm arising from peripheral nerve Schwann cells
Well-defined lesion with smooth remodeling
Enhancing sharply marginated mass, may have internal cystic changes
Fibrous dysplasia: expansile developmental bone lesion
Classically ground-glass matrix (varies depending on amount of fibrous and ossified components)
Enhancing lesion, can be deceptively aggressive on MRI (suspect if very low signal on T1 and T2)
Obtain CT to demonstrate typical characteristics
Mucocele: expanded sinus from chronic obstruction
Variable signal depending on protein content of secretions
No solid enhancement (can see mucosal enhancement around the margins)
Nonaggressive with bone remodeling, MRI highly accurate for distinguishing higher density/proteinaceous secretions that are equivocal on CT from solid masses.
Meningocele and encephalocele: extension of meninges ± brain parenchyma through skull base defect into nasal cavity or ethmoidal labyrinth
CT frequently shows bone defect.
High resolution MRI for confirmation
Heavily T2W high resolution sequences can be helpful for clear demonstration of extension of meninges and/or brain through defect.
Nasal dermal sinus: midline developmental lesion anywhere from nasal tip to anterior skull base at foramen cecum, may be associated with nasal pit or repetitive bouts of meningitis clinically.
Look for tract from nasal lesion to anterior cranial fossa.
May see widened foramen cecum ± bifid crista galli
May have associated dermoid/epidermoid along tract
Lesions may arise from central skull base components, represent inferior extension from above or superior extension from below. Characterization of calcified matrix can help in the diagnosis of cartilaginous lesions and is best seen on CT.
Metastasis: suspect in patient with known malignant neoplasm with new craniofacial pain or cranial neuropathy
Lytic destructive lesion
May have associated soft tissue mass
Look for multiple lesions
Meningioma: in addition to those with a classic appearance, occasionally lesions can be infiltrative, extend to extracranial spaces such as pterygopalatine and infratemporal fossa, or have intraosseous component.
Multiple myeloma: commonly lytic, multiple, may be indistinguishable from extensive metastatic disease with lytic pattern.
Lymphoma: may originate in bone or from dura, wide range of appearances.
Typically enhance uniformly
Can be dural-based
Can involve bone with lytic changes
Extensive soft tissue mass
Relatively restricted diffusion with low apparent diffusion coefficient (ADC) values
Pituitary macroadenoma: may occasionally extend inferiorly into clivus or sphenoid sinus.
Chordoma (clivus): rare, locally aggressive tumor arising from primitive notochord remnants
May present with headaches and diplopia secondary to CN VI involvement in Dorello canal, other nerves can be involved depending on extent.
Expansile destructive midline clival mass, may be near sphenooccipital synchondrosis
Classically high signal on T2W images secondary to high water content
Can also have mix of calcification, hemorrhage, and mucoid material with increased T1 and decreased T2 signal
Enhancing lesion, sometimes in a “honeycomb” pattern
Chondrosarcoma: arises in skull base fissures and synchondroses, most commonly petrooccipital fissure.
Typically eccentric (in contradistinction to chordoma)
Chondroid ring and arc-like calcifications on CT
Heterogeneously enhancing with high T2 signal with scattered hypointense foci (calcifications) on MRI
Langerhans cell histiocytosis: lytic enhancing lesion typically presenting in the first decade of life
Fibrous dysplasia: relatively common expansile bone lesion
Classically ground-glass matrix (varies depending on amount of fibrous and ossified components)
Enhancing lesion, can be deceptively aggressive on MRI (suspect if very low signal on T1 and T2)
Obtain CT to demonstrate typical characteristics
Paget disease: primary metabolic bony disease, often asymptomatic, typically seen in older adults, more common in males
May be multifocal disease, with mixed lytic-sclerotic pattern
Well-defined lytic regions with bone expansion in early phase
“Cotton wool” appearance later in lytic-sclerotic phase
On MRI, hypointense on T1 and heterogeneous enhancement
Aneurysm: cavernous or other ICA segments
Rounded “mass,” low signal/flow void on MRI: must recognize!
Pulsation artifact in phase encoding direction
CT or magnetic resonance (MR) angiography to confirm
Arachnoid granulation: lesions found along dural sinuses and dural surface, typically incidental but in patients with CSF leaks may represent the site of osteodural defect.
Basal cephalocele: uncommon, usually congenital
Persistent craniopharyngeal canal: developmental anomaly resulting in a persistent tract from nasopharynx to pituitary fossa.
Smoothly marginated midline canal between presphenoid and basisphenoid.
Typically incidental finding but may be associated with pituitary abnormalities, cephaloceles, midline craniofacial anomalies, or rarely even tumors arising from tissue within the canal.
Ecchordosis physaliphora: benign nodule of tissue in the dorsal clivus and/or prepontine cistern considered to represent ectopic notochordal remnant.
Benign clival variant of chordoma
Hyperintense on T2, but no enhancement (distinguishes from chordoma)
Often shows restricted diffusion
Spread of malignant tumor along sheath of large nerves
Most commonly along CN V and VII branches
Contiguous spread from the primary tumor site along nerve tracks via epineurium/perineurium distant from primary site, do not confuse with “perineural invasion” at site of primary tumor invasion on histopathology
Often retrograde but can also be antegrade
Important to identify for initial staging and management planning—carefully evaluate major nerve pathways according to primary tumor site
Some head and neck cancer types have particularly high propensity (e.g., adenoid cystic carcinoma [ACC]) but SCC is much more common therefore overall more commonly seen with SCC; can also be seen with desmoplastic melanoma and lymphoma
Subtle but may be seen on CT:
Asymmetry and soft tissue infiltration in PPF, neural foramina; widening of these structures when more advanced
Contrast-enhanced high resolution MRI is the optimal technique for evaluation of perineural spread of tumor.
May also see secondary findings: look for denervation changes
Nerve sheath tumors
Tubular, fusiform, lobular mass along nerve or nerves
Typically larger than perineural tumor spread
No other primary head and neck tumor/malignancy
Skull base meningioma
Homogeneously enhancing dural-based mass
May extend into neural foramina, jugular foramen, or PPF
Invasive fungal sinusitis
Immunocompromised patients, including diabetics
Infiltrating disease but does not follow nerves exclusively
Sarcoidosis
Nerve involvement may mimic perineural tumor spread
Absence of primary head and neck malignancy
May have other associated findings such as dural-based inflammation, leptomeningeal component
Globe (anterior chamber, posterior chamber, lens, vitreous, sclera/choroid/retina)
Intraconal, extraconal fat
Optic nerve and sheath
Ophthalmic artery and vein
Rectus muscles
Fascia arising from orbital rim periosteum
Attaches to outer margins of bony orbit and deep tissues of lids
Separates structures in the orbit from soft tissues in the face (postseptal vs. preseptal)
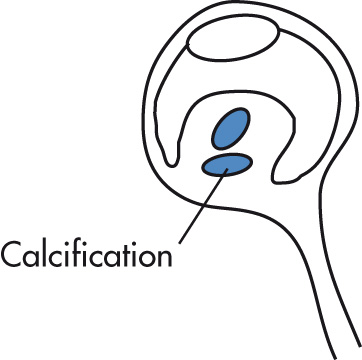
Malignant tumor that arises from neuroectodermal cells of retina. Clinical: leukokoria (white mass behind pupil). Age: <3 years (70%)
30% bilateral, may also be multifocal within one eye
60% sporadic, 40% hereditary, 10% of patients have a positive family history
Intraocular mass
High density (calcification, hemorrhage)
Dense vitreous, common (secondary to hemorrhage)
Calcifications ( Fig. 7.13 ) are common (90%); in absence of calcifications suspect other mass lesions:
Persistent hyperplastic primary vitreous (PHPV)
Retrolental fibroplasia (retinopathy of prematurity); calcium may be seen if advanced
Toxocariasis (no calcification acutely)
Coats disease
MRI findings:
T1: variable, mildly hyperintense compared with vitreous (unless hemorrhage in vitreous)
T2: hypointense relative to vitreous
T1 postcontrast: moderate to marked heterogeneous enhancement
Primary role of imaging is to determine tumor spread:
Scleral breakthrough
Optic nerve extension
Intracranial spread
Tumors may occur bilaterally or trilaterally (bilateral + intracranial, pineal region tumor much more common than suprasellar)
Associated with other malignancies (osteosarcoma most common) later in life; also, risk of postradiation sarcoma in treatment field
Most common (75%) primary ocular malignancy in adults. Arises from pigmented choroidal layer; retinal detachment is common.
Thickening or irregularity of choroid (localized, polypoid, or flat)
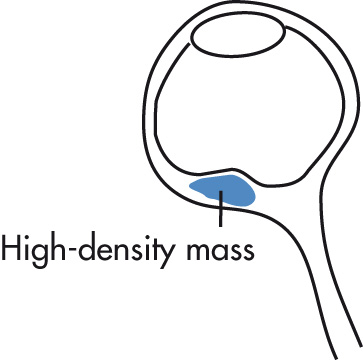
Exophytic, biconvex mass lesion
Usually unilateral, posterior location
Retinal detachment, common
Contrast enhancement
MRI: T1W hyperintense, T2W hypointense (can vary based on pigmentation)
Poor prognostic indicators:
Large tumor size
Lack of pigmentation associated with aggressiveness
Infiltration of the angles, optic nerve, sclera, ciliary body
Caused by persistence of portions of the fetal hyaloid artery and primary vitreous in Cloquet canal. Associated with ocular dysplasias (e.g., Norrie disease = PHPV, seizures, deafness, low intelligence quotient [IQ]). Clinical findings of blindness, leukokoria, and microphthalmia. Unilateral more common. Rare.
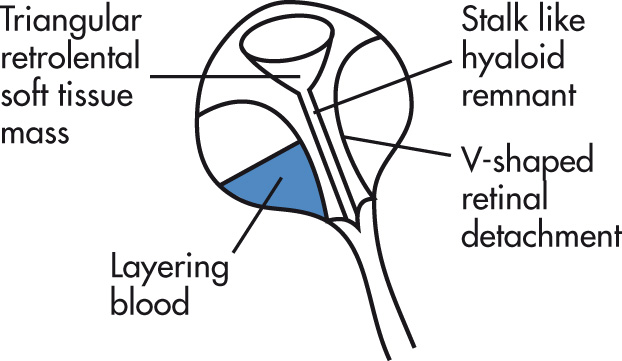
Small globe (microphthalmia)
Vitreous body hyperdensity along the remnant of the hyaloid artery
No calcification rare (in contrast to retinoblastoma)
Complications:
Retinal detachment
Chronic retinal hemorrhage
Toxic retinopathy caused by oxygen treatment (e.g., for hyaline membrane disease [HMD]). A retinal vasoconstriction leads to neovascularization and fibrovascular proliferation of the posterior vitreous and retina. Typically bilateral, retinal detachment common, may have associated hemorrhage, calcification rare except in advanced stages.
Primary vascular abnormality (exudative retinopathy) causing lipoprotein accumulation in retina, telangiectasia, neovascularization, and retinal detachment (pseudoglioma). Age: typically first decade, male predominance. Rare.
Unilateral (90%)
Retinal detachment (V-shaped contour)
Dense vitreous/subretinal exudates
Calcification uncommon
Hyperintense (T1 and T2) proteinaceous, hemorrhagic exudate
Nerve enhancement in advanced disease as a result of secondary glaucoma
Focal calcification in hyaline bodies in the optic nerve head. Usually bilateral and asymptomatic but may be associated with macular degeneration. Blurred disk margins may be mistaken for papilledema. On CT, seen as punctate hyperdensity most commonly at optic nerve head or macula.
Coloboma: focal outpouching involving retina, choroid, iris; caused by deficient closure of fetal optic fissure; located in region of optic disc. Associated with various congenital globe, CNS, and other systemic anomalies.
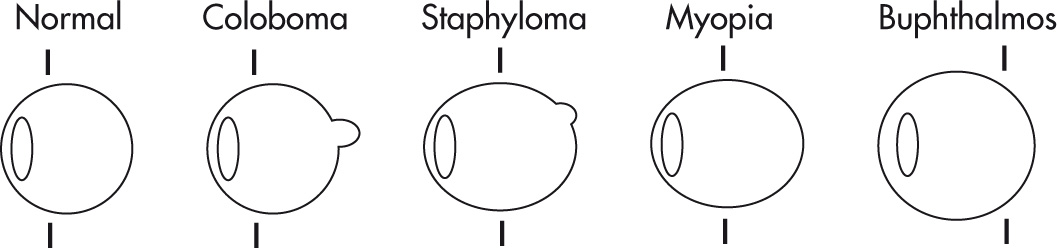
Staphyloma: acquired defect of globe wall with thinning of posterior sclera-uveal rim and protrusion of choroid or sclera. On imaging, see elongated globe with focal deformity, usually posterior, but not as pronounced as coloboma.
Axial myopia: anteroposterior (AP) elongation but no protrusion
Buphthalmos: congenital glaucoma, anterior ocular chamber drainage problem.
Enlarged globe, increased depth of anterior chamber.
Leukokoria refers to a white pupil. Clinical, not a radiologic, finding. Underlying causes:
Retinoblastoma
PHPV
Congenital cataract
Toxocariasis
Other
Sclerosing endophthalmitis
Coats disease
Retrolental fibroplasia
Trauma
Chronic retinal detachment
Separation of the sensory retina from the outer pigmented epithelium. Appears in a characteristic V shape, with the apex of the detachment at the optic disc. The presence of retinal or choroidal detachment may be caused by an ocular mass.
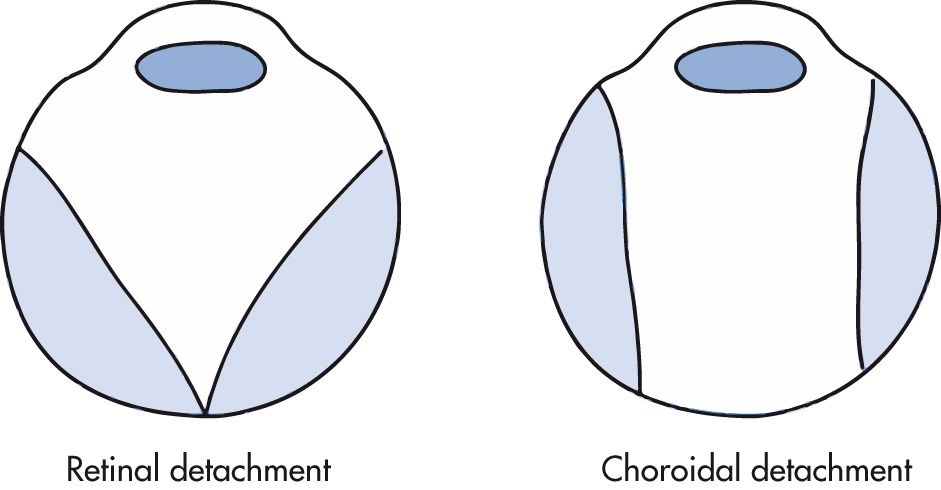
Usually results after ocular surgery, trauma, or uveitis, causing fluid to accumulate in the choroidal space. Characteristic appearance spares the posterior third of the globe (region of the optic disc) in contradistinction with retinal detachment.
Most common cause of diffuse optic nerve enlargement, especially in childhood. Pathology: childhood lesions most commonly low grade astrocytomas (grade I pilocytic astrocytoma > grade II fibrillary astrocytoma). In patients with neurofibromatosis type 1 (NF1), pathology may be perineural arachnoid gliomatosis (PAG) rather than true astrocytoma. In adults, commonly high-grade astrocytomas. Clinical findings include loss of vision, proptosis (bulky tumors). Most common in first decade of life. In NF1 the disease may be bilateral.
Types of tumor growth: tubular, excrescent, fusiform widening of optic nerve
Enlargement of optical canal; >1 mm difference between left and right is abnormal.
Lower CT density than meningioma
Contrast enhancement variable
Calcifications rare (but common in meningioma)
Tumor extension best detected by MRI: chiasm → optic tracts → lateral geniculate body → optic radiation
Optic nerve sheath meningiomas arise from arachnoid rests in meninges covering the optic nerve. Age: fourth and fifth decades (female predominance in adults); younger patients typically have neurofibromatosis type 2 (NF2). Progressive loss of vision.
Mass
Tubular, 65%
Eccentric (exophytic), 25%
Fusiform, surrounding the optic nerve, 10%
Calcification (common)
Enhancement
Intense contrast enhancement
Linear bands of enhancement (nerve within tumor): “tramtrack sign”
Other
Sphenoid bone and/or optical canal hyperostosis in advanced tumors
Visual loss
Pain on eye movement
Afferent papillary defects
Isolated (idiopathic)
Multiple sclerosis (MS)
Neuromyelitis optica (Devic syndrome)
Acute demyelinating encephalomyelitis (ADEM)
Pediatric optic neuritis: rare, may follow viral illness or vaccination, ADEM
40%–60% of patients ultimately develop MS (and 70%–90% of MS patients develop optic neuritis at some point)
Coronal T2W or postgadolinium T1W with fat suppression best for diagnosis
T2W: obliteration of the perioptic space; increased T2 signal of the affected optic nerve
Enhancement of the optic nerve
Orbital cavernous venous malformation (previously/commonly known as cavernous hemangioma ): adults, not a true neoplasm, rather vascular malformation; true capsule, benign, most common vascular orbital mass in adults
Most commonly in lateral intraconal space
Well-defined nonaggressive mass
Hyperintense on T2W images
Progressive enhancement “fill-in”
Orbital infantile hemangioma (capillary hemangioma): benign vascular tumor of infancy, no capsule
Lobular or infiltrative hypervascular, intensely enhancing mass
On T2W images: hyperintense, flow voids may be visible.
Most frequently superficial extraconal location, may have postseptal extension, uncommonly presents as exclusively retrobulbar mass
Grows for <1 year and then typically involutes
Venous, lymphatic, or venolymphatic malformations: vascular malformation with or without lymphatic component
Multiloculated, transspatial lesions
Those with significant lymphatic component tend to be more cystic and have propensity to hemorrhage with fluid-fluid levels.
Those with predominantly venous component tend to have more homogenous enhancement.
Venous varix: distensible venous channel with systemic connection
Dynamic enlargement of lesion with provocative maneuver (e.g., Valsalva)
May not be visible when patient relaxed
May present with thrombosis or hemorrhage
Hemangiopericytoma: uncommon, slow-growing vascular neoplasm that can be benign or malignant
Well-circumscribed or invasive when high grade
May remodel or erode bone
Avidly enhancing
Flow voids common on T2W images
Other vascular lesions: AVM, dural arteriovenous fistula, and cavernous carotid fistula
Common orbital tumor in childhood. Age: typically present in the first two decades.
Low CT attenuation and T1 hyperintensity (fat) are diagnostic.
Contiguous bone scalloping or sclerosis is common.
May contain debris (inhomogeneous MRI signal).
Calcification present occasionally.
Lymphoid
Benign reactive lymphoid hyperplasia
Lymphoma
Epithelial tumors
Benign mixed (pleomorphic) tumor (most common epithelial tumor)
ACC
Mucoepidermoid CA
Malignant mixed tumor (carcinoma ex pleomorphic adenoma)
Most common malignant orbital tumor in childhood. Mean age: 7 years.
Large, aggressive soft tissue mass (intraconal or extraconal)
Metastases to lung and cervical nodes
Adults: breast, lung, renal cell, prostate CA, melanoma. Breast metastasis to the orbit may have retractile fibrotic stroma and paradoxically be associated with enophthalmos. There can also be direct extension of malignant sinonasal tumors to the orbit. Children: rare, neuroblastoma, leukemia.
The orbital septum represents a barrier to infectious spread from anterior to posterior structures. Common causes of orbital infection include spread from infected sinus and less commonly trauma.
Periorbital cellulitis: soft tissue swelling
Postseptal infection (true orbital cellulitis)
Infiltration of extraconal, postseptal fat
Subperiosteal infiltrate or abscess
Lateral displacement of enlarged medial rectus muscle
Stranding of retrobulbar fat if intraconal extension
Proptosis
Evaluate paranasal sinuses for potential source
Bacterial infections can be complicated by cavernous sinus thrombosis.
Orbital pathology (cellular infiltration, deposition of glycoproteins and mucopolysaccharides in the orbit, fibrosis) caused by autoantibodies targeting thyrotropin receptors found in orbit (in addition to thyroid). Clinical: painless proptosis; patients may be euthyroid, hypothyroid, or hyperthyroid. Functional classification (clinical severity and risk):
Mild: eyelid lag and retraction with proptosis in setting of active hyperthyroidism
Moderate: soft tissue inflammation and intermittent myopathy; stabilizes without major sequelae
Severe: rapid and fulminant, greater mass effect, severe sequelae including optic nerve compromise
Treatment: prednisone → radiation therapy → surgical decompression; surgery or 131 iodine (I) for thyroid.
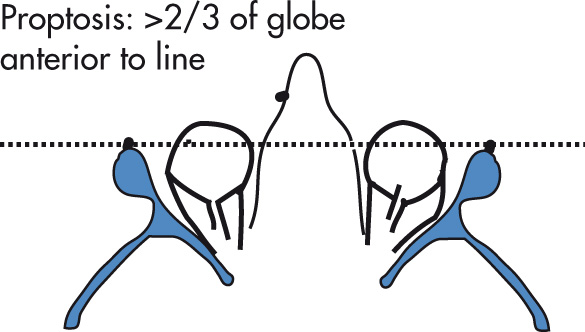
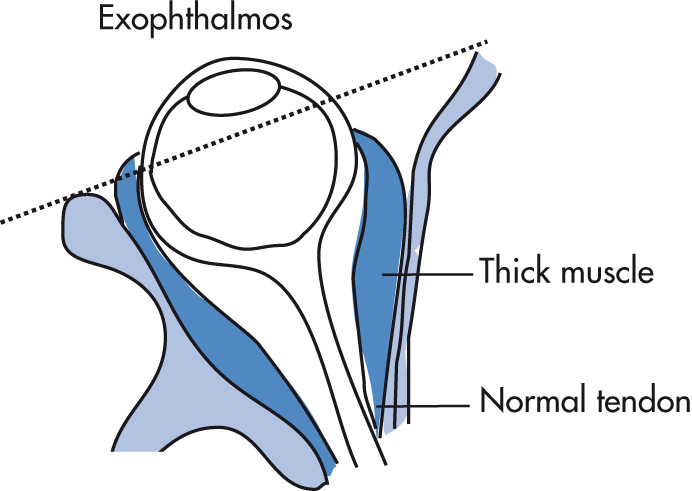
Muscle involvement
Mnemonic for involvement: I'M SLow :
I nferior (most common)
M edial
S uperior
L ateral
Enlargement is maximal in the middle of the muscle and tapers toward the end (infiltrative, not inflammatory disease).
Spares tendon insertions (although may be involved in acute phase)
Often bilateral, symmetric
Other
Straightened or stretched optic nerve
Expansion of orbital fat
Lacrimal gland enlargement
Inflammation of orbital soft tissues of unknown origin
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here