Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is an effective treatment for a wide range of hematologic malignancies, nonmalignant hematologic diseases, and immunologic disorders. Sources of donor stem cells for alloHSCT include human leukocyte antigen (HLA)-matched siblings (MSD), HLA-matched unrelated adult donors (MUD) matched at least at HLA-A, -B, -C, -DRB1, HLA-mismatched unrelated donors, unrelated umbilical cord blood, and HLA half-matched related donors, mismatched at 2 or more HLA antigens (haploidentical donors, HRD). Haploidentical donors are matched at one full haplotype (see later) with various degree of matching in the other haplotype (6 to 10 out of 12 allele match). Historically, the most important consideration in choosing between graft sources has been the degree of match between donor and recipient. A fully HLA-matched sibling has been traditionally the preferred donor because transplants using such donors have been associated with the lowest incidence of treatment-related complications and best overall survival (OS). Unfortunately, only 25% to 30% of patients referred for transplantation have an MSD available. For other patients, a MUD has been the preferred alternative; however, availability of a MUD varies widely based on patient race/ethnicity, from approximately 70% for individuals of Northern European descent to less than 20% for African Americans or Asians. In addition, a longer time to obtain the unrelated donor cells, on average of 3 to 4 months, may expose the patients in urgent need for a transplant to risk of disease progression. Increased HLA mismatching has been associated with increased alloreactivity between donor and recipient T cells, leading, in general, to worse outcomes including higher risks of graft rejection, graft-versus-host disease (GVHD), and non-relapse mortality (NRM). Over the past two decades, significant progress has been made in mitigating alloreactive reactions between the donor and recipient, which has enabled an increase in use of haploidentical donors for transplantation, now the fastest growing source of hematopoietic stem cells, with improved transplant outcomes, similar to HLA-matched donor transplants, and potential to become the alternative donor of choice for patients without a MSD, at least for those in need of an urgent transplant.
There are several advantages of using haploidentical donors for transplantation (see box on The Increasing Popularity of Human Leukocyte Antigen–Haploidentical Donors ):
The number of human leukocyte antigen (HLA)-haploidentical stem cell transplant procedures performed is increasing on a global scale. China is the leader in HLA-haploidentical (“haplo”) stem cell transplants (SCTs) on a per-country basis, perhaps because of the former one-child policy that has nullified the availability of an HLA-matched sibling for many patients. In the past 5 years, the number of haplo transplants performed in Europe and the United States has more than doubled. Reports of successful haplo SCTs are appearing in the published literature from countries with more limited economic resources, such as Brazil, India, and Romania.
There are clinical, practical, and economic reasons for the increasing popularity of HLA-haploidentical SCT. Clinically, results of the procedure have improved dramatically over the past 10 to 15 years, to the point that the outcomes of haplo SCT approach or equal those of HLA-matched sibling or unrelated donor transplantation (see Comparative Outcomes Between Haploidentical and Other Graft Sources). Another major practical advantage of the haplo option is donor availability. A haplo donor can be found for nearly every patient referred for allogeneic SCT, because every biologic child or parent of a patient is HLA haploidentical, and each sibling or half-sibling has a 50% chance of being HLA haploidentical. The likelihood of finding a haplo donor increases further if one is willing to consider second-degree relatives, such as aunts, uncles, nieces, nephews, or cousins, as donors. The wide availability of haplo donors is especially important for members of ethnic minority groups that are underrepresented in registries of volunteer unrelated donors. Relatives, especially parents and children, tend to be highly motivated to donate and can do so more than once if needed to treat graft failure or relapse. The treating center has greater control over the timing of transplants when haplo donors rather than unrelated donors are used. Clinical trials and advances in adoptive cellular therapy of cancer may be easier using related donors because unrelated donor lymphocytes for infusion are regulated by the US Food and Drug Administration as a biologic. Thus an investigational new drug application must be filed for any clinical trial employing the infusion of lymphocytes from unrelated donors, but not for minimally manipulated lymphocytes from related donors.
Finally, the cost of obtaining the graft from haploidentical donors is cheaper compared with stem cells from unrelated adult volunteers or from umbilical cord blood. This, combined with the growing availability of inexpensive methods of graft-versus-host disease prophylaxis for haplo SCT (especially posttransplant cyclophosphamide), makes haploidentical transplants increasingly attractive in countries with limited economic resources and a fixed budget for allogeneic stem cell transplantation. Thus, haploidentical donor transplant as related donor transplants, largely eliminate cost differences compared with HLA-matched related donor transplants.
Near-universal availability of highly motivated donors: Patients have an average of 2.7 potential HLA-haploidentical donors among first-degree relatives;
Rapid availability: As compared with MUDs, a haploidentical donor can be identified, screened, and collected within 2 weeks, and transplant can be performed in 3 weeks;
Low cost of graft acquisition: The costs of graft acquisition from MUD and especially from umbilical cord blood banks can be substantially higher than from related donors, including haploidentical donors ;
Adequate doses of HSCs: An adequate number of HSCs can be obtained from HLA-haploidentical donors for adult recipients, as compared with umbilical cord blood grafts. The number of total nucleated calls infused has been correlated with all transplant outcomes ;
Availability of the donor for repeated donations of HSCs (stem cell boost) in case of poor graft function or therapeutic cells (lymphocytes) to prevent/treat relapse or viral infections;
Potential for a stronger graft-versus-leukemia (GVL) effect: Increase alloreactive reactions generated by higher degree of HLA mismatch may be associated with a stronger GVL effect compared with MSD transplants, with some studies suggesting a lower cumulative incidence of relapse and an improved survival ; however, the full potential of this stronger GVL has not yet been fully realized.
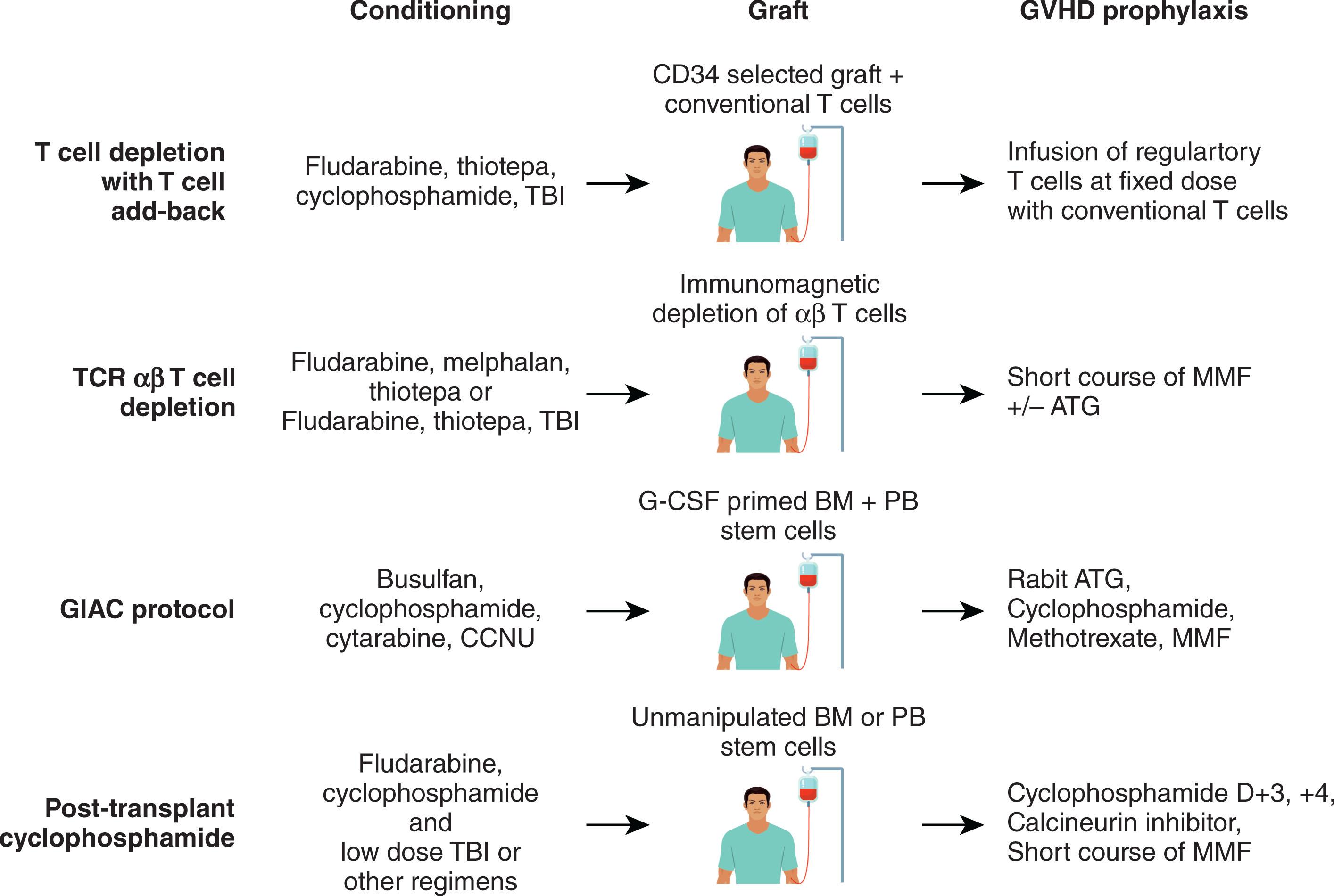
The major challenge in performing haploidentical HSCT (haploSCT) with conventional GVHD prophylaxis has been overcoming the intense bidirectional alloreactive reactions in the host-versus-graft (HVG) and graft-versus-host (GVH) direction, primarily mediated by donor T cells against recipient’s HLA antigens, resulting in high incidence of graft rejection, hyperacute GVHD, and treatment-related mortality. In an analysis of more than 2000 allogeneic transplants performed between 1985 and 1991 and reported to the International Bone Marrow Transplant Registry, compared with MSD transplants and using conventional GVHD prophylaxis, two HLA antigen-mismatched related donor transplants resulted in higher incidence of NRM (55% vs. 21% at 3 years post-transplant), graft failure (16% vs. 1%), moderate-severe acute GVHD (56% vs. 29%), and chronic GVHD (60% vs. 42%). Complete T-cell depletion (TCD) using CD34+ selected grafts has been subsequently used to reduce the incidence of acute GVHD; however, an increased risk of graft rejection and treatment-related mortality secondary to infectious complications emerged. Infusion of “mega-dose” CD34+ cells decreased the incidence of graft failure; however, it could not compensate for the almost complete lack of donor T cells (especially memory T cells) in the graft, and a higher incidence of infectious complications, especially viral infections, persisted, which was the main cause of death in these patients. Consequently, it became apparent that complete T-cell depletion cannot provide better outcomes, and various attempts to maintain different lymphocyte subsets in the graft have been subsequently studied. The Tübingen and Memphis groups introduced CD3 + /CD19 + depletion rather than positive selection of CD34 + cells to produce a graft that retains other cell subsets in the graft (such as NK cells, monocytes, dendritic cells, and other myeloid cells) that might facilitate a faster immune recovery without excessive GVHD, and generate a lower treatment-related mortality. In one study in adults, all but 1 of 29 patients engrafted; however, grades II–IV acute GVHD occurred in 48% of patients, 8 patients (28%) had NRM, 7 died as a result of infection. The 2-year survival was only approximately 20%. However, this approach appeared to be associated with better outcomes in children. In a study of 46 children receiving CD3 + /CD19 + -depleted grafts after myeloablative conditioning, successful engraftment was shown in 88% of patients, and grades II–IV acute GVHD occurred in 20%, grades III–IV acute GVHD in 7%, chronic GVHD in 21%, and NRM in 20%. In this latter study, relapse occurred in 63% of patients after 2 years of follow-up; however, 43% of patients undergoing transplant had active disease at the time of treatment. The increased risk of infection seen after TCD haploSCT may be due to the depletion from the adult donor graft of pathogen-specific memory T cells that provide protection from infections until new donor T cells differentiate from hematopoietic precursors in the recipient thymus. Subsequent research has been focused on infusing grafts containing selected populations of T cells to enhance immune reconstitution without substantially increasing the risk of GVHD. Adding back a fixed number of T cells that have been depleted of alloreactive cells or that have been anergized was proposed, with various degrees of success in improving immune reconstitution post-transplant. Several major advances emerged after year 2000, when graft manipulation focused on partial T-cell depletion of alloreactive T cells and pharmacologic modulation applied early post-transplant, have made major progress in reducing the incidence of infectious complications post-transplant, without increasing the incidence of GVHD and NRM. These maneuvers have significantly improved survival of patients receiving haploidentical transplantation, making this graft source an equal alternative to unrelated donor grafts, with similar outcomes and pattern of immunologic reconstitution post-transplant (see Modern Approaches to Haploidentical Transplantatiom).
A haplotype is a set of genes closely grouped together on a chromosome, inherited as a biologic unit. The HLA locus on chromosome 6p13.2 comprises a set of tightly linked genes encoding molecules that present peptide antigens to T cells. The HLA locus contains three regions: (1) Class I region—encodes the “classical” class I HLA genes HLA-A, HLA-B, and HLA-C, which present antigens to CD8 + T cells, as well as nonclassical HLA-E, HLA-F, and HLA-G molecules; (2) Class II region—encodes HLA-DRB1, HLA-DQB1, and HLA-DPB1, which present antigens to CD4 + T cells, as well as nonclassical class II molecules HLA-DM and HLA-DO; (3) Class III region—encodes molecules not known to be involved in histocompatibility reactions. An HLA haplotype is defined as the set of histocompatibility genes present on chromosome 6 which are transmitted together. Each individual has two HLA haplotypes, one inherited from each parent. An HLA-haploidentical donor is a related donor who shares one full haplotype with the transplant recipient, while the degree of HLA matching on the mismatched haplotype may vary between 0 (full haplotype mismatch, 5/10 HLA matched) to 3 (8/10) (of note a 9/10 HLA related donor is not considered a haploidentical donor). Recently, high-resolution HLA typing is usually performed for three HLA class I genes, HLA-A, HLA-B, and HLA-C, and three class II genes, HLA-DRB1, HLA-DQB1, and HLA-DPB1, to confirm that the donor and recipient share at least at one full haplotype as, rarely, mismatches can occur during meiosis (crossover). By definition, a parent and a child are HLA haploidentical to each other, and each biological sibling or half-sibling of a patient has a 50% chance of being HLA haploidentical to each other. Other potential haploidentical donors include second- or third-degree related donors—aunts, uncles, nieces, and nephews, who each have a 50% chance of being HLA haploidentical, and cousins, who have a 25% chance of being HLA haploidentical with the recipient.
Mismatching of HLA alleles or antigens can occur in the GVH direction only, the HVG direction only, or bidirectionally. When the donor is homozygous for an HLA allele but the recipient is heterozygous at the same genetic locus, there is a mismatch in the GVH direction only. Conversely, when the recipient is homozygous for an HLA allele but the donor is heterozygous, there is a mismatch in the HVG direction only. In general, HLA mismatches in the GVH direction favor GVHD, whereas HLA mismatches in the HVG direction predispose to rejection of the hematopoietic stem cell graft mostly by recipient’s T cells. The number of HLA mismatches between a haploidentical donor and recipient should be expressed as the number of mismatches in the GVH direction, as well as the number of mismatches in the HVG direction. For example, the patient in Fig. 107.1 differs from sibling 2 by four antigens (and alleles) in the GVH direction and by five antigens (and alleles) in the HVG direction, and from sibling 3 by three antigens (vs. four alleles) in the GVH direction and by three antigens (versus five alleles) in the HVG direction. More recently, molecular mismatches evaluating distinct amino acid configurations on the surface of the HLA molecule that could elicit immune responses (eplets) in both HVG and GVH directions are being evaluated, as early data suggest that could impact transplant outcomes.
The biologic mechanisms underlying the high incidence of graft rejection and severe GVHD when crossing major HLA barriers remain to be elucidated; however, two fundamental characteristics of T-cell alloreactivity are likely responsible. The first is the high frequency of T cells reactive against allogeneic HLA molecules. T cells recognize a complex determinant comprising specific amino acid residues of the HLA molecule as well as amino acid residues of the bound peptide. It has been estimated that there are 50,000 to 100,000 copies of each HLA molecule on a cell surface, with as many as 5000 different peptides being presented. Each allogeneic HLA molecule provides as many as 5000 distinct recognition units corresponding to 5000 distinct alloreactive T cells. In contrast, a non-HLA or “minor” histocompatibility antigen consists of a single allelic peptide presented by a single species of HLA molecule. The frequency of T cells reactive to a single minor histocompatibility antigen is on the order of 1 in 50,000 the frequency of anti-HLA alloreactive T cells. The higher frequency of T cells reactive to allogeneic HLA molecules compared to minor histocompatibility antigens corresponds to a higher incidence of graft rejection and GVHD after HLA-haploidentical SCT than after HLA-matched SCT. The second property of alloreactive T cells that contributes to a high incidence of graft failure and GVHD after haploSCT is a significant proportion of HLA-alloreactive memory T cells, even in donors and recipients who have not been exposed to allogeneic HLA molecules through pregnancy or blood transfusions. T cells that are immunized against environmental antigens, especially viruses, can cross-react against allogeneic major histocompatibility complex (MHC) molecules. The phenomenon, in which viral infection triggers cross-reactive memory against allogeneic HLA molecules, is termed heterologous immunity and may be a powerful barrier to the establishment of donor hematopoietic cell chimerism or the induction of tolerance to transplanted organs. Unlike naïve T cells, which are sensitive to chemotherapy-induced apoptosis and to immunologic tolerance induction by antigen without costimulation, memory T cells are more resistant to chemotherapy-induced death, can induce costimulatory signals on antigen-presenting cells that they encounter, and are resistant to tolerance induction by regulatory T cells (Tregs) or to T-cell–depleting antibodies. The presence and substantial number of memory T cells reactive to allogeneic HLA molecules make control of graft rejection and GVHD more challenging after haploidentical versus HLA-matched transplantation.
B cells and NK cells also participate in the immune response to HLA-mismatched tissues. Allogeneic HLA molecules can elicit the formation of alloantibodies. Preexisting donor-specific antibodies (DSA) against HLA molecules are a major risk factor for development of primary graft failure after HLA-haploidentical SCT. Pregnancy and blood transfusions are sensitizing events that can lead to the formation of antibodies against HLA molecules. Pregnancy is the main mechanism of allosensitization, evident by the fact that the highest DSA levels were found in parous women. The prevalence of DSA in parous women has been reported to be as high as 42% ; such sensitization is directed against unshared HLA molecules expressed by their children (father’s HLA antigens).
DSA can be detected by flow cytometry using a Luminex platform using beads coated with single HLA molecules or by complement-dependent cytotoxicity (CDC) testing, in which the patient's serum is mixed with donor lymphocytes in the presence of complement. In one study, a positive crossmatch for anti-donor lymphocytotoxic antibodies was associated with a 2.3-fold increased risk of graft failure. Donors with high DSA levels, especially with complement-binding DSAs and/or a positive CDC crossmatch, are, in general, avoided for transplantation, unless a significant response to desensitization treatment is accomplished, as discussed later in this chapter.
NK cells are a group of lymphoid cells part of the innate immune system capable of recognizing and eliminating malignantly transformed or virally infected cells. NK cells may play a significant role in inducing GVL effects after haploSCT in humans. Unlike T and B cells, they do not express rearranging receptors for antigen, but like CD8 + T cells, they express receptors for HLA class I molecules, including HLA-B and HLA-C, secrete interferon-γ, and kill target cells via granzyme- and perforin-mediated cytotoxicity. Karre and Kiessling initially proposed in the “missing self” hypothesis as mechanism of action for NK cells. It was subsequently found that NK cells are able to detect and rapidly eliminate virally infected or tumor cells that have downregulated cell surface expression of MHC class I molecules to evade the CD8 + T-cell immune response. The molecular basis of NK-cell alloreactivity is incompletely understood; however, it involves a dynamic balance of signals through activating as well as inhibitory receptors on the surface of the NK cells. The killer immunoglobulin-like receptors (KIRs) are encoded by a set of linked genes called the leukocyte receptor complex (LRC) on human chromosome 19q13.4. KIRs contain two or three extracellular Ig-like domains and either a short (S) or a long (L) cytoplasmic tail, which mediate activating or inhibitory signals, respectively. The LRC is marked by significant interindividual variation in KIR gene content as well as significant allelic variation in individual KIR genes. As a consequence, the KIR locus is second only to the HLA locus in the number of polymorphisms, and unrelated individuals are unlikely to share KIR genotypes.
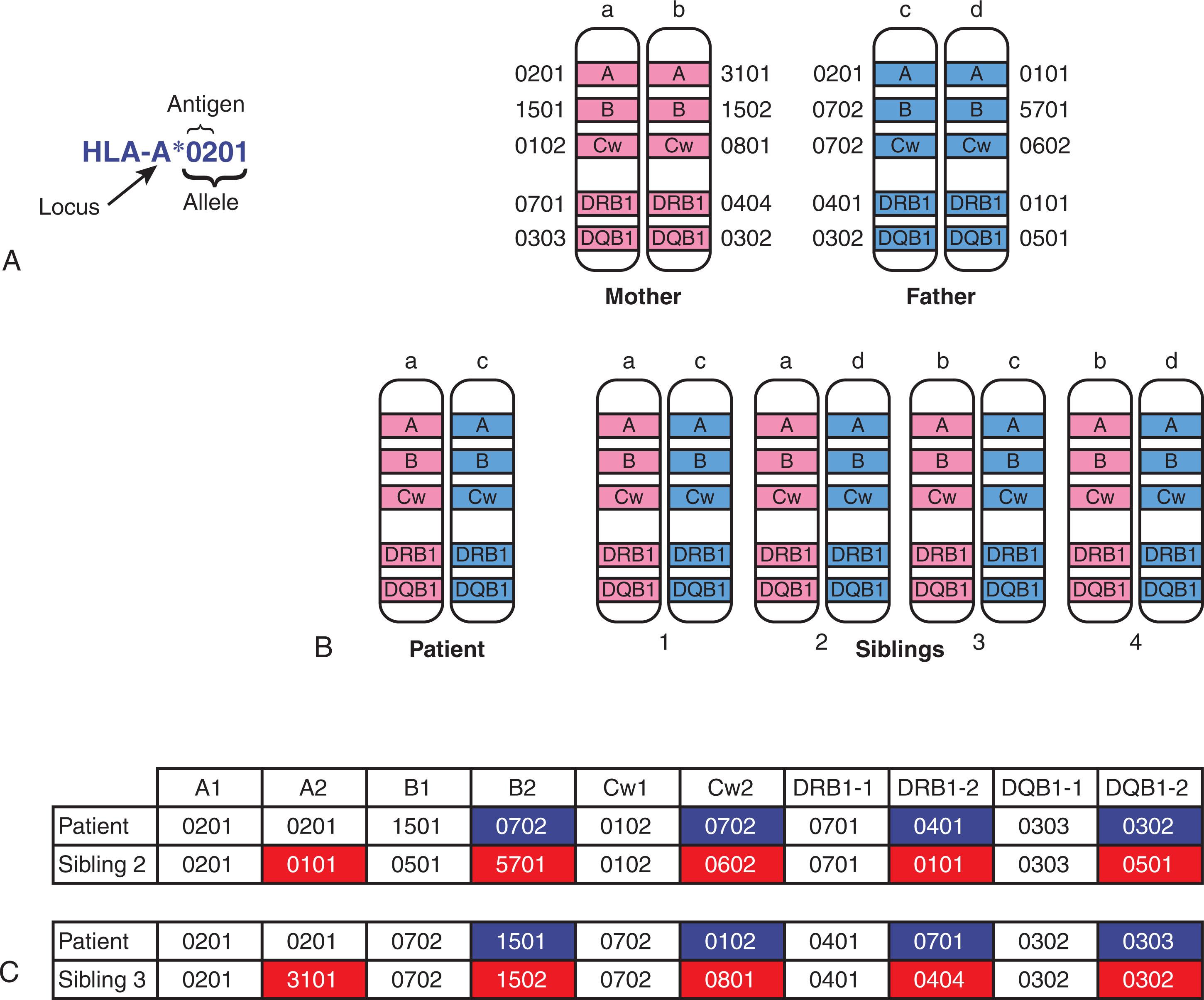
Distinct HLA class I molecules comprise the ligands for specific inhibitory KIRs (iKIRs). An organizing principle of NK-cell biology is that NK-cell self-tolerance is mediated by inhibitory signals delivered by self HLA class I molecules through iKIRs. The ontogeny of receptor expression on individual NK cells is poorly understood, but it is currently thought that each NK cell expresses at least one inhibitory receptor for a self HLA class I molecule. There are four distinct categories of HLA ligands for iKIRs ( Fig. 107.2 ). The C1 group of HLA ligands is characterized by the presence of an asparagine residue at position 80 of the HLA-C molecule and is recognized by either KIR2DL2 or KIR2DL3. The complementary C2 group of HLA ligands is distinguished by a lysine residue at position 80 of the HLA-C molecule and is recognized by KIR2DL1. The Bw4 serologic group is recognized by KIR3DL1. Finally, KIR3DL2 recognizes HLA-3 and HLA-11 molecules. Developing NK cells undergo a host MHC class I–dependent functional maturation process, termed licensing or, more recently, education . Education endows an NK cell with the ability to kill MHC class I–deficient targets but also provides a mechanism for self-tolerance because the same MHC class I ligand that licenses the developing NK cell also inhibits the activity of the mature NK cell. Licensing is not an all-or-none phenomenon, and there are degrees of licensing depending upon the affinity of an iKIR for its HLA ligand. Furthermore, there is evidence that transplanted NK cells can attune themselves to the new HLA environment, indicating that NK-cell alloreactivity is a complex and dynamic phenomenon. Because the genes comprising the LRC and the HLA locus are on chromosomes 19 and 6, respectively, KIR and HLA molecules are inherited independently, so individuals can inherit an iKIR but not its ligand, resulting in an NK cell that cannot be licensed during development. Unlicensed NK cells would be predicted to be poorly functional; unexpectedly, however, they dominate the early response to cytomegalovirus (CMV) infection in mice, and they also may be potent mediators of antibody-dependent cellular cytotoxicity. DNA damage resulting from transplant conditioning or inflammatory conditions such as viral infection may be sufficient to induce stress ligands of NK-cell activation receptors, break tolerance in unlicensed NK cells, and generate autoreactivity, including tumor regression. In contrast to HLA-matched transplants, where donors and recipients express the same ligands for iKIRs, in HLA-haploidentical SCTs, the recipient may lack the HLA ligand for the iKIR on a licensed donor NK cell, releasing the cell from inhibition, and resulting in donor NK-cell alloreactivity. Unlicensed donor NK cells may also be activated by a dominance of stimulatory NK-cell ligands in the inflammatory milieu of a conditioned recipient, but this scenario is not unique to HLA-haploidentical SCT and may also occur after HLA-matched SCT as long as the recipient lacks HLA ligands for iKIR molecules. Although the mechanisms of NK-cell alloreactivity remain to be fully elucidated, there is great interest in harnessing their activity for preventing or controlling relapse of hematologic malignancy after HLA-haploidentical SCT.
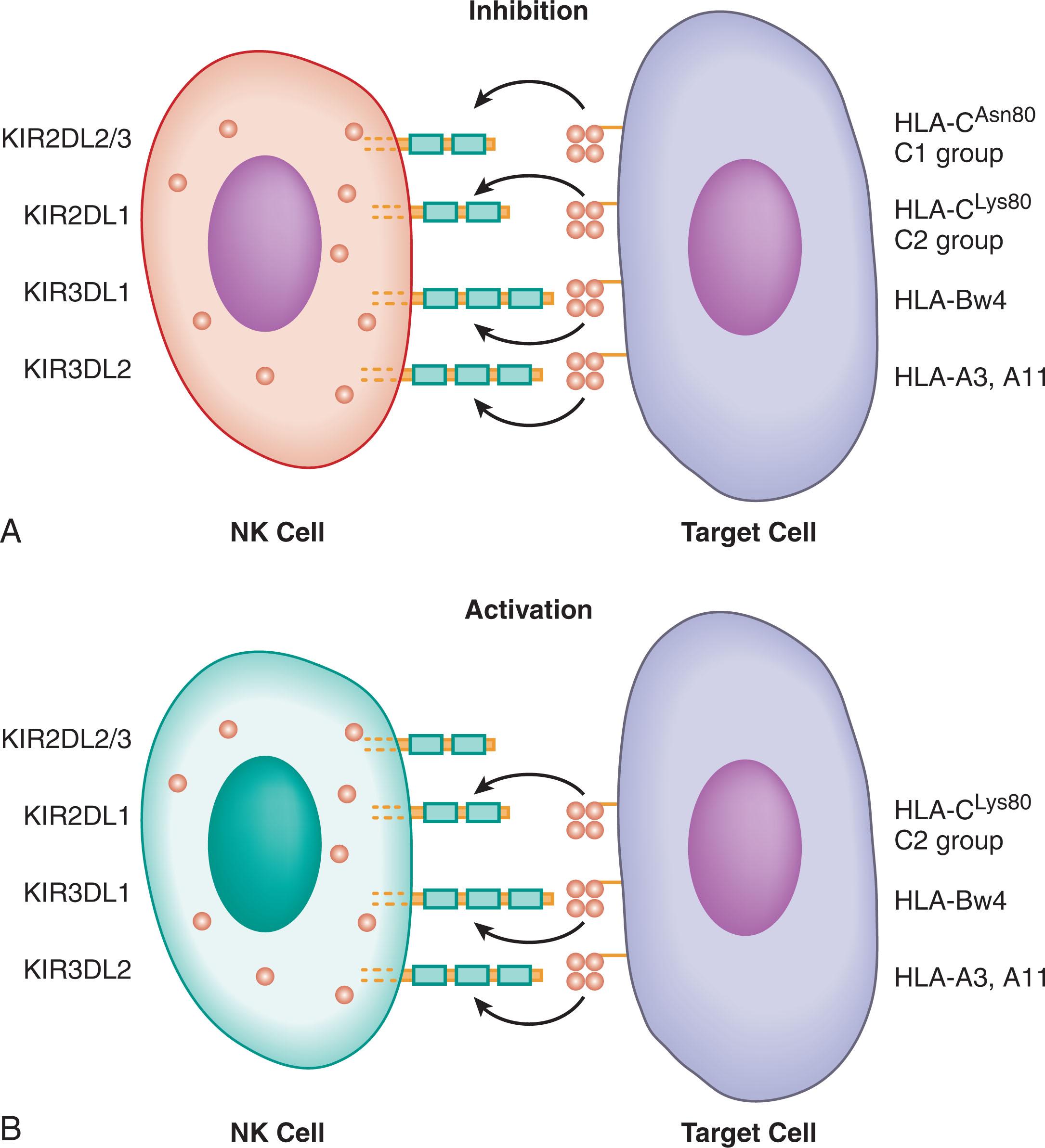
The KIR ligand incompatibility model was first formulated by Ruggeri et al. to account for NK alloreactivity after rigorously T-cell–depleted, stem-cell–enriched, HLA-haploidentical SCT. The model predicts NK cell alloreactivity when the donor expresses, but the recipient lacks, an HLA ligand (HLA group C1, C2, or Bw4) for an iKIR. In this situation, the donor is predicted to contain NK cells that are activated by recipient cells lacking expression of that HLA molecule. Support for the ligand incompatibility model was provided by the ability to generate donor alloreactive NK clones in all 51 ligand incompatible donor—recipient pairs but in none of the 61 donors who were KIR ligand matched with their recipients. NK alloreactivity in the GVH direction was predicted to have three functional consequences : (1) a GVL effect arising from donor NK cytotoxicity against leukemia cells; (2) a decreased rate of graft rejection arising from donor NK-cell killing of host T cells; and (3) a decreased rate of GVHD arising from donor NK-cell elimination of host antigen-presenting cells such as dendritic cells, which are required to initiate GVH reactions. This group (hereinafter the Perugia group) has demonstrated a strong antitumor effect of KIR ligand incompatibility in acute myeloid leukemia (AML) but not in acute lymphoblastic leukemia (ALL) patients undergoing T-cell depleted haploidentical transplantation.
The missing ligand model predicts NK-mediated GVH reactions when the transplant recipient is missing at least one of the three major classes of HLA ligands for iKIR. The missing ligand model differs from the ligand incompatibility model only in that it does not require the presence on donor cells of the HLA ligand that is missing in the recipient. Consequently, donors who are predicted by the ligand incompatibility model to contain alloreactive NK cells against their recipients are a subset of the donors who are predicted by the missing ligand model to contain anti-recipient alloreactive NK cells. The KIR ligand incompatibility and missing KIR ligand models were compared for their ability to predict relapse after T-cell–replete (TCR), unrelated SCT for hematologic malignancies. Among recipients of HLA-mismatched transplants, recipient homozygosity for HLA-B or HLA-C KIR epitopes was used to define “missing” KIR ligands and was associated with a decreased hazard of relapse (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.43 to 0.85; P = .004). The effect was observed in patients with AML, chronic myeloid leukemia, or ALL. The same effect was not observed in HLA-identical transplants. KIR ligand incompatibility was not associated with a decreased risk of relapse in recipients of either HLA-mismatched or HLA-matched grafts.
With novel approaches to haploidentical transplantation, especially using post-transplantation cyclophosphamide, there has been a renewed interest in harnessing antitumor effect of NK cells. Although several large retrospective studies failed to show a survival benefit of a KIR ligand mismatched donor as described by the Perugia group and cannot be recommended now for donor selection, at least for T-cell replete haploidentical transplants (see Selection of Donors for Haploidentical Transplantation), recent data outlined the role of NK cells infused with the graft in prevention of GVHD, relapse, and survival post-transplant.
Early studies of TCD HLA-haploidentical SCT were characterized by lower incidence of GVHD; however, patients experienced a higher incidence of engraftment failure and high NRM, mostly due to infectious complications related to a significant delay in immunologic reconstitution. These outcomes spurred efforts to improve engraftment rate and immune reconstitution after TCD haploSCT.
In order to improve immunologic reconstitution after transplant, the Perugia group proposed administration of a fixed dose of conventional T (Tcons) cells after TCD haploidentical transplantation, and, to prevent development of severe GVHD, infusion of immunomagnetically selected Tregs 4 days before transplant followed by Tcons cells on the same day as the TCD allograft. In the first study of 28 patients only two developed grades II–IV acute GVHD, and both received the highest T-cell doses in the study cohort, while no chronic GVHD was observed. T-cell reconstitution was improved with early expansion of T-cell repertoires and improved pathogen-specific responses were observed. Although none of the patients developed CMV-associated disease, NRM still occurred in 13 patients (50%), 8 of whom died of infectious complications. In an updated report on an expanded cohort of patients the authors showed similar results and suggested that, in both mouse models and human studies, the infusion of Tregs appears to control GVHD without affecting graft-versus-tumor immunity. While relapse rates for patients treated in this study appeared better than in historical controls, NRM remained high, particularly death as a result of infection, despite laboratory evidence of improved immune reconstitution post-transplant.
Viral reactivation is a major cause of morbidity and mortality early, especially after TCD haploidentical transplantation, and, in general, a higher number of viral reactivations has been associated with higher NRM. Hence, prevention and early treatment of viral infections post-transplant is crucial for the success of transplantation. Several groups explored infusion of viral-specific cytotoxic T lymphocytes (CTLs) for the prevention or treatment of viral infections early post-transplant. These T cells expand in vivo following infusion and exert antiviral effects without causing GVHD. Leen and colleagues showed that CTLs against single or multiple viruses that commonly reactivate post-transplant can be safely infused with evidence of antiviral activity in these patients.
Due to the fact that administration of unmodified CD3+ selected donor lymphocytes early post-transplant carries a major risk of development of acute GVHD, infusion of genetically modified donor lymphocytes expressing a suicide gene that could be activated by administration of ganciclovir if GVHD develops has been explored. In a study of 50 patients, the use of this treatment strategy markedly accelerated immune reconstitution. When GVHD did occur, it was promptly and effectively abated by induction of the suicide gene. Although this appeared to be a promising approach, NRM in this study was still high (approximately 40% of the patients). In addition, due to the fact that ganciclovir is a commonly used drug in transplantation for treatment of CMV reactivation, the Baylor group developed an alternative approach using an inducible caspase 9 suicide gene incorporated in donor lymphocytes, which could be eliminated using an inert molecule as dimerizing drug. Administration of this drug resulted in successful elimination of greater than 90% of lymphocytes and promptly stopped the development of GVHD. In a follow-up study, the same group treated 10 haploidentical transplant patients with iCasp9-modified donor lymphocyte infusion (DLI) and showed a favorable effect on immune responses against viral and fungal pathogens. This approach was also studied in conjunction with alpha/beta TCD haploidentical transplantation (see following section), with excellent results.
Acute and chronic GVHD reactions associated with a major HLA disparity between the donor and the recipient have been a main concern since the first introduction of haploidentical donor transplantation. As previously mentioned, while a full HLA haploidentical graft with conventional GVHD prophylaxis has been associated with unacceptable incidence of GVHD, the use of complete T-cell depletion methods resulted in very high treatment-related mortality. These results have emphasized the beneficial effect of donor T lymphocytes and other immune cells in the graft, and led to the development of novel techniques of graft manipulation with the goal to selectively deplete T-cell subgroups most likely responsible for development of GVHD.
The majority of peripheral blood T cells express αβ T-cell receptor (TCR), while approximately 5% express γδ TCR. It has been shown that a donor-derived αβ T-cell subset plays a major role in the pathogenesis of GVHD, unlike innate-like γδ T cells, which are capable of directly recognizing targets in an MHC-independent manner, enabling them to exert anti-tumor activity by directly identifying stress-induced antigens expressed on malignant cells, without recognition of classical MHC-associated alloantigens. Several studies have demonstrated that an increased number of donor-derived γδ T cells following HLA mismatched related allogeneic hematopoietic stem cell transplants is significantly associated with better disease-free survival.
Based on these findings, the immunomagnetic method of ex vivo selective depletion of αβ T cells from mobilized peripheral blood grafts has been pioneered by Chaleff and Handgretinger using a biotinylated anti-TCRαβ antibody, followed by an anti-biotin antibody conjugated to magnetic microbeads, which causes approximately 4 log reduction of αβ T cells, while maintaining in the graft the majority of CD34+ cells, as well as the NK cells and γδ T cells. Several groups have demonstrated that the efficacy in T-cell reduction using this method was comparable to CD34+ positive selection and more effective than CD3+/CD19+ T-cell depletion, whereas recovery of CD34+ cells after depletion is comparable in all three procedures. In addition, results from animal models of transplantation of αβ+ T-cell depleted graft showed rapid engraftment. This method was subsequently combined with depletion of CD19+ B lymphocytes in order to prevent post-transplant EBV-associated lymphoproliferative disease. In the first clinical experience, 23 pediatric patients with advanced hematologic malignancies treated in Tübingen and Rome received TCRαβ+/CD19+ depleted haploidentical peripheral blood grafts, with either reduced-intensity (melphalan, thiotepa, fludarabine, or clofarabine and OKT-3 or ATG) or myeloablative (fractionated TBI, thiotepa, fludarabine, and ATG) conditioning regimen. All patients achieved sustained engraftment, rapid immune reconstitution, and had low incidence of GVHD, despite that no post-transplant pharmacologic GVHD prophylaxis was administered.
Lang et al. reported in abstract format long-term follow-up results of 60 patients (30 adults, 30 pediatric patients) with malignant and nonmalignant diseases who received TCRαβ+/CD19+ depleted peripheral blood stem cell grafts from haploidentical donors as part of a prospective, multi-center, single-arm, phase I/II clinical trial. All patients received reduced-intensity conditioning (RIC) with fludarabine, melphalan, thiotepa, and either ATG or total nodal irradiation, and a short course of mycophenolate mofetil for GVHD prophylaxis. Rapid engraftment was observed with median time to neutrophil and platelet engraftment of 13 and 15 days, respectively. Eight of nine patients who had graft rejection were successfully re-transplanted. None of the patients developed severe acute GVHD, while severe chronic GVHD occurred in only 9%. However, a higher rate of viral reactivation was observed, with three patients dying of adenovirus disease. At 2-year follow-up, OS and DFS were 62% and 53%, respectively, while cumulative incidences of relapse and NRM were 34% and 20%, respectively. Similarly, successful engraftment and lower rate of GVHD have been confirmed in several studies, both in pediatric and adult patients.
Post-transplant immunologic recovery has also been investigated after TCRαβ+/CD19+ depleted haploidentical transplantation. Results from an immunologic reconstitution study of 41 recipients of TCRαβ+/CD19+ depleted haploidentical transplantation showed a significantly higher number of CD3+, CD3+CD4+, and CD56+ cells early post-transplant compared to a historical control group of 36 pediatric patients receiving positive CD34+ selected haploidentical stem cells. In this study, recovery of γδ T cells started at day +7 post-transplant and preceded αβ T-cell reconstitution. Airoldi and colleagues found that γδ T cells are the predominant T-cell population in patients during the first weeks after transplantation, derived not only from cells infused with the graft but also γδ T cells differentiated from donor’s hematopoietic stem cells.
Not only has a rapid increase in number of donor-derived immunologic cell subsets post-transplant been observed, but these cells also might provide a benefit in graft-versus-tumor effect, as evidenced in several studies. Focusing on pediatric patients with acute leukemia, Locatelli et al. reported outcomes of 80 patients of TCRαβ+/CD19+ depleted haploidentical transplant using myeloablative conditioning without post-transplant GVHD prophylaxis, and showed a high GVHD-free, relapse-free survival (GRFS) of 71% at 5 years, comparable to those of children given transplantation from an HLA-identical sibling or a 10/10 MUD treated during the same period of time. Similar results were also reported in other studies, in both pediatric and adult patients with high-risk leukemias.
The benefit of lower incidence of GVHD in TCRαβ+/CD19+ depleted haploidentical transplants may be more important for patients with non-malignant diseases. A prospective study conducted in 23 pediatric patients with non-malignant diseases treated with TCRαβ+/CD19+ depleted haploidentical transplants showed promising outcomes with high engraftment rate and very low incidence of both acute and chronic GVHD. In this study, the cumulative incidence of grade I–II aGVHD was only 13.1%, no chronic GVHD was observed, and transplant-related mortality was only 9%. With a median follow-up of 18 months, 21 of 23 children were alive and disease-free with the 2-year probability of disease-free survival of 91.1%. Considering both primary and secondary graft failure as events, the probability of EFS was 74%. Another study of 14 pediatric patients with thalassemia also reported a high rate of engraftment. In this study the cumulative incidence of engraftment failure was 14%, significantly lower than a historical group of 40 patients with hemoglobinopathies who received CD34+ selected haploidentical grafts (45%). Overall survival and disease-free survival were 78% and 69%, respectively. However, a higher incidence of GVHD was observed in this study despite receiving post-transplant pharmacological prophylaxis up to 60 days (with 28% incidence of grade II–III acute GVHD and 21% chronic extensive GVHD), and viral reactivations, such as CMV, ADV, and BKV, were also common. Although the number of patients treated so far is relatively small and no definitive conclusions can be drawn, it is important to notice that a higher incidence of GVHD has also been seen in adults receiving TCRαβ+/CD19+ depleted haploidentical transplantation in some studies.
Taken together, current available data indicate that TCRαβ+/CD19+ depleted haploidentical transplantation can provide an alternative option for patients without an HLA-matched donor with several studies that reported outcomes comparable to HLA-matched related or unrelated donor transplants. Moreover, the low risk of GVHD without the need of long-term post-transplant immunosuppression may provide a better opportunity to apply cellular therapy post-transplant and help improve quality of life, which could make this type of haploidentical transplant more appealing, especially for pediatric patients. Novel pharmacologic and adoptive cellular therapy approaches to enhance anti-tumor effect and immune reconstitution and improve outcomes after TCRαβ+/CD19+ depleted haploidentical transplantation are being investigated.
Treatment of bone marrow donors with G-CSF before donation increases marrow CD34 + cells and granulocyte-macrophage colony-forming units, reduces total lymphocytes, and reverses the CD4 + /CD8 + T-cell ratio. To enhance engraftment by increasing the dose of transplanted HSCs, 15 patients with high-risk leukemia received myeloablative conditioning with cytarabine, cyclophosphamide, and 1000 cGy TBI, and G-CSF–primed bone marrow from haploidentical donors. GVHD prophylaxis consisted of rabbit ATG (5 mg/kg/day on days −4 through −1), cyclosporine (CsA), methotrexate, and mycophenolate mofetil (MMF). At the time of reporting, all 15 patients had prompt trilineage hematopoietic engraftment; the cumulative incidence of GVHD was 33%; and 9 of 15 patients were alive at a median follow-up of 22 months (range, 13 to 35 months). Subsequently, Lu et al. compared outcomes of 293 patients with leukemia receiving HLA-matched sibling ( n = 158) or HLA-haploidentical related grafts ( n = 135) from G-CSF–primed donors. Patients undergoing haploidentical SCT were conditioned with cytarabine, oral busulfan, cyclophosphamide, and methyl-CCNU (1-[2-chloroethyl]-3-[4-methylcyclohexyl]-1-nitrosourea), received G-CSF primed bone marrow on day 0 ( n = 134) and/or G-CSF–primed peripheral blood on day 1 ( n = 131), and GVHD prophylaxis with ATG 2.5 mg/kg/day on days −4 through −1, CsA, MTX, and MMF. All but two haploidentical SCT patients treated with the GIAC protocol had sustained engraftment of donor neutrophils. The cumulative incidence of acute grades II–IV, grades III–IV, and chronic GVHD in recipients of matched versus mismatched SCT were 32% versus 40% ( P = .13), 11% versus 16%, and 56% versus 55%. Mismatched patients had a higher incidence of CMV antigenemia (65% vs. 39%; P < .001) and hemorrhagic cystitis (35% vs. 13%; P < .001), but not of CMV disease. Two-year relapse rate was 13% versus 18% ( P = .40), NRM 14% versus 22% ( P = .10), LFS 71% versus 64%, and OS 72% versus 71% ( P = .72), in the matched and mismatched cohorts, respectively. In a follow-up report of 157 recipients treated with this approach, recipients of CD3 + T-cell doses higher than the median (1.77 × 10 8 /kg) had a significantly lower NRM, better LFS, and better OS. Two other Chinese groups reported similar results of retrospective studies comparing outcomes of patients who received haploSCT with those of patients who received HLA-matched sibling or HLA-matched unrelated transplants. Moreover, in a prospective, multicenter study comparing haploSCT ( n = 231) versus HLA-matched sibling transplants ( n = 219) using biologic randomization based on donor availability, similar outcomes were noted between the two groups.
Although outcomes are in general very good (especially because patients treated are up to age 60) a higher proportion of patients develop severe acute and mostly chronic GVHD. Several groups have attempted to reduce the incidence of GVHD seen with this protocol. 113–116 In a Korean study in which a modified version of this platform was used with RIC and only G-CSF mobilized PBSC allografts, authors showed a lower incidence of grades II–IV acute (20%) and chronic (34%) GVHD. A different group modified this protocol to use TBI-based conditioning, only GCSF-primed bone marrow, and added basiliximab to enhance GVHD prophylaxis. Although this approach resulted in lower incidence of grades II–IV acute GVHD of 11%, chronic GVHD was still seen in most patients, yet was mostly limited in severity. The Rome Transplant Network employed the same GVHD prophylaxis and reported in 97 patients encouraging results in terms of GVHD control (grades II–IV and grades III–IV acute GVHD of 31% and 9%, respectively, and extensive chronic GVHD of 12%); however, 1-year NRM was 32%.
Survival outcomes of patients with acute leukemia receiving haploSCT with this protocol have been particularly encouraging, owing primarily to lower incidence of relapse in the haploidentical versus HLA matched group (26% vs. 49%; P = .008). In 2009, the Peking University group reported the results of a study of 250 consecutively treated patients with acute leukemia, 108 of whom had AML and 142 of whom had ALL. Survival of patients with AML were particularly favorable, with 3-year OS of 73% and 56% reported for standard-risk and high-risk groups, respectively. However, outcomes of patients with high-risk ALL appeared poor because of higher incidence of NRM (51%) and relapse (49%) at 3 years, presumably due to intense myeloablative conditioning, usually less well tolerated in this group of patients. . Patients with standard-risk ALL, however, had 3-year OS of 60%. Two other studies have confirmed excellent survival of patients with ALL in CR1 treated with haploSCT using this protocol. In another ALL study, patients received consolidation with either 2 years of chemotherapy ( n = 104) or haploSCT ( n = 79) (as preferred by the patients); those who received haploSCT had markedly better 3-year disease-free survival (DFS) (64% vs. 21%), OS (72% vs. 27%), due to a much lower cumulative incidence of relapse (19% vs. 61%) compared with chemotherapy-treated patients. In multivariate analyses, consolidation of remission with a haploSCT was the only factor associated with lower relapse and better OS in these patients.
In all these studies, the extent of HLA disparity did not affect survival post-transplant. However, donor characteristics appeared to impact patient outcomes; lowest NRM and best survival were seen with younger, male donors, and lower incidence of acute GVHD occurred when the donor was the patient's child or an HLA-haploidentical relative who was mismatched for non-inherited maternal HLA antigens (NIMA), while the use of maternal donors was associated with higher incidence of acute and chronic GVHD, and worse survival.
A few studies compared outcomes of unmanipulated haploSCT using the GIAC protocol with post-transplantation cyclophosphamide approach and will be discussed later in this chapter.
The effects of cyclophosphamide (Cy), one of the oldest chemotherapeutic agents, have been studied since the early 1960s. High-dose Cy was found to be effective in prolonging the survival of MHC-mismatched mouse skin allografts only when the drug was given up to the fourth day post-transplant, with the optimal effectiveness of this treatment being at 2 days post-transplant. This work was continued by a number of groups into the mid-1990s, which investigated mechanisms of tolerance induction by Cy. By administering donor spleen cells followed 48 to 72 hours later by administration of cyclophosphamide, long-lasting tolerance to MHC-compatible, but not MHC-incompatible, skin allografts was established. Three primary mechanisms of post-transplant cyclophosphamide (PTCy)-induced tolerance were identified in this model: (1) Direct elimination of host T cells responding to donor antigens in the periphery, (2) intra-thymic clonal deletion of donor-reactive host T cells, and (3) generation of tolerogen-specific host suppressor T cells. Suppressor T cells were found to inhibit responses to both major and minor histocompatibility antigens through active suppression but not clonal deletion of alloreactive T cells. Induction of tolerance by PTCy was disrupted by the administration of CsA or corticosteroids before adoptive cell transfer and cyclophosphamide treatment, while it was not affected by the administration of G-CSF starting the day after PTCy treatment.
PTCy found its greatest applicability in haploidentical transplantation. In the initial experiments in MHC-mismatched mouse models, treatment with PTCy reduced the dose of radiation required to induce reliable engraftment, prevented GVHD, and prolonged survival. Graft failure could be further reduced by the use of antilymphocyte globulin, and radiation in the conditioning regimen could be replaced, at least in part by fludarabine, although high levels of donor chimerism required at least 100 cGy of TBI. The resultant mixed chimeras were tolerant to both donor and host but maintained reactivity against third-party alloantigens in mixed lymphocyte culture. This tolerogenic effect allowed skin and heart allografts from the MHC-mismatched donor strain to survive, whereas MHC-disparate third-party grafts were rejected.
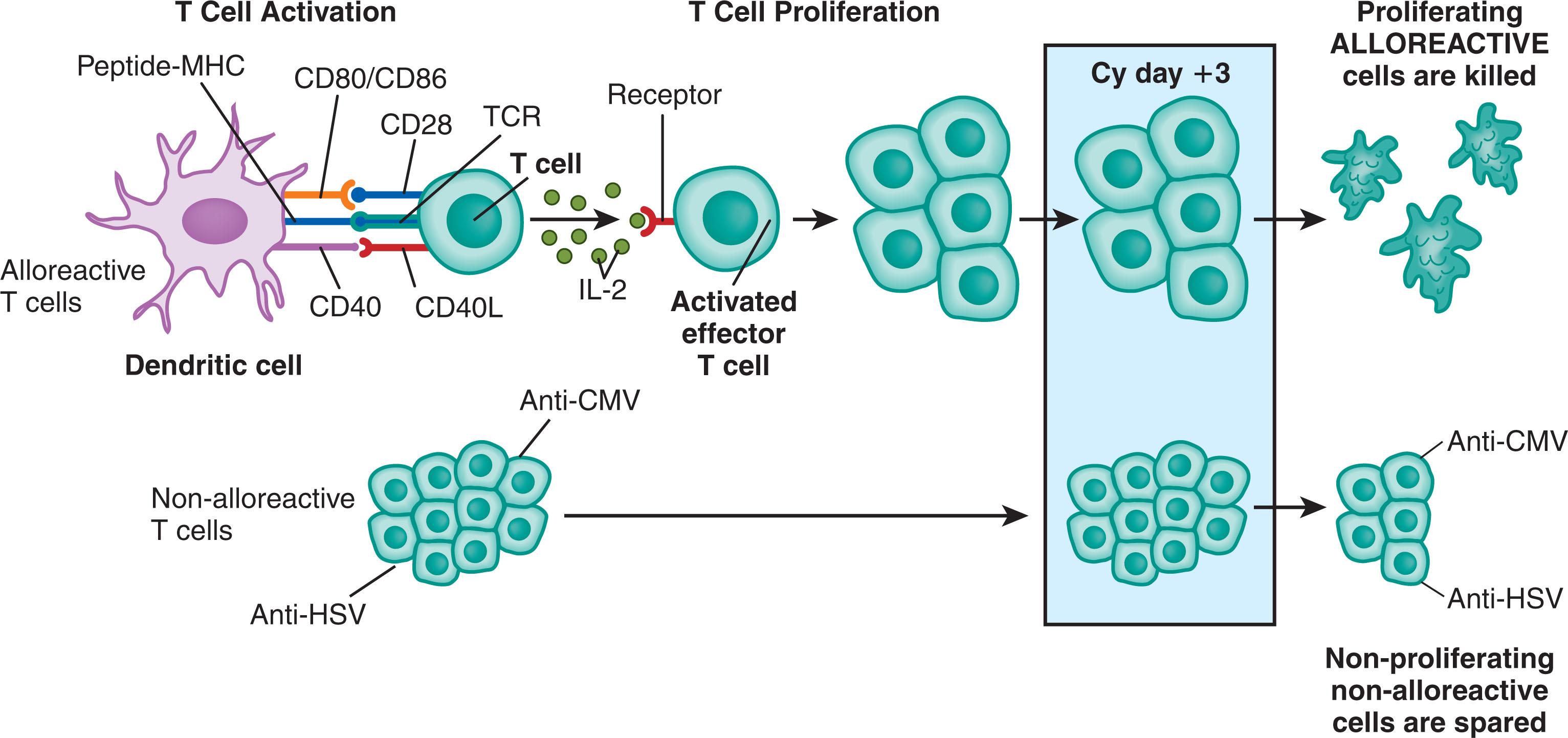
Parallel to the effects seen on host T cells in skin allograft models, in transplant mouse models, treatment with PTCy inhibited GVHD through elimination of alloreactive donor T cells. Donor T cells exposed to antigen on day 0 were largely depleted by PTCy, whereas non-alloreactive donor T cells, which divided more slowly in a lymphopenic environment, were relatively spared ( Fig. 107.4 ). However, elimination of alloreactive donor T cells was necessary but not sufficient for PTCy-induced tolerance. The role of Tregs in inducing, as opposed to maintaining, tolerance is controversial. In mouse models of MHC-matched transplants in which donor CD4 + T cells promote GVHD, donor Tregs were necessary to prevent lethal GVHD after PTCy treatment, an effect consistent with the results from skin allograft models. In mouse models as well as human studies, donor Tregs were resistant to PTCy-induced cytotoxicity, owing to increased expression of aldehyde dehydrogenase, the enzyme primarily responsible for in vivo detoxification of cyclophosphamide. In contrast, PTCy can induce tolerance in alloreactive CD8 + T cells in the absence of CD4 + T cells, including CD4 + Tregs. 135 In addition, other studies have shown that Tregs are not required for cyclophosphamide-induced tolerance of tumor-reactive T cells or for transplant tolerance induced by a different alkylating agent, bendamustine.
In an initial phase I clinical trial of 13 patients done at John Hopkins, patients received a non-myeloablative (NMA) conditioning regimen with fludarabine ± cyclophosphamide and low-dose (200 cGy) TBI, a T-cell replete haploidentical bone marrow graft, and one dose of PTCy 50 mg/kg on day +3 post-transplant. For GVHD prophylaxis, in addition to tacrolimus, MMF was also added starting the day after patients received PTCy and continued for at least 30 days. Approximately 80% of patients who had fludarabine, cyclophosphamide, and 2GyTBI successfully engrafted, and half the patients were alive and in remission after a median follow-up of 284 days. Grade II–IV acute GVHD developed in 6 patients. Application of two doses of cyclophosphamide was found to be more effective in facilitating engraftment and in preventing GVHD. Subsequently, a phase I/II study performed at two institutions enrolled 68 patients, and the majority received two doses of PTCy on day +3 and +4 post-posttransplant. Eighty-seven percent of patients engrafted and achieved complete or near-complete donor chimerism by 1 to 2 months posttransplant. Grades II–IV and grades III–IV acute GVHD occurred in 34% and 6% of patients, respectively. The incidence of extensive chronic GVHD was very low, significantly lower in patients receiving two doses of PTCy (5%). Longer follow-up of an expanded cohort of 210 patients treated according to the JHH protocol confirmed very low incidence of NRM, acute GVHD, and chronic GVHD. The extent of HLA disparity had no negative effects on acute GVHD or progression-free survival (PFS). In addition, a relatively high rate of relapse (55%) was observed, in part reflected by the use of NMA conditioning in patients with acute leukemia and by the advanced disease state of patients who received transplants. Interestingly, a disease risk–stratified analysis of 372 patients performed by the Johns Hopkins group showed that survival outcomes were comparable with those of patients receiving HLA-matched allogeneic stem cell transplantation.
Nevertheless, in an effort to reduce relapse rates post-transplant, several groups explored intensified conditioning with either a melphalan-, total body irradiation (TBI)-, thiotepa-, or busulfan-based regimen for haploidentical transplants in the adult and pediatric population. These studies showed, in general, higher NRM (3% to 21%) lower incidence of relapse (19% to 35%), comparable incidence of grades II–IV aGVHD (12% to 30%), III–IV aGVHD (3% to 10%), and cGVHD (10% to 26%), and at least as good survival as NMA conditioning (1-year DFS 51% to 70%). It is now generally accepted that, as in HLA matched transplants, younger, fit patients with acute leukemia should receive a myeloablative conditioning regimen, while older, less fit individuals and lymphoma patients should be treated with a RIC/NMA conditioning regimen. A summary of commonly employed conditioning regimens for haploSCT with PTCy-based GVHD prophylaxis is presented in Table 107.1 .
| Conditioning Regimen | Study | Type | N | Age Median (Range) | aGVHD gr II-IV | NRM | Relapse rate | PFS | OS | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
|
Prospective, two centers | NMA | 68 | 46 (1–71) | 34% | 15% at 1 year | 51% at 1 year | 26% at 2 years | 36% at 2 years | |
|
Prospective, multicenter, randomized | NMA | 182 | 60 (20–70) | 28% | 11% at 2 years | 48% at 2 years | 41% at 2 years | 57% at 2 years | |
|
Prospective, single center |
|
60 | 45 (20–63) | 28% | 21% at 1 year | 19% at 1 year |
|
|
|
|
Prospective, | MAC | 96 | 42 (1–65) | 11% |
|
|
|
|
|
|
Retrospective, single center | MAC | 82 | 42 (19–61) | 52% | 13% at 4 years | 27% | 60% at 4 years | 67% at 4 years | |
|
Retrospective, single center | MAC | 148 | 47 (17–74) | 24% | 14% | NR | NR |
|
An alternative two-step approach to myeloablative PTCy haploSCT separates the induction of tolerance to donor cells (step 1) from hematopoietic rescue (step 2). Patients received a TBI-based conditioning and peripheral blood T cells at a fixed dose on pre-transplantation day −6, two doses of cyclophosphamide 60 mg/kg/day on days −3 and −2 for tolerance induction (step 1), followed by a CD34-selected PBSC graft from the same donor on day 0. This procedure mimicked the timing of the standard PTCy platform, except that stem cells were spared exposure to cyclophosphamide. Although there were concerns initially with exposure of stem cells to cyclophosphamide, no detrimental effects have been observed long term, now after greater than 10 years of application of Cy in haploSCT. Results for patients who were in remission at the time of transplant showed a low incidence of grades III–IV acute (4%) and chronic (21%) GVHD, NRM of 3.6%, and relapse-related mortality of 19%, leading to a 2-year DFS of 74%.
Although initially a bone marrow graft was used almost exclusively for haploidentical transplantation, several groups have explored the use of peripheral blood stem cells (PBSCs) for haploidentical transplantation with PTCy, and several studies compared these two donor sources for haploidentical transplantation. As seen in a randomized study conducted by the Blood and Marrow Transplant Clinical Trials Network (BMTCTN) in unrelated donor transplantation, the use of peripheral blood graft has been, in general, associated with a slightly higher engraftment rate yet a higher incidence of acute and chronic GVHD, with similar survival in most studies, including in a recent meta-analysis. However, for patients with acute lymphoblastic leukemia, a bone marrow graft should be preferred as it was associated with lower incidence of GVHD and NRM and better DFS and GRFS survival in a European Blood and Marrow Transplant (EBMT) analysis.
PTCy has shown promise as a tolerizing agent in facilitating mismatched hematopoietic stem cell grafts infused during or soon after solid-organ transplantation with the goal to achieve complete tolerance of the solid organ grafts. Leventhal et al. reported on combined kidney and hematopoietic transplants in which fludarabine/cyclophosphamide conditioning was administered before renal transplant and column-selected PBSCs were given the day after renal transplant. Mycophenolate and tacrolimus were started 2 days before kidney transplant and one dose of PTCy (50 mg/kg) was given 3 days post-transplant. According to the latest report, 12 of 19 patients achieved functional tolerance after full donor chimerism as demonstrated by successful cessation of all immunosuppression without graft rejection. This approach suggests that tolerance induction in solid organ transplantation appears to be dependent on achieving sustained donor chimerism. Future studies will explore the use of haploSCT with PTCy after solid organ transplantation to achieve complete tolerance of the transplanted organ to the recipient’s immune system.
With multiple platforms being developed for patients receiving haploidentical transplantation, the natural question is how outcomes of haploidentical transplants using different approaches compare, and how outcomes of haploidentical transplants compare with other donor types. Only a few studies to date have retrospectively compared outcomes using different haploidentical transplantation platforms. In an initial retrospective study of 65 adult patients receiving haploSCT using either PTCy or megadose T-cell–depleted stem cells for GVHD prophylaxis, survival was significantly better for patients receiving PTCy. NRM was markedly lower after TCR graft and PTCy-based GVHD prophylaxis versus TCD haploidentical transplantation (16% vs. 42% at 1 year), with the difference largely a result of lower risk of viral (twofold lower) and fungal (fivefold lower) infections post-transplant. A more rapid immunologic reconstitution was noted in patients receiving PTCy, with similar incidence of aGVHD, lower incidence of cGVHD (7% vs. 18%), similar relapse rate post-transplant, and significantly better survival (64% vs. 30%, P = .02).
Another study compared haploidentical transplants performed with PTCy and GIAC protocol (anti-thymocyte globulin [ATG]) for patients with AML. Patients in the PTCy group ( n = 193) had significantly less severe (grades III–IV) aGVHD than patients in the GIAC group ( n = 115), 5% versus 12%, respectively ( P = .01), with similar incidence of chronic GVHD and relapse incidence. However, recipients of PTCy had a significantly lower incidence of NRM (22% vs. 30%; P = .02) and improved LFS (HR, 1.48; 95% CI, 1.03 to 2.12; P = .03). Taken together, these results suggest that TCR haploSCT with PTCy may the best haploidentical transplant platform to date, yet additional studies are needed, including comparing PTCy with αβTCD.
As noted earlier in this chapter, traditional donor choices in patients receiving conventional GVHD prophylaxis relied on HLA matched donors, as outcomes with haploidentical donors were associated with unacceptable toxicity. HLA-matched sibling donors were preferred, as they were associated with the best outcomes. If an HLA-matched sibling donor was not available, the preferred donor was a well-matched (at least 8/8 allele match at HLA-A, HLA-B, HLA-C, HLA-DRB1) MUD and, in the absence of an HLA-matched sibling or MUD, the choices may be a partially HLA-matched unrelated donor, an unrelated umbilical cord blood, and an HLA-haploidentical first-degree relative. The introduction of PTCy for GVHD prophylaxis has significantly improved transplant outcomes with haploidentical donors, now similar at least with MUDs, and raised questions whether traditional donor choice should continue be used, or if other factors should be considered such as urgency to transplantation. Multiple studies, mostly retrospective, compared outcomes of haploidentical transplants with PTCy with other donor sources; the most relevant are summarized below.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here