Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
These infants are remarkable not only because like foetal versions of Shadrach, Meshach and Abednego, they emerge at least alive from within the fiery metabolic furnace of diabetes mellitus, but because they resemble one another so closely that they might well be related. They are plump, sleek, liberally coated with vernix caseosa, full-faced and plethoric… They convey a distinct impression of having had such a surfeit of both food and fluid pressed upon them by an insistent hostess that they desire only peace so that they may recover from their excesses. And on the second day their resentment of the slightest noise improves the analogy while their trembling anxiety seems to speak of intrauterine indiscretions of which we know nothing. James W. Farquhar
The fetus receives a constant supply of glucose, calcium, and magnesium. Fetal plasma levels are closely regulated by maternal metabolic homeostasis, placental exchange, and fetal regulatory mechanisms. Abruptly at birth, termination of nutrient supply requires profound changes in energy and mineral metabolism. The provision of exogenous nutrients and the mobilization of endogenous fuel and mineral stores determine homeostasis. The result is the potential for rapid changes in plasma glucose and calcium levels during the first days of life. The infant who is premature, growth restricted, stressed, or born to a diabetic mother is at increased risk for problems with homeostasis, hypoglycemia, and hypocalcemia. As we will see, these issues are common and made even more challenging by the fact that most of these infants are asymptomatic. Therefore appropriate screening with protocols that look at identifying these infants along with a management plan is critical and will be reviewed particularly as it relates to diagnosis of hypoglycemia.
An understanding of fetal and neonatal fuel metabolism has emerged from studies in animals and humans. Fetal energy consumption is high, deriving from growth needs and energy storage as well as metabolic maintenance. Maternal glucose crosses the placenta via facilitated diffusion (primarily by the glucose transporters GLUT1 and GLUT3) and serves as the principal energy source for the fetus. There is a linear relationship between maternal and fetal glucose concentrations, with fetal concentrations 60% to 80% of maternal concentrations. This linear relationship is present even during episodes of maternal hyperglycemia secondary to maternal diabetes or glucose infusions. Therefore in high-risk situations associated with maternal hyperglycemia, the neonate is born with approximately 70% of the maternal concentration at delivery.
Under normal circumstances, fetal gluconeogenesis is negligible; however, fetal gluconeogenesis may occur during episodes of prolonged maternal hypoglycemia or starvation. Glucose alone cannot account for the total oxygen consumption of the fetus. Other substrates such as lactate, free fatty acids (FFA), ketones, and amino acids cross the placenta and are potential energy sources for the fetus.
Energy is stored rapidly near term. Fat storage exceeds 100 kcal/day in the ninth month and accounts for 14% of total body weight at term. Glycogen stores, a vital source of energy in the first hours of life, increase toward term to reach about 5% by weight in liver and muscle and up to 4% in heart muscle. These energy stores are compromised by prematurity and by intrauterine growth restriction. Acute perinatal distress or chronic fetal hypoxia can particularly diminish glycogen stores and predispose the infant to hypoglycemia after birth.
Insulin and glucagon do not cross the placenta and are present in the fetus by 12 and 15 weeks, respectively. The fetal insulin response to glucose infusion is poor very early in gestation. At the end of gestation, the insulin response is improved but remains blunted. Fetal blood insulin levels gradually rise toward term, whereas fetal glucagon levels remain low. The resulting high insulin-to-glucagon ratio promotes the accumulation of hepatic glycogen stores and suppresses gluconeogenesis, thus preparing the fetus for birth and maintenance of postnatal glucose homeostasis.
Insulin is an important hormone for fetal growth. The presence of maternal hyperglycemia and fetal hyperinsulinemia, as seen in the infant of a diabetic mother, is associated with macrosomia with elevated liver glycogen and total body fat stores. Macrosomia in the presence of fetal hyperinsulinemia without maternal hyperglycemia is seen in infants with Beckwith-Wiedemann syndrome and in the rare infant with hyperinsulinemic hypoglycemia, which suggests that fetal insulin and not maternal hyperglycemia may be the important growth-promoting factor. Conversely, infants born with pancreatic aplasia and those with transient neonatal diabetes mellitus have little or no insulin present and demonstrate severe intrauterine growth restriction.
At birth, cold stress, work of respiration, and muscle activity cause increased energy demands. Because of the interrupted supply of maternal glucose, the newborn must call on stored fuels to maintain blood glucose levels. This transition at birth is facilitated by increased catecholamine and reversal of the insulin:glucagon ratio with increased glucagon, which promotes lipolysis and glycogenolysis. Decreased insulin levels and increased cortisol levels also facilitate glucose homeostasis at birth. Rapid glycogenolysis causes hepatic glycogen to fall to low levels within 24 hours in a fasted neonate. Gluconeogenesis is important in postnatal glucose homeostasis to supplement glycogenolysis. Lipolysis begins at birth, with the respiratory quotient decreasing from 1.0 in the fetus to less than 0.8 during the first day as most tissues switch to burning fat. Metabolism of FFA and ketones stabilizes blood glucose levels by sparing glucose utilization in heart, liver, muscle, and brain (ketones); and supporting hepatic gluconeogenesis by producing the reduced form of nicotinamide adenine dinucleotide.
The Pediatric Endocrine Society (PES) analyzed available data on this unique period that the first 48 hours is in all mammals, not just human babies. This brief period of hypoglycemia is commonly observed in normal newborns during the transition from fetal to extrauterine life.
Using neuroendocrine and metabolic data, they determined that this 48-hour period was characterized by a relative hyperinsulinism, low ketone levels, inappropriate preservation of glycogen, and mean glucose level at the nadir of 55 to 65 mg/dL. This resembles a known form of congenital hyperinsulinism, causing a lowering of the plasma glucose threshold for suppression of insulin secretion. This 55 to 65 mg/dL range, which is the mean range at the nadir, turns out to be the same level below which adults and older children demonstrate neurogenic symptoms; therefore this observation, along with the rest of the metabolic profile, led the PES to suggest this was the critical range of glucose to maintain. They further argued that this range is where the adult and older children activate mechanisms as seen in the neuroendocrine and metabolic profiles for brain protection. The PES also reported on the fact that by 72 hours or so of life, the glucose levels rise to those similar to levels in older children and adults. To summarize then, the PES using these endocrine-based mechanisms for determining critical levels of glucose found hyperinsulinemia accompanied by suppressed levels of ketones and inappropriately large glycemic responses to glucagon and epinephrine, suggesting the absence of alternative fuels, and the inappropriate preservation of glycogen in a newborn with low glucose levels, all consistent with a hypoketotic hyperinsulinemia.
The American Academy of Pediatrics (AAP) looked at the postnatal glucose homeostasis data and noted at birth that although the infant’s blood glucose concentration is about 70% of the maternal level, it falls rapidly to a low nadir by 1 hour, as low at 20 to 25 mg/dL. This nadir is prevalent in healthy neonates and is seen in all mammalian newborns. These levels are transient and begin to rise over the first hours and days of life. This observation is considered to be part of the normal adaptation for postnatal life that helps establish postnatal glucose homeostasis. Are there advantages to having a lower blood glucose concentration compared with adults the first 48 hours? A decrease in glucose concentration soon after birth might stimulate physiologic processes that are required for survival, including promoting glucose production through gluconeogenesis and glycogenolysis. Also, the decrease in glucose concentration enhances oxidative fat metabolism, stimulates appetite, and may help adapt to fast feed cycles.
The AAP guideline, during the first hours of transition, uses the lower ranges of glucose values, not the mean from fetal and neonatal data. It also emphasizes the clinical examination and condition of the infant. The AAP also looked at neurodevelopmental data to determine if there was any validated level of neuroglycopenia (the critical threshold of plasma glucose at which brain injury occurs).
The fundamental question of how best to manage asymptomatic newborns with low glucose concentrations remains unanswered. Balancing risks of overtreating newborns with low glucose concentrations who are undergoing normal transition following birth against the risks of undertreating those in whom low glucose concentrations are pathologic, dangerous, and/or a harbinger of serious metabolic disease remains a challenge.
In the newborn, basal glucose production and utilization is 4 to 6 mg/kg/min. This high glucose utilization compared with the adult is primarily because of the higher ratio of brain weight to body weight in the newborn infant. During euglycemic conditions, most of the brain’s metabolic needs are met by oxidation of glucose. When the availability of glucose is limited, alternative cerebral fuels such as lactate and ketone bodies may be used. Although these alternative fuels provide some protection to reduce the risk of hypoglycemia-induced brain injury in the newborn, the brain requires a continuous glucose supply; thus these alternative substrates are unable to completely replace glucose as a fuel for brain metabolism.
Blood glucose level at birth is 60% to 80% of the simultaneous maternal plasma concentration. Glucose concentrations normally decrease over 1 to 2 hours and stabilize at a nadir. Over the next 48 hours, the glucose levels increase, and by 72 hours or so, they more resemble adult levels than those levels associated with the first 48 hours of life.
The neurodevelopmental outcome approach is to find the critical threshold of plasma glucose associated with brain injury or where “neuroglycopenia” occurs in the newborn. In the adult, this is 50 mg/dL. Neuroglycopenia is the level at which there is an inadequate supply of glucose for the brain. This level is known for the newborn. This research was profoundly influenced by a multicenter nutrition study from the United Kingdom published in 1988. The authors of the study evaluated blood glucose levels, drawn daily initially and then weekly, until discharge on 661 infants less than 1850 g at birth who were enrolled in a nutrition study looking at early diets and cognitive outcomes. They found that a critical glucose level less than 47 mg/dL would reliably predict adverse outcome. The number of days below this value was strongly related to reduced scores for mental and motor development at 18 months’ corrected age. Similar but less dramatic differences were found when the children were seen again as part of a larger study when the children were 7 to 8 years old. These findings profoundly influenced neonatal care across the developed world ever since. This value, “47” mg/dL, became the worldwide standard and was applied to term healthy appropriate for gestational age (AGA) neonates as the gold standard “critical threshold,” even though this study had no term infants in it. The authors themselves suggested in a letter that there is “difficulty providing causation when an observational approach is used” and remarked that “when such observations generate hypotheses or legitimate clinical concerns, this should stimulate future studies and randomized controlled trials.”
Almost 25 years later from the United Kingdom came a prospective trial including infants less than 32 weeks’ gestation who had blood glucose levels measured daily for the first 10 days of life. A total of 47 had a blood glucose level less than 47mg/dL on at least 3 days of the first 10 days of life. All were matched for appropriate variables with those who never had a value less than 47mg/dL. No differences were found in developmental progress or physical disability at 2 years of age. Some 81% of the cohort were matched again at 15 years of age, and they were almost identical for full-scale IQ. The inclusion of children who had a level less than 47 mg/dL for greater than 4 days and another group less than 37 mg/dL on three occasions did not alter these results. They concluded the study “found no evidence that recurrent low blood glucose levels (<47 mg/dL) in the first 10 days of life pose a hazard to preterm infants.” This study does not imply that low blood glucose levels cannot be damaging in preterm infants.
Studies from the Children with Hypoglycemia and Their Later Development (CHYLD) have added studies with serial follow-up and included use of glucose gel and subcutaneous glucose sensors. A large prospective of at risk-term and late preterm neonates defined hypoglycemia as less than 47 mg/dL. They identified 53% of 404 at-risk infants with hyperglycemia based on the four risk groups (late preterm, small-for-gestational-age [SGA] infants, large-for-gestational-age [LGA] infants, and infants of mothers with diabetes [IDM]). The fact that 53% of patients had glucose less than 47 mg/dL and 19% a level less than 36 mg/dL confirms the decision by the AAP to focus on these patients. They found no increase in risk for neurosensory impairment at 2 years of age with hypoglycemia. They also performed blinded interstitial glucose monitoring, and noted that intermittent blood sampling missed 25% of additional episodes of less than 47mg/dL. Even with aggressive treatment, including dextrose gel, nearly 25% of infants experienced 5 hours of glucose concentration less than 47 mg/dL. Risk of impairment was not increased, even in those infants with hypoglycemia that was unrecognized and therefore not treated (interstitial monitoring). Unexpected was the observation that higher glucose levels after treatment for hypoglycemia tended to be associated with neurodevelopmental impairment. Those infants who spent a larger proportion outside the central range of 54 to 72 mg/dL in the first 48 hours of life had worse outcomes.
Next, 614 term and late preterm infants at risk for hypoglycemia were studied using the same risk factors again as AAP. The study included patients without hypoglycemia and both treated and untreated hypoglycemia. Hypoglycemia again defined as plasma glucose less than 47 mg/dL. Infants were screened and treated with the aim of keeping plasma glucose concentrations greater than 47mg/dL. Surprisingly, there were long and undetected periods of hypoglycemia detected only on interstitial monitoring. Almost one out of four had hypoglycemic episodes not detected on intermittent sampling. Twenty-five percent of those undetected episodes lasted greater than 5 hours during the first week of life. Further studies are underway to determine the utility of continuous glucose monitoring.
Neurosensory impairment or processing difficulty at age 2 was reported among five subgroups, including a reference group who never had hypoglycemia, any episode of hypoglycemia greater than 3 days, or severe hypoglycemia (<36 mg/dL). There was no association between hypoglycemia and neurodevelopmental outcome at age 2 years. However, data on the 4.5-year follow-up demonstrated executive function difficulties in those infants suffering more than one episode of hypoglycemia. This was found only with continuous glucose monitoring, not with intermittent sampling.
Revisiting transitional hypoglycemia is a unique perinatal cohort reported from Arkansas, which included 1400 infants at 10 years of age who had a single glucose level in the first hours of life correlated with fourth grade examinations from across the state. Glucose levels of interest ranged from less than 30 to 45mg/dL. They found that a single episode of hypoglycemia, defined as less than 40 mg/dL that resolved by 3 hours of age, was associated with a 50% reduction in the odds of achieving proficiency in literacy and numeracy at age 10. This group of patients represented all the births during a calendar year, so they were mostly made up of late preterm and term infants. As mentioned earlier, this was a single episode, so their levels were followed by a second value above the cutoff of less than 30, less than 40, and less than 45 mg/dL, respectively. An editorial reviewing this study suggest weaknesses include little information about the management strategies for hypoglycemia and no rates for breastfeeding. Furthermore, they expressed concern whether the exposure group had a single episode of hypoglycemia because only the first two blood glucose levels were measured, and recurrent low glucose levels are common in at-risk infants, even throughout the first week.
As yet, there is no reason to assume that link between transitional neonatal hypoglycemia and subsequent poor academic performance is causal. It is possible that a brief period of hypoglycemia is a marker for other perinatal issues, perhaps including events during intrauterine development.
Should we now consider universal screening of all newborns because the Arkansas study suggests transient hypoglycemia may be associated with poorer academic achievement at 10 years of age? Screening is only justified when you can impact outcome with the result of the screening test. The brief period of hypoglycemia was diagnosed at 90 minutes of age, but the actual result was available 30 minutes after that. The second measurement showing resolution came 70 minutes after the first screen or at 3 hours of age. It is unlikely that any intervention could shorten the exposure to the brief period of “hypoglycemia.”
As mentioned in some of the CHYLD studies, dextrose gel can be a simple and inexpensive therapy administered directly to the buccal mucosa. A Cochrane review that included two trials with 312 infants concluded that treatment of infants with neonatal hypoglycemia with 40% dextrose gel reduces the incidence of mother–infant separation for treatment and increases the likelihood of full breastfeeding after discharge compared with placebo gel. No evidence suggests occurrence of adverse effects during the neonatal period or at 2 years’ corrected age. Oral dextrose gel should be considered first-line treatment for infants with neonatal hypoglycemia.
Also, the application of continuous interstitial glucose monitoring was used in many of the studies reviewed in the section on neurodevelopmental outcomes. A relatively large study included babies greater than 32 weeks who were at risk of hypoglycemia. These infants were all admitted to the neonatal intensive care unit (NICU) and received routine treatment, including intermittent blood glucose measurements and blinded continuous interstitial monitoring. Continuous monitoring was well tolerated in 102 infants. There was good agreement between blood and interstitial glucose concentrations. Low glucose concentrations (<47 mg/dL) were detected in 32 babies (32%) with intermittent blood sampling and in 45 babies (44%) with continuous monitoring. There were 265 episodes of low interstitial glucose concentrations, 215 (81%) of which were not detected with blood glucose measurement. One hundred seven episodes in 34 babies lasted greater than 30 minutes, and 78 (73%) were not detected with blood measurement.
They concluded that continuous interstitial glucose monitoring detects many more episodes of low glucose concentration than blood glucose measurement. The physiologic significance of these previously undetected episodes is unknown.
Several factors need to be considered when interpreting glucose concentrations. First, blood glucose concentrations are 10% to 15% lower than simultaneous plasma concentrations. This is particularly pronounced when the hematocrit is very high. Second, use of capillary samples from unwarmed heels may lead to an underestimation of venous glucose concentration because of stasis. Finally, glucose concentrations decline as much as 18 mg/dL/hour at room temperature while analysis is awaited. Thus all samples should be analyzed immediately or placed on ice.
Although plasma glucose determination in the laboratory using the glucose oxidase reaction is the optimum method, point-of-care (POC) testing using reflectance glucometers offers the advantage of speed and small sample volumes, and permits quick clinical responses. The common thresholds for diagnosis of hypoglycemia with the low ranges of 35 mg/dL to 45 mg/dL and hyperglycemia at 170 mg/dL are at the limits of accuracy for many POC glucose analyzers. These should be looked at as screening devices and not the ultimate test for the diagnoses of hypoglycemia. The sensitivity for detecting hypoglycemia ranges from 80% to 100%, and the negative predictive values range from 80% to 96%, depending on the device and parameters used. Therefore confirmatory plasma glucose concentrations should be measured if hypoglycemia is detected using a POC device or if the infant has symptoms consistent with hypoglycemia, even if the POC test value shows a normal blood glucose concentration.
Hypoglycemia in newborns is often asymptomatic. The most frequent symptoms are jitteriness and cyanosis. Other symptoms include convulsions, hypotonia, coma, poor feeding, apnea, congestive heart failure, high-pitched cry, abnormal eye movements, and temperature instability with hypothermia. In small, sick infants, symptoms may easily be missed. When symptoms are present, the age of onset is most commonly between 24 and 72 hours.
Because these symptoms are nonspecific, they often occur in newborns who are normoglycemic and have other problems. For example, jitteriness, the most common symptom, is found in up to 44% of normal newborns as well as in infants with a variety of other conditions ( Box 11.1 ). Hypoglycemia must, therefore always be confirmed by chemical analysis and by response to treatment.
Metabolic disorders
Hypoglycemia
Hypocalcemia
Hypomagnesemia
Hyponatremia
Hypernatremia
Neonatal drug withdrawal
Opiates
Selective serotonin reuptake inhibitors (SSRIs)
Cocaine
Central nervous system disorders
Malformations
Hypoxic-ischemic encephalopathy
Intracranial hemorrhage
Polycythemia
Sepsis, meningitis
The AAP Committee on Fetus and Newborn Medicine ratified, for another 5 years, their statement on postnatal glucose homeostasis, including an algorithm for screening and management of low glucose levels ( Fig. 11.1 ). Also, a reevaluation of transitional hypoglycemia has been published by the PES.
![Fig. 11.1, Screening for and management of postnatal glucose homeostasis in late-preterm (LPT]; 34–36 6/7 weeks) and term small-for-gestational age (SGA) infants and infants who were born to mothers with diabetes (IDM) /large-for-gestational age (LGA) infants. LPT and SGA (screen 0–24 hours), IDM and LGA greater than or equal to 34 weeks (screen 0–12 hours). IV indicates intravenous. Fig. 11.1, Screening for and management of postnatal glucose homeostasis in late-preterm (LPT]; 34–36 6/7 weeks) and term small-for-gestational age (SGA) infants and infants who were born to mothers with diabetes (IDM) /large-for-gestational age (LGA) infants. LPT and SGA (screen 0–24 hours), IDM and LGA greater than or equal to 34 weeks (screen 0–12 hours). IV indicates intravenous.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/GlucoseCalciumandMagnesium/0_3s20B9780323608541000110.jpg)
A recent editorial called “Imperfect Advise” contrasts the two organizations’ approaches as reviewed earlier in the chapter, and we will offer suggestions on how to combine the advice from both for management. Also, critical to the PES recommendations is to make the diagnoses of persistent hypoglycemic syndromes before discharge.
The AAP clinical report is not inclusive of all preterm infants but focused on late preterm as well as specifically term SGA, LGA, and IDM at-risk patients. Of course, symptomatic infants are all screened. All preterm infants were not included, based on the assumption that the vast majority of more premature infants would be cared for in intermediate care or in the NICU, where routine screening is already in place. The PES expands the list for screening to symptomatic, perinatal stress (birth asphyxia, cesarean section for fetal distress), maternal preeclampsia, meconium aspiration syndrome, premature or postmature, IDM, family history of genetic hypoglycemia, and congenital syndromes or abnormal physical features ( Box 11.2 ).
Neonates at increased risk of hypoglycemia and require glucose screening:
Symptoms of hypoglycemia
Large for gestational age (even without maternal diabetes)
Perinatal stress
birth asphyxia/ischemia; cesarean delivery for fetal distress
maternal preeclampsia/eclampsia or hypertension
intrauterine growth restriction (small for gestational age)
meconium aspiration syndrome, erythroblastosis fetalis, polycythemia, hypothermia
Premature or postmature delivery
Infant of diabetic mother
Family history of a genetic form of hypoglycemia
Congenital syndrome (e.g., Beckwith-Wiedemann), abnormal physical features (e.g., midline facial malformations, microphallus)
Neonates in whom to exclude persistent hypoglycemia before discharge:
Severe hypoglycemia (e.g., episodes of symptomatic hypoglycemia or need for intravenous dextrose to treat hypoglycemia)
Inability to consistently maintain preprandial PG concentration greater than 50 mg/dL up to 48 h of age and greater than 60 mg/dL after 48 h of age
Family history of genetic form of hypoglycemia
Congenital syndromes (e.g., Beckwith-Wiedemann), abnormal physical features (e.g., midline facial malformations, microphallus)
The PES does not offer screening times, nor does it discuss treatment or prevention. Its targets for therapy include less than 50 mg/dL in the first 48 hours, and then if intravenous fluids are required, a value greater than 60 mg/dL should be achieved. It addresses the first 48 hours of life for these recommendations, then emphasizes the need for careful attention to not missing cases of persistent hypoglycemia ( Box 11.2 ).
The AAP guidance applies to only the first 24 hours of life. Actionable ranges of 25 to 40 mg/dL for the first 4 hours of life and then 35 to 45 mg/dL from 4 hours to 24 hours of age are the operational thresholds for the AAP. Glucose levels rise after the first 48 hours of life and should be similar to those of older children by 72 to 96 hours of age. The AAP recommendation for treatment below the actionable range after feeding is based on individual risk assessment and examination of the infant. Target glucose concentrations when intravenous fluids are required should exceed 45 mg/dL.
What is clear with all recommendations is that the greater the glucose threshold set for screening and the more often these tests are done, the more often asymptomatic patients with low glucose levels will be identified. This could result in a neonatal intensive care admission, separation from mother for an asymptomatic infant, and provide a hindrance to successful breastfeeding.
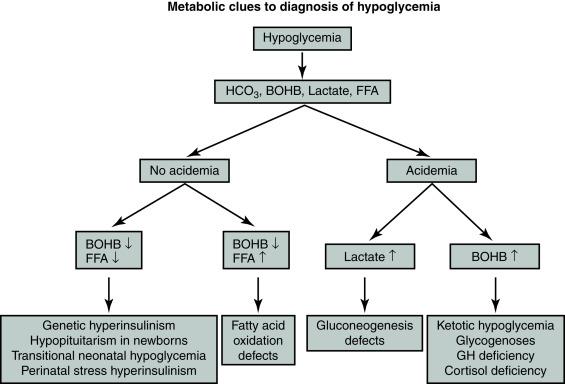
Some neonates can be identified by various clinical features as being high risk for severe hypoglycemia during the first 48 hours after delivery. Also, some of these neonates are at risk for persistent hypoglycemia beyond 48 hours of life ( Box 11.2 ). These include not only the rare infants with genetic hypoglycemic disorders, such as congenital hyperinsulinism or hypopituitarism, but also those with relatively more common prolonged neonatal hyperinsulinism (also referred to as perinatal stress hyperinsulinism) associated with birth asphyxia, intrauterine growth restriction, or toxemia. Fig. 11.2 provides an algorithm showing how the major categories of hypoglycemia may be determined from the critical sample of beta-hydroxybutyrate, FFA, and growth hormone.
The recommendations from the editorial to use the AAP and PES recommendations together include using the AAP guidelines for the first 24 hours and then for 24 to 48 hours to use greater than 45 mg/dL. To increase vigilance to diagnose possible persistent cases of hypoglycemia, consider delaying discharge from the nursery until infants who required intravenous fluids for symptomatic or asymptomatic low glucose levels or those with borderline low glucose levels demonstrate glucose levels after 48 hours greater than 70 mg/dL through several normal feed-fast cycles. More data on the frequency and success of diagnosing persistent hypoglycemia will be necessary to support this strategy.
Hyperglycemia (glucose concentration of >150 mg/dL) is a common, serious problem of very immature infants. Risk factors include low birth weight (especially when <1000 g), earlier gestational age, administration of intravenous glucose infusions (especially glucose infusion rates of >6 mg/kg/min), high illness severity, and glucocorticoid therapy ( Box 11.3 ). Factors contributing to hyperglycemia in premature infants are reduced glucose-induced insulin secretion, immature insulin processing, and increased ratio of GLUT1 to GLUT2 in tissues. Hepatic glucose release may fail to decrease when exogenous glucose is given. Stress from illness increases catecholamine release, which may further elevate glucose levels by inhibiting glucose use and insulin release.
Preterm birth
Intrauterine growth restriction (IUGR)
Increased stress hormone levels
Increased catecholamine infusions
Increased glucocorticoid concentrations (from use of antenatal steroids, postnatal glucocorticoid administration, and stress)
Increased glucagon concentrations
Early intravenous (IV) lipid infusion and high rates of infusion
Higher-than-needed rates of IV glucose infusion
Insufficient pancreatic insulin secretion (preterm and IUGR)
Absence of enteral feedings, leading to diminished “incretin” secretion and action, which limits potential to promote insulin secretion
Hyperglycemia has been associated with increased length of hospitalization, risk of death, and incidence of intraventricular hemorrhage. The mechanism of injury may involve increases in plasma osmolarity (a glucose level of 450 mg/dL is equivalent to an additional 24 mOsm/L) and glucosuria leading to renal water and electrolyte losses and vascular fluid shifts.
Treatment and prevention are accomplished by adjusting the glucose infusion rate to that tolerated by each individual infant. Rates of 4 to 8 mg/kg/min are usually tolerated; however, lower glucose infusion rates may be needed. Glucose infusion rates should be expressed as milligrams per kilogram per minute because variations in either the volume or glucose content of the fluid result in alterations in actual delivery of glucose to the infant. Along with improving nitrogen balance, early amino acid administration at the time of birth in low-birth-weight infants decreases the incidence of hyperglycemia, possibly because of improved insulin release. Occasionally, an insulin infusion starting at 0.01 U/kg/min but increasing to 0.1 U/kg/min if needed is indicated in those infants in whom decreasing the glucose infusion rate is inadequate or improved caloric intake is desired. Frequent monitoring of blood glucose concentration is crucial both to determine the adequacy of therapy and to avoid episodes of hypoglycemia.
Among very low–birth-weight infants, early neonatal hyperglycemia is common and is associated with increased risk of death and major morbidities. Sinclair et al. wished to assess effects on clinical outcomes of interventions for preventing hyperglycemia in very low–birth-weight neonates receiving full or partial parenteral nutrition. They searched for randomized or quasi-randomized controlled trials of interventions for prevention of hyperglycemia in neonates with a birth weight of less than 1500 g or a gestational age of less than 32 weeks. They found only four eligible trials. Two trials compared lower and higher rates of glucose infusion in the early postnatal period. These trials were too small to assess effects on mortality or major morbidities. Two trials, one a moderately large multicenter trial, compared insulin infusion with standard care. Insulin infusion therapy reduced hyperglycemia but increased death before 28 days and was complicated by hypoglycemia. Reduction in hyperglycemia was not accompanied by significant effects on major morbidities; effects on neurodevelopment are awaited. The authors concluded, “There is insufficient evidence from trials comparing lower with higher glucose infusion rates to inform clinical practice. Large randomized trials are needed, powered on clinical outcomes including death, major morbidities, and adverse neurodevelopment.” With regard to insulin infusion, they noted that “the evidence reviewed does not support the routine use of insulin infusions to prevent hyperglycemia in very low-birth-weight neonates. Further randomized trials of insulin infusion may be justified. They should enroll extremely low-birth-weight neonates at very high risk for hyperglycemia and neonatal death.”
Among 580 infants less than 27 weeks’ gestation in Sweden, a daily prevalence of hyperglycemia greater than 180mg/dL (10mmol/L) of up to 30% was observed during the first 2 postnatal weeks, followed by a slow decrease in its occurrence thereafter. Increasing parenteral carbohydrate supply with 1g/kg/day was associated with a 1.6% increase in glucose concentration ( P <.001).
Hyperglycemia was associated with more than double the 28-day mortality risk ( P <.01). Insulin treatment was associated with lower 28- and 70-day mortality when given to infants with hyperglycemia, irrespective of the duration of the hyperglycemic episode ( P <.05).
Neonatal diabetes is a rare disorder. Most infants with neonatal diabetes are born at or near term, with marked intrauterine growth restriction reflecting low levels of insulin and insulin-like growth factor I. Weight loss, dehydration, hyperglycemia, and occasional ketosis usually appear in the first month of life. Seventy to eighty percent of cases involve an abnormality on chromosome band 6q24 caused by uniparental disomy of chromosome 6, duplication of paternal 6q24, or loss of methylation at the differentially methylated region at the 6q24 locus. Treatment is with insulin, with a usual daily dose of 2 to 6 U.
The transient form of the disease usually resolves within a few months. However, many infants subsequently develop diabetes later in childhood or early adulthood. Approximately 50% of patients have permanent neonatal diabetes, and remission never occurs. Permanent neonatal diabetes is associated with activating mutations to the K ATP channel in the pancreatic beta cell, causing the channel to remain open and preventing insulin secretion. Some of these patients are responsive to sulfonylurea, which binds to the sulfonylurea receptor and helps close the K ATP channel. , ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here