Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Know about the major causes of child mortality globally
Know about the most common illnesses in low resource countries: pneumonia, gastroenteritis, malaria, HIV infection, tuberculosis, measles
Know about intervention programmes to improve health: immunization, paediatric life support courses, integrated management of childhood illness (IMCI)
Know about the identification, pathophysiology, clinical features and management of malnutrition
Know about the major causes of neonatal morbidity and death
Be aware of neglected issues in global child health: adolescent health, mental health, neglected tropical diseases
Be aware of vulnerable children: child labour, street children, armed conflict
A baby girl, Aminatta, is born to subsistence farmers in rural Senegal. What is Aminatta's chance of surviving to her 5th birthday?
Senegal's under-5 mortality rate in 2013 was 55 per 1000 live births, compared to UK figures of 5 per 1000 live births and Sierra Leone's 161 per 1000 live births. Millennium Development Goal (MDG) 4 aimed to reduce the under-5 mortality rate by two thirds between 1990 and 2015. In 2013 there were 6.3 million child deaths worldwide, compared to 12 million in 1990. The global under-5 mortality rate (defined as deaths in children under the age of 5 years per 1000 live births) has fallen from 90/1000 in 1990 to 48/1000 in 2012. In sub-Saharan Africa, 1 in 11 children die before their 5th birthday.
Large population size combined with high mortality rates mean almost half (49%) of child deaths occur in just five countries: India, Nigeria, Democratic Republic of Congo, Pakistan, and China.
Data capture and ascertainment of causes of death are fraught with difficulty in countries without proper systems in place for birth and death registration. Of the 12 million deaths in 1990 only 2.7% were medically certified. Many countries use techniques such as verbal autopsy, which involves questioning family members regarding the child's symptoms immediately prior to death in order to retrospectively assign a diagnosis. Data suggest that the major causes of under-5 mortality are pneumonia (13%), preterm birth complications (15%), complications during birth (11%), diarrhoea (9%) and malaria (7%) ( Fig. 33.1 ). Malnutrition is a major underlying cause of death and undernutrition is thought to contribute to 45% of under-5 deaths.
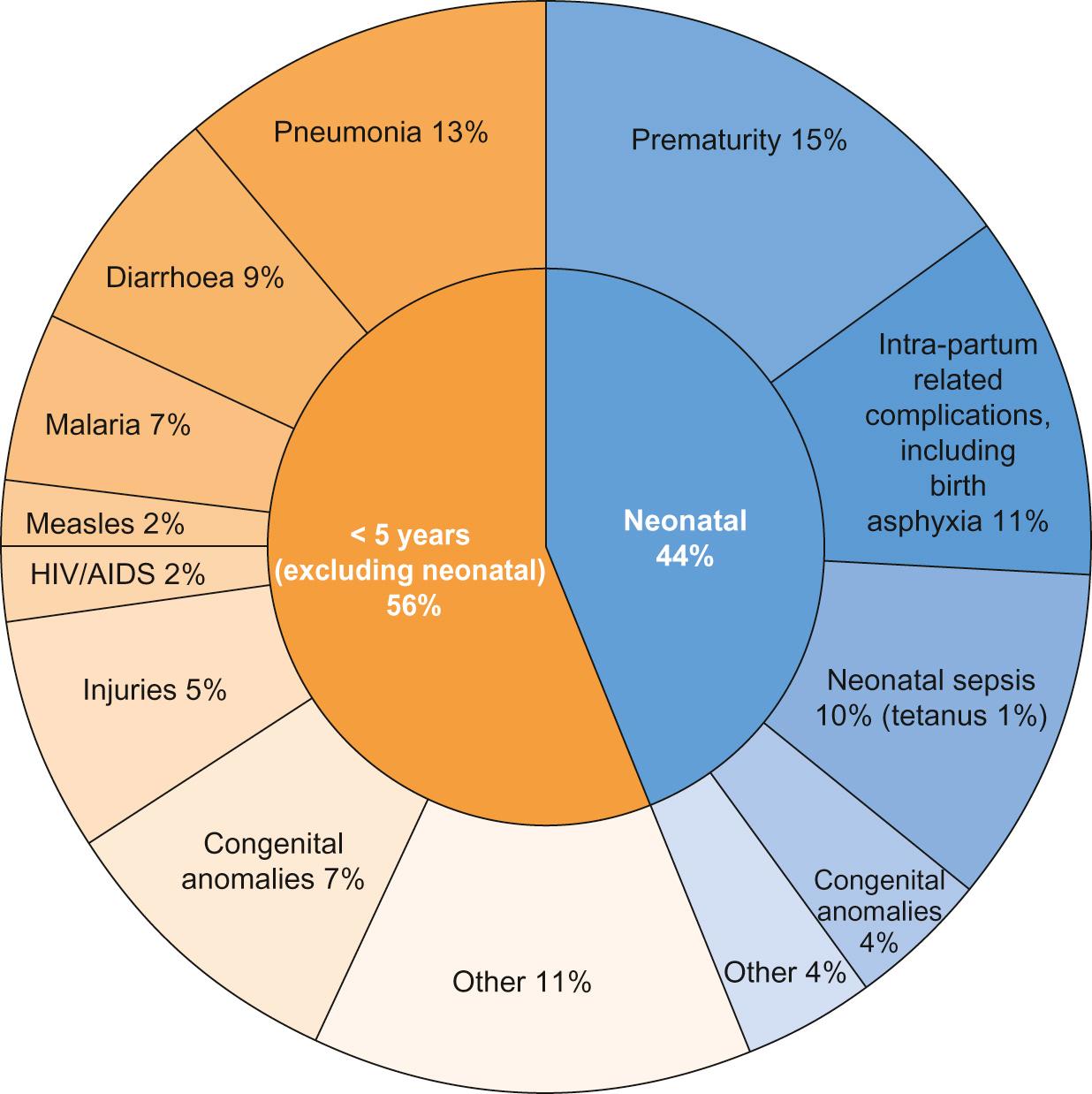
There are important inter-country and inter-regional differences that need to be considered before planning specific public health interventions. For example, malaria is a more significant threat in Africa, where it is responsible for 15% of deaths in children under 5, compared with south-east Asia, where it contributes just 1% to under-5 mortality. Though neonatal causes still account for a substantial proportion of under-5 deaths in most low-resource settings, their contribution varies, e.g. 52% of under-5 deaths in south-east Asia, compared with 30% in Africa. In the post-neonatal period in South and Central America, it is injuries that are the single largest threat to under-5 survival, causing 16% of mortality.
There are marked inter- and intra-country inequalities in child health. In 2012 the under-5 mortality rate was 147/1000 live births in Somalia, 56/1000 in India and 5/1000 in the United Kingdom. Marked inequalities in health also occur within countries. The poorer the child, the more likely they are to be exposed to risk factors for ill health. Unclean water and poor sanitation lead to diarrhoeal disease, while inadequate housing, air pollution and overcrowding promote the spread of respiratory pathogens. Poorer children are more likely to experience worse clinical outcomes for most illnesses compared with children from more wealthy backgrounds. This is partly because children living in poverty are more likely to be underweight and micronutrient deficient, have less resistance to disease, are less likely to be able to reach a health facility and less likely to receive adequate care. As well as poverty, mortality is higher in rural rather than urban areas and to a mother with little or no education. In addition, preventive public health measures such as vaccination, vitamin A supplementation and insecticide-treated net distribution tend to have the worst coverage amongst the poorest populations with the greatest need.
Child health can be measured using a variety of numerical indicators. The most common, showing the rates for sub-Saharan Africa, are displayed in Table 33.1 .
| Indicator | Definition | UK rate | Sub-Saharan Africa rate |
|---|---|---|---|
| Under-5 mortality rate | Under 5 years of age: deaths per 1000 live births | 5 | 98 |
| Infant mortality rate | Under 1 year of age: deaths per 1000 live births | 4 | 64 |
| Neonatal mortality rate | Deaths before 28 days of age per 1000 live births | 3 | 32 |
| Perinatal mortality rate | Stillbirths and early neonatal deaths (deaths before 1 week of age) per 1000 live and stillbirths | 7.6 | Unknown |
| Stillbirth rate | Stillbirths (WHO definition – infants born with no signs of life ≥ 28 weeks' gestation; in UK ≥ 24 weeks' gestation) per 1000 live and stillbirths | 4.9 | Unknown |
Most child deaths are preventable through the scaling up of evidence-based child health interventions, adapted according to country-specific local disease epidemiology, with an emphasis on targeting the most vulnerable children. Educating girls is likely to have a positive impact on reducing child mortality and studies have demonstrated a strong correlation between maternal education and child mortality. However, it has been argued that maternal education may be a proxy measure of socio-economic status; once paternal education and access to piped water and sanitation is taken into consideration, the impact of maternal education has been shown to be less marked, although still significant.
As well as rising wealth in many countries, the reduction in child deaths may be attributed to the implementation of child survival initiatives. Despite impressive reductions in child mortality in some countries, such as Rwanda and Bangladesh, the slowest improvements have been in West and Central Africa and it is unlikely that the MDG4 target will be met globally. The greatest gains in reducing post-neonatal under-5 mortality have occurred with the scaling up and improved coverage of preventative interventions, such as vaccinations and insecticide-treated nets for prevention of malaria. In stark contrast to the progress achieved in reducing post-neonatal under-5 mortality, efforts to reduce neonatal mortality have been hampered by a lack of a continuum between maternal and child care, which is discussed further below.
Individual governments fund and facilitate important global health programmes, often through government organizations, e.g. USAID (United States Agency for International Development) and DFID (UK Department for International Development). However, it is intergovernmental organizations such as the United Nations or the International Labour Organization that often exert the greatest influence through cross-country collaborations and large, well-funded global partnerships. It is under the auspices of the United Nations that the World Health Organization, the World Bank and UNICEF are able to function and to deliver some of the most influential child health initiatives. The field-level implementation of such child health improvement initiatives is often achieved in collaboration with non-governmental organizations (NGOs), who rely on public and donor funding, e.g. Oxfam, and the Malaria Consortium. NGOs may operate internationally (e.g. Médecins Sans Frontières), nationally or regionally. Effective co-ordination between NGOs as well as government programmes are critical factors in determining the success of child health improvement initiatives.
Governmental, intergovernmental and NGO partnerships only form part of the global picture on efforts to improve child health. Public–private partnerships also play an important role in financing and rolling out global health initiatives; GAVI (Global Alliance for Vaccines and Immunization) is a good example of this. Private foundations, such as the Bill and Melinda Gates Foundation, also provide significant investment and funding into global health programmes and research.
Tomoka is a 2-year-old girl living in rural Malawi who has a short history of fever, cough, coryza, rash and diarrhoea. She is taken to a primary healthcare clinic by her aunt. Her mother died recently after an illness characterized by chronic cough and wasting. Tomoka's health card has been lost, which contained details of her past medical history, vaccination status and growth charts.
What are the major threats to her survival? Which intervention programmes would be of greatest benefit for Tomoka's health? These are listed below.
Tomoka's clinical presentation encompasses diarrhoeal disease and possibly pneumonia. They account for the majority of child deaths outside the neonatal period and the global burden is highest in Africa and south-east Asia. Pneumonia and diarrhoea are often considered together as they share common risk factors and programmatic solutions, including tackling poverty, undernutrition, poor hygiene, suboptimal breastfeeding, zinc deficiency as well as improving access to vitamin A and vaccination.
The World Health Organization (WHO) has streamlined its case definitions to stratify the clinical severity of pneumonia and diarrhoeal disease. Pneumonia is now simply classified as ‘pneumonia’ and ‘severe pneumonia’ and diarrhoea is divided into syndromes (acute, persistent, bloody, etc.) with assessment of level of dehydration (none, some and severe). These classifications rely on objective clinical symptoms and signs that can be easily elicited by community health workers and staff working in primary care, facilitating the early referral of appropriate cases to secondary care. Oral rehydration solution (ORS) remains the cornerstone of management of diarrhea, coupled with continued feeding. Effective management of pneumonia relies largely on access to antibiotics. The treatment of hypoxaemia with oxygen has also been shown to reduce pneumonia deaths.
Approximately one third of severe episodes of diarrhoea and pneumonia are preventable by vaccination. Despite increased vaccination coverage, the majority of pneumonia deaths are attributable to vaccine-preventable organisms such as Streptococcus pneumoniae and Haemophilus influenzae type b (Hib). Roll-out of pneumococcal conjugate vaccine (PCV) lags behind Hib vaccine, despite the benefits of PCV even extending to children with viral pneumonia. Rotavirus vaccine continues to be introduced into the immunization schedules of many countries and effective coverage reduces mortality attributable to diarrhoea.
Tomoka's clinical presentation does not immediately suggest malaria but it is important to assess all febrile children who live in endemic areas for malaria. Most deaths from malaria occur in children under 5 and pregnant women. There has been some progress in reducing morbidity and mortality attributable to severe malaria, which is usually caused by Plasmodium falciparum . Plasmodium vivax and ovale cause less severe disease but have a liver hypnozoite stage that requires specific treatment to effect eradication. While Plasmodium malariae is seldom associated with severe disease, it can be associated with nephrotic syndrome. The WHO 2013 malaria report stated there were 627,000 deaths from malaria in 2010, 77% of which were in children under 5. The Roll Back Malaria Partnership has been coordinating international efforts since 1998 to scale up preventative diagnostic and therapeutic interventions with the overarching vision of freeing the world of the burden of malaria. Central to prevention of malaria is the use of long-lasting insecticide-treated bed nets (ITNs), which reduce the vector population of Anopheles mosquitoes. Currently, approximately 40% of children in sub-Saharan Africa sleep under an ITN. Households can be further protected from mosquitoes by indoor residual spraying (IRS) with insecticides. Various candidate malaria vaccines are in development, which, even if partially efficacious, could substantially reduce mortality and morbidity.
The clinical presentation of malaria involves non-specific symptoms such as fever and headache. Clinical assessment alone carries the risks of both over-treatment and under-treatment. It is therefore essential to confirm the diagnosis before initiating treatment. The diagnosis of malaria in low-resource settings has been facilitated by the use of rapid diagnostic tests (RDTs), which are cost effective, require minimal training and can be used by community health workers. The management of acute severe malaria depends not only on prompt access to appropriate antimalarial drugs, but also effective treatment of hypoglycaemia, anaemia and convulsions.
The superiority of artemisinin-based antimalarial treatment over quinine was demonstrated in two large multicentre trials involving more than 6500 children: the SEAQUAMAT study in south-east Asia and the AQUAMAT study in sub-Saharan Africa. The latter trial, a multicentre randomized controlled trial of children with severe malaria, showed significantly reduced mortality (relative risk reduction 22.5%, 95% Confidence Interval (CI) 8.1–36.9; odds ratio for death 0.75, 95% CI 0.63–0.9) and reduced coma and seizures with artemisinin compared with quinine. Since the publication of these landmark studies, the WHO recommends artemisinin combination treatment (ACT) as first line in severe malaria. Artemisinin is combined with partner drugs to delay the development of drug resistance. Nonetheless, artemisinin resistance has emerged in south-east Asia.
Which of the following has been shown to reduce the risk of mother-to-child HIV transmission in low-resource settings? The answer to each may be true (T) or false (F).
Breast and artificial feeding rather than exclusive breastfeeding
Co-existent sexually transmitted diseases in the mother
Delivery by caesarean section
Exclusive formula feeding
Giving all women antiretroviral therapy irrespective of CD4 T-cell count or clinical staging
A. False; B. False; C. True; D. True; E. True.
Exclusive formula feeding reduces the risk of HIV infection, although risk of death from gastroenteritis and other causes may be increased. Delivery by caesarean section reduces the risk but may not be indicated if the mother's viral load is suppressed. Giving all women antiretroviral therapy irrespective of CD4 T-cell count or clinical staging is now WHO recommended policy.
Tomoka's mother died recently after a wasting illness. In a setting with high rates of HIV transmission, suspicion should be raised that Tomoka may have been vertically infected with HIV. Approximately 2 million children globally are currently living with HIV, most of whom acquired the infection perinatally. The rate of new paediatric infections has significantly declined, primarily due to the success of prevention of mother-to-child transmission (PMTCT) interventions. Maternal-to-child transmission of HIV without any of the preventative interventions listed in Box 33.1 can be between 30% and 40%; with intervention, this can be reduced to <1%.
Primary HIV prevention in women of child-bearing age through education, self-determination and condom use.
Universal lifelong antiretroviral therapy for all pregnant women, aiming to suppress viral load before delivery (WHO plan B+).
Treatment of coexistent sexually transmitted infections in the mother.
Caesarian section, although vaginal delivery can be considered if viral load suppressed and mother well. Avoid prolonged rupture of membranes.
A minimum of 6 weeks of postnatal antiretroviral prophylaxis for the newborn.
Exclusive breastfeeding for the first 6 months of life in low-resource settings, where the use of artificial (formula) feeding is associated with greater risks of mortality from gastroenteritis and pneumonia. Thereafter, introduction of complementary foods at 6 months of age with concomitant continuation of breastfeeding until 12 months.
Breast milk pasteurization or ‘flash heating’ has been associated with reduced transmission.
Mixed breast and artificial feeding is the least desirable option, due to the increased mortality associated primarily with gastroenteritis and pneumonia, as well as increased HIV transmission (artificial feeding is associated with increased mucosal inflammation, which can act as a ‘portal of entry’ for the HIV virion in infants co-fed with breast milk).
Support for mothers who choose exclusive artificial feeding, providing this is acceptable, feasible, affordable, sustainable and safe (AFASS criteria)
Tomoka's HIV status should be ascertained as soon as possible. As Tomoka is 2 years old, a positive antibody test would indicate she is HIV-infected. HIV antibody is placentally transferred, and therefore a positive antibody test before the age of 18 months may indicate maternal HIV infection only. HIV proviral polymerase chain reaction (PCR) is the diagnostic test of choice in infants. At birth, HIV PCR has a low sensitivity (due to low viral load) and does not rule out infection. By 3 months, sensitivity is almost 100%. HIV infection is very unlikely if the child has two negative PCRs (one after 3 months) or two negative antibody tests if <12 months or one negative antibody test after 18 months.
Tomoka needs to be assessed for the clinical features and complications of HIV infection. HIV infects CD4+ T cells, macrophages and neuronal cells, amongst others. Primary HIV infection is a mostly mild, mononucleosis-like illness, which resolves spontaneously, with the virus entering a phase of clinical latency. As infection progresses, the CD4+ cell count drops, determining the further clinical course of manifestation of opportunistic infections and malignancies. HIV can cause a chronic encephalopathy with developmental delay and faltering growth. The advance to acquired immune deficiency syndrome (AIDS)-defining diseases in children is highly variable; however, untreated infants and children have a high risk of progression to severe infections and death. In contrast, some vertically infected children may be asymptomatic into their teenage years. The CD4+ cell count and the HIV viral load are therefore the most important laboratory parameters to monitor in HIV-infected children.
A randomized controlled trial (CHER trial) involved 377 HIV-positive infants assigned to receive early antiretroviral therapy (ART) at diagnosis in early infancy, or delayed ART until such time that immunological (CD4+ count) and clinical (WHO Clinical Stage) criteria were met. The study demonstrated a 75% reduction in mortality and disease progression with early antiretroviral therapy (ART) at diagnosis compared with delayed treatment. WHO guidelines now recommend the commencement of lifelong ART for all HIV-infected children and adolescents irrespective of CD4 count. Some countries have not yet incorporated this guidance owing to the cost. However, even prior to this revised WHO guidance, a significant number of HIV-infected children in low-resource settings found themselves within the ‘treatment gap’: in 2011, only 23% of eligible HIV-infected children were receiving ART compared with 51% of adults.
The integration of HIV into the host genome (CD4+ lymphocytes) and subsequent virion replication is reliant on the error-prone reverse transcriptase enzyme. This process provides many opportunities for the virus to develop resistant mutants to ART. To minimize this, highly active antiretroviral therapy (HAART), a combination of a minimum of 3 drugs, is used. First-line antiretroviral therapy involves 2 nucleoside reverse transcriptase inhibitors (usually abacavir and lamivudine) and either a non-nucleoside reverse transcriptase inhibitor (usually efavirenz in children over 3 years of age) or a protease inhibitor (usually lopinavir/ritonavir). Recent data from the ARROW study has shown it is possible to keep children well and virally suppressed in low-resource settings without regular laboratory testing for efficacy (CD4+ cell counts) and toxicity (haematology and biochemistry). The results of this study suggest that ART roll-out and adherence support services should take priority in low-resource settings where there is no comprehensive HIV care programme.
Tomoka's mother had a chronic cough, which may indicate that Tomoka has been infected with tuberculosis (TB). Initial pulmonary tuberculosis is characterized by the primary complex (Ghon focus with local adenitis), which usually resolves with calcification or scarring. Children under 5 years are at high risk of developing primary progressive disease, which can involve dissemination to any organ in the body.
The number of new case notifications of TB in children is rising, despite the overall global fall in TB incidence and prevalence since 2000. In 2012, there were an estimated 530,000 new cases of childhood TB and 74,000 deaths. This increase may represent improved surveillance methods in children rather than a true increase in disease incidence. Despite this, the overall case burden of childhood TB is still likely to be underestimated; case detection of TB in children is difficult due to its non-specific clinical presentation, paucibacillary nature and absence of available diagnostic tests which are both sensitive and specific.
The diagnosis is usually made from a composite of clinical features, radiology, tuberculin skin testing (TST), microscopy and culture of respiratory specimens along with newer PCR methods such as the Xpert MTB/RIF assay, an automated assay to identify Mycobacterium tuberculosis (MTB) DNA and resistance to rifampicin (RIF). The TST relies on the development of a delayed-type hypersensitivity reaction to tuberculin purified protein derivative, which is a combination of proteins from M. tuberculosis , some of which are also present in BCG vaccine. Therefore, children who have received BCG may have a false positive TST, and a different cut-off is used in the UK to allow for this. Interferon-gamma release assays (IGRAs) were developed to circumvent this problem and measure T-cell interferon-gamma production to proteins that are unique to M. tuberculosis and not present in BCG (ESAT-6, CFP-10 and TB7.7). Sensitivity and specificity is less than in adults. Neither the TST nor IGRAs can be reliably used to distinguish between latent and active disease, as they indicate infection by the organism. However, one or both can be used during contact tracing to identify which individuals have been infected. The microbiological confirmation of TB requires culture or PCR. Liquid culture is the most sensitive method of bacteriological confirmation. Almost half of low- and middle-income countries have adopted the WHO guidance recommending the use of Xpert MTB/RIF assay, which is more sensitive than microscopy and can be performed within 100 minutes.
The management of childhood TB is hampered by the lack of paediatric drug preparations, in particular fixed dose combinations. This is especially true for multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB), where there is an urgent need for child-friendly preparations. There are few signs that this will be met in the near future.
Treatment of latent TB is effective at preventing development to active TB disease. Primary chemoprophylaxis is the practice of giving preventative therapy, usually isoniazid, to immunocompetent children who have been exposed to an individual with sputum smear-positive TB for more than 8 hours to prevent development of progressive TB disease, even if there is no initial evidence of TB infection. HIV-infected children are at significantly increased risk of developing TB, although ART markedly reduces this risk. Some authorities recommend offering TB chemoprophylaxis to all children with HIV infection when initially diagnosed, and after each TB exposure. Aside from chemoprophylaxis, the prevention of childhood TB relies mainly on effective case detection and treatment of TB in the adult population.
By coordinating global organizations, the STOP-TB Partnership aims to eliminate TB as a public health problem by 2050. In 2013, the WHO published the first targeted roadmap outlining the steps to ‘end childhood TB deaths’, emphasizing the need for increased investment in the development of effective, novel, child-friendly diagnostics and therapeutics.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here