Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular disease is a major cause of dementia in the elderly.
White matter is vulnerable to ischemic damage with oligodendrocytes targeted by hypoxia.
The neurovascular unit in the white matter includes the oligodendrocytes that myelinate axons.
Microglia impact the health of the oligodendrocytes and influence the endothelial cells.
Understanding the complex impact of cytokines, proteases, and growth factors on the oligodendrocytes could lead to treatments to protect the white matter.
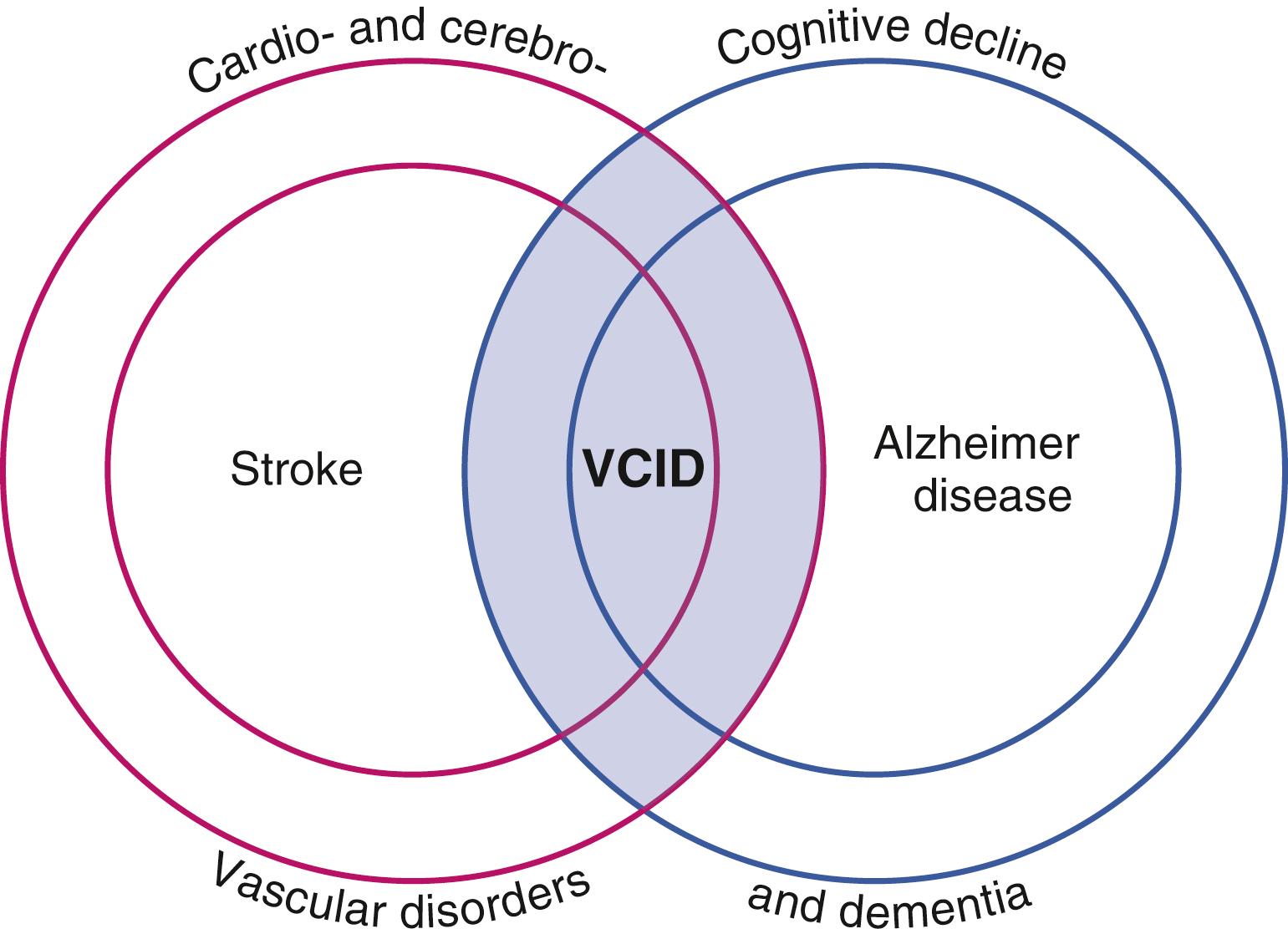
Cerebrovascular factors are significant contributors to cognitive decline during aging. Vascular cognitive impairment and dementia (VCID) is defined as cognitive decline related to impairment of blood supply to the brain ( Fig. 13.1 ). VCID comprises a broad spectrum of disease, including strokes in strategic brain areas, multi-infarct dementia, and subcortical ischemic vascular disease (SIVD). , Vascular dysregulation is also an important component of Alzheimer disease pathophysiology. ,
Although neuronal perturbations in gray matter should ultimately underlie the development of cognitive decline, it is increasingly recognized that white matter mechanisms play a significant role within the broad spectrum of VCID. In SIVD, one of the most common subtypes of VCID, , there is a progressive arteriolosclerosis and fibrohyalinosis in small blood vessels supplying deep white matter, thus resulting in chronic cerebral hypoperfusion that leads to accumulating damage within subcortical and periventricular areas. When damage to the white matter is extensive, the term Binswanger disease is used to define this category of vascular demyelination. ,
White matter vulnerability is based on distribution of the major arteries supplying the deep white matter. End arteries from the base of the brain and the surface supply the periventricular region, forming a watershed. Other vulnerable regions with limited blood flow are gray-white junction beneath the cortex and centrum semiovale. When the cerebral blood flow or oxygen levels are compromised, these regions are impacted. Hypertension, diabetes, and hyperlipidemia predispose the small blood vessels to narrowing of the lumen with thickening of the outer layers by fibrosis. Sleep apnea leads to intermittent hypoxia. Damage to the white matter is visualized as attenuation on computed tomography and hyperintense regions on magnetic resonance imaging (MRI) on the fluid-attenuated inversion recovery (FLAIR) sequences. Because FLAIR white matter hyperintensities can be seen in normal elderly, the diffusion tensor image (DTI) is better for showing damaged fiber tracts. Patients with microstructural injury on DTI have a clinical syndrome involving impaired executive function, increased reflexes, imbalance, and urinary problems. They may have small or lacunar strokes in the white matter and psychiatric symptoms including apathy and depression. These patients differ from patients with Alzheimer disease who have mainly memory and language deficits. Dual pathology of Alzheimer disease and vascular disease accelerates the cognitive decline, suggesting a synergistic effect of inflammation caused by both conditions in many patients with mixed dementia. , The reader is referred to the later sections of this book for detailed clinical and neuroimaging discussions of vascular dementia ( Chapters 18 and 41 ).
In this chapter, we will focus on mechanisms of white matter injury in VCID, and the neurovascular unit will be used as a conceptual framework for dissecting gliovascular signaling in normal and diseased conditions. We begin by surveying the wide range of animal models available for investigating VCID. Then we discuss the molecular and cellular mechanisms underlying the coordinated responses in blood-brain barrier (BBB), extracellular matrix, and crosstalk between oligodendrocyte lineage cells and other components in the neurovascular unit. Finally, we introduce the emerging field of gliome and vasculome mapping as a systematic approach for discovering novel mechanisms and targets for VCID pathophysiology.
Investigating the molecular and cellular mechanisms of white matter injury in VCID is challenging because of its complex clinical substrates. In this regard, the conceptual framework of the neurovascular unit may offer some utility. The concept of the neurovascular unit was first proposed at an NIH workshop in 2003. , Its fundamental premise is that the pathophysiology of stroke, brain injury, and neurodegeneration is mediated by interactions between all cell types in the central nervous system (CNS) (see Chapter 7 ). Under normal conditions, cells from neuronal, glial, and vascular compartments all work together to support brain function. During CNS development, coordinated interactions between vasculature and neural precursors mediate neurogenesis and angiogenesis, and matrix signals from radial glia guide the migration of immature neurons to allow the layered construction of the cortex. In adult brain, signaling from astrocytes and pericytes are essential for maintenance of BBB function. For neural connectivity, astrocytes are required for proper regulation of release-reuptake kinetics of neurotransmitters, whereas microglia are required for dynamic pruning of synapses. Taken together, trophic and matrix signaling between all CNS cells underlie homeostasis within the neurovascular unit. ,
However, under diseased conditions of VCID, coupling between glial, vascular, and neuronal cells is disrupted, and loss of gliovascular signaling contributes to neuronal dysfunction. For example, compromised interaction between endothelium, astrocytes, and pericytes may perturb BBB integrity in white matter, thus amplifying inflammation. In addition, oligodendrocytes are vulnerable to excitotoxicity and oxidative stress that are triggered by cerebral hypoperfusion. Since a single oligodendrocyte myelinates multiple axons, damage to only one oligodendrocyte could cause severe dysfunction in white matter. On the other hand, as a compensatory response, residual oligodendrocyte precursor cells (OPCs) may proliferate and differentiate into mature oligodendrocytes to remyelinate injured axons during the chronic phase of disease. Analyses of postmortem brain tissue from patients with VCID also showed that there was a significant increase of various progenitor cells in the subventricular zone and peri-infarct regions, including cells that were positive for doublecortin (DCX), nestin, and polysialylated neural cell adhesion molecule (PSA-NCAM). In the white matter of VCID patients, OPCs were also increased while mature oligodendrocytes were decreased, indirectly suggesting the presence of oligodendrogenesis as an endogenous response. In this regard, coordinated signaling between multiple cell types in white matter should be required for oligodendrocyte repair and remodeling. In the context of aging white matter, compensatory adaptations may be compromised since many growth factors may be downregulated and cerebral perfusion may be reduced. Ultimately, perturbations in gliovascular signaling may significantly contribute to the increasing risks of VCID with age. Therefore, a deeper understanding of gliovascular mechanisms may allow one to better dissect the pathophysiology of white matter damage in VCID.
For the purpose of probing white matter injury in VCID, integrated approaches utilizing both in vitro and in vivo assessments should be helpful. Subjecting co-cultures of oligodendrocytes and OPCs to different metabolic insults and oxidative and inflammatory stressors may be used to mimic various aspects of white matter damage in VCID ( Box 13.1 ). Using these in vitro systems, a wide spectrum of intra- and intercellular signals has been identified as candidate pathways that may underlie oligodendrocyte damage and regeneration ( Box 13.2 ). Furthermore, several soluble factors (e.g., growth factors, hormones, cytokines) have been identified to mediate the trophic support from astrocyte/endothelium/microglia to oligodendrocyte lineage cells ( Box 13.3 ). The reader is also referred to review articles that summarized mechanisms of oligodendrocyte damage and repair after injury.
| In vitro ischemia model | Oxygen glucose deprivation |
| Metabolic stress | Hypoxia, growth factor deprivation |
| Oxidative stress | H 2 O 2 , glutathione depletion |
| Excitatory amino acid | NMDA receptor agonist, AMPA receptor agonist, ATP |
| Cytokines | TNFα/INFγ co-treatment |
Glutamate efflux from glial cells to accumulate glutamate in extracellular space
AMPA/Kainate receptor activation in oligodendrocytes
NMDA receptor activation in oligodendrocytes
Abnormal Ca 2+ influx in oligodendrocytes
Protease activation that degrades cellular matrix to induce oligodendrocyte death
Adenosine triphosphate mediated toxicity to oligodendrocytes
Accumulation of reactive oxidative species
Neural stem cell to OPC differentiation
Shh, Indian hedgehog, Wnt signaling, Ezh2, Zfp488 145
OPC proliferation
PI3K/Akt pathway, DNMTs, Shh, PDGF, Notch1 148
OPC differentiation to oligodendrocyte bHLH (Olig1/2,149 Ascl1150), HDACs, Shh, Zfp488,152 CNTF, miRNAs ,
| Astrocyte | BDNF | OPC proliferation, OPC differentiation , , |
| PDGF | OPC proliferation | |
| bFGF | OPC proliferation, OPC migration | |
| CNTF | OPC proliferation, OPC differentiation | |
| IGF-1 | OPC proliferation , | |
| Endo-thelium | BDNF | OPC proliferation |
| bFGF | OPC proliferation | |
| VEGF | OPC migration | |
| Microglia | Activin-A | OPC differentiation |
| IGF-1 | OPC proliferation , | |
| CNTF | OPC differentiation |
Although in vitro systems are useful to dissect molecular pathways, they are fundamentally limited in terms of capturing the dynamic complexity of multicellular homeostasis. Therefore, the field has also developed a series of in vivo models to examine gliovascular interactions in white matter. The following subsections summarize the present status of in vivo rodent models of VCID.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here