Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Multinucleated giant cells are seen in small numbers in a diverse range of soft tissue tumors. Similarly, soft tissue tumors with prominent giant cells compose a heterogeneous group of benign, intermediate, and malignant neoplasms. Recognition of the various types of morphologically distinct giant cells may provide clues to the diagnosis. In some cases, the giant cells instead distract the pathologist from paying careful attention to architectural patterns and other constituent cell types that are more helpful in arriving at the correct diagnosis of these tumors.
The multinucleated giant cells in many soft tissue tumors are derived from the monocyte-macrophage lineage. These non-neoplastic giant cells contain cytologically uniform nuclei that lack atypia. In contrast, other soft tissue tumors contain small numbers of distinctive forms of tumor-derived giant cells. Finally, highly atypical, pleomorphic (nondistinctive) tumor giant cells are essentially limited to pleomorphic sarcomas, anaplastic carcinomas, lymphomas (especially anaplastic large cell lymphoma), and occasional malignant melanomas. The group of non-neoplastic giant cells and distinctive tumor giant cells includes Touton giant cells, floret-type giant cells, wreath-like giant cells, multinucleated tumor cells with glassy cytoplasm, and osteoclast-like giant cells. Each of these types of giant cells is described briefly in the following paragraphs ( Table 11.1 and Fig. 11.1 ).
| Type of Giant Cell | Characteristic Tumor Types | Chapters Where Discussed |
|---|---|---|
| Touton giant cell | Xanthogranuloma | 15 |
| Benign fibrous histiocytoma | 15 | |
| Floret-type giant cell | Pleomorphic lipoma | 12 |
| Giant cell fibroblastoma | 15 | |
| Giant cell angiofibroma | 3 | |
| Neurofibroma (rare) | 3 | |
| Wreath-like giant cell | Alveolar rhabdomyosarcoma | 8 |
| Clear cell sarcoma | 3 | |
| Cellular blue nevus | NA | |
| Multinucleated giant cell with glassy cytoplasm | Reticulohistiocytoma | 15 |
| Osteoclast-like giant cell | Nodular fasciitis | 3 and 4 |
| Giant cell tumor of tendon sheath | ||
| Diffuse-type giant cell tumor | 11 | |
| Plexiform fibrohistiocytic tumor | 11 | |
| Giant cell tumor of soft tissue | 11 | |
| Leiomyosarcoma | 3 and 11 | |
| Extraskeletal osteosarcoma | 7 , 11, and 14 | |
| Undifferentiated pleomorphic sarcoma | 7 and 11 | |
| Undifferentiated carcinoma | NA | |
| Pleomorphic tumor giant cell | Undifferentiated pleomorphic sarcoma | 7 |
| Anaplastic carcinomas | NA | |
| Anaplastic large cell lymphoma | NA | |
| Histiocytic sarcoma | 10 |
Touton giant cells, which are histiocytic in nature, contain eosinophilic cytoplasm encircled by a ring of nuclei, which are in turn surrounded by a lipid-laden rim of foamy cytoplasm. Occasionally, hemosiderin may also be seen in the cytoplasm. These giant cells are characteristically found in a few cutaneous lesions, including (juvenile) xanthogranuloma and benign fibrous histiocytoma (see Chapter 15 for details).
Floret-type giant cells are composed of hyperchromatic nuclei arranged in a semicircle surrounding deeply eosinophilic cytoplasm. This type of giant cell is typically found in pleomorphic lipoma, a benign adipocytic tumor of the neck, upper back, and shoulder region (see Chapter 12 ). In addition, this form of giant cell can be found in the pediatric, superficial neoplasm known as giant cell fibroblastoma (discussed in Chapter 15 ) and the solitary fibrous tumor variant previously known as giant cell angiofibroma (see Chapter 3 ), which has a predilection for the soft tissues of the orbit. Neurofibromas may also contain floret-type giant cells in a small subset of cases.
Wreath-like giant cells contain hyperchromatic, peripherally located nuclei surrounding eosinophilic cytoplasm. Typically, this type of giant cell is found in alveolar rhabdomyosarcoma (discussed in Chapter 8 ), clear cell sarcoma (discussed in Chapter 3 ), and cellular blue nevus.
Multinucleated tumor cells with glassy cytoplasm are characteristic of the rare cutaneous lesion reticulohistiocytoma (discussed in Chapter 15 ).
Osteoclast-like giant cells are composed of irregularly distributed, small ovoid nuclei (that resemble those found in histiocytes) within eosinophilic or amphophilic cytoplasm. In most tumors, these giant cells contain 5 to 10 nuclei; however, 20 or more nuclei may occasionally be seen, particularly in giant cell tumor of soft tissue (see later discussion). Osteoclast-like giant cells can sometimes be found in a wide range of soft tissue tumors. For example, prominent osteoclast-like giant cells are seen in about 10% of cases of nodular fasciitis ( Fig. 11.2 ) (see Chapters 3 and 4 ). A relatively small group of soft tissue tumors characteristically contain osteoclast-like giant cells. These latter tumors are the focus of this chapter.
Soft tissue tumors that typically contain osteoclast-like giant cells can be divided into three main groups ( Box 11.1 ):
Tumors associated with joints or arising in the soft tissues adjacent to joints (the tenosynovial giant cell tumors)
Tumors of superficial soft tissue (dermis and subcutis) that usually contain nodules of giant cells admixed with mononuclear cells
Giant cell–rich sarcomas
Giant cell tumor of tendon sheath
Diffuse-type giant cell tumor
Malignant diffuse-type giant cell tumor (sarcoma ex–diffuse-type giant cell tumor)
Plexiform fibrohistiocytic tumor
Giant cell tumor of soft tissue
Leiomyosarcoma
Extraskeletal osteosarcoma
Undifferentiated pleomorphic sarcoma
Attention to the anatomic site, tumor depth (superficial or deep soft tissues), architecture (plexiform, nodular, diffuse), and constituent cells other than the giant cells is helpful in the evaluation of soft tissue tumors containing prominent giant cells. For the tumors in these first two groups in particular, immunohistochemistry is of limited value in differential diagnosis. In contrast, because other malignant neoplasms (especially undifferentiated carcinoma and large cell lymphomas) may easily be confused with giant cell–rich sarcomas, immunohistochemistry is critical to exclude nonmesenchymal neoplasms before an undifferentiated sarcoma is diagnosed (see Chapter 7 ).
Tenosynovial giant cell tumors arise from tendon sheaths, joints, bursae, or adjacent soft tissues. Depending on their location, they can be subclassified clinically into intraarticular and extraarticular subtypes. Based on growth pattern, tenosynovial giant cell tumors can be separated histologically into localized and diffuse subtypes. These variants have significant differences in clinical presentation and prognosis. Rarely, tenosynovial giant cell tumors may be histologically and clinically malignant.
Localized-type tenosynovial giant cell tumor is widely known as giant cell tumor of tendon sheath . In the past, this lesion was referred to as nodular tenosynovitis or benign synovioma . Giant cell tumor of tendon sheath is one of the more common types of soft tissue tumor.
Most giant cell tumors of tendon sheath arise on the fingers (about 85%). Less commonly affected sites include the wrists, feet, ankles, and knees. Very rarely, this tumor type occurs in the elbow or hip. The tumor can occur in people of any age but preferentially affects young to middle-aged adults between 30 and 50 years of age. A female predominance of 2 : 1 has been noted. The tumors are painless, slowly growing nodules, usually in the soft tissue, in close proximity to tendons or interphalangeal joints. Occasionally, these tumors erode nearby bone or involve the overlying skin. Rarely, patients present with multiple discrete tumors, usually along a single tendon sheath, but exceptionally involving separate digits. A history of local trauma has been reported in a subset of cases.
Giant cell tumors of tendon sheath are usually small nodules between 0.5 and 3 cm in size. The uncommon localized intraarticular examples within the ankle, elbow, or hip may be larger. On gross examination, giant cell tumor of tendon sheath is a well-circumscribed, lobulated mass with a white, fibrous cut surface. The presence of yellow and brown areas is variable and depends on the number of xanthoma cells and the extent of hemosiderin deposition.
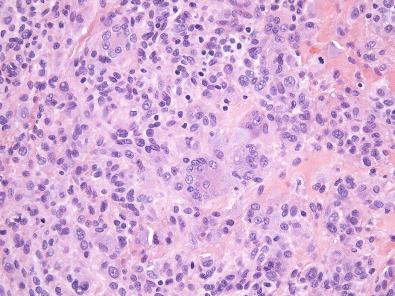
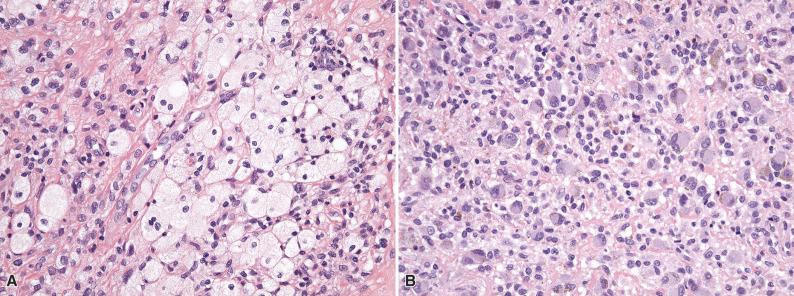
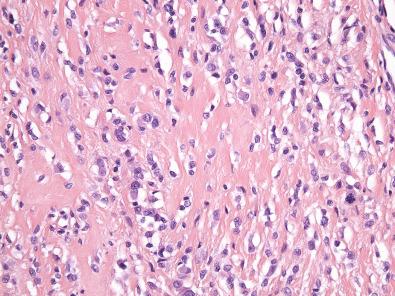
Histologically, giant cell tumor of tendon sheath is surrounded by a fibrous pseudocapsule, and thin bands of fibrous tissue often separate the tumor into nodules. The nodules are usually dominated by small, mononuclear histiocytoid cells with reniform or clefted nuclei, small nucleoli, and pale cytoplasm, which are admixed with larger epithelioid cells with eosinophilic, sometimes glassy cytoplasm and eccentric nuclei, multinucleated osteoclast-like giant cells, foamy macrophages, siderophages, and chronic inflammatory cells ( Figs. 11.3 and 11.4A ). The larger epithelioid cells may contain fine cytoplasmic hemosiderin pigment (see Fig. 11.4B ). In some cases, the histiocytoid cells have a more spindled appearance. The collagenous stroma can be variably hyalinized, and cholesterol clefts may be found. In tumors with prominent stromal hyalinization, the larger epithelioid cells can be more prominent, and only sparse osteoclast-like giant cells may be seen ( Fig. 11.5 ). Cleft-like spaces, which are typically seen in diffuse-type tenosynovial giant cell tumor (see subsequent discussion), are generally absent. Mitotic activity is highly variable and can occasionally be brisk (>5 per 10 high-power fields). In rare cases, focal necrosis and metaplastic bone may be apparent.
In practice, immunohistochemistry plays no real role in the diagnosis of giant cell tumor of tendon sheath. The multinucleated osteoclast-like giant cells and many of the small mononuclear cells usually express CD68 and CD163, reflecting the histiocytic nature of these cells. The larger epithelioid cells may be positive for desmin (which often highlights dendritic cytoplasmic processes), in up to 50% of cases; the number of desmin-positive cells is highly variable but is usually less than 10%. The larger epithelioid cells are often positive for clusterin and podoplanin (D2-40) as well.
Clonal cytogenetic aberrations in giant cell tumor of tendon sheath commonly involve the short arm of chromosome 1 (1p13), with rearrangement of the CSF1 gene in the majority of cases, indicating the neoplastic nature of this tumor type. In a subset of tumors, the fusion partner is COL6A3 , located at 2q37, whereas in a significant number of cases the fusion partner has not yet been identified. Recently, several cases with a novel CSF1 transcript were identified by RNA sequencing, including one with CSF1-S100A10 fusion. However, the CSF1 translocation is only present in a minority of cells within the tumor (i.e., the larger epithelioid cells). These CSF1-producing neoplastic cells are believed to recruit and induce the proliferation of non-neoplastic CSF1 receptor–expressing cells of the monocyte-macrophage lineage. This “landscaping effect” is thought to account for the formation of a tumor mass with the heterogeneous cellular composition of predominantly non-neoplastic cells.
The diagnosis of giant cell tumor of tendon sheath is generally straightforward. The most important differential diagnostic consideration is diffuse-type tenosynovial giant cell tumor (given the much higher risk of local recurrence). Diffuse-type giant cell tumor is distinguished from giant cell tumor of tendon sheath by its lack of sharp circumscription; nodules or sheets of tumor cells infiltrate into surrounding soft tissues. This tumor type most often arises within joints (preferentially the knee and the hip) but can also occur in extraarticular soft tissue. There is considerable histologic overlap between giant cell tumor of tendon sheath and diffuse-type giant cell tumor, although the latter often shows a villous appearance and contains prominent cleft-like spaces and fewer giant cells. Fibroma of tendon sheath occurs at locations similar to those of giant cell tumor of tendon sheath but is histologically quite different, being composed of fascicles of uniform small spindle cells in a collagenous stroma with slit-like blood vessels at the periphery of the tumor. Giant cells may occasionally be seen, but the mononuclear histiocytoid cells and epithelioid cells are absent. When the epithelioid cells in giant cell tumor of tendon sheath are prominent, epithelioid sarcoma may be considered. This differential diagnosis can easily be resolved with immunohistochemistry; epithelial membrane antigen (EMA) and keratins are consistently positive in epithelioid sarcoma but are negative in giant cell tumor of tendon sheath. In addition, loss of SMARCB1 (INI1) is specific for epithelioid sarcoma in this differential diagnosis. The presence of desmin expression in the epithelioid cells may raise the possibility of a myogenic tumor (such as rhabdomyosarcoma). However, giant cell tumor of tendon sheath is negative for other muscle markers such as smooth muscle actin (SMA), caldesmon, and myogenin. Awareness of this occurrence can help avoid diagnostic confusion.
Giant cell tumor of tendon sheath is benign, with a low risk of nondestructive local recurrence (5% to 20%). Conservative local excision is adequate treatment.
Diffuse-type tenosynovial giant cell tumor is also widely known clinically as pigmented villonodular (teno)synovitis (PVNS). However, diffuse-type giant cell tumor is a clonal neoplasm with a significant potential for destructive local recurrence, as well as a small risk of metastasis (not an inflammatory process). Therefore, the misleading designation PVNS should be discouraged.
According to location, diffuse-type (tenosynovial) giant cell tumors can be divided into intraarticular tumors (which often extend into adjacent soft tissue) and entirely extraarticular examples. The most common site of intraarticular diffuse-type giant cell tumor is the knee (about 70% of cases), followed by the hip (10%). Rarely, the temporomandibular joint or the spinal facet joints are involved. Extraarticular diffuse-type giant cell tumors most commonly arise in the periarticular soft tissues around the knee, hip, or foot. The fingers, toes, wrists, and elbows are rarely affected. Entirely intramuscular tumors and subcutaneous examples are rarely observed. Diffuse-type giant cell tumor preferentially occurs in patients younger than 40 years of age with a slight female predominance. Patients often present with a long duration of symptoms, including pain, swelling, tenderness, and limitation of motion of the affected joint. The presence of hemarthrosis and joint effusion is not unusual. Radiographically, the tumor appears as an ill-defined mass, which may be associated with degenerative alterations of the affected joint. On magnetic resonance imaging, the tumor shows decreased signal intensity in T1- and T2-weighted images.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here