Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Human pregnancy begins with the fusion of an egg and a sperm within the female reproductive tract, but extensive preparation precedes this event. First, both male and female sex cells must pass through a long series of changes ( gametogenesis ) that convert them genetically and phenotypically into mature gametes , which are capable of participating in the process of fertilization. Next, the gametes must be released from the gonads and make their way to the upper part of the uterine tube, where fertilization normally takes place. Finally, the fertilized egg, now properly called an embryo , must enter the uterus, where it sinks into the uterine lining ( implantation ) to be nourished by the mother. All these events involve interactions between the gametes or embryo and the adult body in which they are housed, and most are mediated or influenced by parental hormones. This chapter focuses on gametogenesis and the hormonal modifications of the body that enable reproduction to occur.
Gametogenesis is typically divided into four phases: (1) the extraembryonic origin of the germ cells and their migration into the gonads, (2) an increase in the number of germ cells by mitosis, (3) a reduction in chromosomal number by meiosis, and (4) structural and functional maturation of the eggs and spermatozoa. The first phase of gametogenesis is identical in males and females, whereas distinct differences exist between the male and female patterns in the last three phases.
Primordial germ cells (PGCs), the earliest precursors of gametes, arise and are specified ∗
∗ In embryological parlance, specification refers to the commitment of a cell to a particular fate, in this case, a gamete.
outside the gonads in the epiblast (see p. 400) of the pre-gastrula human embryo. Responding to the growth factor bone morphogenetic protein (BMP, see p. 43), a very small number of cells, as few as six in the mouse, produce the regulatory molecule BLIMP1 (B lymphocyte–induced maturation protein-1). BLIMP1 is not only heavily involved in the specification of PGCs, but it also coordinates PGC-specific gene expression by repressing the genetic program that would otherwise lead to the development of these cells into somatic cells, such as epithelial cells or neurons. PGCs become highly mobile and make their way into the epithelium that lines the hindgut/yolk sac region of the 3-week embryo ( Figure 1.1A ). A week later, they begin to leave the gut wall and enter the dorsal mesentery on their way toward settling in the gonads ( Figure 1.1B ). In the mouse, an estimated 100 PGCs leave the hindgut/yolk sac region, and through mitotic multiplication (6 to 7 rounds of cell division) about 4000 PGCs enter the primitive gonads.
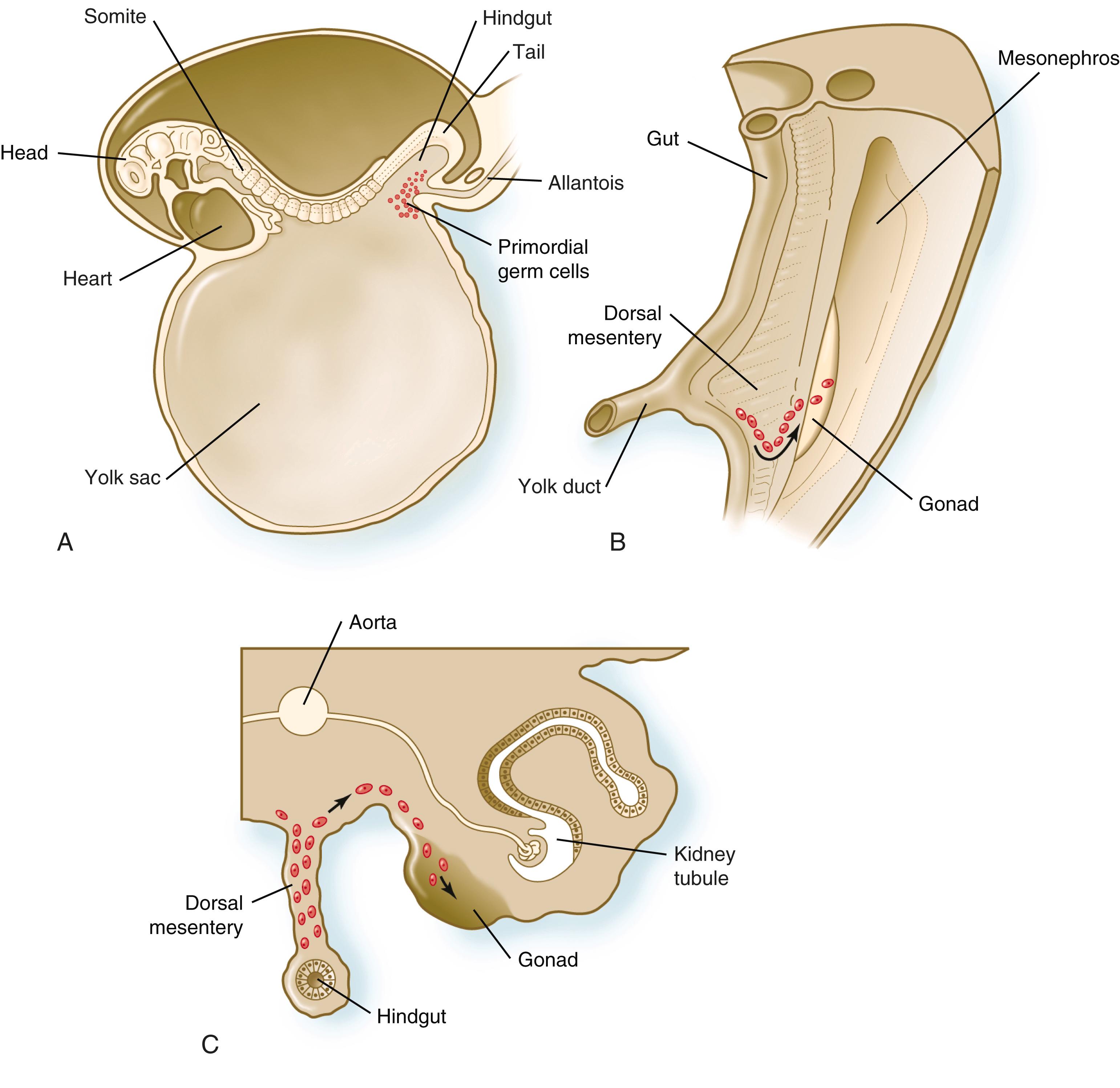
The passage of PGCs through the dorsal mesentery involves molecular cues, cellular properties, and properties of the substrate over which they migrate. Gradients of two signaling systems, stromal cell–derived factor 1 (SDF1) and stem cell factor (SCF, or Steel factor), coming from the gonads and dorsal mesentery, interact with surface receptors on the PGCs to guide a directional migration toward the gonadal primordia. The PGCs themselves assume a shape and surface properties that are characteristic of migrating cells. Most of them form an interconnected chain during their passage through the dorsal mesentery. Their passage through the dorsal mesentery is facilitated by the presence of fibronectin in the extracellular matrix during the time of their passage. Once they arrive in the gonadal primordia ( Figure 1.1C ), they begin to interact with the stromal cells as they begin their long progression toward forming eggs and sperms.
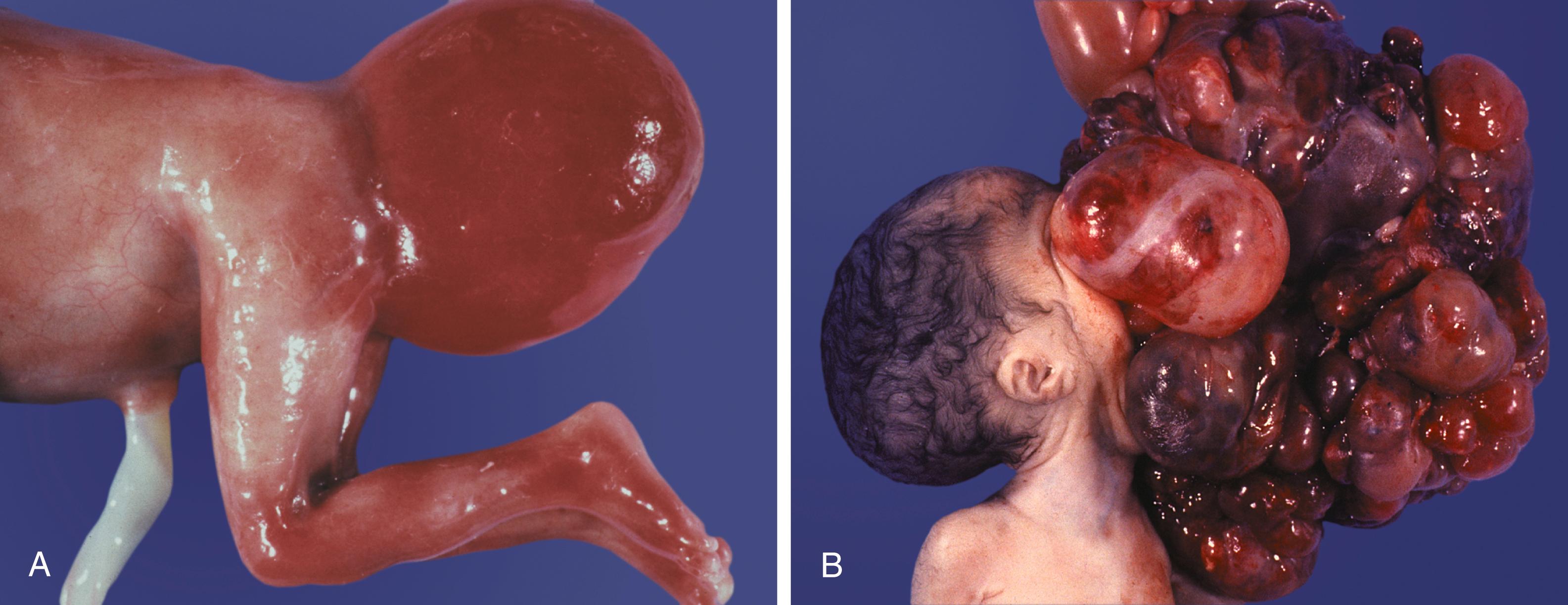
Misdirected PGCs that lodge in extragonadal sites usually die, but if such cells survive, they may develop into teratomas . Teratomas are bizarre growths that contain scrambled mixtures of highly differentiated tissues, such as skin, hair, cartilage, and even teeth ( Figure 1.2 ). They are found in the mediastinum, the sacrococcygeal region, and the oral region.
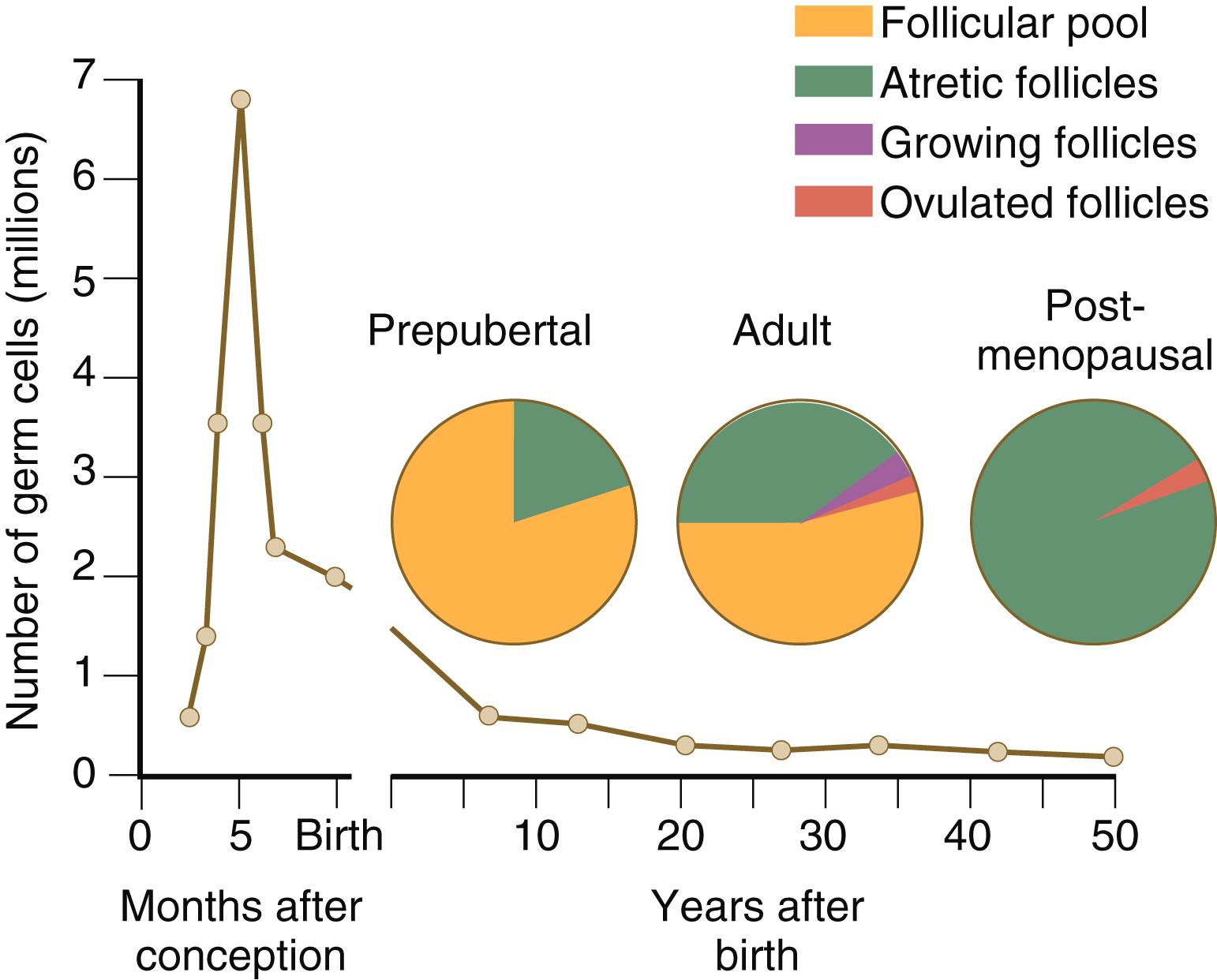
After they arrive in the gonads, the PGCs begin a phase of rapid mitotic proliferation. In a mitotic division, each germ cell produces two diploid progeny that are genetically equal. Through several series of mitotic divisions, the number of PGCs increases exponentially from hundreds to millions. The pattern of mitotic proliferation differs markedly between male and female germ cells. Oogonia , as mitotically active germ cells in the female are called, go through a period of intense mitotic activity in the embryonic ovary from the second through the fifth month of pregnancy in the human. During this period, the population of germ cells increases from only a few thousand to nearly 7 million ( Figure 1.3 ). This number represents the maximum number of germ cells that is ever found in the ovaries. Shortly thereafter, numerous oogonia undergo a natural degeneration called atresia . Atresia of germ cells is a continuing feature of the histological landscape of the human ovary until menopause.
Spermatogonia , which are the male counterparts of oogonia, follow a pattern of mitotic proliferation that differs greatly from that in the female. Mitosis also begins early in the embryonic testes, but ceases early in the second trimester of pregnancy and does not resume until adolescence. In contrast to female germ cells, male germ cells maintain the ability to divide throughout postnatal life. The seminiferous tubules of the testes are lined with a germinative population of spermatogonia. Beginning at puberty, subpopulations of spermatogonia undergo periodic waves of mitosis. The progeny of these divisions enter meiosis as synchronous groups. This pattern of spermatogonial mitosis continues throughout life.
The biological significance of meiosis in humans is similar to that in other species. Of primary importance are (1) reduction of the number of chromosomes from the diploid (2n) to the haploid (1n) number so that the species number of chromosomes can be maintained from generation to generation, (2) independent reassortment of maternal and paternal chromosomes for better mixing of genetic characteristics, and (3) further redistribution of maternal and paternal genetic information through the process of crossing-over during the first meiotic division.
Meiosis involves two sets of divisions ( Figure 1.4 ). Before the first meiotic division, deoxyribonucleic acid (DNA) replication has already occurred, so at the beginning of meiosis, the cell is 2n, 4c. (In this designation, n is the species number of chromosomes, and c is the amount of DNA in a single set [n] of chromosomes.) The cell contains the normal number (2n) of chromosomes, but as a result of replication, its DNA content (4c) is double the normal amount (2c).
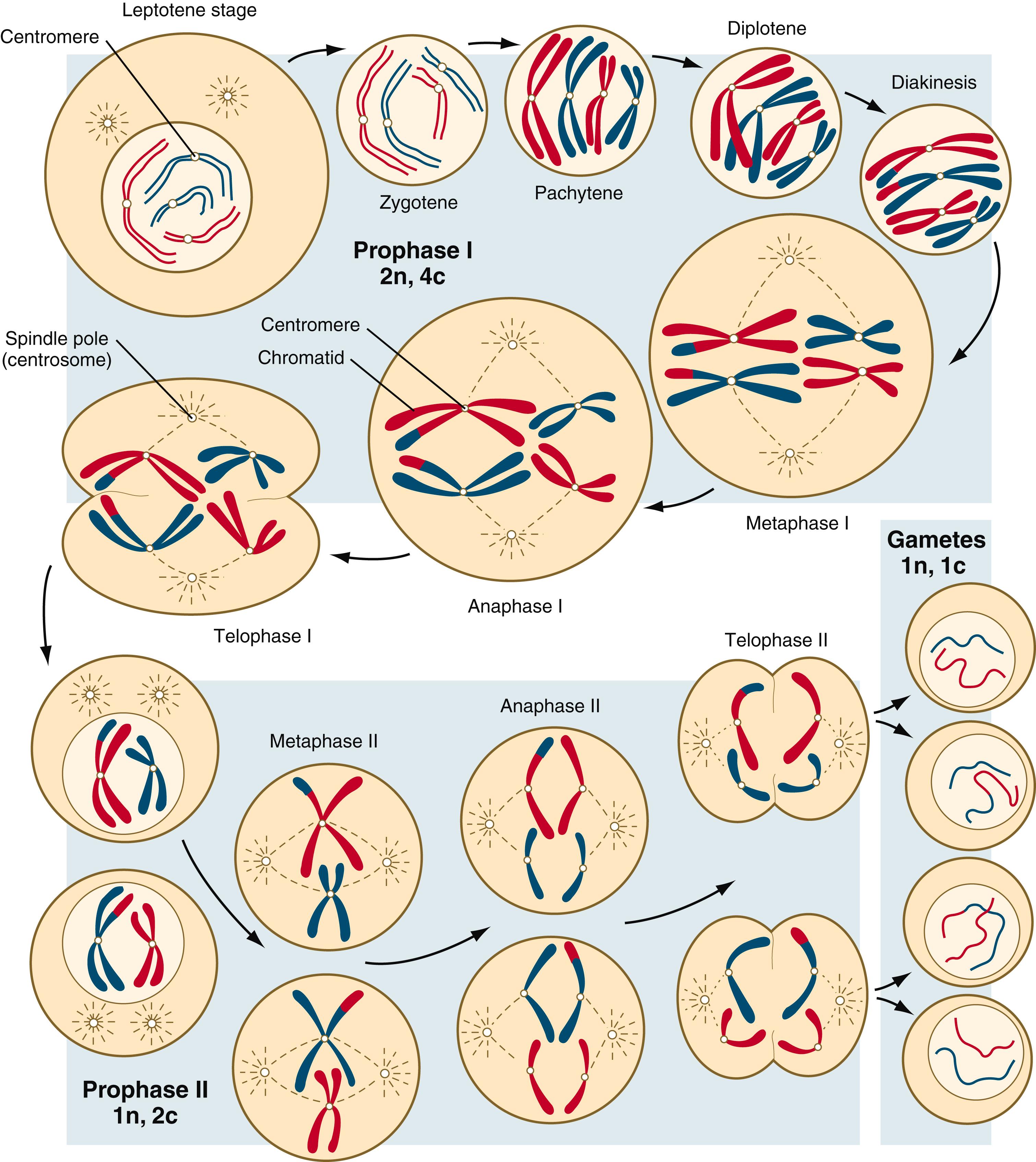
The first meiotic division, often called the reductional division , is characterized by a prolonged prophase, which is divided into several stages (see Figure 1.4 ). In the brief leptotene phase, homologous chromosomes, each consisting of paired sister chromatids , begin to pair up and condense. The zygotene phase is characterized by the side-by-side joining together of portions of homologous chromosomes ( synapsis ) through the formation of a synaptonemal complex , consisting of several protein elements. The homologous chromosomes, firmly joined by synaptonemal complexes, further condense in the pachytene phase. In the diplotene phase, the synaptonemal complexes begin to degrade, and parts of the homologous chromosomes are slightly separated. Within the chromatids, parts of the DNA may uncoil into broad loops, which allow some transcription to occur. Diakinesis , the last component of prophase I, involves the further condensation of the chromosomes and preparation for metaphase.
A major component of the first meiotic division is genetic recombination —the exchange of genetic material between the two homologous chromosomes. One form of recombination, which typically occurs early in prophase, involves short segments and occurs through a mechanism similar to that of DNA repair. The other, called crossing-over , consists of the exchange of segments between the two chromosomes during the pachytene stage. Crossing-over even occurs in the sex chromosomes. This takes place in a small region of homology between the X and Y chromosomes. Crossing-over is not a purely random process. Rather, it occurs at sites along the chromosomes known as hot spots . Their location is based on configurations of proteins that organize the chromosomes early in meiosis. One such protein is cohesin , which helps hold sister chromatids together during division. Hypermethylation of histone proteins in the chromatin indicates specific sites where the DNA strands break and are later repaired after crossing-over is completed. Another protein, condensin , is important in compaction of the chromosomes, which is necessary for both mitotic and meiotic divisions to occur.
During metaphase of the first meiotic division, the chromosome pairs ( tetrads ) line up at the metaphase (equatorial) plate so that at anaphase I, one chromosome of a homologous pair moves toward one pole of the spindle, and the other chromosome moves toward the opposite pole. This represents one of the principal differences between a meiotic and a mitotic division. In a mitotic anaphase, the kinetochore (centromere ∗
∗ The centromere is the region that links two homologous chromosomes, whereas the kinetochore is a set of proteins in the centromere that constitutes the actual linkage mechanism.
) between the sister chromatids of each chromosome splits after the chromosomes have lined up at the metaphase plate, and one chromatid from each chromosome migrates to each pole of the mitotic spindle. This activity results in genetically equal daughter cells after a mitotic division, whereas the daughter cells are genetically unequal after the first meiotic division. Each daughter cell of the first meiotic division contains the haploid (1n) number of chromosomes, but each chromosome still consists of two chromatids (2c) connected by a kinetochore. No new duplication of chromosomal DNA is required between the first and second meiotic divisions because each haploid daughter cell resulting from the first meiotic division already contains chromosomes in the replicated state.
The second meiotic division, called the equational division , is similar to an ordinary mitotic division except that before division the cell is haploid (1n, 2c). When the chromosomes line up along the equatorial plate at metaphase II, the kinetochores between sister chromatids divide, allowing the sister chromatids of each chromosome to migrate to opposite poles of the spindle apparatus during anaphase II. Each daughter cell of the second meiotic division is truly haploid (1n, 1c).
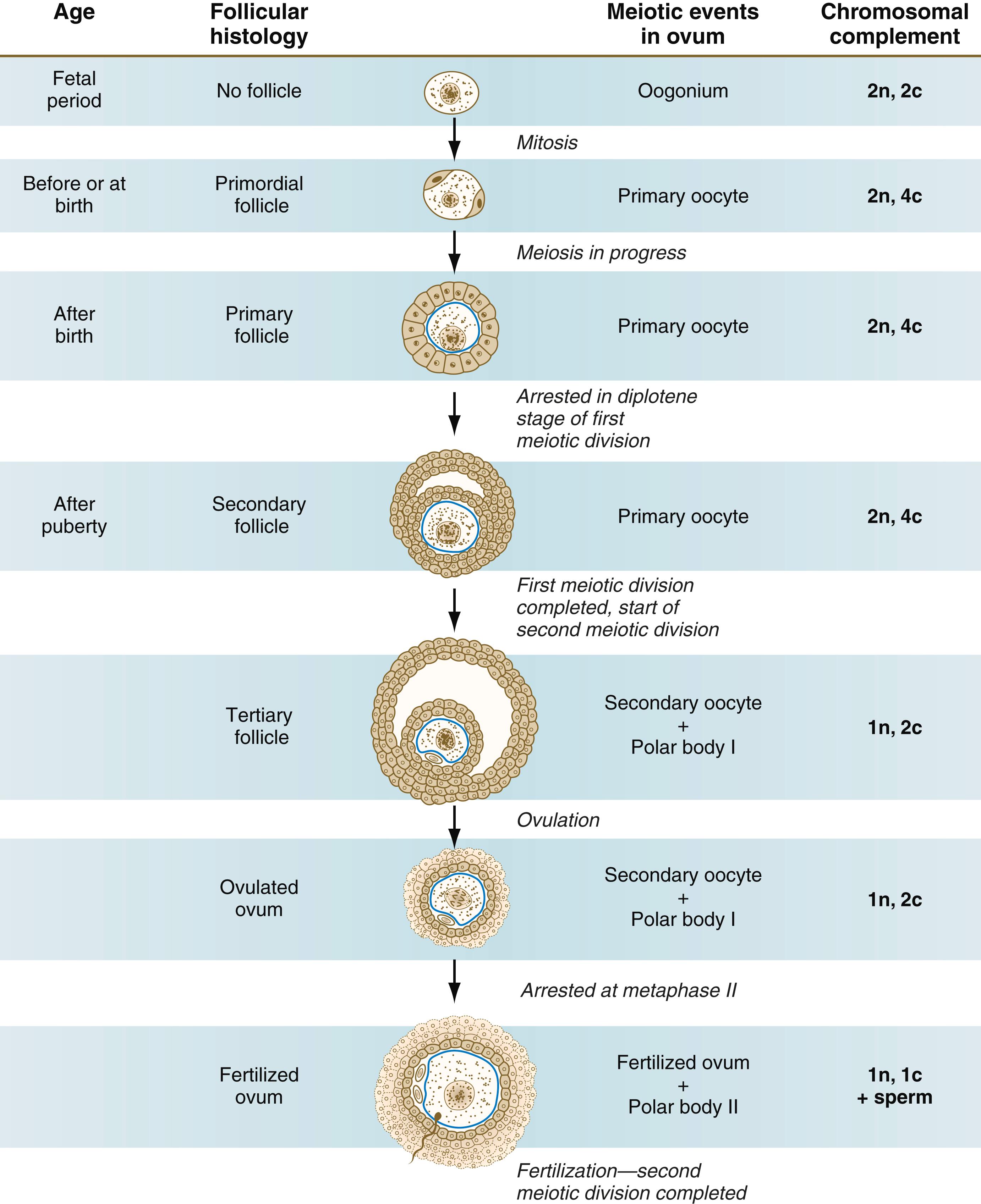
The period of meiosis involves other cellular activities in addition to the redistribution of chromosomal material. When the oogonia enter the first meiotic division late in the fetal period, they are called primary oocytes .
Meiosis in the human female is a very leisurely process. As the primary oocytes enter the diplotene stage of the first meiotic division in the early months after birth, the first of two blocks in the meiotic process occurs ( Figure 1.5 ). The suspended diplotene phase of meiosis is the period when the primary oocyte prepares for the needs of the embryo. In oocytes of amphibians and other lower vertebrates, which must develop outside the mother’s body and often in a hostile environment, it is highly advantageous for the early stages of development to occur very rapidly so that the stage of independent locomotion and feeding is attained as soon as possible. These conditions necessitate a strategy of storing up the materials needed for early development well in advance of ovulation and fertilization because normal synthetic processes would not be rapid enough to produce the materials required for the rapidly cleaving embryo. In such species, yolk is accumulated, the genes for producing ribosomal ribonucleic acid (rRNA) are amplified, and many types of RNA molecules are synthesized and stored in an inactive form for later use.
RNA synthesis in the amphibian oocyte occurs on the lampbrush chromosomes , which are characterized by many prominent loops of spread-out DNA on which messenger RNA (mRNA) molecules are synthesized. The amplified genes for producing rRNA are manifested by the presence of 600 to 1000 nucleoli within the nucleus. Primary oocytes also prepare for fertilization by producing several thousand cortical granules, which are of great importance during the fertilization process (see Chapter 2 ).
The mammalian oocyte prepares for an early embryonic period that is more prolonged than that of amphibians and that occurs in the nutritive environment of the maternal reproductive tract. Therefore it is not faced with the need to store as great a quantity of materials as the eggs of lower vertebrates. As a consequence, the buildup of yolk is negligible. Evidence indicates, however, a low level of ribosomal RNA (rRNA) amplification (two to three times) in diplotene human oocytes, a finding suggesting that some degree of molecular advance planning is also required to support early cleavage in the human. The presence of 2 to 40 small (2-μm) RNA-containing micronuclei (miniature nucleoli) per oocyte nucleus correlates with the molecular data.
Human diplotene chromosomes do not appear to be arranged in a true lampbrush configuration, and massive amounts of RNA synthesis seem unlikely. The developing mammalian (mouse) oocyte produces 10,000 times less rRNA and 1000 times less mRNA than its amphibian counterpart. Nevertheless, there is a steady accumulation of mRNA and a proportional accumulation of rRNA. These amounts of maternally derived RNA seem to be enough to move the fertilized egg through the first couple of cleavage divisions, after which the embryonic genome takes control of macromolecular synthetic processes. With all its activity during early meiosis, the nucleus swells and is commonly referred to as the germinal vesicle.
Because cortical granules play an important role in preventing the entry of excess spermatozoa during fertilization in human eggs (see p. 32), the formation of cortical granules (mainly from the Golgi apparatus) continues to be one of the functions of the diplotene stage that is preserved in humans. Approximately 4500 cortical granules are produced in the mouse oocyte. A higher number is likely in the human oocyte.
Unless they degenerate, all primary oocytes remain arrested in the diplotene stage of meiosis until puberty. During the reproductive years, small numbers (10 to 30) of primary oocytes complete the first meiotic division with each menstrual cycle and begin to develop further. The other primary oocytes remain arrested in the diplotene stage, some for 50 years.
With the completion of the first meiotic division hours before ovulation, two unequal cellular progeny result. One is a large cell called the secondary oocyte . The other is a small cell called the first polar body (see Figure 1.5 ). The secondary oocytes begin the second meiotic division, but again the meiotic process is arrested, this time at metaphase. The stimulus for the release from this meiotic block is fertilization by a spermatozoon. Unfertilized secondary oocytes fail to complete the second meiotic division. The second meiotic division is also unequal; one of the daughter cells is relegated to becoming a second polar body. The first polar body may also divide during the second meiotic division. Formation of both the first and second polar bodies involves highly asymmetric cell divisions. The asymmetrical meiotic divisions of the oocyte leading to small polar bodies are designed to preserve as much cytoplasm in the egg as possible. These meiotic divisions are also accomplished without the mediation of centrosomes. This avoids an excess of centrosomes in the fertilized egg because at fertilization, the sperm supplies the centrosomal material in the form of centrioles, which organize the mitotic spindle of the zygote. To a large extent, this is accomplished by displacement of the mitotic spindle apparatus toward the periphery of the oocyte through the actions of the cytoskeletal protein actin (see Fig. 2.8).
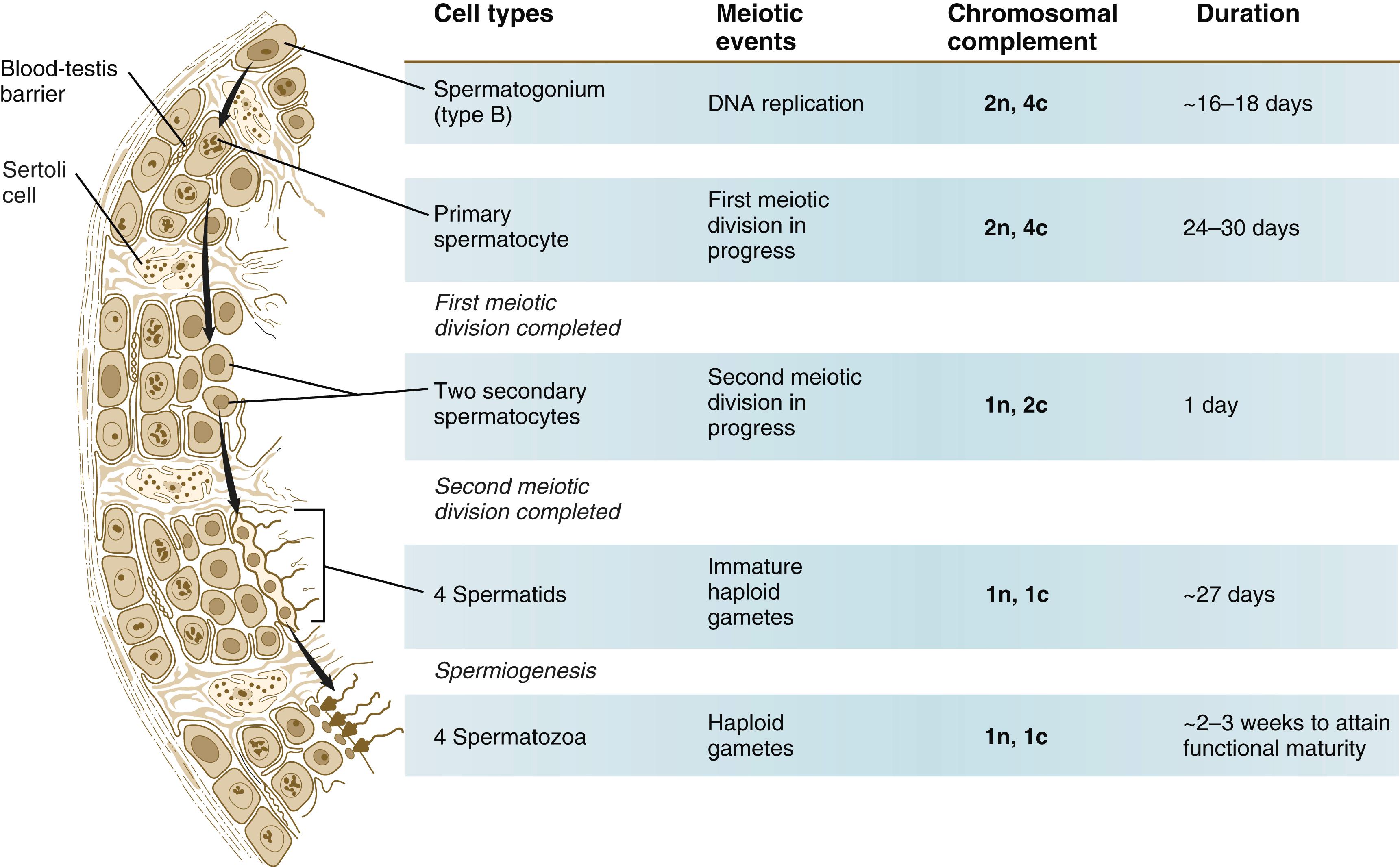
Meiosis in the male does not begin until after puberty. In contrast to the primary oocytes in the female, not all spermatogonia enter meiosis at the same time. Large numbers of spermatogonia remain in the mitotic cycle throughout much of the reproductive lifetime of males. When the progeny of a spermatogonium have entered the meiotic cycle as primary spermatocytes , they spend several weeks passing through the first meiotic division ( Figure 1.6 ). The result of the first meiotic division is the formation of two secondary spermatocytes , which immediately enter the second meiotic division. Approximately 8 hours later, the second meiotic division is completed, and four haploid (1n, 1c) spermatids remain as progeny of the single primary spermatocyte. The total length of human spermatogenesis is approximately 74 days.
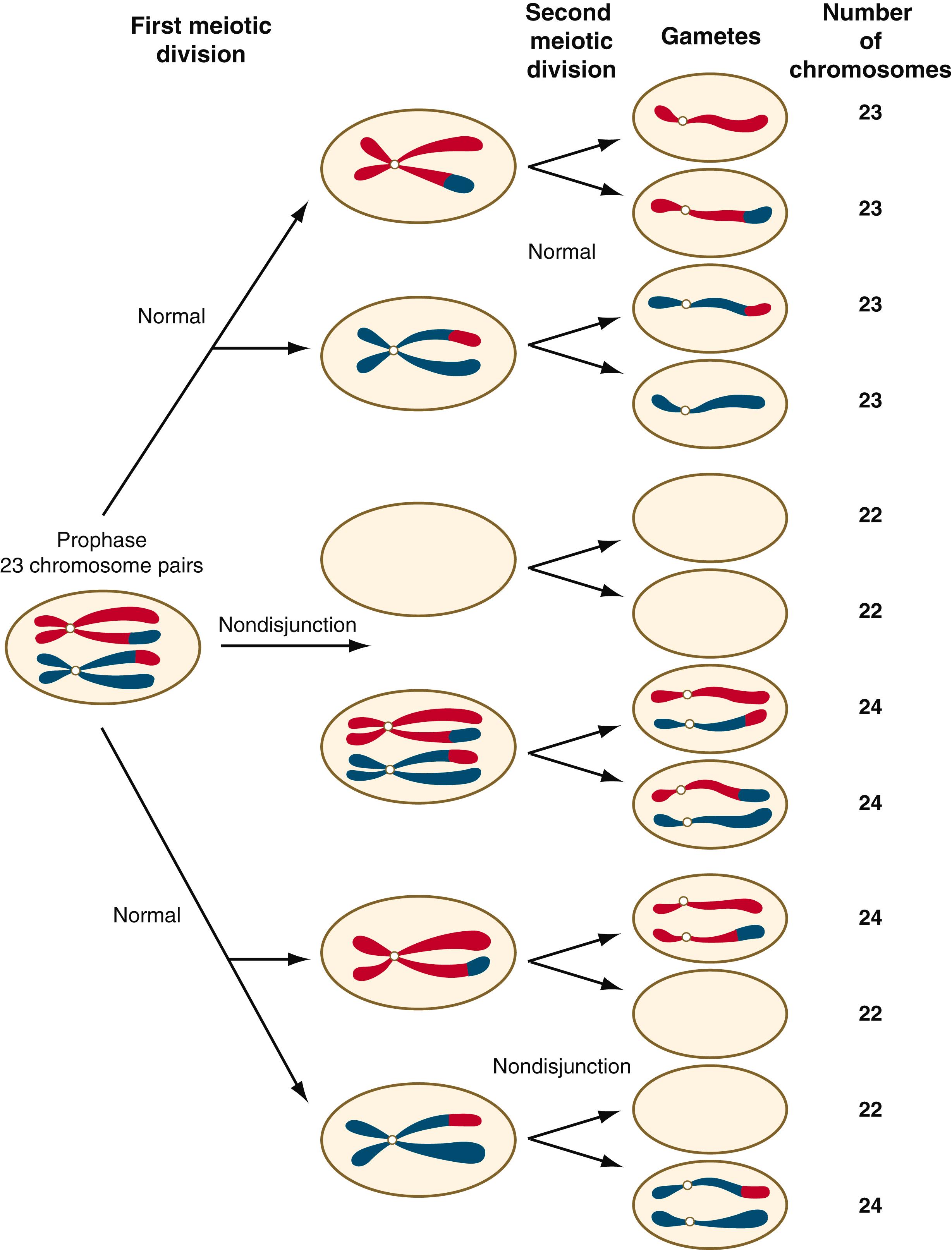
Disturbances that can occur during meiosis and result in chromosomal aberrations are discussed in Clinical Correlation 1.1 and Figure 1.7 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here