Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The kidneys, renal pedicle, and adrenal glands are located in the perirenal space, which is bound by the anterior and posterior renal fascia (Gerota fascia; for anatomy, see the section Retroperitoneum ).
Renal artery
Renal vein
Collecting system and ureter
Lymphatics
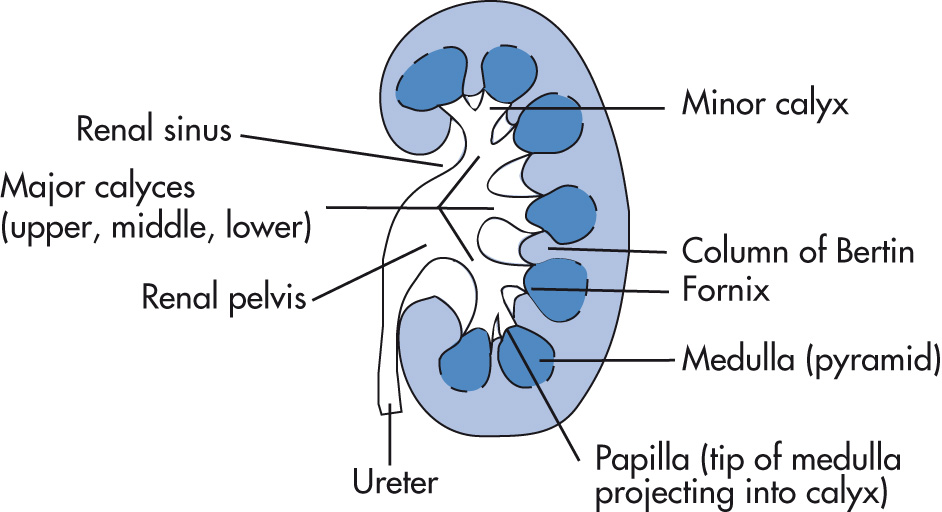
Minor calyces: most kidneys have 10–14 minor calyces.
Major calyces
Renal pelvis: may be completely within the renal sinus or partially “extrarenal.”
Kidneys are 3 to 4 lumbar vertebral bodies in length, 12–14 cm long and 5–7 cm wide.
Intravenous pyelograms (IVPs) overestimate the true renal length because of magnification and renal engorgement from osmotic diuresis. Ultrasound (US) often underestimates the true renal length because of technical difficulties in imaging the entire kidney.
Left and right kidney size should not vary more than 1 cm.
Right kidney is 1–2 cm lower than the left kidney and slightly more lateral.
Renal axis parallels axis of psoas muscles.
Today, IVPs are rarely used, but are covered for historical purposes. The bolus administration ensures maximum concentration of contrast media in the kidney. Indication: healthy ambulatory patients (screening type urography [e.g., for urinary tract infection (UTI)]), trauma.
Kidney, ureter, bladder (KUB)
Inject 100 mL of 30% contrast
1-min and 5-min radiograph of both kidneys
10-min KUB and both obliques
Coned bladder view
Postvoid KUB
With drip infusion, the nephrogram persists longer, allowing more time for nephrotomography and special views, if necessary. Today, this is much less commonly performed than the bolus technique or computed tomography (CT). If calcifications are seen on the renal outlines, scout oblique radiographs can be obtained to determine the exact location relative to the kidneys.
KUB and preliminary tomogram of kidneys (starting at 8 cm from back)
Drip infusion of 300 mL of Urovist 14% (or Conray-30) or 150 mL of Isovue 300 (or Omnipaque 300)
Obtain tomograms after 150 mL has been given. Usually 7 to 9 cuts are obtained.
Postinfusion KUB and both oblique views
Coned bladder view
Postvoid KUB
| Body Weight (kg) | Dose of Contrast | |
|---|---|---|
| Bolus Injection | ||
| Children | 5–25 | 0.5 mL/kg |
| 25–50 | 50 mL | |
| Adult | >50 | 100 mL (28–40 g iodine) |
| Infusion Technique | ||
| Children | Contraindicated | |
| Adult | 300 mL (33–45 g iodine) | |
A catheter is placed in the distal ureter via cystoscopy and contrast is administered via the catheter by hand injection to opacify the collecting system (no parenchymal opacification). This technique is primarily used for percutaneous ureterolithotomy (PUL) access and difficult nephrostomy placements.
Prednisone, 50 mg PO or IV, 13 hours, 7 hours, and 1 hour before contrast injection
Diphenhydramine, 50 mg PO or IV, 30–60 minutes before contrast media injection
Single bolus CT technique
Phase 1: Noncontrast CT of the abdomen and pelvis, including KUB
Single bolus (100–150 mL, 300–320 mg of iodine/mL) of IV contrast material at 2–4 mL/s
Early images of kidney in nephrographic phase
Excretory phase images (5 min following injection) through KUB
Split bolus CT technique:
Phase 1: noncontrast CT of abdomen and pelvis. Immediately thereafter administer 30 mL of IV contrast and wait 5–10 min.
Phase 2: place patient back on scanner and inject 100 mL of contrast at 2–3 mL/s, and scan patient prone from top of kidneys to pubic symphysis after 100 s.
Phase 2: excretory phase (5 min) through the kidneys is obtained at the same time as the 100-s scan. The initial bolus of 30 mL should now be making its way through the collecting system.
Noncontrast CT of the abdomen and pelvis using 5 mm slice thickness
Coronal and sagittal reconstructions with 3 mm slice thickness
Phase 1: noncontrast CT images of abdomen
Phase 2: corticomedullary phase, 25–30 s after contrast injection, through kidneys
Phase 3: nephrographic phase, 60–80 s after injection
Refers to percutaneous drainage of renal collecting system by catheter placement. Complications: 2%–4% (extravasation of contrast, bleeding)
Hydronephrosis (acute, subacute obstruction)
Pyonephrosis
Preprocedure workup:
Check coagulation status.
Cefazolin 2 g IV < 120 kg; 3 g IV > 120 kg and gentamicin 120 mg IV × 1
Penicillin-allergic patients: vancomycin as above and aztreonam 2 g IV
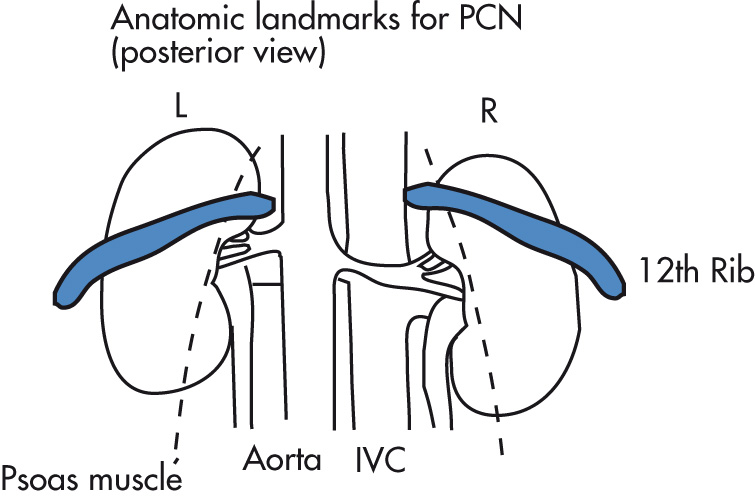
Review all radiographs and determine on plain film where kidneys are located, especially in relation to colon, spleen, and pleural reflections.
Local lidocaine. Under US guidance, place a 20-gauge introducer system into a posterior, lower pole calyx.
The inner needle stylet can be removed and free flow of urine checked. Aspirate a small amount for cultures and to mildly decompress the system when urine is obtained.
Attach connector tubing and inject a small amount of contrast to confirm positioning at the tip of a calyx. Detach the connector tubing carefully to ensure that the needle stays in place.
Use the microwire to gain access to the renal pelvis. Remove the introducer needle, and insert the plastic sheath that comes with the set.
Place a 0.038 Amplatz wire through the sheath into the renal collecting system and down the ureter. Depending on the anatomy/obstruction, it may be necessary to use different wire combinations.
Dilate skin up to 12 Fr.
Pass 8–10-Fr percutaneous nephrostomy (PCN) catheter over guidewire. Remove stiffener and guidewire. Coil pigtail. Inject contrast to check position of catheter within the renal pelvis. Cut thread.
In the setting of infection, complete evaluation of the ureter via an antegrade injection is best deferred to a second visit.
One pelvis drains upper pole calyces; the other drains the middle and lower pole calyces. The two pelvises join proximally to the ureteropelvic junction (UPJ). Incidence: 10% of population. No complications.
Duplications join distal to UPJ and proximal to bladder: Y-shaped ureters. No complications.
See Chapter 11 .
The two kidneys are connected across the midline by an isthmus. This is the most common fusion anomaly (other fusion anomalies: cross-fused ectopia, pancake kidney). The isthmus may contain parenchymal tissue with its own blood supply or consist of fibrous tissue.
UPJ obstruction, 30%
Ureteral duplication, 10%
Genital anomalies
Other anomalies: anorectal, cardiovascular, musculoskeletal anomalies
Obstruction, infection, calculus formation in 30%
Increased risk of renal malignancies, especially Wilms tumor
Increased risk of traumatic injury
Abnormal axis of each kidney with lower calyx more medial than upper calyx
Bilateral malrotation of renal pelvises in anterior position
Isthmus lies anterior to aorta and inferior vena cava (IVC) but behind inferior mesenteric artery (IMA)
Persistent fetal lobulation: scalloped appearance of renal outline; adjacent calyx is normal
Junctional parenchymal defect: fusion defect in upper pole of kidney that does not represent a scar; echogenic linear defect extends from sinus; commonly seen in pediatric patients
Septum of Bertin (upper pole 90%, bilateral 60%); associated with bifid renal pelvis
Dromedary hump: parenchymal prominence in left kidney; results from compression of adjacent spleen
Lobar dysmorphism: abnormally oriented lobe between upper and middle calyces; always points to posterior calyx, which is key to diagnosis on CT or IVP. By US this entity is indistinguishable from column of Bertin.
Aberrant papilla: papilla indents infundibulum or pelvis rather than minor calyx
Linear vascular impressions on renal pelvis and infundibula
Ureteral spindle: dilatation of middle third of ureter at crossing of iliac vessels
Sinus lipomatosis: large amount of fat in renal sinus
Cross-fused ectopia: the ectopic kidney is inferior
Nephroptosis (floating or wandering kidney): acquired condition with excessive descent of the kidney in erect position; differs from congenital pelvic kidney in that the paired renal arteries arise from their typical anatomic location.
Cortical cysts
Simple cysts
Complicated (complex) cysts
Localized cystic disease of the kidney
Medullary cystic disease (MCD)
Polycystic renal disease
Infantile polycystic disease
Adult polycystic disease
Multicystic dysplastic kidney (MCDK)
Multilocular cystic nephroma (MLCN)
Cysts associated with systemic disease
Tuberous sclerosis
Von Hippel-Lindau (VHL) disease
Miscellaneous cysts
Hydatid
Acquired cysts in uremia
Extraparenchymal cysts
Parapelvic cysts
Perinephric cysts
Simple cysts (in >50% of population >50 years) probably arise from obstructed tubules or ducts. They do not, however, communicate with the collecting system. Most commonly asymptomatic; rare findings include hematuria (from cyst rupture), hypertension (HTN), and cyst infection. Mass effect from large cysts may cause dull ache or discomfort.
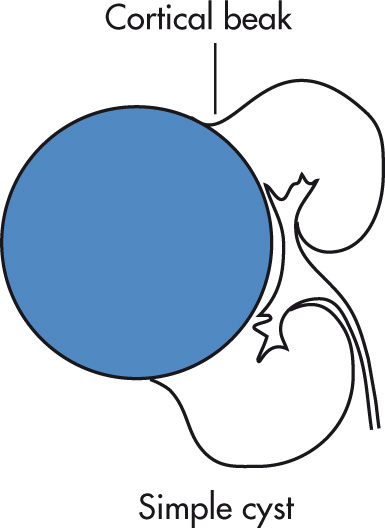
IVP
Lucent defect
Cortical bulge
Round indentations on collecting system
“Beak sign” can be seen with large cysts.
US
Anechoic
Enhanced through-transmission
Sharply marginated, smooth walls
Very thin septations may occasionally be seen.
CT
Smooth cyst wall
Sharp demarcation from surrounding renal parenchyma
Water density (<10–15 HU) that is homogeneous throughout lesion
No significant enhancement after IV contrast administration (small increases of <5 HU, however, can be seen likely due to technical factors, not true enhancement)
Cyst wall too thin to be seen by CT (with small cysts, volume-averaging may create the false appearance of a thick wall)
MRI
Indications (rather than CT): renal insufficiency, solitary kidney, contrast allergy, patients with complex cystic renal masses needing multiple follow-up examinations
End-expiration images are preferred because they are more reproducible in optimizing image coregistration and subtraction algorithms if the patient can hold his or her breath.
Simple renal cysts are uniformly hypointense on T1W and hyperintense on T2W images with no perceptible enhancement after gadolinium. MRI can characterize extremely small renal cysts.
Internal components of cystic masses and subtle enhancement are better appreciated by MRI than by CT.
True renal cysts should always be differentiated from hydronephrosis, calyceal diverticulum, and peripelvic cysts.
Differentiate renal cyst from hypoechoic renal artery aneurysm using color Doppler US.
Cysts that contain calcium, septations, and irregular margins (complicated cysts) need further workup.
Complicated cysts are cysts that do not meet the criteria of simple cysts and thus require further workup.
Category 1: benign simple cyst
Simple cysts contain low-attenuation (0–20 HU) fluid and a hairline-thin smooth wall.
Cysts do not contain septations, calcifications, or enhancing nodular soft tissue.
Category 2: minimally complicated cysts that are benign but have certain radiologic findings of concern.
Includes septated (paper-thin septations) cystic, minimally calcified cysts, high-density cysts.
Fine calcification or a short segment of slightly thickened calcification may be present in the wall or septa.
Hyperattenuating cysts contain more fluid than water attenuation (i.e., >20 HU). In general, cysts that measure between 20 and 40 HU are proteinaceous cysts and will show findings of a simple cyst using US; those with attenuations over 40–50 HU are likely to be hemorrhagic cysts and will be complex under US. When a hyperattenuating renal mass is encountered on an unenhanced CT scan, the probability of the mass being benign is more than 99% as long as the attenuation is 70 HU or more and the mass is homogeneous.
Category 2F (the “F” means to follow) lesions cannot be considered benign without some period of observation.
Slightly more complicated (e.g., may contain multiple, hairline-thin septa that demonstrate perceived [not measurable] enhancement).
May have minimal smooth thickening of the wall or septa.
May contain thick irregular or nodular calcification.
No enhancing soft tissue components.
Hyperattenuating renal masses that exhibit all the features of hyperattenuating cysts but are larger than 3 cm and are completely intrarenal are also included in this category.
Recommended follow-up is to perform a CT or MR examination at 6 months, followed by yearly studies for a minimum of 5 years.
Category 3: complicated cystic lesions that exhibit some radiologic features seen in malignancy
This category includes multiloculated cystic nephroma, multiloculated cysts, complex septated cysts, chronically infected cysts, heavily calcified cysts, and cystic renal cell carcinoma (RCC).
Because these entities are difficult to separate radiologically, surgery is usually performed.
Category 4 lesion: clearly malignant lesions with large cystic component.
Irregular margins, solid vascular elements
| Category | US Features | Workup |
|---|---|---|
| Type 1: Simple cyst | Round, anechoic, thin wall, enhanced through-transmission | None |
| Type 2: Mildly complicated cyst | Thin septation, calcium in wall | CT or US follow-up |
| Type 3: Indeterminate lesion | Multiple septa, internal echoes, mural nodules | Partial nephrectomy, biopsy |
| Thick septa | CT follow-up if surgery is high risk | |
| Type 4: Clearly malignant | Solid mass component | Nephrectomy |
Septations
Thin septa within cysts are usually benign.
Thick or irregular septa require workup.
Calcifications
Thin calcifications in cyst walls are usually benign.
Milk of calcium: collection of small calcific granules in cyst fluid: usually benign
Thick wall
Usually require surgical exploration
Increased CT density (>15 HU) of cyst content
Vast majority are benign.
High density is usually due to hemorrhage, high protein content, and/or calcium.
50% appear as simple cysts using US.
The remainder of patients require a further imaging workup (to exclude soft tissue mass) or occasionally cyst puncture (analyze fluid, inject contrast).
Diagnosis
Complex, high-density cyst (type 2) ≥3 cm
Evaluate fluid for character, as well as cytology
After aspiration, inject contrast (“cystogram”) and obtain multiple projections so that all surfaces of the wall are smooth.
Therapy
Most commonly performed for large cyst obstructing collecting system or causing dull, aching pain or, rarely, HTN (Page kidney)
If the cyst is simple and the fluid is clear, yellow, and free-flowing, laboratory analysis is not necessary. Bloody or brownish fluid should be sent for cytology.
If a symptomatic cyst recurs after aspiration, percutaneous ablation may be considered to avoid surgery.
Place a 20-gauge needle into cyst and measure total volume aspirated. Some interventional radiologists prefer a small pigtail catheter.
Inject contrast to exclude communication with the collecting system, which would preclude alcohol ablation.
Inject absolute ethanol, 25% of the volume of cyst fluid aspirated.
Leave ethanol in place 15–20 min, turning the patient to different positions to maximize wall contact of alcohol with different surfaces.
Aspirate residual ethanol.
Not a true cyst but a calyceal diverticulum, which may be communicating or closed off
Contains layering calcific granules (calcium carbonate)
No pathologic consequence
Originates from renal parenchyma but expands into the renal sinus
May cause compression of the collecting system
Originates from sinus structures, most likely lymphatic in origin
May be indistinguishable from hydronephrosis on US, requiring an IVP or CT for definitive diagnosis
Attenuated, stretched infundibula
IVP differential diagnosis (DDx): renal sinus lipomatosis
Located beneath the renal capsule
Not true cysts; represent extravasated urine trapped beneath renal capsule (pseudocysts, uriniferous cysts, urinoma)
Benign, acquired unilateral condition characterized by multiple cysts of varying size. Cysts, separated by normal renal parenchyma, may occupy the entire kidney or be more localized. Calcification of cyst walls may be present but the cyst wall has to be thin; it does not compromise renal function. No surgery necessary.
Spectrum of diseases characterized by tubulointerstitial fibrosis. Patients usually present with azotemia and anemia and subsequently progress to end-stage failure.
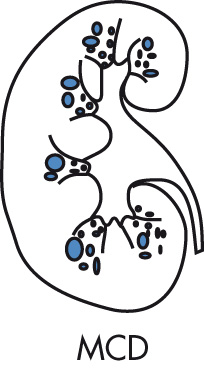
Familial nephronophthisis, 70%, autosomal recessive (AR)
Juvenile type, onset at age 3–5 years (most common); adult type
Adult MCD, 15%, autosomal dominant (AD)
Renal-retinal dysplasia, 15%; recessive associated with retinitis pigmentosa
Small kidneys (as opposed to large kidneys in polycystic disease)
Multiple small (<2 cm) cysts in medulla
Cysts may be too small to resolve by imaging, but their multiplicity will result in increased medullary echogenicity and apparent widening of central sinus echoes.
Cortex is thin and does not contain cysts.
No calcifications
Cystic dilatation of collecting tubules, as well as nephrons (unlike MCD and infantile polycystic kidney disease in which only the collecting tubules are involved). AD trait (childhood type is AR). Incidence: 0.1% (most common form of cystic kidney disease; accounts for 10% of patients on chronic dialysis). Slowly progressive renal failure. Symptoms usually begin in 3rd or 4th decade, but clinical onset is extremely variable, ranging from palpable cystic kidneys at birth to multiple cysts without symptoms in old age. Enlarged kidneys may be palpable. Treatment is with dialysis and transplant. No increased risk of malignancy.
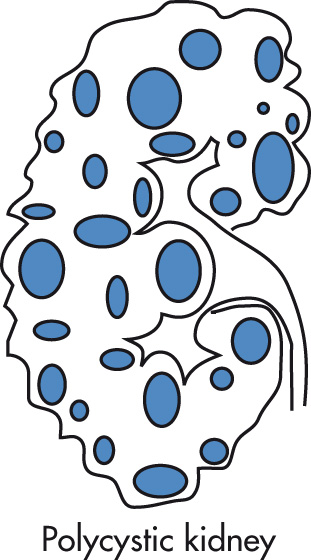
Hepatic cysts, 70%
Intracranial berry aneurysm, 20%
Cysts in pancreas and spleen, <5%
Kidneys are enlarged and contain innumerable cysts, creating a bosselated surface.
Calcification of cyst walls is common.
Pressure deformities of calyces and infundibula
IVP: “Swiss-cheese” nephrogram
Cysts have variable signal characteristics.
CT: hypodense, hyperdense (hemorrhage, protein, calcium)
T1-weighted (T1W): some cysts contain clear, watery fluid (hypointense); others contain blood and protein (hyperintense); layering in some cysts is caused by cellular debris.
Hepatic cysts
40% of patients with end-stage renal disease develop renal cysts. Incidence increases with time on dialysis so that incidence of UCD is 90% in patients on dialysis for 5 years. Associated complications include:
Increased incidence of RCC
Hemorrhage of cysts
Cysts can regress after successful transplantation.
Renal parenchymal tumors
Renal cell adenocarcinoma, 80%
Wilms tumor, 5%
Adenoma (thought to represent early RCC)
Oncocytoma
Nephroblastomatosis
Mesoblastic nephroma
Mesenchymal tumors
Angiomyolipoma (AML)
Malignant fibrous histiocytoma (MFH)
Hemangioma
Other rare tumors
Renal pelvis tumors
Transitional cell carcinoma (TCC) <10%
Squamous cell carcinoma
Other malignant tumors: undifferentiated adenocarcinoma tumors
Benign tumors: papilloma > angioma, fibroma, myoma, polyp
Secondary tumors
Metastases
Lymphoma
Synonyms: RCC, hypernephroma, clear cell carcinoma, malignant nephroma
Hematuria, 50%
Flank pain, 40%
Palpable mass, 35%
Weight loss, 25%
Paraneoplastic syndrome: HTN (renin), erythrocytosis (erythropoietin), hypercalcemia (PTH), gynecomastia (gonadotropin), Cushing syndrome (ACTH)
Clear cell type (65% of RCC). Cell origin: proximal tubule. Cytogenetic abnormalities: chromosome 3p deletions, mutations of VHL gene (tumor suppressor gene)
Papillary cell type (chromophil) (15%). Cell origin: proximal tubule. Cytogenetic abnormalities: trisomies of chromosomes 3q, 7, 12, 16, 17, 20; loss of Y chromosome
Chromophobe cell type (10%). Cell origin: intercalated cell of cortical collecting duct. Cytogenetic abnormalities: monosomies of chromosomes 1, 2, 6, 10, 13, 17, and 21; –hypodiploidy
Oncocytoma (5%). Cell origin: intercalated cell of cortical collecting duct. Cytogenetic abnormalities: loss of chromosomes 1 and Y
Unclassified cell type (5% of RCC). Sarcomas, collecting duct tumors, others
Tobacco use
Long-term phenacetin use
VHL disease (bilateral tumors)
Chronic dialysis (>3 years)
Family history
| Clear cell RCC | Von Hippel–Lindau disease; tuberous sclerosis |
| Papillary RCC type 1 | Hereditary papillary renal cell cancer |
| Papillary RCC type 2 | Hereditary leiomyoma and renal cell carcinoma |
| Chromophobe and oncocytic neoplasms | Birt–Hogg–Dubé |
| Medullary carcinoma | Sickle cell trait |
5-year survival: stage I = 81%, stage II = 74%, stage III = 53%, stage IV = 8%
Tumors often have atypical behavior:
Late recurrence of metastases: 10% recur 10 years after nephrectomy.
Some patients survive for years with untreated tumor.
Spontaneous regression of tumor has been reported but is very rare.
Imaging findings
Mass lesion: renal contour abnormality, calyceal displacement
Large variability in signal characteristics on noncontrast CT and MRI depending on the degree of hemorrhage and necrosis
Contrast enhancement is usually heterogeneous; strong contrast enhancement (>15 HU)
Calcification, 10%
Cystic areas (2%–5% are predominantly cystic)
Filling defects (clots, tumor thrombus) in collecting system and renal veins.
US appearance
Hyperechoic: 70% of tumors >3 cm, 30% of tumors <3 cm
Hypoechoic
Angiography
95% of tumors are hypervascular.
Caliber irregularities of tumor vessels are typical (encasement).
Prominent AV shunting, venous lakes
Angiography may be useful for detection in complicated and equivocal cases:
Small tumors
Underlying abnormal renal parenchyma
VHL disease
Preoperative embolization
MRI
Venous invasion of tumor is better diagnosed by MRI.
Using a 0.1-mmol/kg dose of gadolinium (Gd)-chelate, the percentage of enhancement suggestive of RCC is 15%, obtained after 2–4 min of contrast administration (1.5T).
DDx for enhancing renal lesions: RCC, oncocytoma, and AML. The likelihood of RCC increases with size and extension beyond kidney into Gerota fascia.
Stage I: tumor confined to kidney
Stage II: extrarenal (may involve adrenal gland) but confined to Gerota fascia
Stage III: A: venous invasion (renal vein); B: lymph node metastases; C: both
Stage IV: A: direct extension into adjacent organs through Gerota fascia; B: metastases
Lungs, 55%
Liver, 25%
Bone, 20% (classic lesions are lytic, expansile)
Adrenal, 20%
Contralateral kidney, 10%
Other organs, <5%
Radical nephrectomy (entire contents of Gerota fascia are removed)
Ablation (radiofrequency, microwave, cryo)
Partial nephrectomy
Chemotherapy
Radiotherapy is used for palliation only.
Radiofrequency, cryoablation, and microwave ablation are noninvasive options for treating RCC. High-frequency alternating current is applied to a metallic applicator placed within the tumor. The surface area of the electrode is small, which results in a high current density at the electrode surface resulting in heat. When tissues reach temperatures greater than 50°C, they undergo necrosis.
Comorbidities and contraindications to conventional or laparoscopic surgery
Refusal of conventional surgery
Compromised renal function; patients who have undergone a nephrectomy
High risk of recurrence (e.g., VHL disease)
Tumors >3 cm can be difficult to treat with current ablation technology. Larger tumors often require multiple overlapping ablations, which increase the risk of complications.
Tumors >5 cm have a higher incidence of recurrence.
Complete ablation of central tumors is difficult owing to heat sink effect of large vessels within the renal hilum. Also, ablating central tumors poses the risk of injury to the collecting system or ureter.
Anterior location of tumors can lead to colonic injury. This can be avoided with hydrodissection.
Tumors are ablated under general anesthesia or deep conscious sedation.
CT is the preferred modality for renal tumor ablation because of high spatial resolution and its ability to image structures that need to be avoided. US can also be used for superficial and peripheral exophytic lesions.
Pain may last for several days to weeks.
Postablation syndrome: fever, malaise, and body aches. Severity is related to volume of tissue ablated.
Hemorrhage, often self-limited
Injury to ureter, central collecting system, or adjacent organs
Most solid and complex cystic masses suspicious for primary renal malignancy are ultimately surgically removed. Established criteria for renal mass biopsy include:
Renal mass presenting in a patient with other primary malignancy to rule out metastasis
Rule out focal pyelonephritis
Comorbid disease increases risk for surgery (e.g., solitary kidney)
Unresectable mass, confirmation of diagnosis before systemic therapy
Confirmation of diagnosis before percutaneous ablation
Hyperdense homogeneously enhancing mass, which may be a lipid-poor AML
Bosniak category 3 lesion
Multiple solid masses
Incidence of renal involvement in lymphoma is 5% (non-Hodgkin lymphoma [NHL] > Hodgkin disease) at diagnosis and 30% at autopsy. Three patterns of involvement:
Direct extension from retroperitoneal disease (common)
Hematogenous dissemination (common)
Primary renal lymphoma (i.e., no other organ involvement) is rare, because kidneys do not have primary lymphatic tissue.
Multiple lymphomatous masses (hypoechoic, hypodense), 50%
Diffuse involvement of one or both kidneys
Adenopathy
Incidence: 20% of cancer patients at autopsy. Common primary lesions are lung, breast, and colon cancer and melanoma.
Hamartomas containing fat, smooth muscle, and blood vessels. Small lesions are not treated; large and symptomatic lesions are resected or embolized. Unlikely to bleed if <4 cm. Complication: tumors may spontaneously bleed because of their vascular elements.
Tuberous sclerosis: 80% of patients with tuberous sclerosis have AML, typically multiple, bilateral lesions. However, <40% of patients with AML have tuberous sclerosis. In the absence of tuberous sclerosis, 5% of all AML patients will have multiple, bilateral AML.
Lymphangiomyomatosis
Fat appears hypodense (CT), hyperechoic (US), and hyperintense (T1W). The presence of fat in a renal lesion is virtually diagnostic of AML. There have been only a few case reports of fat in RCC or in oncocytomas. Five percent do not demonstrate fat on CT. Caveat: be certain that fat associated with a large mass is not trapped in renal sinus or peripheral fat.
Predominance of blood vessels
Strong contrast enhancement
T2W hyperintensity
Predominance of muscle: signal intensity (SI) similar to that of RCC
AMLs do not contain calcifications; if a lesion does contain calcification, consider other diagnosis, such as RCC.
Angiography: tortuous, irregular, aneurysmally dilated vessels are seen in 3%. Presence depends on the amount of angiomatous tissue. Predominantly myxomatous AMLs may be hypovascular.
Best described as adenocarcinoma with no metastatic potential. Usually detected at autopsy.
These tumors arise from oncocytes (epithelial cell) of the proximal tubule. Although the majority of lesions are well differentiated and benign, these tumors are usually resected because of their malignant potential and the difficulty in differentiating them from RCC preoperatively. Can consider performing biopsy followed by observation in poor surgical candidates. Represent 5% of renal tumors.
Central stellate scar (CT) and spoke wheel appearance (angiography) are typical but not specific (also seen in adenocarcinoma).
Well-defined, sharp borders
Radiographically impossible to differentiate from RCC
Secretion of renin causes HTN, hypernatremia, and hypokalemia (secondary aldosteronism). Most patients undergo angiography as part of the workup for HTN. Tumors appear as small hypovascular masses. Rare.
Most tumors arising from the renal pelvis are malignant with transitional cell cancer being the most common tumor. Papillomas are the most common benign tumor.
No malignant potential
20% are associated with uroepithelial malignancies elsewhere, most commonly in bladder.
Tumors are often multifocal: 40%–80% of patients have bladder TCC. However, only 3% of patients with bladder TCC later develop upper tract TCC.
Radiographic finding: irregular filling defect
Obliteration of renal sinus fat and infiltration of renal parenchyma (faceless kidney)
60% recurrence on ipsilateral side
50% have lung metastases.
Staging
Stage I: mucosal lamina propria involved
Stage II: into, but not beyond, muscular layer
Stage III: invasion of adjacent fat/renal parenchyma
Stage IV: metastases
Represent 5% of renal pelvis tumors and <1% of all renal tumors
Frequently associated with leukoplakia or chronic irritation (nephrolithiasis, schistosomiasis)
Uncommon yet distinct epithelial neoplasm of the kidney.
Aggressive malignancy derived from the renal medulla, possibly from the distal collecting ducts of Bellini.
Propensity for showing infiltrative growth, which differs from the typical expansible pattern of growth exhibited by most renal malignancies.
By US, cortical RCC may be hypoechoic to normal renal parenchyma, isoechoic to renal parenchyma, hyperechoic to renal parenchyma, but hypoechoic to renal sinus fat, or isoechoic to renal sinus fat.
By CT, the lesions are medullary in location and have an infiltrative appearance. The reniform contour of the kidney is maintained.
By renal angiography, the tumors are usually hypovascular.
By MR imaging, the tumors are hypointense on T2W images.
Solid or cystic?
Does it arise from parenchyma or collecting system?
Size and exact location
Staging if malignant
Variant vascular or collecting system anatomy
RCC in a solitary kidney
Significant risk factors that predispose to the development of renal failure later in life (e.g., stone disease, chronic infection, vesicoureteric reflux)
Solitary renal tumors <7 cm
Tumors confined to kidney
Location that will not require extensive collecting system or vascular reconstruction
Elective indication
Most common pathogen is Escherichia coli. Less common organisms include other gram-negative bacteria: Proteus, Klebsiella, Enterobacter, Pseudomonas, Neisseria , and Trichomonas vaginalis. “Sterile pyuria” refers to increased urinary white blood cell count (WBC) without being able to culture pathogens. Common causes of sterile pyuria:
Tuberculosis (TB)
Fungal infections
Interstitial nephritis
Glomerulonephritis
Urinary obstruction (e.g., benign prostatic hyperplasia, calculi)
Vesicoureteral reflux
Pregnancy (dilatation of ureters)
Diabetes mellitus
Immune deficiency
Instrumentation
Abscess formation
Xanthogranulomatous pyelonephritis (XGP)
Emphysematous pyelonephritis
Scarring and renal failure
Acute bacterial infection of the kidney and urinary tract (Proteus, Klebsiella, E. coli). Medical treatment is usually initiated without imaging studies. Role of imaging studies:
Define underlying pathology
Obstruction
Reflux
Calculus
Rule out complications
Abscess
Emphysematous pyelonephritis
Determine presence of chronic changes such as scarring
Diabetes mellitus (DM)
Immunosuppression
Obstruction
Focal type (lobar nephronia)
Diffuse type: more severe and extensive
Imaging studies (IVP, CT, US) are normal in 75%. In the remaining 25%, there are nonspecific findings:
Renal enlargement (edema)
Loss of the corticomedullary differentiation (edema)
IVP findings:
Delay of contrast excretion
Narrowing of collecting system (edema)
Striated nephrogram
Ridging of uroepithelium
Areas of decreased perfusion by contrast-enhanced CT
Focal areas of hypodensity in lobar nephronia
Complications: abscess, scarring
Infected renal collecting system usually because of obstruction, calculi, 50% > tumor strictures > postoperative strictures. Penicillin and other antibiotics are sufficient in 35% of cases; remainder of patients require nephrectomy (depending on underlying cause).
US
Best study to differentiate pyonephrosis from uninfected hydronephrosis
Echoes within collecting system
Urine/debris levels
Dense shadowing because of gas in collecting system
Poor through-transmission
CT
Best study to show cause and level of obstruction, as well as complications
Dilated collecting system
Allows detection of perinephric or renal abscess
Interventional procedures
Aspiration for culture and sensitivity (definitive diagnostic study)
Penicillin
Formal antegrade pyelography should be deferred to a second visit so as not to cause sepsis.
Usually caused by gram-negative bacteria, less commonly by Staphylococcus or fungus (candidiasis). Underlying disease: calculi, obstruction, diabetes, AIDS.
Well-delineated focal renal lesion
Central necrosis (no enhancement with IV contrast)
Thickened, hyperemic abscess wall with contrast enhancement
Perinephric inflammatory involvement:
Thickening of Gerota fascia
Stranding of perirenal fat mass
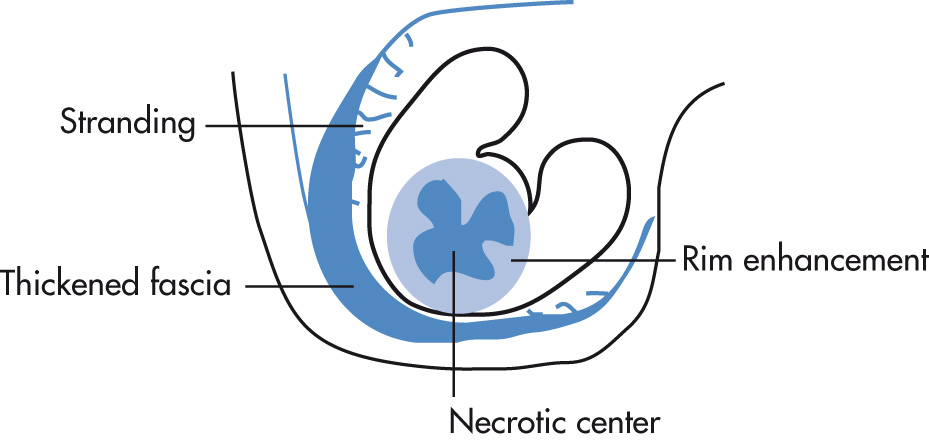
Retroperitoneal spread of abscess
Renocolic fistula
Results most commonly from high-grade ureteral obstruction and infected kidney. Nonrenal causes include duodenal perforation, diverticular abscess, Crohn disease, infected pancreatic fluid collections, and spinal TB, which may spread and cause perirenal as well as psoas abscess. Treatment is with percutaneous drainage.
Most commonly caused by gram-negative bacteria in patients with DM; less commonly in nondiabetics with obstruction. Spectrum includes:
Emphysematous pyelonephritis: gas in renal parenchyma and collecting system. Mortality: 60%–80%.
Emphysematous pyelitis: gas in collecting system (“air pyelogram”). Mortality: 20%.
Gas in collecting system and/or renal parenchyma
Gas may extend to Gerota fascia (high mortality)
Nephrectomy
In poor surgical candidates or in patients with focal disease, percutaneous drainage has been used as a temporizing or, occasionally, definitive therapy.
Chronic suppurative form of renal infection characterized by parenchymal destruction and replacement of parenchyma with lipid-laden macrophages. Diffuse form, 90%; focal form, 10%. Ten percent of patients have DM. Rare.
Large or staghorn calculus (thought to cause obstruction and inflammatory response), 75%; in the remaining 25% of patients, XGP is due to UPJ obstruction or ureteral tumors.
Enlarged, nonexcreting kidney
Multiple nonenhancing low-attenuation masses (–10 to 30 HU): xanthomatous masses. The masses may extend beyond the kidney into the perinephric space. There may be a thin peripheral ring of enhancement.
Fine calcifications may be present in xanthomatous masses.
Thickened Gerota fascia
May be associated with psoas abscess
Also known as replacement fibrolipomatosis, replacement represents extreme form of renal sinus lipomatosis in which infection, long-term hydronephrosis, and calculi are associated with severe renal parenchymal atrophy. Calculi and inflammation are present in >70% of cases.
Enlarged renal outline, fatty lucent mass, and staghorn calculus
IVP shows poorly functioning or nonfunctioning kidney.
On US, kidney is enlarged but has a preserved shape. Residual hypoechoic renal parenchymal rim is surrounded by hyperechoic areas of fatty proliferation in both renal hilum and perinephric space.
CT is the imaging modality of choice to best demonstrate fatty characteristics.
DDxs for this condition include XGP (on IVP only), fat-containing tumors such as AMLs, lipoma, and liposarcoma. CT can aid in differentiating XGP because CT shows attenuation values of –5 to +15 HU in XGP, in contrast to replacement lipomatosis where the fatty tissues measure –100 HU. Fat-containing tumors usually produce a mass effect and show renal function, unlike replacement lipomatosis.
The genitourinary (GU) tract is the second most common site of tuberculous involvement after the lung. GU disease is typically due to hematogenous spread. Clinical findings include history of pulmonary TB, pyuria, hematuria, and dysuria.
Renal
Ureteral
Bladder
Seminal vesicles (SVs), epididymis
Distribution
Unilateral involvement is more common (70%) than bilateral (30%).
Size
Early, kidneys are enlarged
Later, kidneys are small
Autonephrectomy (nonfunctioning kidney)
Parenchyma
Parenchymal calcifications, 70%
Calcification may take on multiple forms: curvilinear, mottled, or amorphous; “putty kidney” results when calcification has homogeneous, ground-glass appearance.
Papillary necrosis; papillae may be irregular, necrotic, or sloughed
Tuberculoma
Parenchymal scarring, 20%
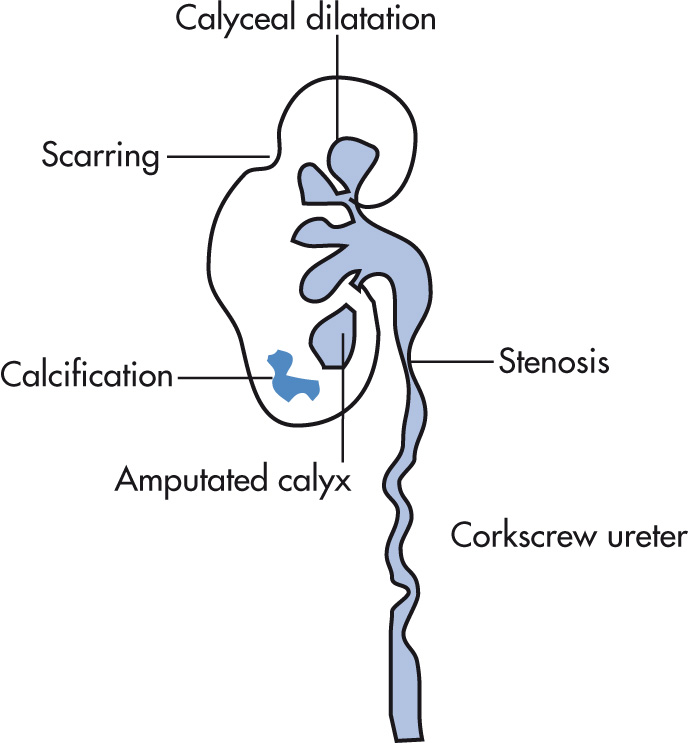
Collecting system
Mucosal irregularity
Infundibular stenosis, hiked-up pelvis with narrowed pelvis pointing up
Amputated calyx
Corkscrew ureter: multiple infundibular and ureteral stenoses (hallmark finding)
“Purse-string” stenosis of renal pelvis
“Pipestem ureter” refers to a narrow, rigid, aperistaltic segment
Renal calculi, 10%
Most common renal fungal infections (coccidioidomycosis, cryptococcosis less common). Common in patients with DM.
Multiple medullary and cortical abscesses
Papillary necrosis because of diffuse fungal infiltration
Fungus balls in collecting system (mycetoma) cause filling defects on IVP; nonshadowing echogenic foci on US
Hydronephrosis secondary to mycetoma
Scalloping of ureters (submucosal edema)
AIDS-related renal abnormalities are seen in most AIDS patients during the course of their illness. AIDS nephropathy refers to irreversible renal failure in 10% of patients and is seen in end-stage disease.
Increased cortical US echogenicity, 70%; (tubulointerstitial abnormalities)
Renal enlargement without hydronephrosis, 40%
Focal hypoechoic (US)/low-attenuation (CT) lesions (infection, tumor), 30%
Acute tubular necrosis (ATN)
Interstitial nephritis
Focal nephrocalcinosis
Infection: Cytomegalovirus (CMV), aspergillus, toxoplasmosis, Pneumocystis jiroveci Frenkel 1999, histoplasmosis, Mycobacterium avium-intracellulare (MAI)
Tumors: increased incidence of RCC, lymphoma, Kaposi sarcoma (KS)
Prostatitis: bacterial, fungal, viral
Prostate abscess
Testicular atrophy: common
Infection: bacterial, fungal, viral
Tumors: germ cell tumors, lymphoma
Renal calcifications can be located in renal parenchyma (nephrocalcinosis), in abnormal tissue (e.g., dystrophic calcification in cysts, tumors), or in the collecting system (e.g., nephrolithiasis, calculi).
Incidence: 5% of population; 20% at autopsy. Recurrence of stone disease, 50%. Symptoms in 50% of patients during first 5 years of stone presence. Predisposing conditions: Calyceal diverticula, Crohn disease, some diversions, stents, renal tubular acidosis, hypercalcemia, and hypercalciuria. The radiographic density of a calculus depends mainly on its calcium content:
Calcium calculi (opaque), 75%
Calcium oxalate
Calcium phosphate
Struvite calculi (opaque), 15%
Magnesium ammonium phosphate: “infection stones” (represent 70% of staghorn calculi, remainder are cystine or uric acid calculi); struvite is usually mixed with calcium phosphate to create “triple phosphate” calculi.
Cystine calculi (less opaque)
Cystinuria, 2%
Nonopaque calculi (on KUB)
Uric acid (gout, treatment of myeloproliferative disorders), 10%
Xanthine (rare)
Mucoprotein matrix calculi in poorly functioning, infected urinary tracts (lucent even on CT); rare
Protease inhibitor indinavir used in HIV treatment can result in radiolucent stones (even on CT).
Calculus (determine size, number, location)
Radiopaque calculus, 90%
Radiolucent calculi are best detected by CT
Renal calculi can be detected by US: hyperechoic focus (calculus), posterior shadowing; calculi 3 mm or less may not be detected.
| Calculus | Phlebolith | |
|---|---|---|
| Shape | Any shape | Round, smooth |
| 90% homogeneously opaque | Central lucency | |
| Location | Along projected tract of ureter | In true pelvis (below distal ureter) |
IVP
Delayed and persistent nephrogram because of ureteral obstruction
Column of opacified urine extends in ureter from renal pelvis to lodged calculus (diminished or absent peristalsis).
Ureter distal to calculus is narrowed (edema, inflammation); may create false impression of stricture.
Ureter proximal to calculus is minimally dilated and straightened: columnization; degree of dilatation has no relation to stone size.
“Steinstrasse:” several calculi are bunched up along the ureter (common after lithotripsy).
Halo appearance (edema) around distal ureter may resemble appearance of ureterocele (pseudoureterocele) or bladder carcinoma. Thickness of radiolucent halo of pseudoureterocele is typically >2 mm as opposed to ureterocele.
CT
CT detects most calculi regardless of calcium content. The exceptions are matrix stones.
Dedicated CT protocol for stone search is performed; rarely need to follow with contrast-enhanced CT (CECT) to differentiate stone in ureter from phlebolith. CECT may obscure a calcified ureteral calculus because it may blend in with high-density contrast material.
Dual energy CT: Acquisition of CT data from two different energy spectra. Most urinary calculi, regardless of composition, appear as opaque densities by conventional CT. In dual energy CT, the differences in x-ray attenuation properties at high and low kVp allow more accurate renal stone differentiation between uric acid- and calcium-containing stones. Stones that appear more dense on water decomposition images than iodine decomposition images have a significant uric acid component.
Location: three narrow sites in the ureter at which calculi often lodge
UPJ: junction of renal pelvis and ureter proper
At crossing of ureter with iliac vessels
UVJ: insertion of ureters into bladder
Forniceal rupture (pyelosinus backflow); inconsequential in isolation if urine is uninfected; chronic leak may result in periureteral/retroperitoneal fibrosis.
Chronic calculous pyelonephritis
XGP in the presence of staghorn calculus
Squamous metaplasia (leukoplakia); more common in pyelocalyceal system and upper ureter than lower ureter or bladder. Cholesteatoma may result from desquamation of keratinized epithelium.
Small renal calculi (<2.5 cm): extracorporeal lithotripsy
Large renal calculi (>2.5 cm): percutaneous removal
Upper ureteral calculi: extracorporeal lithotripsy
Lower ureteral calculi: ureteroscopy
Best results with calcium oxalate and uric acid stones and calculi <2.5 cm. Larger calculi are better treated by percutaneous removal.
Contraindications for ESWL include:
Patient not eligible for anesthesia
Severe bleeding
Pregnancy
UTI
Nonfunctioning kidneys
Gross obesity
Small children
Tall patients (>200 cm)
Distal obstruction
Calyceal neck stenosis
UPJ obstruction
Prostatic enlargement
Renal artery aneurysm
Complications of ESWL
Intrarenal; subscapular and perinephric hematoma
Decrease in effective renal plasma flow
Large stones requiring initial debulking (e.g., staghorn calculus)
Calculi not responding to ESWL (e.g., cysteine stones)
Body habitus precludes ESWL
Patients with certain types of pacemakers
Renal artery aneurysms
Calculi >5 cm
Usually dystrophic calcification
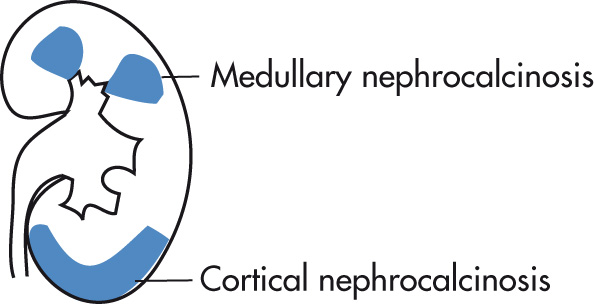
Chronic glomerulonephritis
Cortical necrosis (because of ischemia)
Pregnancy
Shock
Infection
Toxins: methoxyflurane, ethylene glycol
AIDS-related nephropathy
Glomerular sclerosis
Punctate calcifications MAI
Uncommon causes
Rejected renal transplants
Chronic hypercalcemia
Oxalosis
Alport syndrome
Peripheral calcifications (medullary pyramids are spared)
Tramline calcifications are classic: interface of necrotic cortex and viable subcapsular cortex
Columns of Bertin may be calcified.
US: hyperechoic cortex
HPT (hypercalciuria, hypercalcemia), 40%
Renal tubular acidosis (RTA), 20%
Medullary sponge kidney, 20%
Papillary necrosis
Lasix in infancy
Other causes
Nephrotoxic drugs (amphotericin B)
Chronic pyelonephritis
Oxalosis may produce both medullary and cortical nephrocalcinosis.
Bilateral, stippled calcification of medullary pyramids
Calcifications may extend peripherally.
US: hyperechoic medulla
Congenital condition in which there are too many enlarged calyces (20–25; normal is 10–14). Associated with hypoplastic pyramids, resulting in polyclonal, faceted calyces rather than the blunting that is seen with obstruction. There is no obstruction, and the remainder of the collecting system is normal. Renal parenchyma and renal function are normal. The cause is not known; there may be congenital underdevelopment of pyramids, “burnt-out” fetal obstruction, reflux or abnormal branching of collecting system. Associated with megaureter.
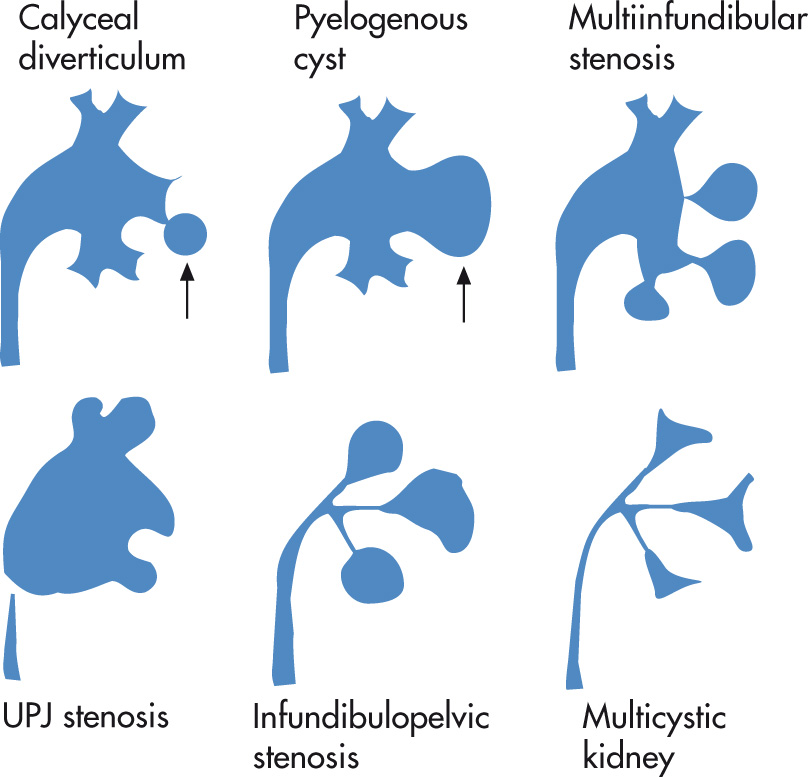
Spectrum of diseases characterized by hypoplasia or aplasia of the upper collecting system:
Calyceal diverticulum
Pyelogenous cyst
Multiinfundibular stenosis
UPJ stenosis
Infundibulopelvic stenosis
Multicystic kidney
Outpouching of calyx into corticomedullary region. May also arise from renal pelvis or an infundibulum. Usually asymptomatic, but patients may develop calculi.
Type I: originates from minor calyx
Type II: originates from infundibulum
Type III: originates from renal pelvis
Cystic lesion connects through channel with collecting system.
If the neck is not obstructed, diverticula opacify retrograde from the collecting system on delayed CT.
May contain calculi or milk of calcium, 50%
Fragmented calculi after ESWL may fail to pass because of a narrow neck. Percutaneous stone retrieval may be indicated.
Cortical divot may overlie diverticulum.
RPN represents an ischemic coagulative necrosis involving variable amounts of pyramids and medullary papillae. RPN never extends to the renal cortex.
Ischemic necrosis
DM
Chronic obstruction, calculus
Sickle cell disease
Analgesics
Necrosis caused by infections
TB
Fungal
Papillae
Enlargement (early)
Small collection of contrast medium extends outside the interpapillary line in partial necrosis.
Contrast may extend into central portion of papilla in “medullary type” RPN.
Eventually contrast curves around papilla from both fornices, resulting in “lobster-claw” deformity.
Sequestered, sloughed papillae cause filling defects in collecting system: “ring sign.”
Tissue necrosis leads to blunted or clubbed calyces.
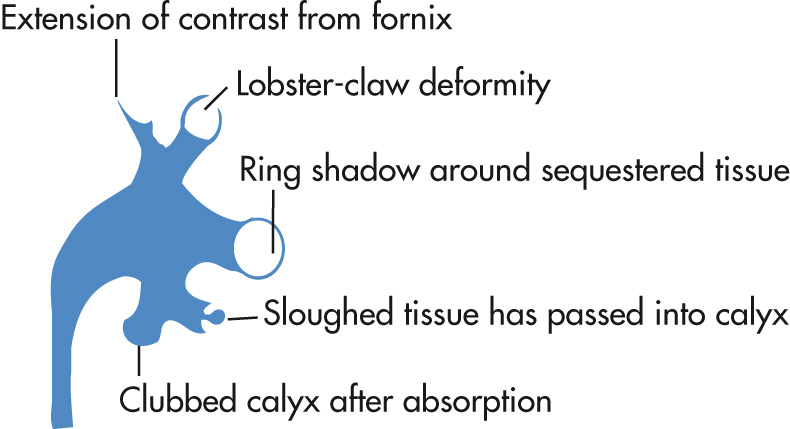
Multiple papillae affected in 85%. Rim like calcification of necrotic papilla occurs.
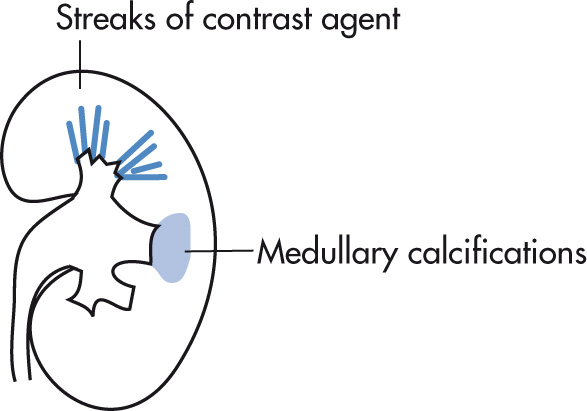
Dysplastic dilatation of renal collecting tubules (ducts of Bellini). Cause: developmental. Usually detected in young adults (20–40 years) as an incidental finding. Usually there are no signs, but there can be urine stasis, UTI, calculi, and hematuria. In 10%, progressive renal failure may develop. Relatively common (0.5% of IVP). May involve one or both kidneys or be confined to a single papilla.
Hemihypertrophy
Associated with Beckwith-Wiedemann syndrome
Congenital pyloric stenosis
Ehlers-Danlos syndrome
Other renal abnormalities: cortical renal cysts, horseshoe kidney, renal ectopia, autosomal-dominant polycystic kidney disease (ADPKD), RTA
Striated nephrogram (contrast in dilated collecting ducts), “brush like” appearance
Cystic tubular dilatation usually 1–3 mm; occasionally larger, usually too small for CT resolution
Punctate calcifications in medullary distribution (located in dilated tubules), 50%
Differentiate on IVP from “papillary blush,” a normal variant, representing amorphous enhancement without tubular dilation, streaks, or globules; nephrocalcinosis; or pyramidal enlargement. Papillary blush is also an inconstant finding on successive IVPs.
Calculi
Tumor
Previous surgery (ligation, edema, clot)
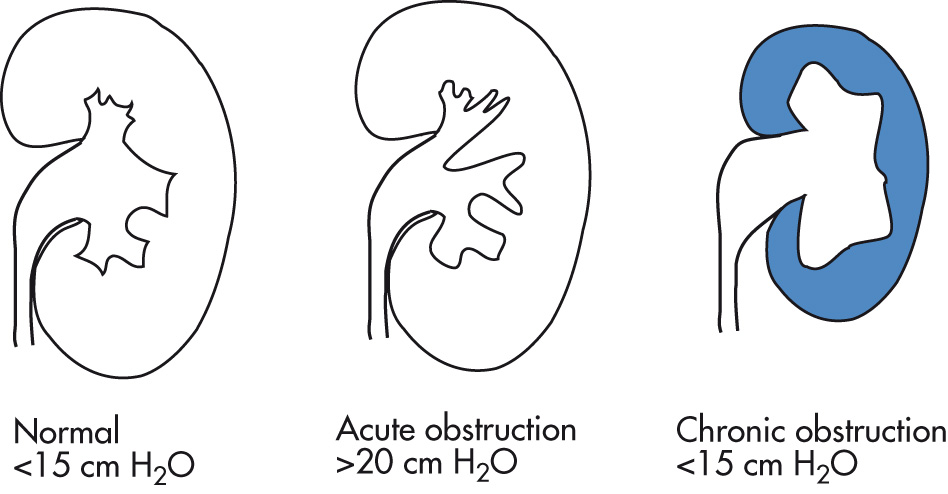
IVP
Kidney
Delayed nephrogram (peak enhancement at >30 min after IV injection, slow fading)
Delayed renal (peak) density may be higher than in normal kidney.
Faint radial striations of nephrogram
Negative pyelogram: nephrogram with delayed pyelogram resulting in dilated unopacified calyces outlined by opacified parenchyma
Dunbar crescents (caliceal crescents): thin rings or crescents at interface with calyx and parenchyma resulting from contrast in dilated collecting ducts; disappear when collecting system is completely opacified
Atrophy of renal parenchyma in chronic obstruction: “rim nephrogram” or “shell nephrogram”
Collecting system
Blunting of forniceal angles
Dilatation of ureter and pelvis; decreased or absent peristalsis
Backflow
US
Sensitivity for detection of chronic obstruction: 90%
Sensitivity for detection of acute obstruction: 60%
Common causes of false-positive examinations:
Extrarenal pelvis
Peripelvic cyst
Vessels: differentiate with color Doppler
Vesicoureteral reflux, full bladder
High urine flow (overhydration, furosemide)
Corrected long-standing obstruction with residual dilatation
Prune-belly syndrome
Common causes of false-negative examinations:
US performed early in disease before dilatation has occurred
Distal obstruction
Pressure-flow study for determining ureteral obstruction or resistance in dilated, nonrefluxing upper urinary tracts. Useful test particularly in patients with surgically corrected obstruction having residual dilatation and/or symptoms. Because the test is invasive and time-consuming, it is usually reserved for cases with equivocal diuretic renograms.
Catheterize bladder and inject contrast to exclude refluxing megaureter.
Obtain percutaneous access to collecting system with 20-gauge needle.
Connect extension tubing, three-way stopcock, and manometer to both antegrade needle and bladder catheter. The bases of both manometers have to be set at the same level as the tip of the antegrade needle.
Connect perfusion pump to antegrade needle and bladder catheter.
Obtain spot and overhead radiographs during perfusion (intermittent fluoroscopic monitoring).
Pressures in collecting system and bladder are recorded during delivery of known flow rates (5, 10, 15 mL/min), and pressure differences are calculated.
Pressure difference of >15 mm is abnormal, and test should be terminated.
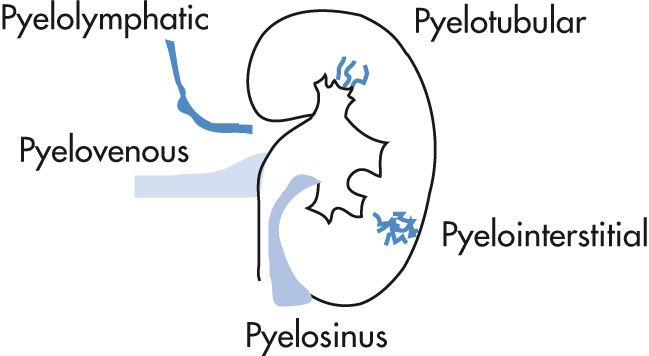
Backflow of contrast material from collecting system into renal or perirenal spaces. Usually caused by increased pressure in collecting system from retrograde pyelography or ureteral obstruction.
Pyelosinus backflow (forniceal rupture): extravasation along infundibula, renal pelvis, ureter
Pyelotubular backflow (no rupture): backflow into terminal collecting ducts; thin streaks with fanlike radiation from the papillae
Pyelointerstitial backflow: extravasation into parenchyma and subcapsular structures; more amorphous than pyelotubular backflow
Pyelolymphatic backflow: dilated lymphatic vessels (may occasionally rupture): thin irregular bands extending from hilum or calyces
Pyelovenous backflow: contrast in interlobar or arcuate veins; rarely seen because venous flow clears contrast material rapidly: renal vein extends superiorly from renal hilum.
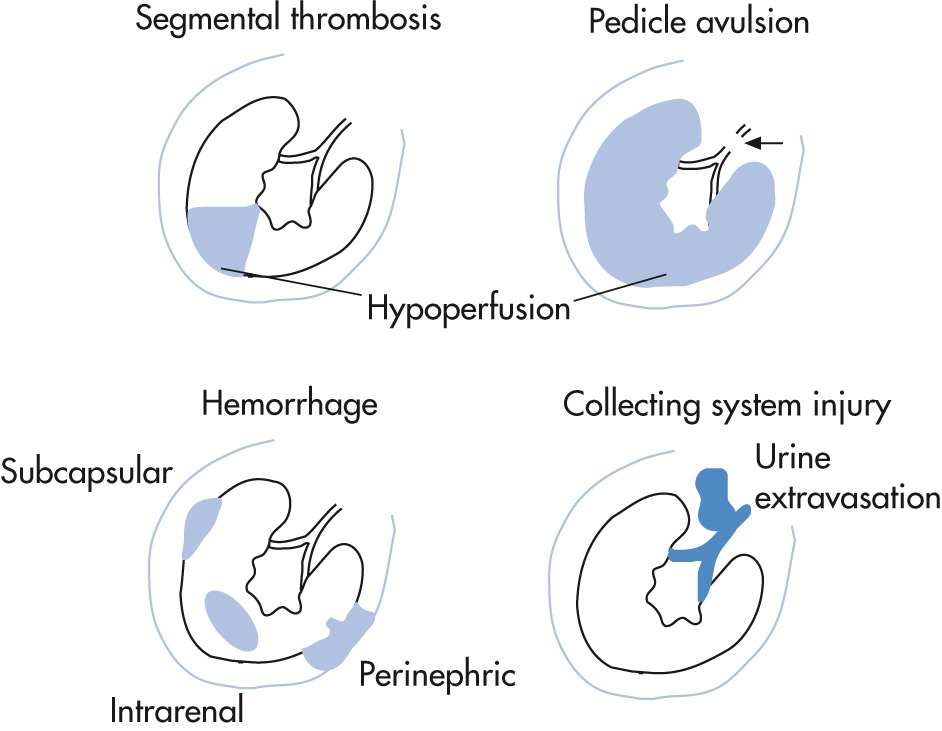
Spectrum of renal injury in trauma:
Renal infarction
Segmental branch
Vascular pedicle avulsion
Hemorrhage (renal laceration, rupture)
Intraparenchymal
Extraparenchymal
Ruptured collecting system
Blunt trauma, 70%–80%
Penetrating trauma, 20%–30%
Minor injuries (conservative treatment), 85%
Hematomas
Contusion (any injury that results in hematuria)
Small lacerations
Subsegmental renal infarcts
Moderate injuries, 10% (management controversial; 15%–50% will eventually require surgery)
Urine leak
Laceration communicating with collecting system
Major injuries (surgical treatment), 15%
Multiple renal lacerations (rupture)
Pedicle injury avulsion, thrombosis
The optimum type of imaging study depends on stability of patient and symptoms (hematuria, blood at meatus, multiple bony fractures):
CT is the study of choice.
One-shot IVP: visualization of both kidneys excludes pedicle avulsion
Indications for angiography:
Nonvisualization of kidney on IVP in patient with abdominal trauma
Persistent hematuria in a patient with abdominal trauma
Hypotension or hypertension or persistent hematuria after an interventional urologic procedure
RVT may be caused by many conditions:
Adults: tumor > renal disease > other causes (nephrotic syndrome, postpartum, hypercoagulable states)
Infants: dehydration, shock, trauma, sepsis, sickle cell disease
Renal vein
Absence of flow (US, CT, MRI)
Intraluminal thrombus
Renal vein dilatation proximal to occlusion
Renal venography: amputation of renal vein
Magnetic resonance venography (MRV) or conventional venography: studies of choice
Kidneys
Renal enlargement
US: hypoechoic cortex (early edema); hyperechoic cortex after 10 days (fibrosis, cellular infiltrates) with preserved corticomedullary differentiationm (CMD); late phase (several weeks): decreased size, hyperechoic kidney with loss of CMD
IVP: little opacification, prolonged nephrogram, striated nephrogram (stasis in collecting tubules); intrarenal collecting system is stretched and compressed by edema
CT may show low-attenuation thrombus in renal vein or simple renal enlargement with collaterals; prolongation of CMD
Loss of CMD
Scintigraphy ( 99m Tc DTPA): absent or delayed renal perfusion and excretion, alternatively may be delayed and reveal a large kidney
Chronic thrombosis
Small kidneys
Collateral veins may cause pelvic and ureteral notches by extrinsic compression.
Rare acquired hemolytic disorder. The renal cortex appears hypointense on T2/T2* because of hemosiderin deposition.
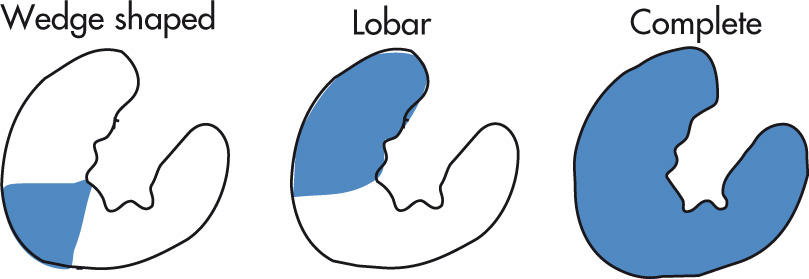
Renal infarcts may be focal and wedge shaped or larger, involving the anterior or posterior kidney or entire kidney. CECT or IVP may show thin, enhancing rim from capsular arteries.
Trauma to renal vessels
Embolism
Cardiac causes (e.g., atrial fibrillation, endocarditis)
Catheter
Thrombosis
Arterial
Venous
Transplant donor evaluation is most commonly done by CT or MRI and involves the following steps:
Location of kidneys. It is important that both kidneys are located in their normal retroperitoneal locations. Pelvic and horseshoe kidneys are associated with complex anomalous vascular and collecting systems, making them difficult to use for transplantation.
The presence of solid and complex cystic renal masses must be excluded. The contralateral kidney should be evaluated in the donor to exclude any neoplasm.
Number of renal arteries supplying the kidneys. Transplant surgeons prefer single arterial anastomosis in the recipient; the presence of multiple renal arteries increases the donor organ warm ischemia time and also increases the complexity of the operation.
Left renal arteries that branch within 2 cm of the aorta are difficult to transplant because there is not a sufficient length of the main trunk for clamping and anastomosis in the recipient.
Accessory renal arteries to the lower pole of the kidneys must be identified. These arteries may supply the renal pelvis and proximal ureter; accidental injury to this vessel can predispose to ureteral ischemia and possible compromise of ureteral anastomosis.
Aberrant renal venous anatomy.
Collecting system anomalies (duplication).
Morphology of normal transplanted kidney:
Well-defined kidney, elliptical contour (i.e., not enlarged)
CMD should be present but may not always be very well defined.
Cortical echogenicity should be similar to liver echogenicity.
The central echo complex should be well defined.
Normal perfusion and excretion by scintigraphy (MAG 3 , DTPA)
Resistive index (P sys – P diast /P sys ) should be <0.7 by Doppler US
ATN
Rejection
Cyclosporine toxicity
Arterial or venous occlusion
Urinary leak
Urinary obstruction
Most common form of acute, reversible renal failure in transplant patients, usually seen within 24 hours. Other causes of ATN are:
Renal ischemia, 60%
Surgery, transplant, other causes
Pregnancy related
Nephrotoxins, 40%
Radiographic contrast material, now controversial. Risk is probably real in patients with GFR ≤30
Aminoglycosides
Antineoplastic agents
Hemoglobin, myoglobin
Chemicals: organic solvents, HgCl 2
Smooth large kidneys
Normal renal perfusion (MAG 3 angiography)
Diminished or absent opacification after IV contrast administration
Persistent dense nephrogram at late time points, 75%
Variable US features:
Increased cortical echogenicity with normal corticomedullary junction
Increased echogenicity of pyramids
Increased renal size, 90% (in chronic rejection, size is decreased)
Thickened cortex may be hypoechoic or hyperechoic
Large renal pyramids, edematous uroepithelium
Indistinct corticomedullary junction
Focal hypoechoic areas in cortex and/or medulla, 20%
Increased cortical echogenicity, 15%
Decrease or complete absence of central echo complex echogenicity
Resistive index >0.7 by Doppler US; nonspecific
| Cause | Flow | Excretion |
|---|---|---|
| ATN | Normal | Reduced |
| Rejection | Reduced | Reduced |
| Vascular compromise | Reduced | Reduced |
ATN is the only renal process with normal renal flow but reduced excretion.
Hyperacute rejection has decreased flow but excretion on delayed images (opposite to ATN).
Cyclosporine toxicity has similar pattern as ATN but occurs later in the posttransplant period.
ATN rarely occurs beyond 1 month after transplant.
Cyclosporine toxicity is uncommon within first month after transplant.
MAG 3 results in better quality images in transplant patients with renal insufficiency compared with DTPA.
RVT: most occur in first 3 days after transplantation.
Renal artery occlusion or stenosis. Anastomotic stenosis is treated with angioplasty with up to 87% success rate.
Infarction
Pseudoaneurysm of anastomosis: surgical treatment
AV fistula: usually from renal biopsy; if symptomatic, embolization is performed.
Ureterovesical anastomosis obstruction may result from edema, stricture, ischemia, rejection, extrinsic compression, or compromised position of kidney.
Perirenal fluid collections occur in 40% of transplants. The collections persist in 15%.
Lymphocele: in 10%–20% of transplants at 1–4 months posttransplant. Usually inferomedial to kidney; linear septations are detectable in 80%. Most lymphoceles are inconsequential; if large and symptomatic or obstructing, percutaneous sclerosis with tetracycline or povidone-iodine may be tried.
Abscess: develops within weeks; complex fluid collection; fever
Urinoma: develops during first month; near UVJ; may be “cold” on nuclear medicine study if leak is not active at time of examination; may be associated with hydronephrosis
Hematoma: immediate postoperative period; hyperechoic by US; pain, hematocrit drop
Ureter does not insert in the normal location in the trigone of the bladder (see Chapter 11 ). Incidence: male-female ratio 1 : 6.
UTI
Obstruction
Incontinence
80% have complete ureteral duplication.
30% have a ureterocele (“cobra head” appearance on IVP).
Males: ureter inserts ectopically into the bladder > prostatic urethra > SVs, vas deferens, ejaculatory ducts
Females: ectopic ureter commonly empties into postsphincteric urethra, vagina, tubes, perineum
Ureter passes behind IVC and exits between aorta and IVC. Medial looping at L2-L3 level is seen on IVP. May result in ureteral narrowing and obstruction.
Ureteral notching (vascular impression), dilatation, or obstruction as a result of ovarian vein thrombosis or varices. Usually associated with pregnancy. Normally the right gonadal vein crosses the ureter to drain into the IVC and the left gonadal vein drains into the left renal vein.
Asymptomatic ureteral and/or pyelocalyceal cysts 2–4 mm in diameter (may be up to 2 cm), usually related to infection or calculi. Radiographically, there are multiple small intraluminal filling defects (cysts originate from degenerated uroepithelial cells). Most common in 6th decade, usually unilateral. May resolve with treatment of underlying infection or remain unchanged for months or years. Not a premalignant condition.
Outpouchings of 1–2 mm produced by outward proliferation of epithelium into lamina propria. Associated with inflammation. Fifty percent eventually develop a uroepithelial malignancy.
Congenital blind-ending ureter. Probably caused by aborted attempt at duplication.
Rare inflammatory condition that most commonly affects the bladder. Yellow-brown subepithelial plaques consist of mononuclear histiocytes that contain Michaelis-Gutmann bodies. On IVP, multiple mural filling defects with flat or convex border are seen, giving a cobblestone appearance. Obstruction is a rare complication.
Ureteral involvement is less common than bladder and collecting system involvement.
Benign tumors
Epithelial: inverted papilloma, polyp, adenoma
Mesodermal: fibroma, hemangioma, myoma, lymphangioma
Fibroepithelial polyp: mobile long intraluminal mass, ureteral intussusception
Malignant tumors
Epithelial: TCC, SCC, adenocarcinoma
Mesodermal: sarcoma, angiosarcoma, carcinosarcoma
50% of patients will develop bladder cancer.
75% of tumors are unilateral.
5% of patients with bladder cancer will develop ureteral cancer.
Intraluminal filling defect
Goblet sign: retrograde pyelogram demonstrates dilated ureteral segment distal to obstruction with filling defect and meniscus.
Bergman coiled catheter sign: on retrograde pyelogram, the catheter is typically coiled in dilated portion of ureter just distal to the lesion.
Sites of metastatic spread of primary ureteral neoplasm (at autopsy):
Retroperitoneal lymph nodes, 75%
Liver, 60%
Lung, 60%
Bone, 40%
Gastrointestinal tract, 20%
Peritoneum, 20%
Other (<15%): adrenal glands, ovary, uterus
Ureter(s) drain into isolated ileal segment, which serves as an isoperistaltic conduit (not as a reservoir). May or may not have a surgical “intussusception” of conduit. Fifty percent of patients have hydronephrosis immediately postoperatively; the hydronephrosis should resolve by 3 months. Common locations of strictures are at the ureteroileal anastomosis and where the left ureter enters the peritoneum.
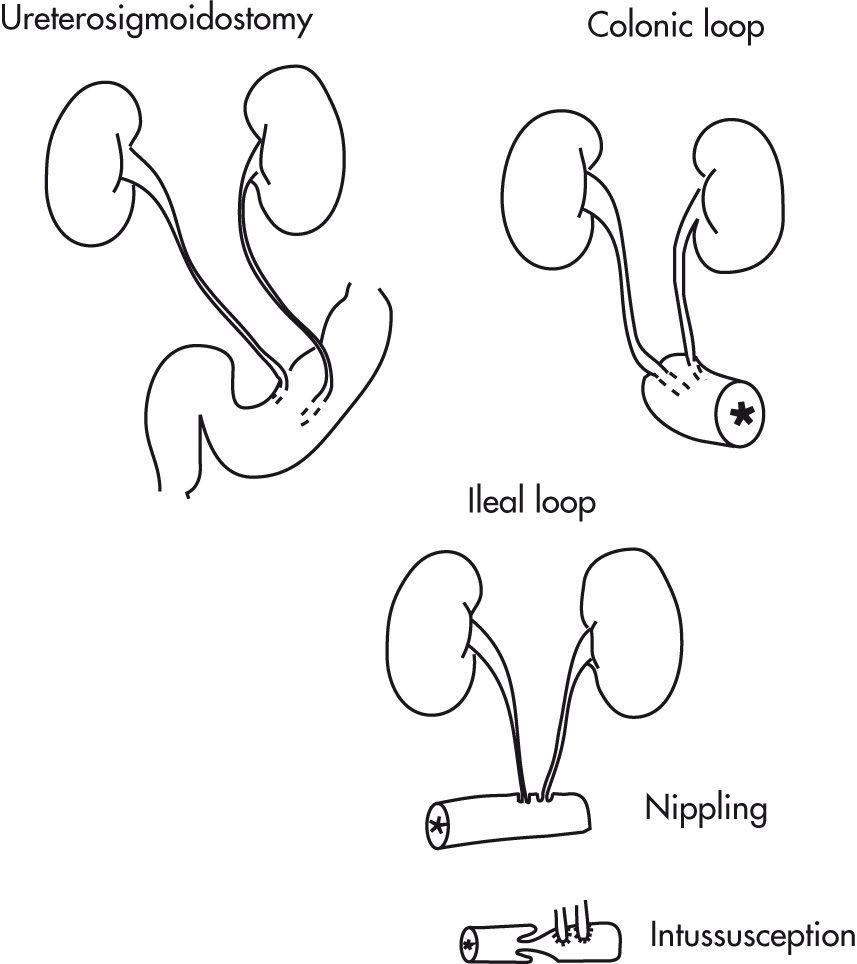
Ureter(s) drain into isolated colon segment. Ureters are tunneled submucosally for antireflux.
Distal ureter(s) drain into sigmoid colon; ureters are tunneled for antiperistalsis. Procedure is largely surpassed by ileal conduit nowadays because of complications (e.g., pyelonephritis, reflux, hyperchloremic acidosis, high risk of colon cancer).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here