Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The Centers for Disease Control and Prevention regularly revises its treatment protocols for sexually transmitted infections (STIs). This information may be accessed online at www .cdc.gov/publications .
Pediculosis pubis, an infestation by the crab louse Phthirus pubis, is characterized by constant itching, predominantly vulvar involvement, and the finding of eggs and lice by visual inspection. It may be treated by topical application of 1% permethrin cream rinse (Nix) or 1% lindane shampoo (Kwell).
Scabies, an infection by the itch mite Sarcoptes scabiei, is characterized by intermittent pruritus, most commonly in the hands, wrists, breasts, vulva, and buttocks. It may be treated by a topical application of 5% permethrin cream or 1% lindane lotion or 30 g of cream.
Genital herpes is a recurrent incurable STI. Approximately 80% of individuals are unaware that they are infected. It is usually transmitted by individuals who are asymptomatic and unaware that they have the infection at the time of transmission.
Nonspecific tests for syphilis, the VDRL and rapid plasma reagin, have a 1% false-positive rate; therefore specific tests such as the Treponema pallidum immobilization, fluorescent-labeled Treponema antibody absorption, and microhemagglutination assay for antibodies to T. pallidum must be used when a positive nonspecific test result is encountered.
In women in the reproductive age range, bacterial vaginosis represents approximately 50% of vaginitis cases and Candida and Trichomonas infections represent approximately 25% each. HIV acquisition is increased in women with bacterial vaginosis and Trichomonas vaginalis infection.
T. vaginalis is a highly contagious STI. It is the most prevalent nonviral, nonchlamydial STI in women. An asymptomatic female patient in whom Trichomonas has been identified in the lower female genital urinary tract should definitely be treated.
Symptoms that suggest cervical infection include vaginal discharge, deep dyspareunia, and postcoital bleeding. Most women who have lower reproductive tract infections caused by Chlamydia trachomatis or Neisseria gonorrhoeae do not have mucopurulent cervicitis. The corollary is that most women who have mucopurulent cervicitis are not infected by C. trachomatis or N. gonorrhoeae.
Acute pelvic inflammatory disease (PID) is usually caused by a polymicrobial infection of organisms ascending from the vagina and cervix, traveling along the mucosa of the endometrium to infect the mucosa of the oviduct. It should be diagnosed with a minimum of suspicion and treated with broad-spectrum antibiotics with the knowledge that overtreatment is preferable to missed diagnosis.
Approximately one in four women with acute PID experiences further medical sequelae, including recurrent acute PID, ectopic pregnancy, and chronic pelvic pain.
For clarity of presentation, discussion of infectious diseases of the female genital tract is divided into those of the lower genital tract, the vulva, vagina, and cervix, and those of the upper genital tract, the endometrium and fallopian tubes; however, the female genital tract has anatomic and physiologic continuity. One must keep in mind that infectious agents that colonize and involve one organ often infect adjacent organs to understand the pathophysiology and natural history of infectious diseases of the genital tract.
The symptoms caused by infections of the lower genital tract produce the most common conditions seen by gynecologists. Therefore the initial focus of this chapter is on clinical presentation and the differential diagnosis of vulvitis, vaginitis, and cervicitis.
Toxic shock syndrome (TSS) and syphilis are also discussed in this chapter. Although the most devastating pathologic processes from these diseases occur in sites other than the genital tract, they often obtain entry into the body through the vulvar, rectal, vaginal, or cervical epithelium.
Many of the infections discussed in this chapter may be acquired through sexual contact and are termed sexually transmitted infections (STIs). STIs often coexist—for example, Chlamydia trachomatis and Neisseria gonorrhoeae. When one disease is suspected, appropriate diagnostic methods must be used to detect other infections.
The Centers for Disease Control and Prevention (CDC) regularly revises management protocols for infections of the female genital tract. Recommendations and medications in this edition are based on the 2015 CDC guidelines. Readers are urged to consult updates in the online CDC guidelines ( http://www.cdc.gov ) because bacterial sensitivities and epidemiologic concerns may lead to changes in treatment protocols.
Similar to skin elsewhere on the body, the vulvar area is subject to primary and secondary bacterial, viral, parasitic, or fungal infections and is sensitive to hormonal and allergic influences. The skin of the vulva is composed of a stratified squamous epithelium containing hair follicles and sebaceous, sweat, and apocrine glands. The subcutaneous tissue of the vulva also contains specialized structures such as the Bartholin glands. Vulvar pruritus accounts for approximately 10% of outpatient gynecology visits . Pruritis may be infectious or have other causes.
Short-duration vulvar pruritis: causes include infection or contact dermatitis.
Long-duration vulvar pruritis: causes infection or contact dermatitis and vulvar dystrophies such as lichen sclerosis, lichen planus, lichen simplex chronicus, and psoriasis.
Mucinous secretions from Bartholin glands provide moisture for the epithelium of the vestibule and open the vagina at 5 and 7 o’clock, in the groove between the hymen and the labia minora. Because they drain via narrow ducts approximately 2 cm long, approximately 2% of adult women develop enlargements of one or both glands. The most common cause is cystic dilation of the Bartholin duct typically caused by distal obstruction secondary to nonspecific inflammation or trauma with subsequent continued glandular fluid secretion resulting in the cystic dilation.
Most women with Bartholin duct cysts are asymptomatic. The cysts vary in size and can be up to 8 cm in diameter; they are usually unilocular, unilateral, tense, and nonpainful. In chronic or recurrent cysts there occasionally are multiple compartments.
In contrast, an abscess of a Bartholin gland ( Fig. 23.1 ) tends to develop rapidly over 2 to 4 days with significant acute pain and tenderness with signs of a classic abscess: erythema, acute tenderness, edema, and, occasionally, cellulitis of the surrounding subcutaneous tissue. Without therapy, most abscesses tend to rupture spontaneously by the third or fourth day. Bartholin gland abscesses are often polymicrobial, reflecting the normal flora of the vagina, and rarely are caused by an STI.
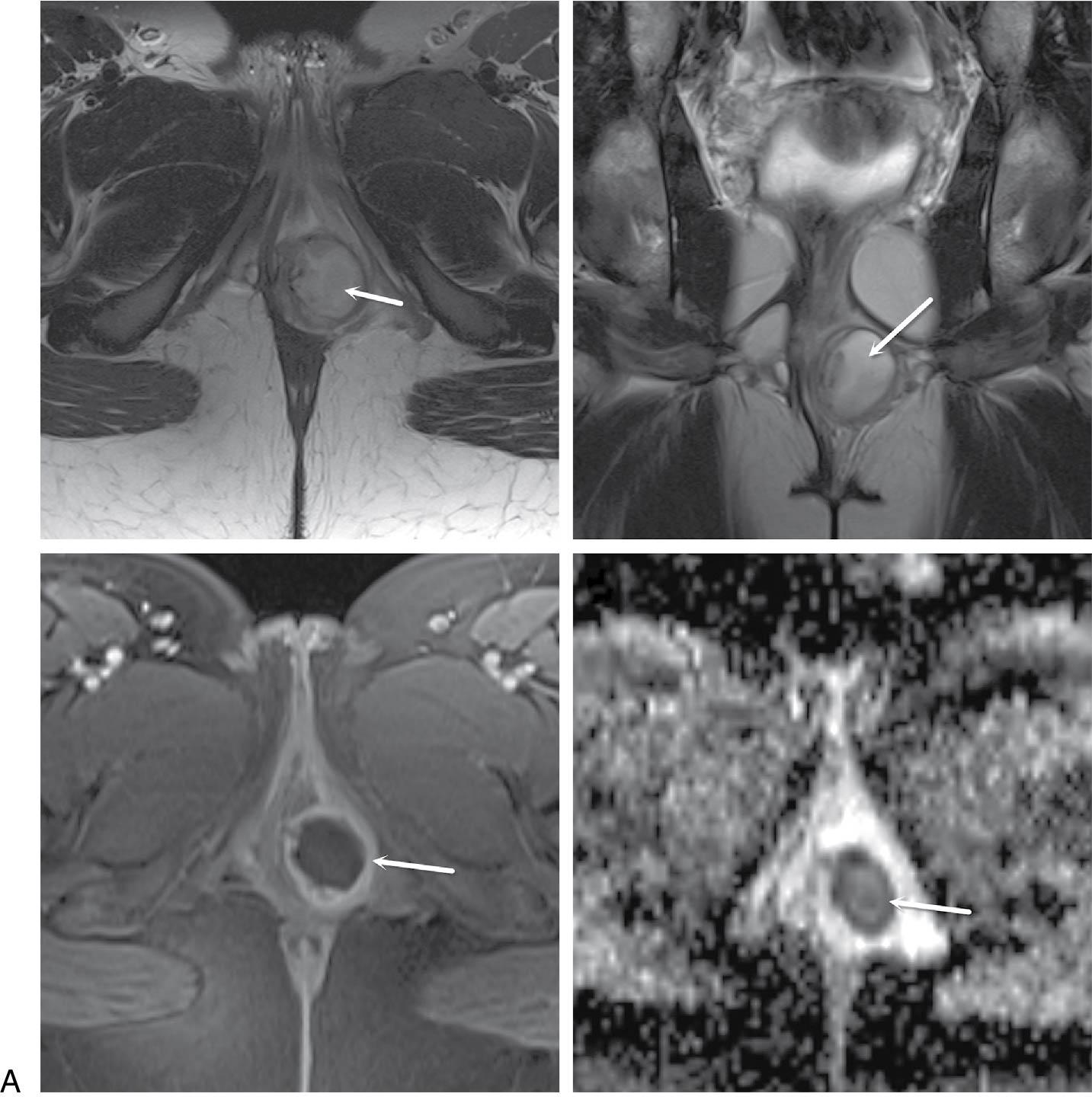
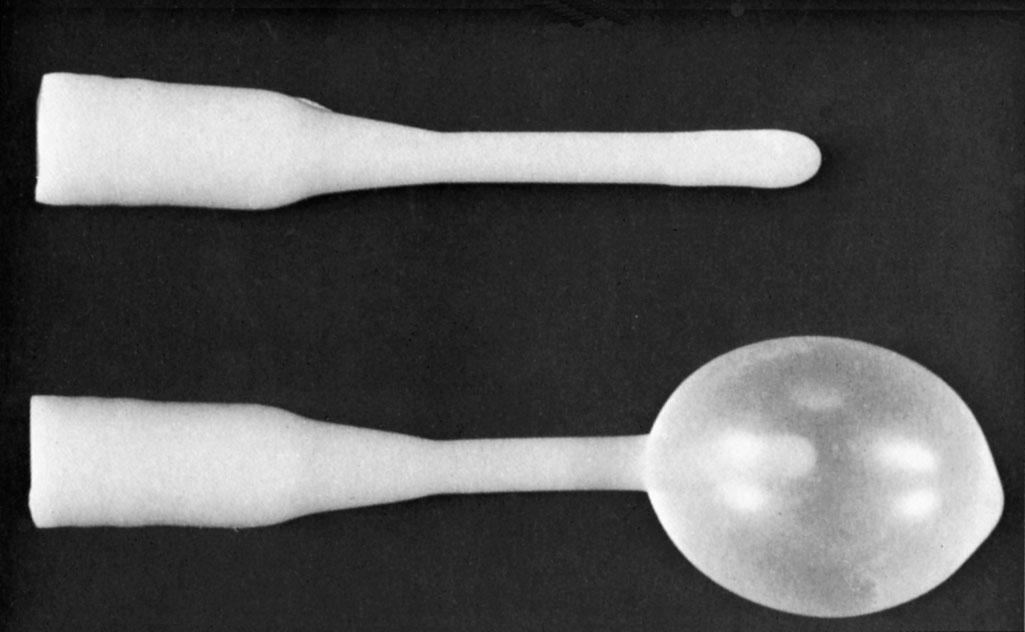
Treatment depends on symptoms. Asymptomatic cysts in women younger than 40 years do not need treatment. Simple incision and drainage often leads to recurrence; hence, marsupialization to develop a fistulous tract from the dilated duct to the vestibule is the surgical treatment of choice, with a 5% to 10% recurrence rate. An alternative surgical approach is to insert a Word catheter, a short catheter with an inflatable Foley balloon, through a stab incision into the abscess and leave it in place for 4 to 6 weeks ( Fig. 23.2 ), enabling a tract of epithelium to form. All the procedures mentioned may be performed with local anesthesia. Antibiotics are not necessary unless there is an associated cellulitis surrounding the Bartholin gland abscess.
Excision of a Bartholin duct and gland is indicated for persistent deep infection, multiple recurrences of abscesses, or recurrent enlargement of the gland in women older than 40 years. Excision requires regional block or general anesthesia and can be challenging because of the rich vascular supply to the region and risk for intraoperative hemorrhage, hematoma formation, fenestration of the labia, postoperative scarring, and associated dyspareunia. Excision of a Bartholin gland for recurrent infection should be performed when the infection is quiescent. Bartholin’s gland carcinoma is exceedingly rare; hence, routine recommendation for excision in women older than 40 with persistent enlargement may not be merited. Drainage and biopsy may be sufficient.
The differential diagnosis of vulvar cysts includes mesonephric cysts of the vagina (generally more anterior and cephalad in the vagina) and epithelial inclusion cysts (more superficial). Rarely, a lipoma, fibroma, hernia, vulvar varicosity, or hydrocele may be confused with a Bartholin duct cyst.
Vulvar pruritis may be caused by animal parasites, the two most common being the crab louse and the itch mite. Ideally, early diagnosis and treatment are of the utmost importance in controlling parasitic infection.
Pediculosis pubis is an infestation by the crab louse, Phthirus pubis, which is a different species from the body or head louse. Lice in the pubic hair are the most contagious of all STIs, with more than 90% of sexual partners becoming infected after a single exposure, and although usually transmitted by close contact, nonsexual transmission of pubic lice via towels or bedding has been documented. P. pubis is generally confined to the hairy areas of the vulva. The incubation period for pediculosis is approximately 30 days.
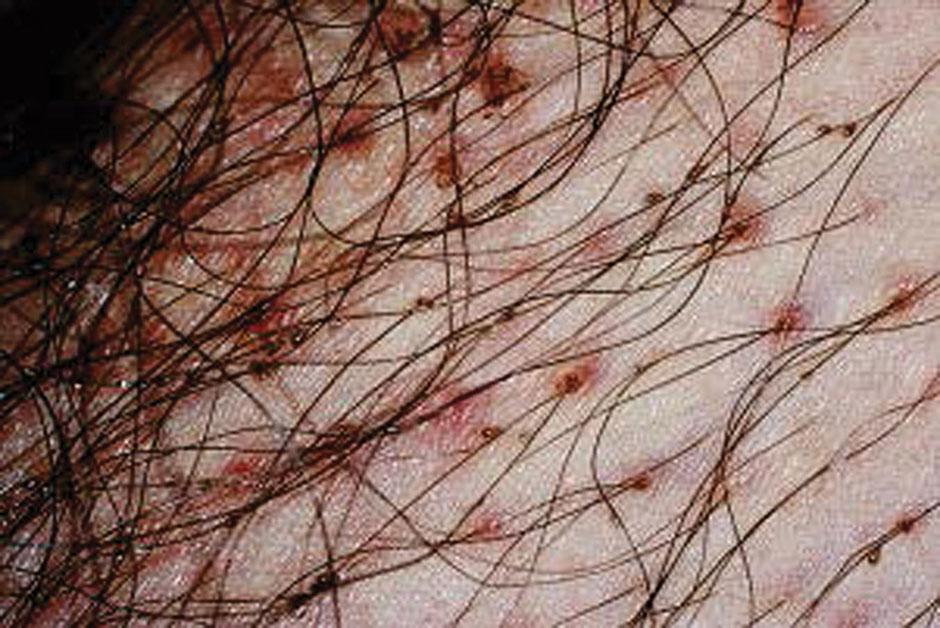
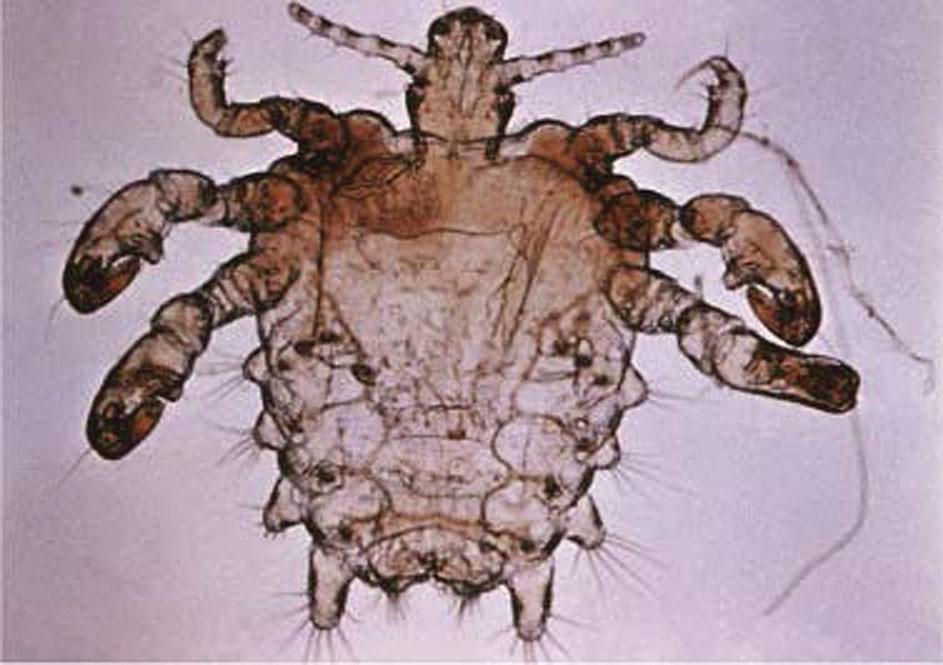
The predominant clinical symptom is constant pubic pruritus caused by allergic sensitization. Initial sensitization typically takes several weeks to develop but may occur as rapidly as 5 days after initial infection. After a reinfection, pruritus may occur within 24 hours. Examination of the vulvar area without magnification demonstrates eggs and adult lice and pepper grain feces adjacent to the hair shafts ( Fig. 23.3 ). The vulvar skin may become secondarily irritated or infected by constant scratching. For definitive diagnosis, one can make a microscopic slide by scratching the skin papule with a needle and placing the crust under a drop of mineral oil. The louse’s body looks like that of a miniature crab, with six legs that have claws on them ( Fig. 23.4 ).
Scabies is a parasitic infection of the itch mite, Sarcoptes scabiei, also transmitted by close contact. Scabies is widespread over the body, without a predilection for hairy areas . Unlike the crab louse, an itch mite travels rapidly over skin and may move up to 2.5 cm in 1 minute. Mites are able to survive for only a few hours away from the warmth of skin.
The predominant clinical symptom of scabies is severe but intermittent itching. Generally, more intense pruritus occurs at night. Initial symptoms usually present approximately 3 weeks after primary infestation. Scabies may present as papules, vesicles, with the pathognomonic sign of scabies the burrow in the. Although any area of the skin may be infected, the hands, wrists, breasts, vulva, and buttocks are the most common ( Fig. 23.5 ). A handheld magnifying lens is helpful for examining suspicious areas. Microscopic slides may be made using mineral oil and a scratch technique Mites lack lateral claw legs but have two anterior triangular hairy buds. Scabies has been termed the great dermatologic imitator, and the differential diagnosis includes almost all dermatologic diseases that cause pruritus. The treatment of pediculosis pubis or scabies involves an agent that kills both the adult parasite and the eggs.
Treatment for pediculosis pubis and scabies can change, so consult the CDC treatment guidelines for updates . The therapy recommended by the CDC’s 2015 guidelines for pediculosis pubis involves the use of permethrin 1% cream rinse, applied to affected areas and washed off after 10 minutes, or pyrethrins, with piperonyl butoxide applied to the affected area and washed off after 10 minutes. None of these should be applied to the eyelids. Retreatment may be necessary if lice are found or if eggs are observed at the hair-skin junction. Patients with pediculosis pubis should be evaluated for other STIs and evaluated after 1 week if symptoms persist. Those who do not respond to one of the recommended regimens should be retreated with an alternative regimen.
To treat scabies, the CDC recommends permethrin 5% cream applied to all areas of the body from the neck down and washed off after 8 to 14 hours or ivermectin, 200 μg/kg orally, repeated in 2 weeks, if necessary. Alternative regimens include lindane 1%, 1 oz of lotion or 30 g of cream applied thinly to all areas of the body from the neck down and thoroughly washed off after 8 hours. Resistance to lindane has been reported in some parts of the United States. Lindane is not recommended as first-line therapy because of toxicity. It should only be used as an alternative if the woman cannot tolerate other therapies or if they have failed. Lindane should not be used immediately after a bath or shower and should not be used by persons who have extensive dermatitis, women who are pregnant or lactating, or children younger than 2 years. Patients with scabies have intense pruritus that may persist for many days after effective therapy. An antihistamine will help alleviate this symptom. Similar to pediculosis pubis, women should be examined 1 week after initial therapy and retreated with an alternative regimen if live mites are observed.
To avoid reinfection by pediculosis pubis or scabies, treatment should be prescribed for sexual contacts within the previous 6 weeks and other close household contacts. Those with close physical contact should be treated at the same time as the infected woman, regardless of whether they have symptoms. Bedding and clothing should be decontaminated (i.e., machine washed, machine dried using the heat cycle, or dry cleaned) or removed from body contact for at least 72 hours. Fumigation of living areas is not necessary. Importantly, women and physicians should not confuse the 1% cream rinse of permethrin dosage recommended for pubic lice with the 5% permethrin cream recommended for scabies.
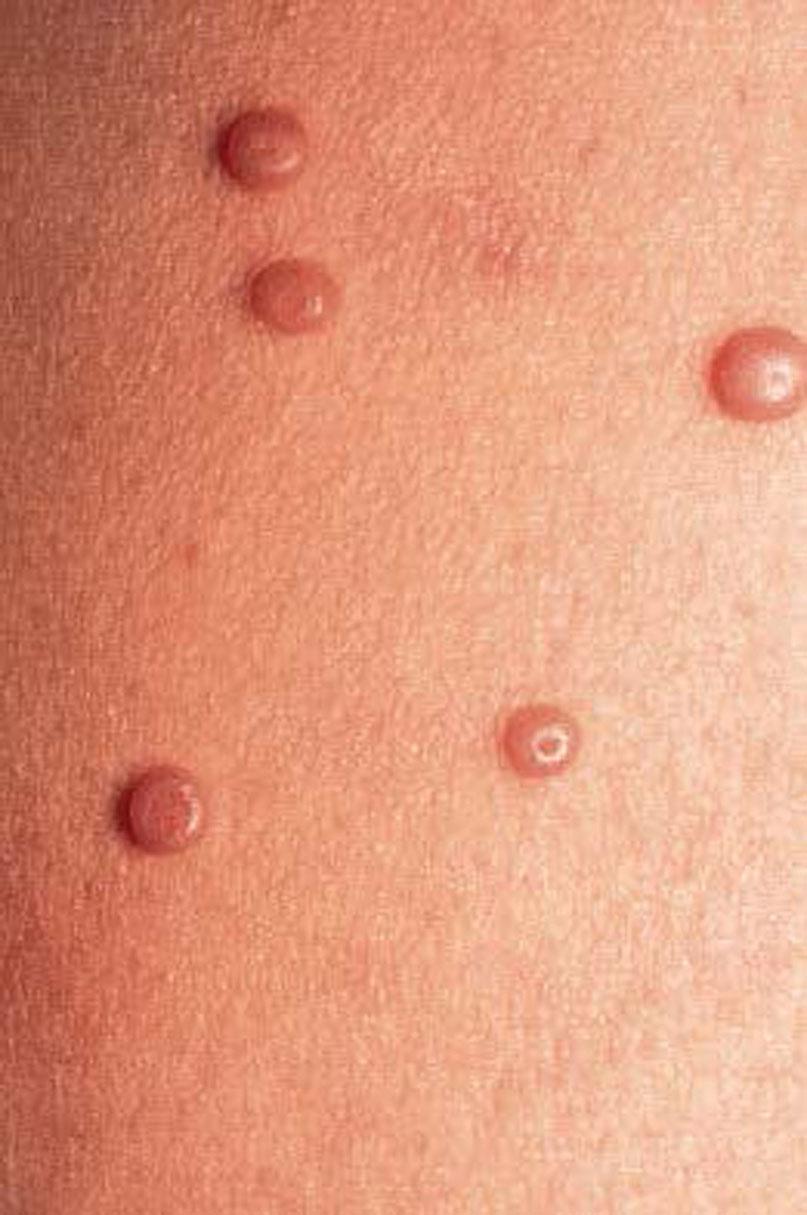
Molluscum contagiosum is a poxvirus spread by direct skin-to-skin contact resulting in flesh-colored, dome-shaped papules with an umbilicated center. Lesions can be spread by autoinoculation, during contact sports, or by fomites on bath sponges or towels. The incubation period is 2 to 7 weeks. In adults it is primarily an asymptomatic disease of the vulvar skin, and, unlike most STIs, it is only mildly contagious. Widespread infection in adults is most closely related to underlying cellular immunodeficiency, such as in human immunodeficiency virus (HIV) infection. It can also occur in the setting of chemotherapy or corticosteroid administration.
Diagnosis is made by the characteristic appearance of the lesions: The small nodules or domed papules of molluscum contagiosum are usually 1 to 5 mm in diameter ( Fig. 23.6 ). Close inspection reveals that many of the more mature nodules have an umbilicated center. The major complication of molluscum contagiosum is bacterial superinfection. The umbilicated papules may resemble furuncles when secondarily infected.
Molluscum contagiosum is usually a self-limiting infection and spontaneously resolves after a few months in immunocompetent individuals; however, treatment of individual papules will decrease sexual transmission and autoinoculation of the virus. After injection of local anesthesia, the caseous material is evacuated and the nodule excised with a sharp dermal curette. The base of the papule is chemically treated with ferric subsulfate (Monsel solution) or 85% trichloroacetic acid. Alternative methods are cantharidin, a chemical blistering agent; imiquimod; or cryotherapy.
In immunocompromised individuals, treatment is more difficult. In women with HIV, there have been multiple reports of recalcitrant molluscum lesions resolving only after initiating highly active antiretroviral therapy (HAART).
Herpes, granuloma inguinale (donovanosis), lymphogranuloma venereum, chancroid, and syphilis may all present as ulcerations in the genital area; however, their causes, disease courses, and treatments are different. Table 23.1 lists some of their major characteristics. Physicians must always consider the possibility of more than one STI concurrently infecting an individual.
| Type | |||||
|---|---|---|---|---|---|
| Parameter | Syphilis | Herpes | Chancroid | Lymphogranuloma Venereum | Donovanosis |
| Incubation period | 2-4 wk (1-12 wk) | 2-7 days | 1-14 days | 3 days-6 wk | 1-4 wk (up to 6 mo) |
| Primary lesion | Papule | Vesicle | Papule or pustule | Papule, pustule, or vesicle | Papule |
| Number of lesions | Usually one | Multiple, may coalesce | Usually multiple, may coalesce | Usually one | Variable |
| Diameter (mm) | 5-15 | 1-2 | 2-20 | 2-10 | Variable |
| Edges | Sharply demarcated Elevated, round or oval |
Erythematous | Undermined, ragged, irregular | Elevated, round or oval | Elevated, irregular |
| Depth | Superficial or deep | Superficial | Excavated | Superficial or deep | Elevated |
| Base | Smooth, nonpurulent | Serous, erythematous | Purulent | Variable | Red and rough (beefy) |
| Induration | Firm | None | Soft | Occasionally firm | Firm |
| Pain | Unusual | Common | Usually very tender | Variable | Uncommon |
| Lymphadenopathy |
|
Firm, tender, often bilateral | Tender, may suppurate, usually unilateral | Tender, may suppurate, loculated, usually unilateral | |
Genital herpes is a recurrent viral infection that is incurable and highly contagious, with 75% of sexual partners of infected individuals contracting the disease. Although among the most prevalent STIs, 80% of infected individuals are unaware that they are infected. Asymptomatic shedding that leads to transmission may occur as frequently as once in 5 days . Excellent online patient education and support can be found www.ashastd.org .
There are two distinct types of herpes simplex virus (HSV), type 1 (HSV-1) and type 2 (HSV-2). Genital HSV-1 transmitted from orolabial lesions to the vulva during oral-genital contact or from genital to genital to contact with a partner with genital HSV-1, is the most commonly acquired genital herpes in women younger than 25 years.
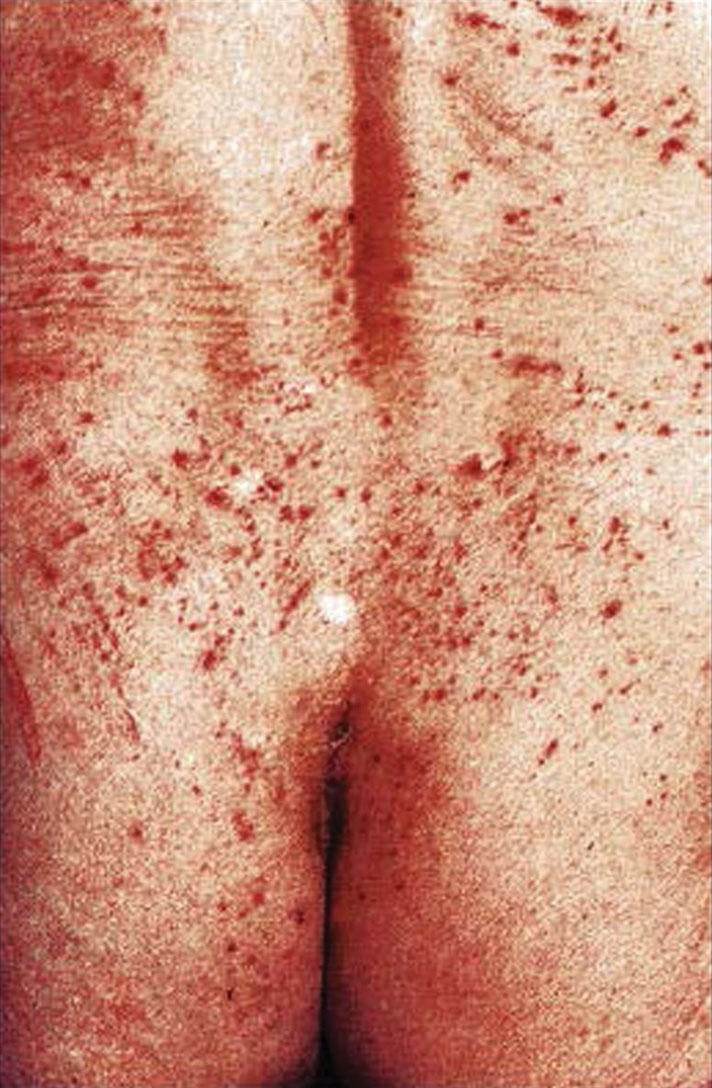
Although subclinical primary herpes infection is common, when the primary infection is symptomatic, both local and systemic disease manifestations occur. Local effects include vulvar skin paresthesia preceding eruption of multiple painful vesicles, which progress to shallow, superficial ulcers over a large area of the vulva. Patients may experience multiple ulcer crops for 2 to 6 weeks, which heal without scarring ( Fig. 23.7 ). Viral shedding may occur for 2 to 3 weeks, and during primary infections, positive cultures for herpesvirus may be obtained from lesions in 80% of women. Severe vulvar pain, tenderness, and inguinal adenopathy and simultaneous involvement of the vagina and cervix are common ( Fig. 23.8 ).
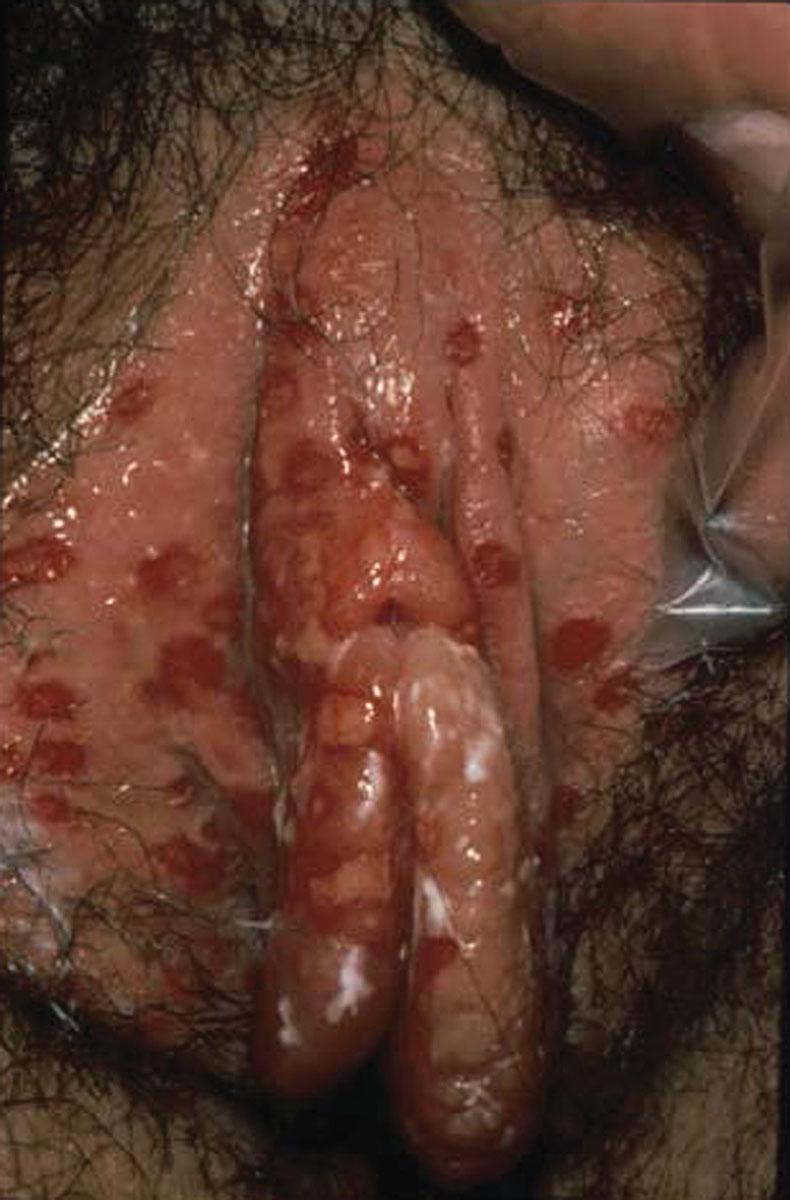
Systemic symptoms, including general malaise and fever, are experienced by 70% of women during the primary infection. Primary infections of the urethra and bladder may result in acute urinary retention, necessitating catheterization. The symptoms of vulvar pain, pruritus, and discharge peak between days 7 and 11 of the primary infection. The typical woman experiences severe symptoms for approximately 14 days.
Recurrent genital herpes is a local disease with less severe symptoms typically with unilateral involvement lasting an average of 7 days. In the first year of HSV-2 infection, 80% of women experience a recurrence, and if the primary HSV-2 infection was severe, the recurrences will occur approximately twice as often. With an initial HSV-1 pelvic infection, there is a 55% chance of a recurrence within 1 year, with the average rate of recurrence slightly less than one episode per year.
A common feature of recurrence is a prodromal phase of sacroneuralgia, vulvar burning, tenderness, and pruritus for a few hours to 5 days before vesicle formation. Extragenital sites of recurrent infection are common. The herpesvirus resides in a latent phase in the dorsal root ganglia of S2, S3, and S4.
The diagnosis of genital herpes is often made clinically by simple inspection. Viral cultures are useful in primary episodes, when culture sensitivity is 80%, but less useful in recurrent episodes. The most accurate and sensitive technique for identifying herpesvirus is the polymerase chain reaction (PCR) assay. Serologic tests determine whether a woman was infected with herpesvirus in the past. The Western blot assay for antibodies to herpes is the most specific method but is not widely available and is difficult to perform. HSV serologic testing should be considered for persons presenting for an STI evaluation, especially for those with multiple sex partners or HIV infection and at increased risk for HIV acquisition. Screening for HSV-1 or HSV-2 in the general population is not indicated .
Enzyme-linked immunoassay (ELISA) and immunoblot tests are available for HSV-1 and HSV-2. Rapid serologic point-of-care tests are available for HSV-2 antibodies. Appropriate screening tests for other STIs should be obtained because they may coexist with herpes.
Treatment of HSV-1 or HSV-2 may be used for three different clinical scenarios summarized in Table 23.2 : primary episode, recurrent episode and daily suppression. Daily suppressive therapy is recommended when the woman has six or more episodes annually or for psychological distress. It is important for patients to be aware that asymptomatic viral shedding can occur even when on daily suppressive therapy.
| Antiviral Agent | |||
|---|---|---|---|
| Indication | Valacyclovir | Acyclovir | Famciclovir |
| First clinical episode | 1000 mg bid, 7–10 days | 400 mg tid; or 200 mg five times/day, 7–10 days | 250 mg tid, 7–10 days |
| Recurrent episodes | 1000 mg daily, 5 days; or 500 mg bid, 3 days | 400 mg tid, 5 days; 800 mg bid, 5 days; or 800 mg tid, 2 days |
|
| Daily suppressive | 1000 mg daily (≥10 recurrences/year) or 500 mg daily (≤9 recurrences/year) | 400 mg bid | 250 mg bid |
In serodiscordant couples a prospective placebo-controlled randomized trial has demonstrated that daily use of valacyclovir for suppression in the seropositive partner results in significantly fewer cases of HSV acquisition in the seronegative partner. Regular use of condoms in serodiscordant couples also decreases transmission but is not 100% protective. Women who are HSV seronegative are three times as likely to acquire HSV infection from seropositive male partners compared with seronegative males acquiring HSV from infected female partners.
The CDC recommends that acyclovir or other suppressive drugs be discontinued after 12 months to determine the subsequent rate of recurrence for each individual woman. Acyclovir is a drug with minimal toxicity, and reports have documented its safety in patients receiving daily therapy for as long as 6 years and with valacyclovir or famciclovir for 1 year; however, even without HSV suppressive treatment, clinical recurrences tend to dramatically decrease in number.
A vaccine would be the logical approach for optimum prevention of herpes. Research is ongoing.
Granuloma inguinale, also known as donovanosis, is a chronic, ulcerative, bacterial infection of the skin and subcutaneous tissue of the vulva. Granuloma inguinale is common in tropical climates such as New Guinea and the Caribbean Islands, but fewer than 20 cases are reported each year in the United States. Spread through both sexual and close nonsexual contact, it is not highly contagious, and chronic exposure is usually necessary with a variable incubation period from 1 to 12 weeks. It is caused by an intracellular, gram-negative, nonmotile, encapsulated rod, Klebsiella granulomatis, which is difficult to culture on standard media. There are no U.S. Food and Drug Administration (FDA)–approved molecular tests for the detection of K. granulomatis DNA. Serologic tests are nonspecific.
The disease course is as follows:
The initial nodule progresses into a painless, slowly progressing ulcer surrounded by granulation tissue.
The ulcer has a beefy red appearance, bleeds easily when touched, and is painless and without regional adenopathy.
Multiple nodules are typically present, resulting in ulcers that grow and coalesce.
Chronic disease eventually destroys the normal vulvar architecture with scarring and lymphatic obstruction, producing marked enlargement of the vulva.
The disease is usually clinically diagnosed in endemic areas but may also be established by identifying Donovan bodies in smears and specimens taken from the ulcers ( Fig. 23.9 ). Donovan bodies appear as clusters of dark-staining bacteria with a bipolar (safety pin) appearance found in the cytoplasm of large mononuclear cells. The differential diagnosis includes lymphogranuloma venereum, vulvar carcinoma, syphilis, chancroid, genital herpes, amebiasis, and other granulomatous diseases.
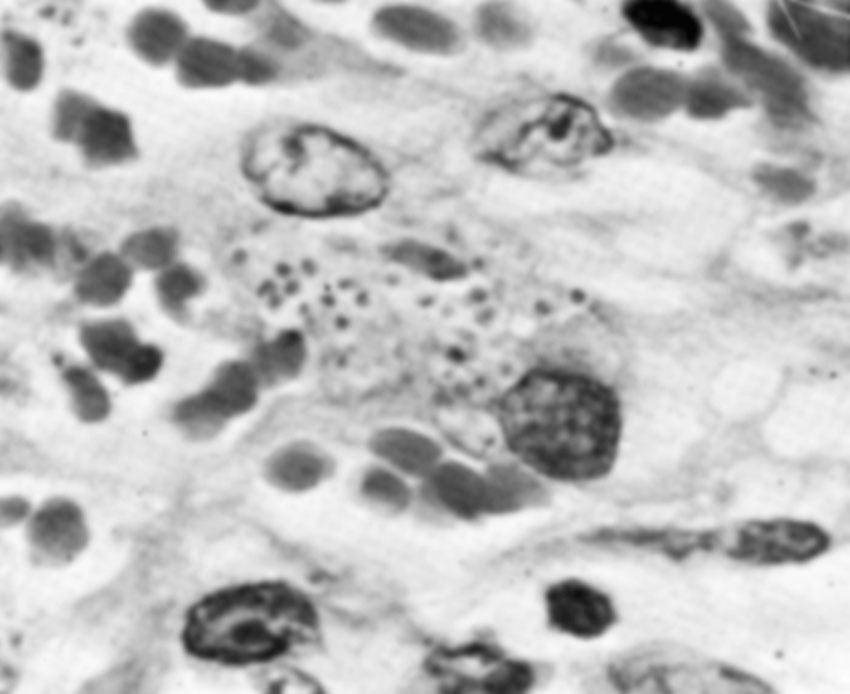
A wide range of oral broad-spectrum antibiotics may be used to manage granuloma inguinale. The CDC recommends azithromycin 1 g orally once a week or 500 mg daily for 3 weeks and until all lesions have healed. See guidelines for alternative antibiotic regimens. Rarely, medical therapy fails and surgical excision is required. Coinfection with another sexually transmitted pathogen is a distinct possibility. Sex partners of women who have granuloma inguinale should be examined if they have had sexual contact during the 60 days preceding the onset of symptoms.
Lymphogranuloma venereum (LGV) is a chronic infection of lymphatic tissue produced by Chlamydia trachomatis found most commonly in the tropics with fewer than 150 new cases reported each year in the United States, most of which occur in men . In women the vulva is the most common site of infection, but the urethra, rectum, and cervix may also be involved. This STI is caused by serotypes L1, L2, and L3 of C. trachomatis. Serologic studies in high-risk populations have found that subclinical infection is common. The incubation period is between 3 and 30 days.
There are three distinct phases of vulvar and perirectal LGV.
Primary infection: A shallow, painless ulcer is present that heals rapidly without therapy typically located on the vestibule or labia but occasionally in the periurethral or perirectal region.
Secondary phase: 1 to 4 weeks later, painful inguinal and perirectal adenopathy is present, with 50% of patients developing systemic symptoms, including general malaise and fever. Without treatment, the infected nodes become increasingly tender, enlarged, matted together, and adherent to overlying skin, forming a bubo ( Fig. 23.10 ) (tender lymph nodes), which is a double genitocrural fold, or groove sign.
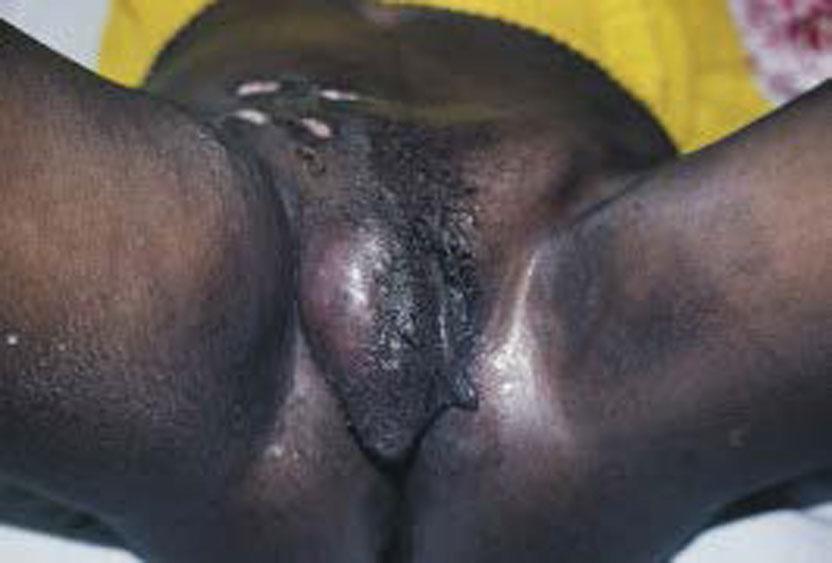
Tertiary phase: Extensive tissue destruction of the external genitalia and anorectal region may occur during the tertiary phase. This tissue destruction and secondary extensive scarring and fibrosis may result in elephantiasis, multiple fistulas, and stricture formation of the anal canal and rectum.
Diagnosis is established by detecting C. trachomatis by culture, direct immunofluorescence, or nucleic acid detection from the pus or aspirate from a tender lymph node. Chlamydia serologic testing (complement fixation titers >1:64) can support the diagnosis in the appropriate clinical context. In the absence of specific LGV diagnostic testing, patients should be treated based on the clinical presentation, including proctocolitis or genital ulcer disease with lymphadenopathy. The differential diagnosis of LGV includes syphilis, chancroid, granuloma inguinale, bacterial lymphadenitis, vulvar carcinoma, genital herpes, and Hodgkin disease.
The CDC recommends doxycycline, 100 mg twice daily for at least 21 days, as the preferred treatment, with alternative treatments listed on CDC website. Antibiotic therapy cures the bacterial infection and prevents further tissue destruction, but fluctuant nodes should be aspirated to prevent sinus formation. Rarely, incision and drainage of infected nodes are necessary to alleviate inguinal pain. The late sequelae of the destructive tertiary phase of LGV often require extensive surgical reconstruction. It is important to administer antibiotics during the perioperative period.
Chancroid is a sexually transmitted, acute, ulcerative disease of the vulva, common in developing countries but infrequent in the United States , caused by Haemophilus ducreyi, a highly contagious, small, nonmotile, gram-negative rod. Epidemiologic studies have suggested that chancroid tends to occur in clusters and may account for a substantial portion of genital ulcer cases when present; however, difficulty in making the diagnosis may cause underreporting. The clinical importance of chancroid has been enhanced by reports that the genital ulcers of chancroid facilitate the transmission of HIV infection . The incubation period is short, usually 3 to 6 days. Tissue trauma and excoriation of the skin must precede initial infection because H. ducreyi is unable to penetrate and invade normal skin.
The initial lesion is a small papule and is always painful and tender.
Within 48 to 72 hours, the papule evolves into a pustule and subsequently ulcerates.
Ulcers are usually in the vestibule, shallow, extremely painful, with a characteristic ragged edge, and have a dirty, gray, necrotic, foul-smelling exudate and lack induration at the base (the soft chancre).
Within 2 weeks of an untreated infection, 50% of women develop acutely tender inguinal adenopathy, a bubo, which is typically unilateral.
Fluctuant nodes should be treated by needle aspiration to prevent rupture or by incision and drainage if larger than 5 cm.
A definitive diagnosis requires the identification of H. ducreyi on special culture media that are not widely available and no FDA-approved PCR test for H. ducreyi is available in the United States, but this testing can be performed by clinical laboratories that have developed their own PCR test and conducted a Clinical Laboratory Improvement Amendments (CLIA) verification study. Chancroid can be diagnosed clinically in a woman with painful vulvar ulcers after excluding other common STIs that produce vulvar ulcers, including genital herpes, syphilis, LGV, and donovanosis.
Because of antibiotic resistance to tetracyclines and sulfonamides, the CDC recommends the following: azithromycin, 1 g orally in a single dose, or ceftriaxone, 250 mg intramuscularly (IM) in a single dose or ciprofloxacin, 500 mg orally twice daily for 3 days; or erythromycin base, 500 mg orally three times daily for 7 days. Sexual partners should be treated in a similar fashion. Approximately 10% of women whose ulcers initially heal have a recurrence at the same site. Women with HIV infection have an increased rate of failure of the standard treatments for chancroid and therefore often require more prolonged therapy. Coinfection with another ulcer causing an STI should be considered, especially in women lacking an appropriate response to treatment.
Syphilis is a chronic, complex systemic disease that is commonly underreported. It remains one of the most important STIs in the United States. Early syphilis is a cofactor in the transmission and acquisition of HIV, and 25% of new syphilis cases occur in persons coinfected with HIV. Even with mandatory screening, congenital syphilis continues to be a public health problem. Syphilis should be included in the differential diagnosis of all genital ulcers and cutaneous rashes of unknown origin, and all women diagnosed with syphilis should be screened for HIV.
Syphilis facts include the following:
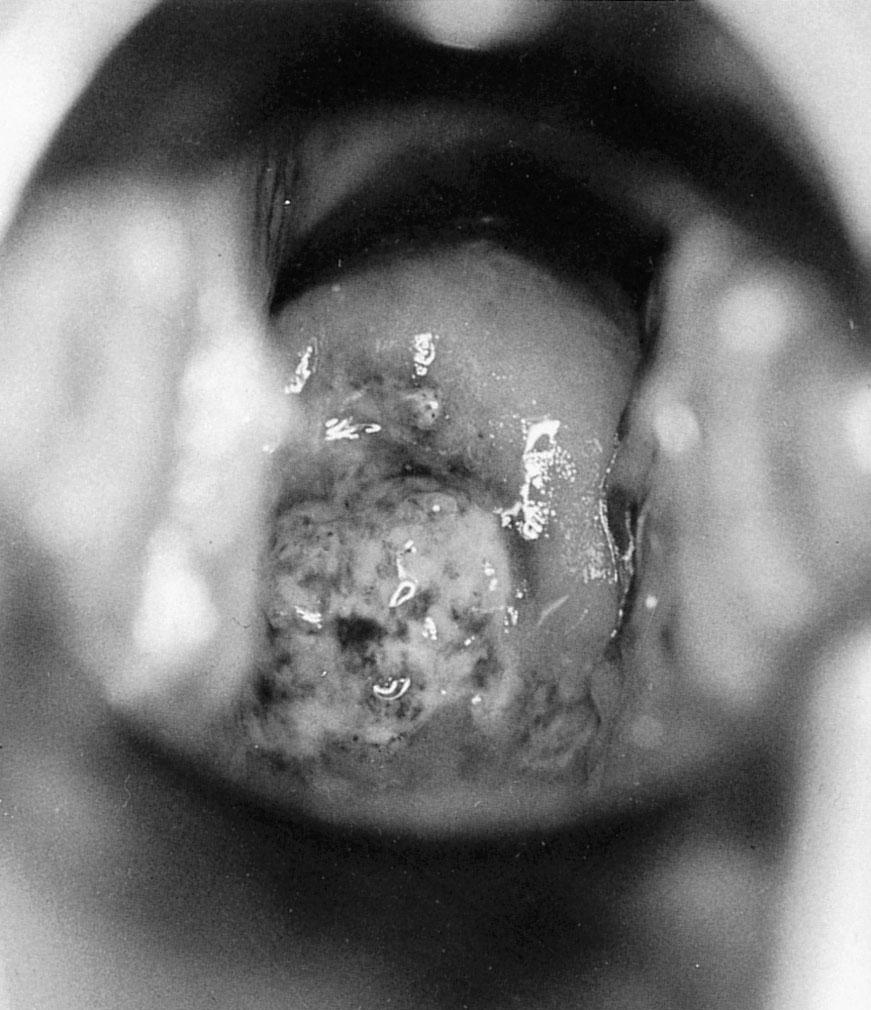
Etiologic agent: the spirochete Treponema pallidum, which penetrates skin or mucous membranes
Detection: noncultivable. Can be diagnosed via dark-field microscopy, direct fluorescent antibody tests ( Fig. 23.11 ), or serologic testing.
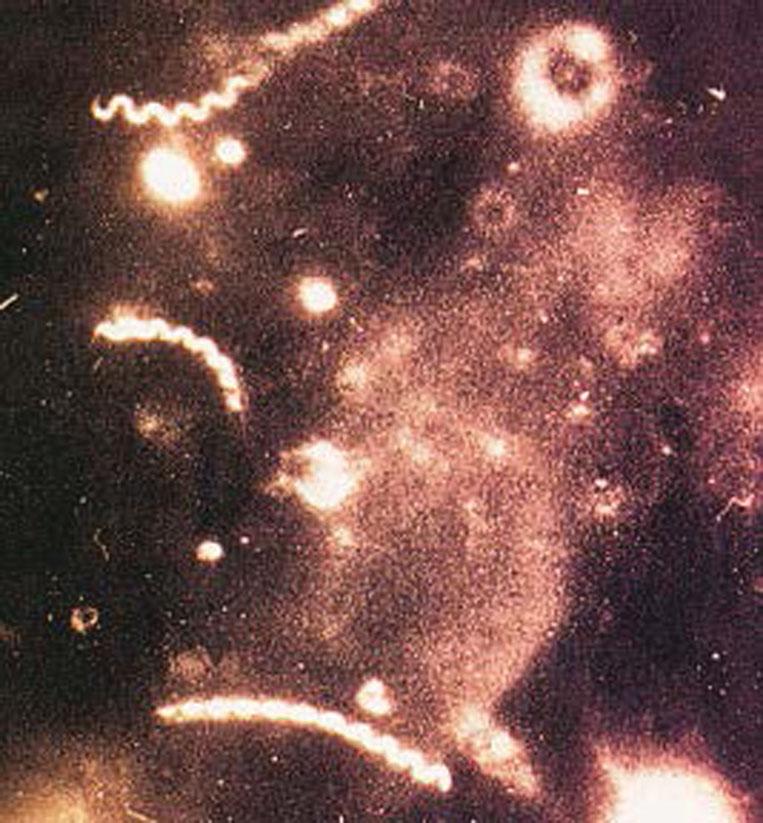
Incubation period: 10 to 90 days, with an average of 3 weeks.
Moderately contagious: 3% to 10% of patients contract the disease from a single sexual encounter with an infected partner; 30% of individuals become infected during a 1-month exposure to a sexual partner with primary or secondary syphilis. Patients are contagious during primary and secondary stages and probably for the first year of latent syphilis.
Transmission: via kissing or touching a person who has an active lesion on the lips, oral cavity, breast, or genitals. Case transmission can occur with oral-genital contact.
Presumptive diagnosis and screening of syphilis rely on two types of serologic tests: the nonspecific nontreponemal and the specific antitreponemal antibody tests . The nontreponemal tests, such as the Venereal Disease Research Laboratory (VDRL) slide test and the rapid plasma reagin (RPR) card test, are inexpensive and easy to perform and are used as screening tests for the disease. They become positive 4 to 6 weeks after exposure and also are a useful index of treatment response because quantitative nontreponemal antibody titers usually correlate with the activity of the disease. Approximately 1% of patients have technical or biologic false-positive results, usually associated with extremely low titers (<1:8). A false-negative result also is a possibility, occurring in approximately 1% to 2% of tests. This negative reaction occurs in women in whom there is an excess of anticardiolipin antibody in the serum, termed the prozone phenomenon. Because serologic testing relies on a humoral immune response to infection, women with immunocompromise also may have false-negative tests.
A nonspecific positive test result is confirmed by a specific antitreponemal test, which is more sensitive but may produce false-positive results, especially in women with lupus erythematosus ( Table 23.3 ). The standard for antitreponemal tests are the fluorescent-labeled Treponema antibody absorption (FTA-ABS) test and the microhemagglutination assay for antibodies to T. pallidum (MHA-TP). A woman with a positive reactive treponemal test usually will have this positive reaction for her lifetime, regardless of treatment or activity of the disease.
| Biologic False-Positive Reaction | ||
|---|---|---|
| Cause | Acute | Chronic |
| Physiologic | Pregnancy | Advanced age, multiple blood transfusions |
| Infectious | Varicella, vaccinia, measles, mumps, infectious mononucleosis, herpes simplex, viral hepatitis, HIV seroconversion illness, cytomegalovirus, pneumococcal pneumonia, Mycoplasma pneumoniae, chancroid, lymphogranuloma venereum, psittacosis, bacterial endocarditis, scarlet fever, rickettsial infections, toxoplasmosis, Lyme disease, leptospirosis, relapsing fever, rat bite fever | HIV, tropical spastic paraparesis, leprosy, * tuberculosis, malaria, * lymphogranuloma venereum, trypanosomiasis, * kala-azar * |
| Vaccinations | Smallpox, typhoid, yellow fever | |
| Autoimmune disease | Systemic lupus erythematosus, discoid lupus, drug-induced lupus, autoimmune hemolytic anemia, polyarteritis nodosa, rheumatoid arthritis, Sjögren syndrome, Hashimoto thyroiditis, mixed connective tissue disease, primary biliary cirrhosis, chronic liver disease, idiopathic thrombocytopenic purpura | |
| Other | IV drug use, advanced malignancy hypergammaglobulinemia, lymphoproliferative disease | |
* Biologic false-positive reaction resolves with resolution of infection.
Clinically, syphilis is divided into primary, secondary, latent, and tertiary stages.
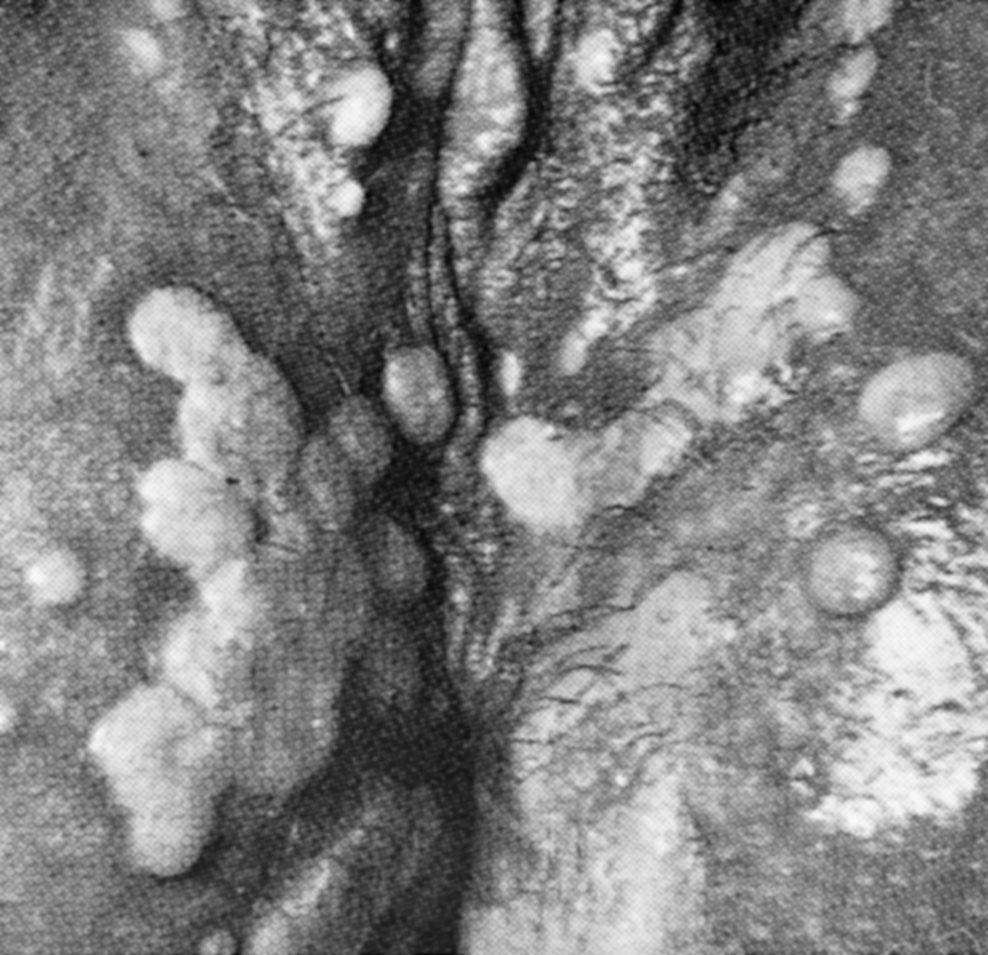
Approximately 2 to 3 weeks after exposure painless papule appears at inoculation site and soon ulcerates to produce the classic chancre—a painless ulcer, 1 to 2 cm, with a raised indurated margin and a nonexudative base ( Fig. 23.12 ). The chancre is typically solitary, painless, and found on the vulva, vagina, or cervix, with nontender and firm regional adenopathy during the first week of clinical disease. Within 2 to 6 weeks, the painless ulcer heals spontaneously; hence, many women do not seek treatment, a feature that enhances the likelihood of transmission. Syphilis is not often diagnosed in the primary stage in women.
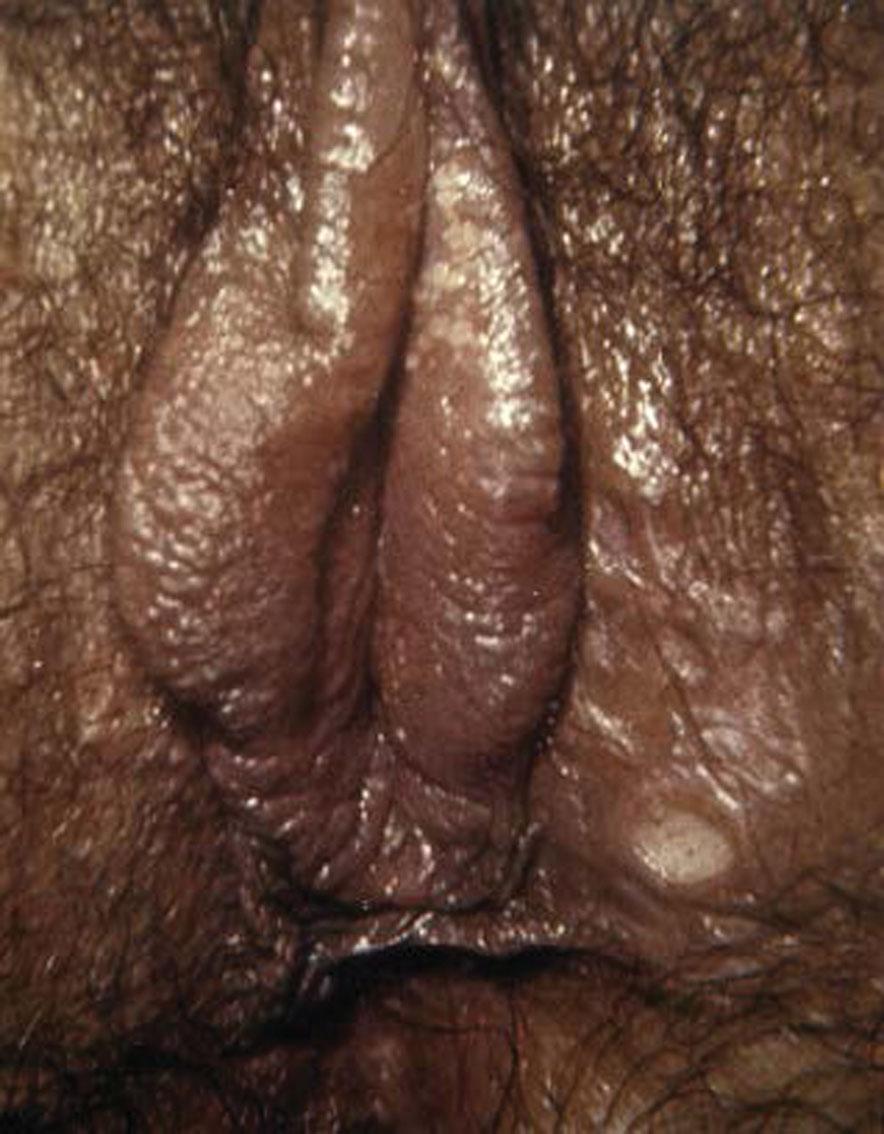
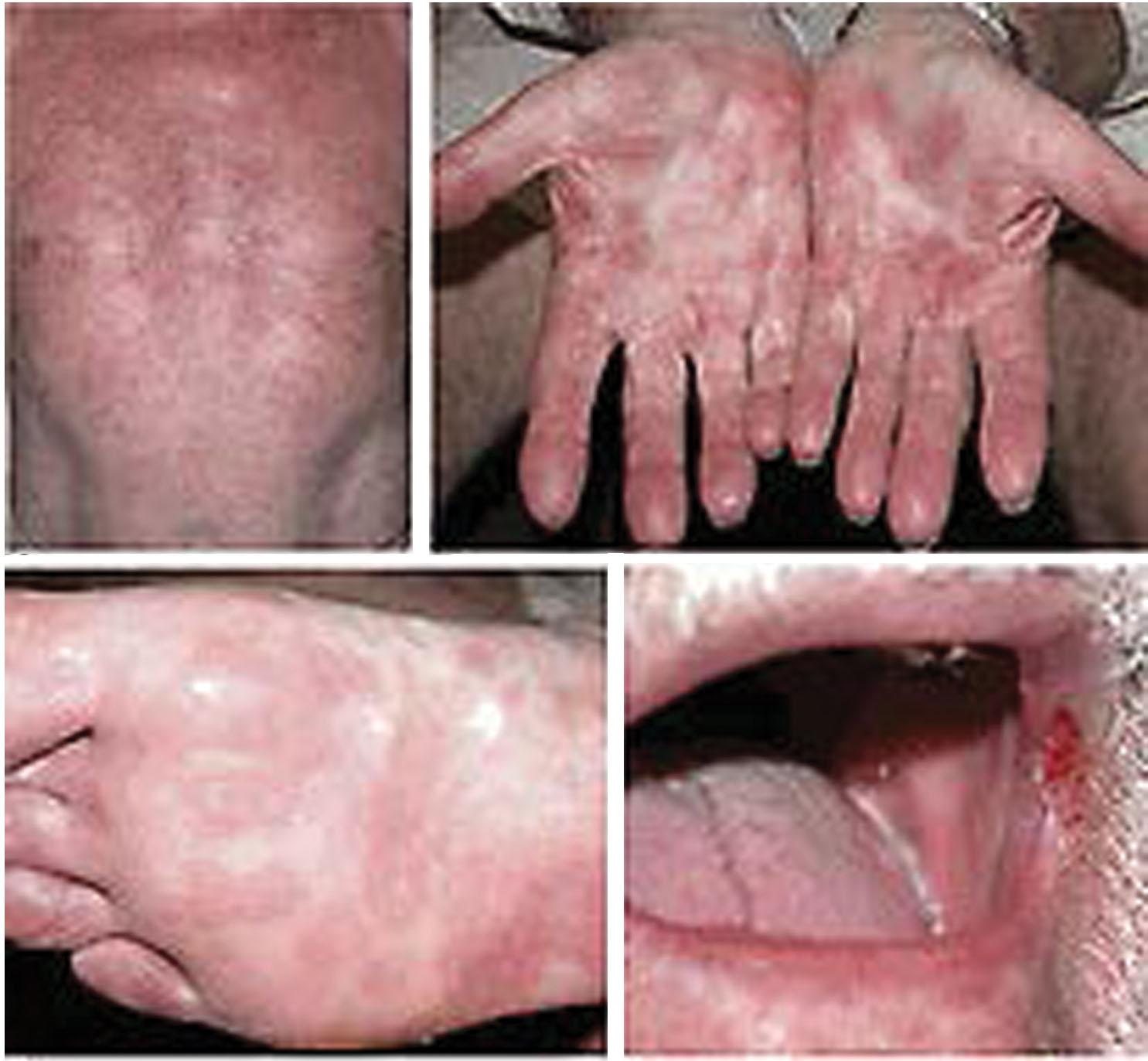
Hematogenous dissemination of the spirochetes leads to systemic disease that develops in approximately 25% of patients between 6 weeks and 6 months (average, 9 weeks) after the primary chancre if primary syphilis is untreated. An untreated attack of secondary syphilis lasts 2 to 6 weeks and produces a multitude of systemic symptoms, such as rash, fever, headache, malaise, lymphadenopathy, and anorexia. The classic rash of secondary syphilis is red macules and papules over the palms of the hands and the soles of the feet ( Fig. 23.13 ). Vulvar lesions of condyloma latum are large, raised, flattened, grayish white areas ( Fig. 23.14 ). On wet surfaces of the vulva, soft papules often coalesce to form ulcers that are larger than herpetic ulcers and are not tender unless secondarily infected. A woman with syphilis is most infectious during the first 1 to 2 years of disease, with decreasing infectivity thereafter.
Positive serologic testing without symptoms or signs of disease follows the secondary stage and varies in duration from 2 to 20 years. This is when most women are diagnosed with syphilis, which is detected via positive blood tests. Early latent syphilis is an infection of 1 year or less and women are infectious during the first year of latent syphilis. All other cases are referred to as late latent or latent syphilis of unknown duration. Women with latent syphilis who have been sexually active should have a pelvic examination to discover potential lesions involving the vagina or cervix.
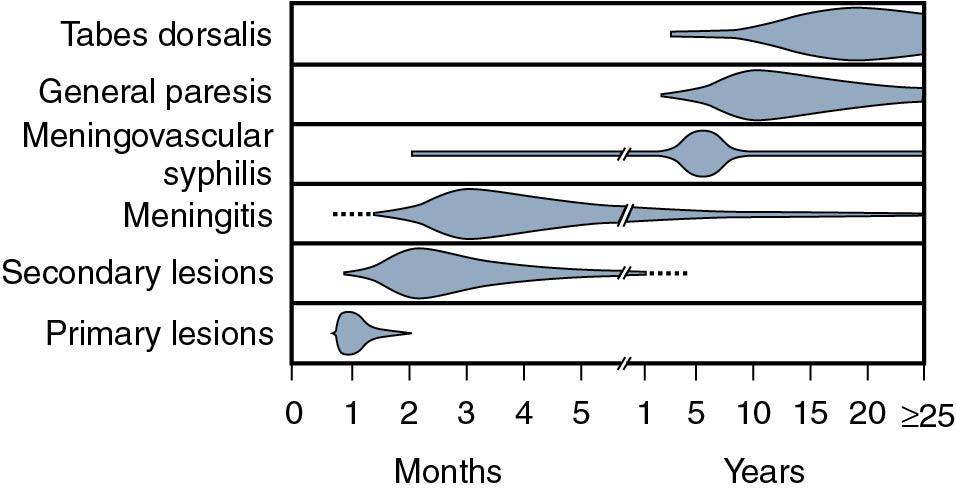
Tertiary syphilis develops in approximately 33% of patients who are not appropriately treated during the primary, secondary, or latent phases of the disease ( Fig. 23.15 ) and is devastating in its potentially destructive effects on the central nervous, cardiovascular, and musculoskeletal systems. The manifestations of late syphilis include optic atrophy, tabes dorsalis, generalized paresis, aortic aneurysm, and gummas of the skin and bones. A gumma is similar to a cold abscess, with a necrotic center and the obliteration of small vessels by endarteritis.
T. pallidum is exquisitely sensitive to penicillin; parenteral penicillin G is the drug of choice for syphilis . Because of the slow replication time of the spirochete, blood levels must be maintained for 7 to 14 days. Box 23.1 lists CDC standard treatment protocols for syphilis. Approximately 60% of women develop an acute febrile reaction associated with flulike symptoms such as headache and myalgia within the first 24 hours after parenteral penicillin therapy for early syphilis. This response is known as the Jarisch-Herxheimer reaction.
Recommended regimen: Benzathine penicillin G, 2.4 million U IM, one dose
Alternative regimen (penicillin-allergic nonpregnant patients): Doxycycline, 100 mg orally bid for 2 wk or tetracycline, 500 mg orally qid for 2 wk
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here