Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cerebrovascular malformations are a major cause of intracranial hemorrhage in young adults and children, resource-intensive to manage, and poorly understood with respect to mechanisms and risk factors.
Most cases of brain arteriovenous malformations (AVMs) are sporadic, but they can occur in Mendelian diseases, notably hereditary hemorrhagic telangiectasia (HHT) caused by autosomal dominant mutations in transforming growth factor beta (TGF-β)/bone morphogenetic protein 9 (BMP-9) signaling pathway genes: endoglin (ENG) , ACVRL1/ALK-1 , and SMAD4, and in capillary malformation—arteriovenous malformation (CM-AVM) caused by mutations in EPHB4-RAS-ERK signaling pathway genes: RASA1 and EPHB4.
Animal models for brain AVM have been established through combining conditional deletion of HHT genes with angiogenic stimulation. Current models mimic aspects of the human disease and display arteriovenous shunting, a hallmark feature of AVMs.
Cerebral cavernous malformations (CCMs) are low-flow, angiographically occult lesions often associated with neurologic morbidity and hemorrhage. Familial CCM often displays multiple lesions, shows autosomal dominant inheritance, and is caused by mutations in three genes: KRIT1 , MGC4067 , and PDCD10.
Somatic mutations occur in most cerebrovascular malformations, including CCM, CM-AVM, vascular malformation-capillary malformation (VMCM), Sturge Weber syndrome (SWS), and most recently in sporadic AVM.
Dural arteriovenous fistulas (DAVFs) are acquired lesions, resulting from trauma and/or venous thrombosis, and characterized by venous hypertension.
Different cerebrovascular malformations share commonalities in signaling pathways affecting angiogenesis, vascular remodeling, inflammation, and response to injury.
This chapter is dedicated in memory of our collaborator and mentor, William L. Young, MD, for his seminal contributions to research on brain AVM and other vascular malformations. The authors gratefully acknowledge collaborators of the UCSF Brain AVM Study Project http://avm.ucsf.edu and the Brain Vascular Malformation Consortium; NIH grant support (R01NS034949, R01 NS099268, R01NS027713, R01HL122774, P01NS044155, U54NS065705); and the Michael Ryan Zodda Foundation.
This chapter focuses on the vascular biology and genetics of brain arteriovenous malformations (AVMs) and cerebral cavernous malformations (CCMs); other cerebrovascular malformations are also discussed briefly. Despite their relative rarity, these malformations pose common challenges: they are resource-intensive to manage effectively, have high probability of serious neurologic morbidity, and lack primary medical therapy for treatment.
Each disease is characterized by distinct vascular malformations and a unique spectrum of clinical and phenotypic outcomes, for which biological risk factors are poorly understood. The identification of these risk factors would improve patient surveillance and management. Since there are no specific medical therapies for these diseases, better understanding of the molecular etiology and pathophysiology holds promise for design of pharmacologic treatments. Appropriate treatment (efficacy) trials will require risk stratification for selection and surrogate outcomes for trial development. Therefore, better biomarkers are needed, especially for assessing risk of hemorrhage.
Brain AVMs represent a rare but important source of neurologic morbidity in young adults. The population prevalence is 10–18 per 100,000 adults, , with a new detection rate of 1.3 per 100,000 person-years estimated from a pooled meta-analysis of six studies. The basic morphology is a vascular mass called the nidus, a complex tangle of abnormal, dilated channels, not clearly artery or vein, with intervening gliosis, that directly shunts blood from the arterial to venous circulations without a true capillary bed, typically with high flow.
Seizures, mass effect, and headache are causes of associated morbidity, but prevention of new or recurrent intracranial hemorrhage (ICH) is the primary rationale to treat AVMs, usually with some combination of surgical resection, embolization, and stereotactic radiotherapy. The risk of spontaneous ICH in the untreated course of brain AVM patients is estimated at 2%–4% per year overall and 1%–2% per year for unruptured AVMs. Other than non-specific control of symptoms, e.g., headache and seizures, primary medical therapy is still lacking.
The genesis of AVMs has been enigmatic. There are no known environmental or epidemiologic risk factors for AVM susceptibility, with the possible exception of essential hypertension. Although AVMs have been detected in utero, considering the high utilization of prenatal ultrasound, there is remarkably little evidence for the common belief that AVMs are congenital lesions arising during embryonic development (Vein of Galen lesions are an interesting counterexample but probably represent a different disease process), and the etiologic mechanism is likely more complex. The mean age at presentation (detection) is around 40 years of age, with a normal distribution. Epidemiologic data suggests that the hemorrhagic behavior of most AVMs undergoes some fundamental change during childhood. Data from a large cohort of 1581 sporadic AVM cases suggests an increase in ICH rates around 10 years of age, perhaps in response to changing hormonal levels. Others have noted higher ICH rates in women of childbearing age who harbor an AVM and during pregnancy or the postpartum period, , although others have found no increased risk. ,
There are multiple reports of AVMs that grow or regress and of local AVM regrowth after treatment, almost exclusively during childhood. AVMs occasionally arise de novo after a normal angiogram and regrow after resection, either de novo, from a retained fragment, or from a lesion treated with radiotherapy. , Although such events are relatively rare in clinical practice (1%–5%; higher for radiotherapy), they support the hypothesis of active vascular changes in the lesion. Perhaps these observations of regrowth or de novo appearance are extreme cases of a continuum of behaviors of actively, albeit slowly, growing lesions. Consistent with this hypothesis, endothelial proliferation in surgically resected AVM tissue was seven-fold higher than in control tissue (structurally normal temporal lobe). The scarce data available on longitudinal assessment of AVM growth after detection suggest that approximately 50% of cases display interval growth. Although the relationship of such growth with clinical risk profile (e.g., hemorrhagic risk) remains unknown, one plausible target for therapy might be to slow lesion growth over time.
Recently, somatic mutations in RAS/MAPK (mitogen-activated protein kinase) pathway genes have been shown to be associated with intracranial and extracranial AVMs. Somatic mutations are not inherited and do not occur de novo in the germline, but arise during development in a somatic cell and are subsequently found in a subset of cells in each affected individual. Nikolaev et al. first reported the presence of low-allelic representation (<5%) but recurrent, somatic activating mutations in KRAS (c.35G>A p.G12D, c.35G>T p.G12V, c.183A>T, p.Q61H), using whole exome sequencing of DNA from sporadic brain AVM tissue. These mutations were not present in DNA from paired blood samples and were confirmed in 62% of brain AVM lesion samples using digital droplet PCR. Recurrent KRAS mutations were subsequently reported to be present in 90% of brain and 100% of spinal cord AVM samples from a Chinese population, and in 29% in a third study. Somatic mutations in MAP2K1 and BRAF , other members of the RAS/MAPK pathway, have also been reported in extracranial AVM lesions, , suggesting a potential common signaling pathway for treatment of both intracranial and extracranial AVMs. Mutations in these genes are potent drivers of tumorigenesis and result in increased ERK (extracellular signal-regulated kinase) activity. , Specifically, expression of mutant KRAS G12V in endothelial cells (EC) in vitro induced increased ERK1/2 phosphorylation, but not AKT or p38 phosphorylation, suggesting that mutant KRAS specifically activates the MAPK-ERK pathway in sporadic brain AVM. KRAS G12V expression also resulted in increased expression of a number of angiogenesis and NOTCH signaling genes (both important in AVM and described in more detail below) and enhanced migratory behavior, which was reversed by inhibition of MAPK-ERK signaling. These exciting findings implicate a new pathway for study in brain AVM. Further work is needed to understand the interplay between the MAPK-ERK pathway, vascular endothelial growth factor (VEGF) or other angiogenic pathways, and transforming growth factor beta (TGF-β) pathways in AVM pathogenesis.
A prominent feature of the AVM phenotype is the relative overexpression of VEGF at both the messenger RNA (mRNA) and protein level. Animal models suggest that VEGF may contribute to the hemorrhagic tendency. , Other upstream factors that may contribute to AVM formation include homeobox (HOX) transcription factors, such as excess expression of pro-angiogenic HOXD3, or deficient expression of anti-angiogenic HOXA5. , The vascular phenotype of AVM tissue may be explained, in part, by an inadequate recruitment of peri-endothelial support structures, which is mediated by angiopoietins/TIE-2 signaling and platelet derived growth factor B (PDGF-B)/PDGF receptor β (PDGFRβ) signaling. For example, angiopoietin-2 (ANG-2), which allows loosening of cell-to-cell contacts, is overexpressed in the perivascular region of AVMs and PDGFRβ expression is reduced in brain AVMs of an animal model. ,
A key downstream consequence of VEGF and ANG-2 signaling, contributing to angiogenesis, is matrix metalloproteinase (MMP) expression. MMP-9 expression, in particular, is an order of magnitude higher in AVM than in control tissue, , and levels of naturally occurring MMP inhibitors, such as tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-3, are also increased. Additional inflammatory markers that are overexpressed include myeloperoxidase (MPO) and interleukin 6 (IL-6), which are highly correlated with MMP-9. , MMP-9 expression is correlated with the lipocalin-MMP-9 complex, which suggests neutrophils as a major source. In a subset of unruptured, non-embolized AVMs, neutrophils (MPO), macrophages/microglia (CD68+) were prominent in the vascular wall and intervening stroma of AVM tissue. T and B lymphocytes were also present in human brain AVM lesions. Unlike macrophages, the load and location of T lymphocytes (CD3+) were not associated with iron deposition, an indicator of microhemorrhage, suggesting the possibility of an independent cell-mediated immunologic mechanism in brain AVM pathogenesis. Elevated immunoglobulin (Ig) levels have also been reported in brain AVM tissue. Whole genome expression profiling of blood from ruptured and unruptured brain AVM patients identified over 1490 genes differentially expressed, with over-representation of genes in 8 pathways including MAPK, VEGF, Wnt signaling, and several inflammatory pathways.
NOTCH signaling plays a critical role in normal vasculogenesis and angiogenesis, as well as in abnormal vascular remodeling. Increased expression of NOTCH-1 and downstream target HES-1 was observed in brain AVM tissue compared to control vessels. NOTCH-1 ligands, DLL4 and JAGGED-1, were increased in AVM tissue and expressed in smooth muscle cells, whereas HES-1 was expressed predominantly in nuclei of ECs. NOTCH-1 was also observed at higher levels in ruptured vs. unruptured brain AVM cases. NOTCH-4 levels were also significantly increased, and NOTCH-4 variants were present at a higher frequency in brain AVM cases compared to controls. These data suggest that NOTCH signaling is activated in human brain AVM disease, consistent with that observed in animal models described below.
Endothelial progenitor cells (EPCs) may incorporate into the AVM vessel wall, mediating pathologic angiogenesis and vascular remodeling. EPCs, identified as CD133 and KDR double-positive cells, are increased in brain and spinal cord AVM nidus compared to epilepsy and basilar artery control tissue. Increased expression of SDF-1 in AVM vessel wall could be responsible for the EPC homing. In addition to EPCs, other bone-marrow (BM) derived cells have also been implicated in AVM pathogenesis, such as CD68 + macrophages. , BM-derived monocytes/macrophages may provide critical repair functions in response to injury and/or provide guidance involving NOTCH signaling during angiogenesis. ,
The majority of brain AVMs are sporadic; however, some evidence supports a familial component to the AVM phenotype, and that genetic variation is relevant to the disease course. Approximately 5% of AVMs occur in the context of rare inherited vascular disorders. A schematic of potentially relevant pathways suggested by these Mendelian forms of the disease, human tissue investigations, and animal models (see next section) is shown in Fig. 12.1 .
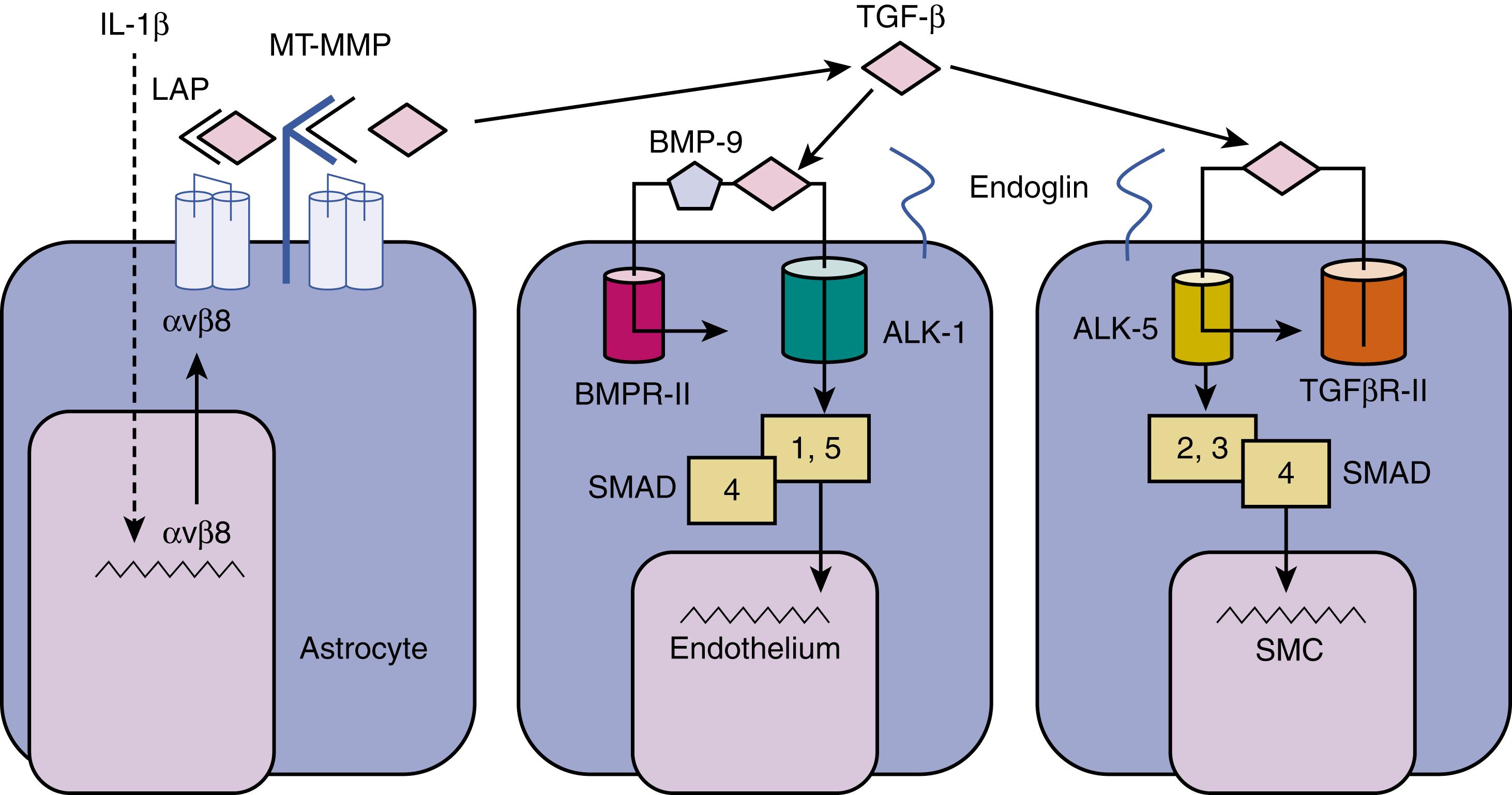
AVMs in various organs, including the brain, are highly prevalent in patients with hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disorder of mucocutaneous fragility. The two main subtypes of HHT (HHT1 and HHT2) are caused by loss-of-function mutations in two genes originally implicated in TGF-β signaling pathways (see Fig. 12.1 ). The first is endoglin ( ENG ), which encodes an accessory protein of TGF-β receptor complexes. The second is activin-like kinase 1 ( ALK-1 or ACVRL1 ), which encodes a transmembrane kinase that participates in TGF-β signaling. There are hundreds of reported mutations in the ALK-1 and ENG genes; the functional effect appears to be effective haploinsufficiency.
The signaling pathways for ALK-1 and ENG are complex and interrelated, and the relative importance and cellular specificity are controversial. ENG interacts with TGFBR2 (the type II TGF-β receptor) as well as with type I TGF-β receptors, ALK-1 and ALK-5, and can bind ligands besides TGF-β, including activins and bone morphogenetic protein (BMP) family members. ,
BMP-9 may be the physiologically relevant endothelial signaling ligand for ALK-1; ENG can potentiate the signal, which suggests a key interaction for HHT pathogenesis. , , Mice lacking Alk-1 ( Acvrl1 ) form systemic arteriovenous fistulas (AVFs) from fusion of major arteries and veins in early vascular development. EC-specific ablation of Alk-1 causes vascular malformations during development and in adult mice, whereas mice harboring an EC-specific knockout of Alk-5 or Tgfbr2 do not show any perturbation in vascular morphogenesis. ,
A third gene implicated in AVM pathogenesis is SMAD4 , encoding a downstream participant in TGF-β and BMP signaling. SMAD4 is mutated in a combined syndrome of juvenile polyposis and HHT. Two additional independent loci, termed HHT3 and HHT4, have been reported, , but the underlying genes have yet to be identified.
More recently, mutations in BMP-9 have been reported to cause a vascular-anomaly syndrome with phenotypic overlap with HHT. In addition, germline mutations in RASA1 and EPHB4 cause two forms of multifocal capillary malformations associated with AVMs (CM-AVM); , these patients also share several phenotypic features that overlap with HHT.
Since defects in ENG or ALK-1 signaling cause AVMs in HHT, by inference this common pathway may also contribute to sporadic brain AVM development. A potential mechanism for the role of this pathway in AVM pathogenesis would include the requirement of ALK-1 for normal EC maturation. , Disruption of this signaling pathway would impair EC maturation, leading to inappropriate EC migration and proliferation. This is consistent with the view that aberrant EC migration and proliferation is an early stage in the development of an AVM. However, another view is that TGF-β/ALK-1 induces and TGF-β/ALK-5 inhibits cell migration and proliferation. More work is needed with in vivo model systems to settle these apparent contradictions.
ENG and ALK-1 levels are reduced in HHT1 and HHT2 ECs. Both ALK-1 and ENG are expressed predominantly in ECs, but ENG is also expressed in other cell types, notably in monocytes. Monocytic ENG appears to be critical for vascular repair in other organs, for example, the heart. ENG expression appears grossly normal in both sporadic and HHT brain AVMs. In one small series, ALK-1 expression was decreased in sporadic AVM. The expression and functionality of these proteins in normal postnatal brain and in sporadic AVMs needs more elucidation. , , , ,
ENG and ALK-1 may contribute to disease via mechanisms other than canonical TGF-β signaling in endothelium, smooth muscle, or in BM-derived cells. ENG and ALK-1 signaling may modulate endothelial nitric oxide synthase (eNOS) activation, thereby influencing local regulation of vascular tone and integrity. , Studies in Eng +/- mice suggest that impaired arterial myogenic responses and Eng-deficient ECs produce less nitric oxide (NO) and generate more eNOS-derived superoxide (O 2 − ). Treatment with an O 2 − scavenger reversed the vasomotor abnormalities in Eng +/− arteries, suggesting that uncoupled eNOS activity and the resulting impaired myogenic response represent early events in HHT1 pathogenesis related to oxidative stress. A loss of local microvascular flow regulation alone may lead to the development of arteriovenous shunts, as predicted by computational modeling studies. There are conflicting data regarding the nature of the vasomotor response, but this hemodynamic mechanism has been demonstrated in the pulmonary circulation.
As a class, HHT AVMs have distinguishing morphologic characteristics such as smaller size, higher incidence of single hole fistulas, and higher incidence of cortical location. However, HHT and sporadic AVMs are generally similar and cannot be distinguished individually by their angioarchitecture. , Brain AVM multiplicity is highly predictive of HHT diagnosis and rarely seen in sporadic disease. HHT brain AVMs are also more frequently diagnosed before rupture, but this probably reflects more aggressive screening in HHT patients rather than any underlying biologic difference.
Brain AVMs are approximately 10 times more common in patients with HHT1/ ENG mutations (≈20%) than those with HHT2/ ALK-1 mutations (≈2%). Compared with the prevalence of sporadic lesions in the normal population, ENG or ALK-1 mutation confers approximately a 1000- and 100-fold increased risk, respectively, of developing a brain AVM. The greatly elevated risk of brain AVM in HHT suggests that germline sequence variants of these and other genes in shared pathways may likewise pose a significant risk for sporadi c brain AVM development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here