Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In summary overview, long-chain fatty acids (LCFAs), whether monomeric or in the form of monoglycerides, represent the major substrate for the generation of triglyceride- rich lipid droplets (LDs) and in turn for the assembly of triglyceride-rich lipoproteins. Apical uptake of LCFAs occurs predominantly in the proximal small intestine (duodenum, jejunum), although the ileum has the capacity to absorb LCFA and expresses all the requisite fatty acid and cholesterol transporter genes. Uptake across the intestinal brush border is facilitated by micellar solubilization of LCFA, which promotes their access to the apical surface and microvillus membrane. Diffusion across the microvillus membrane is both passive (i.e., along a concentration gradient) and also at least in part carrier mediated ( Fig. 49.1 ). Among the apical membrane proteins implicated in LCFA uptake are the multiligand transporter CD36 and the multifunctional glycoprotein milk fat globule-epidermal growth factor 8 (Mfge8). Earlier work implicated a role for CD36 in intestinal LCFA uptake. The impact of this pathway in humans, however, has yet to be established. More recently, new insight into the mechanisms involved in CD36-mediated regulation of apical lipid transport has emerged from findings linking a dynamic signaling pathway in which Mfge8 coordinates LCFA uptake through pathways including integrin-dependent phosphorylation of key intermediates (Akt and mTOR), which in turn promote translocation of CD36 from intracellular vesicular pools back to the apical membrane ( Fig. 49.1 ). This apical membrane distribution of CD36 is proposed to promote uptake of LCFA, which are then reassembled into triglyceride-rich LDs in the apical pole of the enterocyte as a temporary storage pool destined for chylomicron production ( Fig. 49.1 ). Further work has shown that Mfge8 also plays a key role in the mobilization of these LDs by promoting hydrolysis upon interacting with key heterodimeric integrins ( Fig. 49.1 ), which then promote triglyceride hydrolase activity. Other LD-associated proteins have been implicated in regulating postprandial lipid absorption, including comparative gene identification-58 (CGI-58), where genetic ablation led to accumulation of large apical LDs and impaired intestinal lipid delivery into the systemic circulation. These new findings together point to a revised model of intestinal lipid absorption in which cytoplasmic LDs represent a metabolically dynamic organelle from which lipid substrates are mobilized for chylomicron formation as well as participating in other metabolic processes (reviewed in Ref. ).
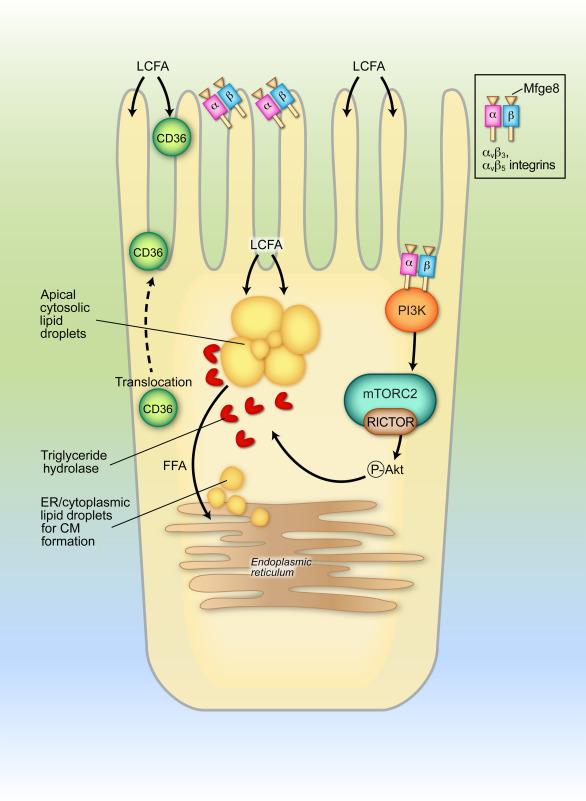
Once dietary lipids (LCFA, sterols, and fat-soluble vitamins) have been transferred into apical domains of small intestinal enterocytes, the process of their vectorial delivery into the systemic circulation via the intestinal lymph is coordinated through the assembly of large, vesicular particles called chylomicrons. A similar but not identical process occurs in the liver, where endogenous and exogenously derived lipids are assembled into slightly smaller particles called very low-density lipoproteins (VLDLs). Two dominant genes regulate the initiation of intestinal chylomicron assembly, specifically apolipoprotein B (apoB) and microsomal triglyceride transfer protein (MTTP). ApoB is the structural protein surrounding nascent intestinal lipoproteins, which are formed following the transfer of bulk membrane lipids mediated by MTTP. Details of the structure, function, and genetic regulation of these two genes are discussed below.
ApoB is a structurally unique, obligate integral component of the surface of VLDL and chylomicrons. The APOB gene is located on the short arm of human chromosome 2 and in the syntenic locus of mouse chromosome 12. The liver and small intestine represent the major sites for apoB gene expression, although apoB is also expressed at low levels by human placenta, cardiomyocytes, and kidney proximal tubules. The full-length apoB protein (ApoB100) is a (Mr 550 kDa) hydrophobic polypeptide composed of 4536 amino acid residues. Human apoB100 contains three amphipathic α-helical domains alternating with two β-strand domains in an NH 2 -α 1 -β 1 -α 2 -β 2 -α 3 -COOH configuration. A truncated form of apoB (ApoB48) is generated in the small intestine through posttranscriptional RNA editing (described below) in which the translated apoB48 protein comprises the first two domains (α 1 -β 1 -) and a portion of the third domain (α 2 ). This structural configuration is organized with a single copy of apoB48 on each lipoprotein particle. The β 1 and β 2 domains likely exist in the form of belt-like structures wrapped around the particle and are predicted to be irreversibly associated with the lipid core of the lipoprotein, whereas the α-helices of the α-domains, which are similar to those found in the “exchangeable” apolipoproteins such as apoAI and apoE, are thought to confer reversible lipid-binding properties. The α 1 domain is a highly disulfide-bonded globular domain, containing 7 of the 8 paired disulfide bonds found in apoB100. Most of the plasma apoB-containing particles are metabolized via the low-density lipoprotein (LDL) receptor-mediated pathway after they are secreted into the blood stream. The LDL receptor-recognition sites for apoB reside within the β 2 domain of apoB100 and are thus absent in apoB48. As a result, uptake of apoB48-containing particles (chylomicron remnants) is mediated by the interaction of apoE with the LDL receptor-related protein 1 and other lipoprotein receptors.
As noted above, mammalian small intestine synthesizes a truncated species of apoB, referred to as apoB48. The underlying mechanism of apoB48 production is tissue-specific posttranscriptional C-to-U RNA editing of the primary transcript that introduces a translational stop codon. The net result is that intestinal chylomicron assembly, and triglyceride secretion is coordinated through apoB48 production while hepatic VLDL assembly and secretion is coordinated through apoB100 production. The functional impact and presumed survival advantages of apoB48 production are discussed below.
New insights have recently emerged into the molecular machinery required for intestinal apoB C-to-U RNA editing. The minimal requisite components for C-to-U RNA editing include Apobec-1 and one or more RNA-binding proteins, which together promote site-specific cytosine deamination in selected RNA targets. Apobec-1, the catalytic deaminase, is required for C-to-U RNA editing because its genetic deletion in mice eliminates C-to-U RNA editing of intestinal apoB as well as eliminating C-to-U RNA editing of all other targets. Moreover, the conditional rescue of Apobec-1 in Apobec-1 intestine-only transgenic mice restores C-to-U RNA editing of apoB and the other known targets to physiological levels. However, while Apobec-1 is essential for C-to-U RNA editing both in vivo and in vitro, apobec-1 alone is not sufficient. C-to-U editing of apoB RNA requires an auxiliary RNA-binding cofactor, Apobec-1 complementation factor, ACF. ACF was identified as the requisite auxiliary factor for Apobec-1-dependent C-to-U RNA editing of apoB in 2000 by two groups, based on its ability to promote in vitro RNA editing of a synthetic apoB RNA template. New information suggests that, while ACF indeed functions in vitro to promote site-specific RNA deaminase activity of Apobec-1, ACF appears completely dispensable to C-to-U RNA editing in vivo. Germline deletion of ACF in mice was early embryonic lethal (E 3.5 days), precluding attempts to discern a role in C-to-U RNA editing of apoB. However, a recently developed conditional ACF-knockout mouse (using a Cre driver active after E 6.5 days) yields viable offspring with no ACF in any tissue, yet with no change in C-to-U RNA editing of apoB or of any other Apobec-1-dependent target. Thus, while ACF binds apoB RNA and Apobec-1 and may be sufficient to mediate Apobec-1 deaminase activity on a synthetic RNA template in vitro, it is not required for Apobec-1 activity in vivo. Rather, another RNA-binding protein (RBM47) functionally related to ACF may be the dominant auxiliary factor for RNA editing. RBM47 was identified in a genetic screen for developmentally regulated genes involved in gut development in which RBM47-null mice exhibited neonatal lethality and stunted growth. In addition, RBM47-null mice exhibit decreased (although not zero) intestinal apoB RNA editing as well decreased C-to-U RNA editing of other Apobec-1-dependent RNA targets. Further studies demonstrated that recombinant RBM47 is sufficient to mediate Apobec-1-dependent deaminase activity on a synthetic apoB RNA template in vitro (similar to that shown for ACF). These data together suggest that RBM47 is both necessary and sufficient for Apobec-1-dependent C-to-U RNA editing, both in vitro and in vivo and strongly points to its role as a dominant cofactor for intestinal apoB RNA editing. More recent work has suggested a key role for RBM47 in supporting tissue growth, implying a broader function for this RNA-binding protein, beyond apoB RNA editing.
The tissue-specific distribution of apoB mRNA editing and its restriction in humans to intestinal apoB reflects the tissue and cell-specific distribution of Apobec-1, which is developmentally regulated and expressed in enterocytes and subepithelial cells throughout the luminal gastrointestinal tract. ACF expression, like that of RBM47, is found in almost all tissues in humans, rats and mice. Intestinal apoB mRNA editing is developmentally regulated, with a progressive increase in the proportion of intestinal apoB48 mRNA culminating in greater than 90% editing in postnatal mammalian enterocytes. These observations strongly suggest that RNA editing plays an important role in the developmental regulation of key pathways in intestinal lipid metabolism.
As alluded to above, many (more than 50) additional C-to-U RNA editing targets (beyond apoB RNA) of Apobec-1 have been identified, principally within 3′ untranslated regions of RNAs that contain the canonical apobec-1-binding site, an UUUN[A/U]U motif embedded within an A + U rich sequence. Accordingly, there is increasing interest in the possibility that both Apobec-1 and its RNA binding partners (ACF, RBM47) exhibit other important functions, beyond intestinal lipid metabolism. For example, crossing Apobec-1 −/− mice into Apc min /+ mice resulted in a dramatic attenuation of intestinal polyposis, suggesting that Apobec-1 may represent a genetic modifier of other dominant pathways, including those involved in intestinal malignancy. Other studies have demonstrated a role for ACF in modulating cytokine (specifically interleukin 6) mRNA stability and in the regulation of liver growth. More recent studies identified RBM47 as a selectable metastatic trait in breast cancer strongly supporting the possibility that Apobec-1, RBM47, and ACF each have a range of targets distinct from apoB mRNA, whose biological implications have yet to be clearly delineated.
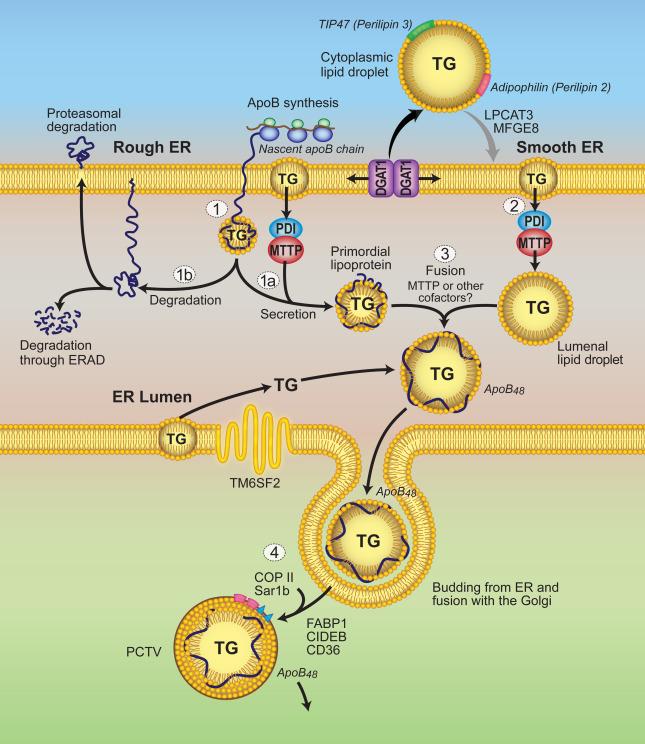
Initiation of apoB-containing lipoprotein formation ( Fig. 49.2 ) requires cotranslational lipidation of apoB via the lipid transfer function of MTTP. MTTP is neutral lipid transfer protein found in the ER lumen of apoB-producing cells, principally enterocytes and hepatocytes but also in cardiomyocytes, placenta, retinal, and proximal renal tubular epithelial cells as well as in cells that do not produce apoB, such as CD1-restricted T cells. MTTP exists as a 97 kDa protein monomer, but full functionality in vivo requires formation of a heteromeric complex with a ubiquitously expressed 55 kDa resident ER chaperone, protein disulfide isomerase (PDI). The 97 kDa subunit of MTTP is responsible for the lipid binding and transfer activity of the MTTP complex, while the PDI subunit contains a signature KDEL ER-retention sequence that functions to retain MTTP at the site of apoB translocation. Recent studies have revealed a role for PDI in regulating the activity of MTTP (under conditions of ER stress) and in turn modulating the ability of the liver to secrete VLDL. These findings raise the possibility that a parallel pathway may be relevant to regulating intestinal chylomicron production. MTTP is a member of the large lipid transfer protein family, including insect, nematode, and vertebrate MTTPs, the vitellogenins and also apoB. The ancestral orthologs of MTTP exhibit similar secondary and tertiary structure and interact with PDI to promote apoB secretion. Mammalian MTTP exhibits functional transfer of all lipid classes, including triglycerides, cholesteryl esters, free cholesterol, and phospholipids (PLs), but triglyceride transfer activity is highest in mammalian MTTP, perhaps providing a functional advantage in the assimilation of lipid-rich nutrients particularly during suckling. MTTP is necessary and (in the presence of apoB) sufficient for lipid export and lipoprotein secretion, as evidenced by the ability to convert nonlipoprotein secreting cell lines to apoB-lipoprotein-secreting cells when the large MTTP subunit is coexpressed with lipid-binding competent forms of apoB. On the other hand, inactivation of MTTP function using pharmacologic inhibitors or conditional genetic deletion, selectively blocks apoB-lipoprotein secretion from hepatocytes and enterocytes. Furthermore, truncating or missense MTTP mutations result in abetalipoproteinemia (ABL), an autosomal recessive disorder characterized by the absence of apoB-containing lipoproteins in serum and lipid accumulation in the liver and intestine. ABL is discussed in detail in a later section. Together, these studies have established unequivocally the role of MTTP in the assembly and secretion of triglyceride-rich lipoproteins as required steps in intestinal lipid transport.
Several studies have examined the regulation of MTTP in enterocytes, and important conclusions have emerged regarding the complexity and physiological implications for the regulation of lipid absorption and plasma lipoprotein homeostasis. Among the most intriguing findings were that intestinal MTTP exhibits diurnal regulation in concert with the diurnal changes in plasma triglyceride levels. Other findings have extended these observations with the demonstration that this diurnal regulation is mediated by CLOCK genes and requires the transcriptional repressor SHP (short heterodimeric partner). More recent studies have demonstrated that the circadian rhythm of MTTP expression may be regulated by Forkhead (FoxO) transcription factors A2 and O1 acting in concert with intestinal apolipoprotein A-IV. These findings have important implications for our understanding of how nutritional and visual cues might prime the intestine to accommodate rapid fluxes in dietary lipid availability. Leptin signaling has also been implicated in the regulation of intestinal MTTP, specifically the role of leptin receptor and melanocortin 4 receptor pathways. Yet other work has related regulation of ER stress to nutrient modulation of MTTP expression with the finding that inositol requiring enzyme 1β-knockout mice exhibit increased intestinal MTTP and augmented lipid mobilization, along with increased chylomicron secretion. More recent work has demonstrated that microRNA-30c (miR-30c) is a potent repressor of MTTP expression in mouse liver, principally by binding to regions within the 3′ untranslated region of MTTP mRNA. These authors also demonstrated miR-30C also suppressed lipogenesis and reduced diet-induced hyperlipidemia and atherosclerosis. Although miR-30C is also expressed in the small intestine as well as other tissues (including heart and testis), its role in regulating intestinal MTTP activity or lipid export from this or other tissues is yet to be determined experimentally.
Assembly of VLDL and chylomicrons requires coordination of apoB synthesis and fusion with LDs and newly synthesized lipids in a fixed temporal sequence ( Fig. 49.2 ). A two-step model most plausibly defines the process for apoB containing lipoprotein synthesis and secretion ( Fig. 49.2 ), in which apoB is first assembled into a lipid-poor primordial lipoprotein particle in the rough ER and then acquires additional triglycerides through bulk addition of neutral lipids smooth ER to form mature triglyceride-rich lipoprotein particles. Depending on lipid availability and MTTP activity, apoB-containing lipoprotein particles may be secreted as dense VLDL, large triglyceride-rich VLDL, or chylomicrons.
The first step of apoB-containing lipoprotein assembly is important in governing the number of lipoprotein particles produced and is influenced by physiological variations in the rates and efficiency of secretion of newly synthesized apoB. The apoB gene is constitutively expressed, and apoB synthesis rates are relatively stable both in vitro and in vivo. For example, oleic acid supplementation in human hepatoma cells (HepG2 cells) does not affect either apoB mRNA abundance or the synthesis of apoB100, yet rapidly stimulates apoB100 secretion. Intestinal apoB synthesis is also unchanged in rat enterocytes following either acute or chronic dietary lipid challenge. Thus, the rate of secretion of apoB-containing lipoproteins is likely regulated at a posttranslational level in which (rather than altering de-novo synthesis) apoB secretion efficiency is determined by the extent to which the nascent apoB protein escapes presecretory degradation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here