Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In Chapter 1 , we discussed the architecture of the normal human genome. Here we build on that knowledge to discuss the genetic basis of human diseases.
Genetic disorders are far more common than is widely appreciated. The lifetime frequency of genetic diseases is estimated to be 670 per 1000. Furthermore, the genetic diseases encountered in medical practice represent only the tip of the iceberg, that is, those with less extreme genotypic errors that permit full embryonic development and live birth. It is estimated that 50% of spontaneous abortuses during the early months of gestation have a demonstrable chromosomal abnormality; there are, in addition, numerous smaller detectable errors and many other genetic lesions that are only now being recognized thanks to advances in DNA sequencing. About 1% of all newborn infants possess a gross chromosomal abnormality, and serious disease with a significant genetic component develops in approximately 5% of individuals younger than age 25 years. How many more mutations remain hidden?
Before discussing specific aberrations that may cause genetic diseases, it is useful to note that human genetic disorders can be broadly classified into three categories .
Disorders related to mutations in single genes with large effects. These mutations cause the disease or predispose to the disease and, with some exceptions like hemoglobinopathies, are typically not present in the normal population. Such mutations and their associated disorders are highly penetrant, meaning that the presence of the mutation is associated with the disease in a large proportion of individuals. Because these diseases are caused by single gene mutations, they usually follow the classic Mendelian pattern of inheritance and are also referred to as Mendelian disorders. A few important exceptions to this rule are noted later.
Study of single genes and mutations with large effects has been extremely informative in medicine, since a great deal of what is known about several physiologic pathways (e.g., cholesterol transport, chloride secretion) has been learned from analysis of single-gene disorders. Although informative, these disorders are generally rare unless they are maintained in a population by strong selective forces (e.g., sickle cell anemia in areas where malaria is endemic; Chapter 14 ).
Chromosomal disorders . These arise from structural or numerical alteration in the autosomes and sex chromosomes. Like monogenic disease, they are uncommon but associated with high penetrance.
Complex multigenic disorders . These are far more common than diseases in the previous two categories. They are caused by interactions between multiple variant forms of genes and environmental factors. Such variations in genes are common within the population and are also called polymorphisms . Each such variant gene confers a small increase in disease risk, and no single susceptibility gene is necessary or sufficient to produce the disease. It is only when several such polymorphisms are present in an individual that disease occurs—hence the term multigenic or polygenic . Thus, unlike mutant genes with large effects that are highly penetrant and give rise to Mendelian disorders, each polymorphism has a small effect and is of low penetrance. Since environmental interactions are important in the pathogenesis of these diseases, they are also called multifactorial disorders. In this category are some of the most common diseases that afflict humans, including atherosclerosis, diabetes mellitus, hypertension, and autoimmune diseases. Even normal traits such as height and weight are governed by polymorphisms in several genes.
The following discussion describes mutations that affect single genes, which underlie Mendelian disorders, followed by transmission patterns and selected samples of single-gene disorders.
A mutation is defined as a permanent change in the DNA. Mutations that affect germ cells are transmitted to the progeny and can give rise to inherited diseases. Mutations that arise in somatic cells understandably do not cause hereditary diseases but are important in the genesis of cancers and some congenital malformations.
General principles relating to the effects of gene mutations follow.
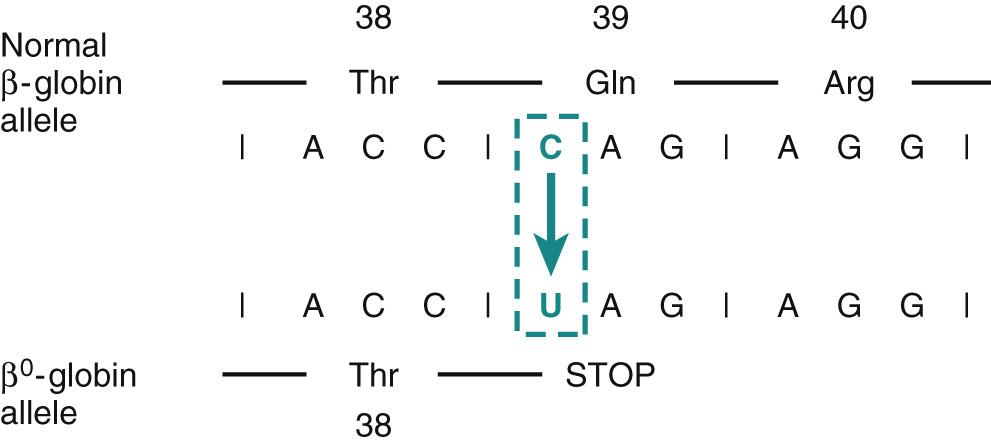
Point mutations within coding sequences. A point mutation is a change in which a single base is substituted with a different base. It may alter the code in a triplet of bases and lead to the replacement of one amino acid by another in the gene product. Because these mutations alter the meaning of the sequence of the encoded protein, they are often termed missense mutations. If the substituted amino acid is biochemically similar to the original, typically it causes little change in the function of the protein, and the mutation is called a “conservative” missense mutation. On the other hand, a “nonconservative” missense mutation replaces the normal amino acid with a biochemically different one. An excellent example of this type is the sickle mutation affecting the β-globin chain of hemoglobin ( Chapter 14 ). Here the nucleotide triplet CTC (or GAG in mRNA), which encodes glutamic acid, is changed to CAC (or GUG in mRNA), which encodes valine. This single amino acid substitution alters the physicochemical properties of hemoglobin, giving rise to sickle cell anemia. Besides producing an amino acid substitution, a point mutation may change an amino acid codon to a chain terminator, or stop codon (nonsense mutation). Taking again the example of β-globin, a point mutation affecting the codon for glutamine (CAG) creates a stop codon (UAG) if U is substituted for C ( Fig. 5.1 ). This change leads to premature termination of β-globin gene translation, and the short peptide that is produced is rapidly degraded. The resulting deficiency of β-globin chains can give rise to a severe form of anemia called β 0 -thalassemia ( Chapter 14 ).
Mutations within noncoding sequences. Deleterious effects may also result from mutations that do not involve the exons. Recall that transcription of DNA is initiated and regulated by promoter and enhancer sequences ( Chapter 1 ). Point mutations or deletions involving these regulatory sequences may interfere with binding of transcription factors and thus lead to a marked reduction in or total lack of transcription. Such is the case in certain forms of hereditary anemias called thalassemias ( Chapter 14 ). In addition, point mutations within introns may lead to defective splicing of intervening sequences. This, in turn, interferes with normal processing of the initial mRNA transcripts and results in a failure to form mature mRNA. Therefore translation cannot take place, and the gene product is not synthesized.
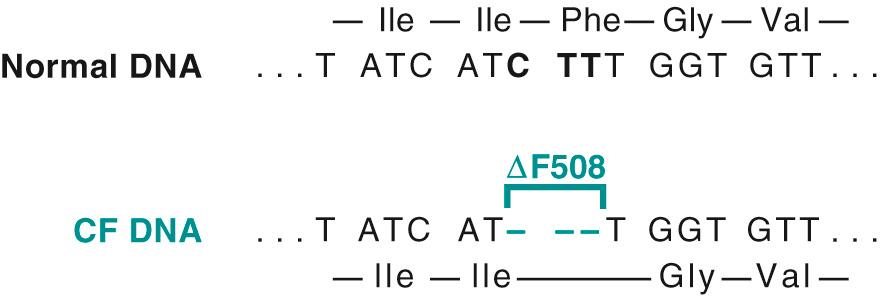
Deletions and insertions. Small deletions or insertions involving the coding sequence can have two possible effects on the encoded protein. If the number of base pairs involved is three or a multiple of three, the reading frame will remain intact, and an abnormal protein lacking or gaining one or more amino acids will be synthesized ( Fig. 5.2 ). If the number of affected coding bases is not a multiple of three, this will result in an alteration of the reading frame of the DNA strand, producing what is referred to as a frameshift mutation ( Figs. 5.3 and 5.4 ). Typically the result is the incorporation of a variable number of incorrect amino acids followed by truncation resulting from a premature stop codon.
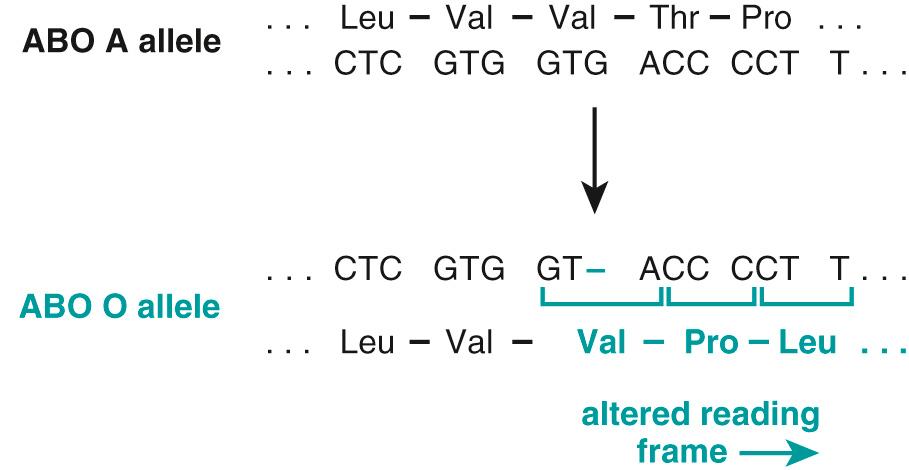
Alterations in protein-coding genes other than mutations. In addition to alterations in DNA sequence, coding genes also can undergo structural variations, such as copy number changes— amplifications or deletions —or translocations that result in aberrant gain or loss of protein function. As with mutations, structural changes may occur in the germline or be acquired in somatic tissues. In many instances, pathogenic germline alterations involve a contiguous portion of a chromosome rather than a single gene, such as in the 22q microdeletion syndrome, discussed later. With the widespread availability of next-generation sequencing (NGS) technology for assessing genome-wide DNA copy number variation at very high resolution, potentially pathogenic structural alterations have now been discovered in common disorders such as autism. Cancers often contain somatically acquired structural alterations, including amplifications, deletions, and translocations. The so-called Philadelphia chromosome—translocation t(9;22) between the BCR and ABL genes in chronic myeloid leukemia ( Chapter 13 )—is a classic example.
Alterations in noncoding RNAs . It is worth noting that in the past, the major focus of gene hunting was discovery of genes that encode proteins. We now know that a very large number of genes do not encode proteins but produce transcripts—so-called noncoding RNAs (ncRNAs)—that serve important regulatory functions. Although many distinct families of ncRNAs exist, the two most important examples—small RNA molecules called microRNAs (miRNAs) and long noncoding RNAs (lncRNAs)—are discussed in Chapter 1 .
Trinucleotide-repeat mutations. Trinucleotide-repeat mutations belong to a special category of genetic anomaly. These mutations are characterized by amplification of a sequence of three nucleotides. Although the specific nucleotide sequence that undergoes amplification differs in various disorders, almost all affected sequences share the nucleotides guanine (G) and cytosine (C). For example, in fragile X syndrome (FXS), prototypical of this category of disorders, there are 250 to 4000 tandem repeats of the sequence CGG within the regulatory region of a gene called familial mental retardation 1 (FMR1). In normal populations the number of repeats is small, averaging 29. Such expansions of the trinucleotide sequences prevent normal expression of the FMR1 gene, thus giving rise to intellectual disability. Another distinguishing feature of trinucleotide-repeat mutations is that they are dynamic (i.e., the degree of amplification increases during gametogenesis). These features, discussed in greater detail later, influence the pattern of inheritance and the phenotypic manifestations of the diseases caused by this class of mutation.
To summarize, mutations can interfere with gene expression at various levels. Transcription may be suppressed by gene deletions and point mutations involving promoter sequences. Abnormal mRNA processing may result from mutations affecting introns or splice junctions or both. Translation is affected if a nonsense mutation creates a stop codon (chain termination mutation) within an exon. Finally, some pathogenic point mutations may lead to expression of normal amounts of a dysfunctional protein.
Against this background, we now turn our attention to the three major categories of genetic disorders: (1) disorders related to mutant genes of large effect, (2) diseases with multifactorial inheritance, and (3) chromosomal disorders. To these three well-known categories must be added a heterogeneous group of single-gene disorders with nonclassic patterns of inheritance. This group includes disorders resulting from triplet-repeat mutations, those arising from mutations in mitochondrial DNA (mtDNA), and those in which the transmission is influenced by genomic imprinting or gonadal mosaicism. Diseases within this group are caused by mutations in single genes, but they do not follow the Mendelian pattern of inheritance. These are discussed later in this chapter.
It is beyond the scope of this book to review normal human genetics. Some fundamentals of DNA structure and regulation of gene expressions were described in Chapter 1 . It is important here to clarify several commonly used terms— hereditary, familial, and congenital. Hereditary disorders, by definition, are derived from one's parents and are transmitted in the germline through the generations and therefore are familial. The term congenital simply implies “born with.” Some congenital diseases are not genetic (e.g., congenital syphilis). Not all genetic diseases are congenital; individuals with Huntington disease, for example, begin to manifest their condition only after their 20s or 30s.
Virtually all Mendelian disorders are the result of mutations in single genes that have large effects. It is not necessary to detail Mendel's laws here, since every student in biology, and possibly every garden pea, has learned about them at an early age. Only some comments of medical relevance are made.
It is estimated that every individual is a carrier of several deleterious genes; most of these genes are recessive and therefore do not have serious phenotypic effects. About 80% to 85% of these mutations are familial. The remainder represent new mutations acquired de novo by an affected individual.
Most mutations in autosomal genes produce partial expression in the heterozygote and full expression in the homozygote. Sickle cell anemia is caused by substitution of normal hemoglobin (HbA) by hemoglobin S (HbS). When an individual is homozygous for the mutant gene, all the hemoglobin is of the abnormal, HbS, type, and even with normal saturation of oxygen the disorder is fully expressed (i.e., sickling deformity of all red cells and hemolytic anemia). In the heterozygote, only a proportion of the hemoglobin is HbS (the remainder being HbA), and therefore red cell sickling occurs only under unusual circumstances, such as exposure to lowered oxygen tension. This is referred to as the sickle cell trait to differentiate it from full-blown sickle cell anemia.
Although Mendelian traits are usually described as dominant or recessive, in some cases both of the alleles of a gene pair contribute to the phenotype—a condition called codominance. Histocompatibility and blood group antigens are good examples of codominant inheritance.
A single mutant gene may lead to many end effects, termed pleiotropism; conversely, mutations at several genetic loci may produce the same trait (genetic heterogeneity). Sickle cell anemia is an example of pleiotropism. In this hereditary disorder, not only does the point mutation in the gene give rise to HbS, which predisposes the red cells to hemolysis, but also the abnormal red cells tend to cause a logjam in small vessels, inducing, for example, splenic fibrosis, organ infarcts, and bone changes. The numerous differing end-organ derangements all are related to the primary defect in hemoglobin synthesis. On the other hand, profound childhood deafness, an apparently homogeneous clinical entity, results from many different types of autosomal recessive mutations. Recognition of genetic heterogeneity not only is important in genetic counseling but also is relevant in the understanding of the pathogenesis of some common disorders, such as diabetes mellitus.
Mutations involving single genes typically follow one of three patterns of inheritance: autosomal dominant, autosomal recessive, and X-linked. The general rules that govern the transmission of single-gene disorders are well known; only a few salient features are summarized. Single-gene disorders with nonclassic patterns of inheritance are described later.
Autosomal dominant disorders are manifested in the heterozygous state, so at least one parent of an index case is usually affected; both males and females are affected, and both can transmit the condition. When an affected person marries an unaffected one, every child has one chance in two of having the disease. In addition to these basic rules, autosomal dominant conditions are characterized by the following:
With every autosomal dominant disorder, some proportion of patients do not have affected parents. Such patients owe their disorder to new mutations involving either the egg or the sperm from which they were derived. Their siblings are neither affected nor at increased risk for disease development. The proportion of patients who develop the disease as a result of a new mutation is related to the effect of the disease on reproductive capability. If a disease markedly reduces reproductive fitness, most cases would be expected to result from new mutations. Many new mutations seem to occur in germ cells of relatively older fathers.
Clinical features can be modified by variations in penetrance and expressivity. Some individuals inherit the mutant gene but are phenotypically normal. This is referred to as incomplete penetrance. Penetrance is expressed in mathematical terms. Thus 50% penetrance indicates that 50% of those who carry the gene express the trait. In contrast to penetrance, if a trait is seen in all individuals carrying the mutant gene but is expressed differently among individuals, the phenomenon is called variable expressivity. For example, manifestations of neurofibromatosis type 1 range from brownish spots on the skin to multiple skin tumors and skeletal deformities. The mechanisms underlying incomplete penetrance and variable expressivity are not fully understood, but they most likely result from effects of other genes or environmental factors that modify the phenotypic expression of the mutant allele. For example, the phenotype of a patient with sickle cell anemia (resulting from mutation at the β-globin locus) is influenced by the genotype at the α-globin locus because the latter influences the total amount of hemoglobin made ( Chapter 14 ). The influence of environmental factors is exemplified by individuals heterozygous for familial hypercholesterolemia (FH). The expression of the disease in the form of atherosclerosis is conditioned by the dietary intake of lipids.
In many conditions the age at onset is delayed; symptoms and signs may not appear until adulthood (as in Huntington disease).
The molecular mechanisms of autosomal dominant disorders depend on the nature of the mutation and the type of protein affected. Most mutations lead to the reduced production of a gene product or give rise to a dysfunctional or inactive protein. Whether such a mutation gives rise to dominant or recessive disease depends on whether the remaining copy of the gene is capable of compensating for the loss. Thus, understanding the reasons why particular loss-of-function mutations give rise to dominant versus recessive disease patterns requires an understanding of the biology. Many autosomal dominant diseases arising from deleterious mutations fall into one of a few familiar patterns:
Diseases involved in regulation of complex metabolic pathways that are subject to feedback inhibition. Membrane receptors such as the low-density lipoprotein (LDL) receptor provide one such example; in FH, discussed later, a 50% loss of LDL receptors results in a secondary elevation of cholesterol that, in turn, predisposes to atherosclerosis in affected heterozygotes.
Key structural proteins, such as collagen and cytoskeletal elements of the red cell membrane (e.g., spectrin). The biochemical mechanisms by which a 50% reduction in the amounts of such proteins results in an abnormal phenotype are not fully understood. In some cases, especially when the gene encodes one subunit of a multimeric protein, the product of the mutant allele can interfere with the assembly of a functionally normal multimer. For example, the collagen molecule is a trimer in which the three collagen chains are arranged in a helical configuration. Each of the three collagen chains in the helix must be normal for the assembly and stability of the collagen molecule. Even with a single mutant collagen chain, normal collagen trimers cannot be formed, and hence there is a marked deficiency of collagen. In this instance the mutant allele is called dominant negative because it impairs the function of a normal allele. This effect is illustrated by some forms of osteogenesis imperfecta, characterized by marked deficiency of collagen and severe skeletal abnormalities ( Chapter 26 ).
Less common than loss-of-function mutations are gain-of-function mutations, which can take two forms. Some mutations result in an increase in normal function of a protein, for example, excessive enzymatic activity. In other cases, mutations impart a wholly new activity completely unrelated to the affected protein's normal function. The transmission of disorders produced by gain-of-function mutations is almost always autosomal dominant, as illustrated by Huntington disease ( Chapter 28 ). In this disease the trinucleotide-repeat mutation affecting the Huntington gene (see later) gives rise to an abnormal protein, called huntingtin, that is toxic to neurons, and hence even heterozygotes develop a neurologic deficit.
Table 5.1 lists common autosomal dominant disorders. Many are discussed more logically in other chapters. A few conditions not considered elsewhere are discussed later in this chapter to illustrate important principles.
| System | Disorder |
|---|---|
| Nervous | Huntington disease Neurofibromatosis Myotonic dystrophy Tuberous sclerosis |
| Urinary | Polycystic kidney disease |
| Gastrointestinal | Familial polyposis coli |
| Hematopoietic | Hereditary spherocytosis von Willebrand disease |
| Skeletal | Marfan syndrome a Ehlers-Danlos syndrome (some variants) a Osteogenesis imperfecta Achondroplasia |
| Metabolic | Familial hypercholesterolemia a Acute intermittent porphyria |
a Discussed in this chapter. Other disorders listed are discussed in appropriate chapters in the book.
Autosomal recessive traits make up the largest category of Mendelian disorders. They occur when both alleles at a given gene locus are mutated. These disorders are characterized by the following features: (1) The trait does not usually affect the parents of the affected individual, but siblings may show the disease; (2) siblings have one chance in four of having the trait (i.e., the recurrence risk is 25% for each birth); and (3) if the mutant gene occurs with a low frequency in the population, there is a strong likelihood that the affected individual (proband) is the product of a consanguineous marriage. The following features generally apply to most autosomal recessive disorders and distinguish them from autosomal dominant diseases:
The expression of the defect tends to be more uniform than in autosomal dominant disorders.
Complete penetrance is common.
Onset is frequently early in life.
Although new mutations associated with recessive disorders do occur, they are rarely detected clinically. Since the individual with a new mutation is an asymptomatic heterozygote, several generations may pass before the descendants of such a person mate with other heterozygotes and produce affected offspring.
Many of the mutated genes encode enzymes. In heterozygotes, equal amounts of normal and defective enzyme are synthesized. Usually the natural “margin of safety” ensures that cells with half the usual complement of the enzyme function normally.
Autosomal recessive disorders include almost all inborn errors of metabolism. The various consequences of enzyme deficiencies are discussed later. The more common of these conditions are listed in Table 5.2 . Most are presented elsewhere; a few prototypes are discussed later in this chapter.
| System | Disorder |
|---|---|
| Metabolic | Cystic fibrosis Phenylketonuria Galactosemia Homocystinuria Lysosomal storage diseases a α 1 -Antitrypsin deficiency Wilson disease Hemochromatosis Glycogen storage diseases a |
| Hematopoietic | Sickle cell anemia Thalassemias |
| Endocrine | Congenital adrenal hyperplasia |
| Skeletal | Ehlers-Danlos syndrome (some variants) a Alkaptonuria |
| Nervous | Neurogenic muscular atrophies Friedreich ataxia Spinal muscular atrophy |
a Discussed in this chapter. Many others are discussed elsewhere in the text.
All sex-linked disorders are X-linked, and almost all are recessive. Several genes are located in the male-specific region of Y; all of these are related to spermatogenesis. Males with mutations affecting the Y-linked genes are usually infertile, and hence there is no Y-linked inheritance. As discussed later, a few additional genes with homologs on the X chromosome have been mapped to the Y chromosome, but only a few rare disorders resulting from mutations in such genes have been described.
X-linked recessive inheritance accounts for a small number of well-defined clinical conditions. The Y chromosome, for the most part, is not homologous to the X chromosome, and so mutant genes on the X chromosome do not have corresponding alleles on the Y chromosome. Thus, males are said to be hemizygous for X-linked mutant genes, so these disorders are expressed in males. Other features that characterize these disorders are as follows:
An affected male does not transmit the disorder to his sons, but all daughters are carriers. Sons of heterozygous women have, of course, one chance in two of receiving the mutant gene.
A heterozygous female usually does not express the full phenotypic change because of the paired normal allele. Because of the random inactivation of one of the X chromosomes in the female, however, females have a variable proportion of cells in which the mutant X chromosome is active. Thus it is remotely possible for the normal allele to be inactivated in most cells, permitting full expression of heterozygous X-linked conditions in females. Much more commonly, the normal allele is inactivated in only some of the cells, and thus a heterozygous female expresses the disorder partially. An illustrative condition is glucose-6-phosphate dehydrogenase (G6PD) deficiency. Transmitted on the X chromosome, this enzyme deficiency, which predisposes to red cell hemolysis in patients receiving certain types of drugs ( Chapter 14 ), is expressed principally in males. In females, a proportion of the red cells may be derived from precursors with inactivation of the normal allele. Such red cells are at the same risk for undergoing hemolysis as the red cells in hemizygous males. Thus, females are not only carriers of this trait but also are susceptible to drug-induced hemolytic reactions. Because the proportion of defective red cells in heterozygous females depends on the random inactivation of one of the X chromosomes, however, the severity of the hemolytic reaction is almost always less in heterozygous women than in hemizygous men. Most of the X-linked conditions listed in Table 5.3 are covered elsewhere in the text.
| System | Disease |
|---|---|
| Musculoskeletal | Duchenne muscular dystrophy |
| Blood | Hemophilia A and B Chronic granulomatous disease Glucose-6-phosphate dehydrogenase deficiency |
| Immune | Agammaglobulinemia Wiskott-Aldrich syndrome |
| Metabolic | Diabetes insipidus Lesch-Nyhan syndrome |
| Nervous | Fragile X syndrome a |
a Discussed in this chapter. Others are discussed in appropriate chapters in the text.
There are only a few X-linked dominant conditions. They are caused by dominant disease-associated alleles on the X chromosome. These disorders are transmitted by an affected heterozygous female to half her sons and half her daughters and by an affected male parent to all his daughters but none of his sons, if the female parent is unaffected. Vitamin D–resistant rickets and Alport syndrome are examples of this type of inheritance.
Autosomal dominant disorders are characterized by expression in heterozygous state; they affect males and females equally, and both sexes can transmit the disorder.
Enzyme proteins are not affected in autosomal dominant disorders; instead, receptors and structural proteins are involved.
Autosomal recessive diseases occur when both copies of a gene are mutated; enzyme proteins are frequently involved. Males and females are affected equally.
X-linked disorders are transmitted by heterozygous females to their sons, who manifest the disease. Female carriers usually are protected because of random inactivation of one X chromosome.
Mendelian disorders result from alterations involving single genes. The genetic defect may lead to the formation of an abnormal protein or a reduction in the output of the gene product. Virtually any type of protein may be affected in single-gene disorders and by a variety of mechanisms ( Table 5.4 ). To some extent the pattern of inheritance of the disease is related to the kind of protein affected by the mutation. For this discussion, the mechanisms involved in single-gene disorders can be classified into four categories: (1) enzyme defects and their consequences; (2) defects in membrane receptors and transport systems; (3) alterations in the structure, function, or quantity of nonenzyme proteins; and (4) mutations resulting in unusual reactions to drugs.
| Protein Type/Function | Example | Molecular Lesion | Disease |
|---|---|---|---|
| Enzyme | Phenylalanine hydroxylase | Splice-site mutation: reduced amount | Phenylketonuria |
| Hexosaminidase A | Splice-site mutation or frameshift mutation with stop codon: reduced amount | Tay-Sachs disease | |
| Adenosine deaminase | Point mutations: abnormal protein with reduced activity | Severe combined immunodeficiency | |
| Enzyme inhibitor | α 1 -Antitrypsin | Missense mutations: impaired secretion from liver to serum | Emphysema and liver disease |
| Receptor | Low-density lipoprotein receptor | Deletions, point mutations: reduction of synthesis, transport to cell surface, or binding to low-density lipoprotein | Familial hypercholesterolemia |
| Vitamin D receptor | Point mutations: failure of normal signaling | Vitamin D–resistant rickets | |
| Transport | |||
| Oxygen | Hemoglobin | Deletions: reduced amount | α-Thalassemia |
| Defective mRNA processing: reduced amount | β-Thalassemia | ||
| Point mutations: abnormal structure | Sickle cell anemia | ||
| Ion channels | Cystic fibrosis transmembrane conductance regulator | Deletions and other mutations: nonfunctional or misfolded proteins | Cystic fibrosis |
| Structural | |||
| Extracellular | Collagen | Deletions or point mutations cause reduced amount of normal collagen or normal amounts of defective collagen | Osteogenesis imperfecta |
| Ehlers-Danlos syndromes | |||
| Fibrillin | Missense mutations | Marfan syndrome | |
| Cell membrane | Dystrophin | Deletion with reduced synthesis | Duchenne/Becker muscular dystrophy |
| Spectrin, ankyrin, or protein 4.1 | Heterogeneous | Hereditary spherocytosis | |
| Hemostasis | Factor VIII | Deletions, insertions, nonsense mutations, and others: reduced synthesis or abnormal factor VIII | Hemophilia A |
| Growth regulation | Rb protein | Deletions | Hereditary retinoblastoma |
| Neurofibromin | Heterogeneous | Neurofibromatosis type 1 |
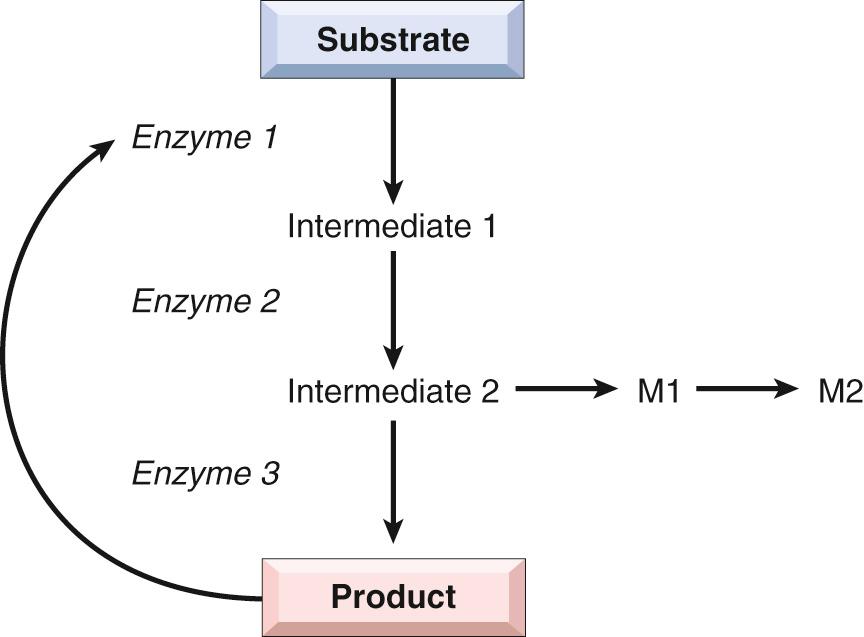
Mutations may result in the synthesis of an enzyme with reduced activity or a reduced amount of a normal enzyme. In either case, the consequence is a metabolic block. Fig. 5.5 provides an example of an enzyme reaction in which the substrate is converted by intracellular enzymes, denoted as 1, 2, and 3, into an end product through intermediates 1 and 2. In this model the final product exerts feedback control on enzyme 1. A minor pathway producing small quantities of M1 and M2 also exists. The biochemical consequences of an enzyme defect in such a reaction may lead to three major consequences:
Accumulation of the substrate, depending on the site of block, may be accompanied by accumulation of one or both intermediates. Moreover, an increased concentration of intermediate 2 may stimulate the minor pathway and thus lead to an excess of M1 and M2. Under these conditions tissue injury may result if the precursor, the intermediates, or the products of alternative minor pathways are toxic in high concentrations. For example, in galactosemia, the deficiency of galactose-1-phosphate uridyltransferase ( Chapter 10 ) leads to the accumulation of galactose and consequent tissue damage. Excessive accumulation of complex substrates within the lysosomes as a result of deficiency of degradative enzymes is responsible for a group of diseases generally referred to as lysosomal storage diseases.
An enzyme defect can lead to a metabolic block and a decreased amount of end product that may be necessary for normal function. For example, a deficiency of melanin may result from lack of tyrosinase, which is necessary for the biosynthesis of melanin from its precursor, tyrosine, resulting in the clinical condition called albinism. If the end product is a feedback inhibitor of the enzymes involved in the early reactions (in Fig. 5.5 it is shown that the product inhibits enzyme 1), the deficiency of the end product may permit overproduction of intermediates and their catabolic products, some of which may be injurious at high concentrations. A prime example of a disease with such an underlying mechanism is Lesch-Nyhan syndrome ( Chapter 26 ).
Failure to inactivate a tissue-damaging substrate is best exemplified by α 1 -antitrypsin deficiency. Individuals who have an inherited deficiency of serum α 1 -antitrypsin are unable to inactivate neutrophil elastase in their lungs. Unchecked activity of this protease leads to destruction of elastin in the walls of lung alveoli, leading eventually to pulmonary emphysema ( Chapter 15 ).
As we discussed in Chapter 1 , biologically active substances have to be actively transported across the cell membrane. In some cases, transport is achieved by receptor-mediated endocytosis. A genetic defect in a receptor-mediated transport system is exemplified by familial hypercholesterolemia (FH), in which reduced synthesis or function of LDL receptors leads to defective transport of LDL into the cells and secondarily to excessive cholesterol synthesis by complex intermediary mechanisms. In cystic fibrosis the transport system for chloride and bicarbonate ions in exocrine glands, sweat ducts, lungs, and pancreas is defective. By complex mechanisms not fully understood, impaired anion transport leads to serious injury to the lungs and pancreas ( Chapter 10 ).
Genetic defects resulting in alterations of nonenzyme proteins often have widespread secondary effects, as exemplified by sickle cell disease. The hemoglobinopathies, sickle cell disease being one, all of which are characterized by defects in the structure of the globin molecule, best exemplify this category. In contrast to the hemoglobinopathies, the thalassemias result from mutations in globin genes that affect the amount of globin chains synthesized. Thalassemias are associated with reduced amounts of structurally normal α-globin or β-globin chains ( Chapter 14 ). Other examples of genetic disorders involving defective structural proteins include osteogenesis imperfecta (defect in collagen, Chapter 26 ), hereditary spherocytosis (spectrin, Chapter 14 ), and muscular dystrophies (dystrophin, Chapter 27 ).
Certain genetically determined enzyme deficiencies are unmasked only after exposure of the affected individual to certain drugs. This special area of genetics, called pharmacogenetics, is of considerable clinical importance. The classic example of drug-induced injury in the genetically susceptible individual is associated with a deficiency of the enzyme G6PD. Under normal conditions, G6PD deficiency does not result in disease, but on administration, for example, of the antimalarial drug primaquine, a severe hemolytic anemia results ( Chapter 14 ). In recent years an increasing number of polymorphisms of genes encoding drug-metabolizing enzymes, transporters, and receptors have been identified. In some cases these genetic factors have a major impact on drug sensitivity and adverse reactions. It is hoped that advances in pharmacogenetics will lead to patient-tailored therapy, an example of personalized medicine.
With this overview of the biochemical basis of single-gene disorders, we now consider selected examples grouped according to the underlying defect.
Several diseases caused by mutations in genes that encode structural proteins are listed in Table 5.4 . Many are discussed elsewhere in the text. Only Marfan syndrome and Ehlers-Danlos syndromes (EDSs) are discussed here because they affect connective tissue and hence involve multiple organ systems.
Marfan syndrome is a disorder of connective tissues, manifested principally by changes in the skeleton, eyes, and cardiovascular system. Its prevalence is estimated to be 1 in 5000. Approximately 70% to 85% of cases are familial and transmitted by autosomal dominant inheritance. The remainder are sporadic and arise from new mutations.
Marfan syndrome results from an inherited defect in an extracellular glycoprotein called fibrillin-1 . There are two fundamental mechanisms by which loss of fibrillin leads to the clinical manifestations of Marfan syndrome: loss of structural support in microfibril-rich connective tissue and excessive activation of transforming growth factor (TGF)-β signaling. Each of these is discussed below.
Fibrillin is the major component of microfibrils found in the extracellular matrix ( Chapter 1 ). These fibrils provide a scaffold on which tropoelastin is deposited to form elastic fibers. Although microfibrils are widely distributed in the body, they are particularly abundant in the aorta, ligaments, and the ciliary zonules that support the lens; these tissues are prominently affected in Marfan syndrome. Fibrillin occurs in two homologous forms, fibrillin-1 and fibrillin-2, encoded by two separate genes, FBN1 and FBN2, mapped on chromosomes 15q21.1 and 5q23.31, respectively. Mutations of FBN1 underlie Marfan syndrome; mutations of the related FBN2 gene are less common, and they give rise to congenital contractural arachnodactyly, an autosomal dominant disorder characterized by skeletal abnormalities. Mutational analysis has revealed close to 1000 distinct mutations of the FBN1 gene in individuals with Marfan syndrome. Most of these are missense mutations that give rise to abnormal fibrillin-1. These can inhibit polymerization of fibrillin fibers (dominant negative effect). Alternatively, the reduction of fibrillin content below a certain threshold weakens the connective tissue (haploinsufficiency).
TGF-β bioavailability . While many clinical manifestations of Marfan syndrome can be explained by changes in the mechanical properties of the extracellular matrix resulting from abnormalities of fibrillin, several others such as bone overgrowth and myxoid changes in mitral valves cannot be attributed to changes in tissue elasticity. It is now established that fibrillin-1 controls the bioavailability of TGF-β. Reduced or altered forms of fibrillin-1 give rise to abnormal and excessive activation of TGF-β, since normal microfibrils sequester TGF-β. Excessive TGF-β signaling, in turn, leads to inflammation, has deleterious effects on vascular smooth muscle development, and increases the activity of metalloproteases, causing loss of extracellular matrix. This schema is supported by two sets of observations.
First, gain-of-function mutations in the TGF-β type II receptor give rise to a related syndrome, called Marfan syndrome type 2 (MFS2). Furthermore, patients with germline mutations in one isoform of TGF-β, called TGF-β3, present with an inherited predisposition to aortic aneurysm and other cardiovascular manifestations similar to those found in patients with classic Marfan syndrome.
Second, angiotensin receptor II blockers, which inhibit TGF-β activity, markedly reduce the aortic root diameter in mouse models of Marfan syndrome. Clinical trials are under way to evaluate the efficacy of angiotensin receptor blockade in patients with Marfan syndrome.
Skeletal abnormalities are the most striking feature of Marfan syndrome. Typically the patient is unusually tall with exceptionally long extremities and long, tapering fingers and toes. The joint ligaments in the hands and feet are lax, suggesting that the patient is double-jointed; typically the thumb can be hyperextended back to the wrist. The head is commonly dolichocephalic (long-headed) with bossing of the frontal eminences and prominent supraorbital ridges. A variety of spinal deformities may appear, including kyphosis, scoliosis, or rotation or slipping of the dorsal or lumbar vertebrae. The chest is classically deformed, presenting either pectus excavatum (deeply depressed sternum) or a pigeon-breast deformity.
Ocular changes take many forms. Most characteristic is bilateral subluxation or dislocation (usually outward and upward) of the lens, referred to as ectopia lentis. This abnormality, resulting from weakening of ciliary zonules, is so uncommon in persons who do not have this disease that the finding of bilateral ectopia lentis should raise the suspicion of Marfan syndrome.
Cardiovascular lesions are the most life-threatening features of this disorder. The two most common lesions are mitral valve prolapse, which occurs in 40% to 50% of cases and, of greater importance, dilation of the ascending aorta due to cystic medionecrosis. Histologically the changes in the media are virtually identical to those found in cystic medionecrosis not related to Marfan syndrome ( Chapter 12 ). Loss of medial support results in progressive dilation of the aortic valve ring and the root of the aorta, giving rise to severe aortic incompetence. Weakening of the media predisposes to an intimal tear, which may initiate an intramural hematoma that cleaves the layers of the media to produce aortic dissection. After cleaving the aortic layers for considerable distances, sometimes back to the root of the aorta or down to the iliac arteries, the hemorrhage often ruptures through the aortic wall.
Although mitral valve lesions are more frequent, they are clinically less important than aortic lesions. Loss of connective tissue support in the mitral valve leaflets makes them soft and billowy, creating a so-called floppy valve ( Chapter 12 ). Valvular lesions, along with lengthening of the chordae tendineae, frequently give rise to mitral regurgitation. Similar changes may affect the tricuspid and, rarely, the aortic valves. Echocardiography greatly enhances the ability to detect the cardiovascular abnormalities and is therefore extremely valuable in the diagnosis of Marfan syndrome. The great majority of deaths are caused by rupture of aortic dissections, followed in importance by cardiac failure.
While the lesions just described typify Marfan syndrome, it must be emphasized that there is great variation in the clinical expression of this genetic disorder. Patients with prominent eye or cardiovascular changes may have few skeletal abnormalities, whereas others with striking changes in body habitus have no eye changes. Although variability in clinical expression may be seen within a family, interfamilial variability is much more common and extensive. Because of such variations, the clinical diagnosis of Marfan syndrome is currently based on the so-called revised Ghent criteria. These take into account family history, cardinal clinical signs in the absence of family history, and presence or absence of fibrillin mutation. In general, major involvement of two of the four organ systems (skeletal, cardiovascular, ocular, and skin) and minor involvement of another organ is required for diagnosis.
The variable expression of the Marfan defect is best explained on the basis of the many different mutations that affect the fibrillin locus, which number around 1000. This genetic heterogeneity also poses formidable challenges in the diagnosis of Marfan syndrome. The evolving high throughput sequencing technologies discussed later in this chapter may overcome this problem in the future.
EDSs comprise a clinically and genetically heterogeneous group of disorders that result from some mutations in genes that encode collagen, enzymes that modify collagen, and less commonly other proteins present in the extracellular matrix. Other disorders resulting from mutations affecting collagen synthesis include osteogenesis imperfecta ( Chapter 26 ), Alport syndrome ( Chapter 20 ), and epidermolysis bullosa ( Chapter 25 ).
Biosynthesis of collagen is a complex process ( Chapter 1 ) that can be disturbed by genetic errors that may affect any one of the numerous structural collagen genes or enzymes necessary for posttranscriptional modifications of collagen. Hence the mode of inheritance of EDSs encompasses all three Mendelian patterns. On the basis of clinical and molecular characteristics, six variants of EDS have been recognized. These are listed in Table 5.5 . More recently, next-generation sequencing has revealed other subgroups bringing the total to 11 molecular types. Although individually rare, the collective frequency of EDSs is 1 in 5000 births. It is beyond the scope of this book to discuss each variant individually; instead, the important clinical features common to most variants are summarized, and clinical manifestations are correlated with the underlying molecular defects in collagen synthesis or structure.
| EDS Type a | Clinical Findings | Inheritance | Gene Defects |
|---|---|---|---|
| Classic (I/II) | Skin and joint hypermobility, atrophic scars, easy bruising | Autosomal dominant | COL5A1, COL5A2 |
| Hypermobility (III) | Joint hypermobility, pain, dislocations | Autosomal dominant | Unknown |
| Vascular (IV) | Thin skin, arterial or uterine rupture, bruising, small joint hyperextensibility | Autosomal dominant | COL3A1 |
| Kyphoscoliosis (VI) | Hypotonia, joint laxity, congenital scoliosis, ocular fragility | Autosomal recessive | Lysyl hydroxylase |
| Arthrochalasia (VIIa, VIIb) | Severe joint hypermobility, skin changes (mild), scoliosis, bruising | Autosomal dominant | COL1A1, COL1A2 |
| Dermatosparaxis (VIIc) | Severe skin fragility, cutis laxa, bruising | Autosomal recessive | Procollagen N -peptidase |
a EDS types were previously classified by Roman numerals. Parentheses show previous numerical equivalents.
As might be expected, tissues rich in collagen, such as skin, ligaments, and joints, are frequently involved in most variants of EDS. Because the abnormal collagen fibers lack adequate tensile strength, skin is hyperextensible, and the joints are hypermobile. These features permit grotesque contortions, such as bending the thumb backward to touch the forearm and bending the knee forward to create almost a right angle. It is believed that most contortionists have one of the EDSs. A predisposition to joint dislocation, however, is one of the prices paid for this virtuosity. The skin is extraordinarily stretchable, extremely fragile, and easily bruised. Minor injuries produce gaping defects, and surgical repair or intervention is accomplished with great difficulty because of the lack of normal tensile strength. The basic defect in connective tissue may lead to serious internal complications. These include rupture of the colon and large arteries (vascular EDS), ocular fragility with rupture of cornea and retinal detachment (kyphoscoliosis EDS), and diaphragmatic hernia (classic EDS).
The biochemical and molecular bases of these abnormalities are known in all but one form of EDS—the so-called hypermobility type. Some of the EDS types are described briefly because they offer some insights into the perplexing clinical heterogeneity of these disorders. Perhaps the best characterized is the kyphoscoliosis type , the most common autosomal recessive form of EDS. It results from mutations in the PLOD1 gene encoding lysyl hydroxylase, an enzyme necessary for hydroxylation of lysine residues during collagen synthesis. Affected patients have markedly reduced levels of this enzyme. Because hydroxylysine is essential for intermolecular and intramolecular cross-linking of collagen fibers, a deficiency of lysyl hydroxylase results in the synthesis of collagen that lacks normal structural stability.
The vascular type of EDS results from abnormalities of type III collagen. This form is genetically heterogeneous because at least three distinct types of mutations affecting the COL3A1 gene encoding collagen type III can give rise to this variant. Some affect the rate of synthesis of pro-α1 (III) chains, others affect the secretion of type III procollagen, and still others lead to the synthesis of structurally abnormal type III collagen. Some mutant alleles behave as dominant negatives (see discussion under “Autosomal Dominant Disorders”) and thus produce severe phenotypic effects. These molecular studies provide a rational basis for the pattern of transmission and clinical features that are characteristic of this variant. First, because vascular-type EDS results from mutations involving a structural protein (rather than an enzyme protein), an autosomal dominant pattern of inheritance would be expected. Second, because blood vessels and intestines are known to be rich in collagen type III, an abnormality of this collagen is consistent with severe structural defects (e.g., vulnerability to spontaneous rupture) in these organs. Unlike many other EDS subtypes, the skin is not usually hyperextensible.
In two forms of EDS—arthrochalasia type and dermatosparaxis type—the fundamental defect is in the conversion of type I procollagen to collagen. This step in collagen synthesis involves cleavage of noncollagen peptides at the N terminus and C terminus of the procollagen molecule. This is accomplished by N-terminal–specific and C-terminal–specific peptidases. The defect in the conversion of procollagen to collagen in the arthrochalasia type has been traced to mutations that affect one of the two type I collagen genes, COL1A1 and COL1A2 . As a result, structurally abnormal pro-α1 (I) or pro-α2 (I) chains that resist cleavage of N-terminal peptides are formed. In patients with a single mutant allele, only 50% of the type I collagen chains are abnormal, but because these chains interfere with the formation of normal collagen helices, heterozygotes manifest the disease. In contrast, the related dermatosparaxis type is caused by mutations in the ADAMTS2 gene that encodes procollagen-N-peptidase, essential for the cleavage of procollagens. Because in this case the disease is caused by an enzyme deficiency, it follows an autosomal recessive form of inheritance.
Finally, in the classic type of EDS, molecular analysis suggests that genes other than those that encode collagen may also be involved. In close to 90% of cases, mutations in the genes for type V collagen ( COL5A1 and COL5A2 ) have been detected. Surprisingly, in the remaining cases, no other collagen gene abnormalities have been found despite clinical similarity with the classic type EDS. It is suspected that in some cases, genetic defects that affect the biosynthesis of other extracellular matrix molecules that influence collagen synthesis indirectly may be involved. One example is a classic EDS-like condition caused by mutation in the TNXB gene encoding tenascin-X, a large multimeric protein that interacts with fibrillar types I, III, and V collagens.
To summarize, the common thread in EDSs is some abnormality of collagen. These disorders, however, are extremely heterogeneous. At the molecular level, a variety of defects, varying from mutations involving structural genes for collagen to those involving enzymes that are responsible for posttranscriptional modifications of mRNA, have been detected. Such molecular heterogeneity results in the expression of EDSs as clinically variable disorders with several patterns of inheritance.
Marfan syndrome is caused by a mutation in the FBN1 gene encoding fibrillin, which is required for structural integrity of connective tissues and regulation of TGF-β signaling.
The major tissues affected are the skeleton, eyes, and cardiovascular system.
Clinical features may include tall stature, long fingers, bilateral subluxation of lens, mitral valve prolapse, aortic aneurysm, and aortic dissection.
There are several variants of EDS, all characterized by defects in collagen synthesis or assembly. Each of the variants is caused by a distinct mutation involving one of several collagen genes or genes that encode other ECM proteins like tenascin-X.
Clinical features may include fragile, hyperextensible skin vulnerable to trauma; hypermobile joints; and ruptures involving the colon, cornea, or large arteries. Wound healing is poor.
Familial hypercholesterolemia (FH) is caused most commonly by mutations in the gene encoding the receptor for LDL, resulting in inadequate removal of plasma LDL by the liver. Mutations in the LDL receptor gene (LDLR) account for 80% to 85% of cases of FH. Much less commonly, FH is caused by mutations in two other genes involved in clearance of plasma LDL. They encode (1) apolipoprotein B-100 (ApoB), the ligand for LDL receptor on the LDL particle (5% to 10% cases), and (2) proprotein convertase subtilisin/kexin type 9 (1% to 2% cases). This enzyme, better known by its abbreviation PCSK9 , reduces expression of LDL receptors by downregulating their recycling and consequent degradation in lysosomes. Each of these three types of mutations impairs hepatic clearance of LDL and increases serum levels of cholesterol, giving rise to premature atherosclerosis and a greatly increased risk of myocardial infarction. The functions of these genes in cholesterol metabolism are discussed below.
FH caused by LDL receptor mutations is one of the most frequently occurring Mendelian disorders. Heterozygotes with one mutant gene, representing about 1 in 200 individuals, have from birth a twofold to threefold elevation of plasma cholesterol level, leading to tendinous xanthomas and premature atherosclerosis in adult life ( Chapter 11 ). Homozygotes, having a double dose of the mutant gene, are much more severely affected and may have fivefold to sixfold elevations in plasma cholesterol levels. Skin xanthomas and coronary, cerebral, and peripheral vascular atherosclerosis may develop in these individuals at an early age. Myocardial infarction may occur before age 20 years. Large-scale studies have found that FH is present in 3% to 6% of survivors of myocardial infarction.
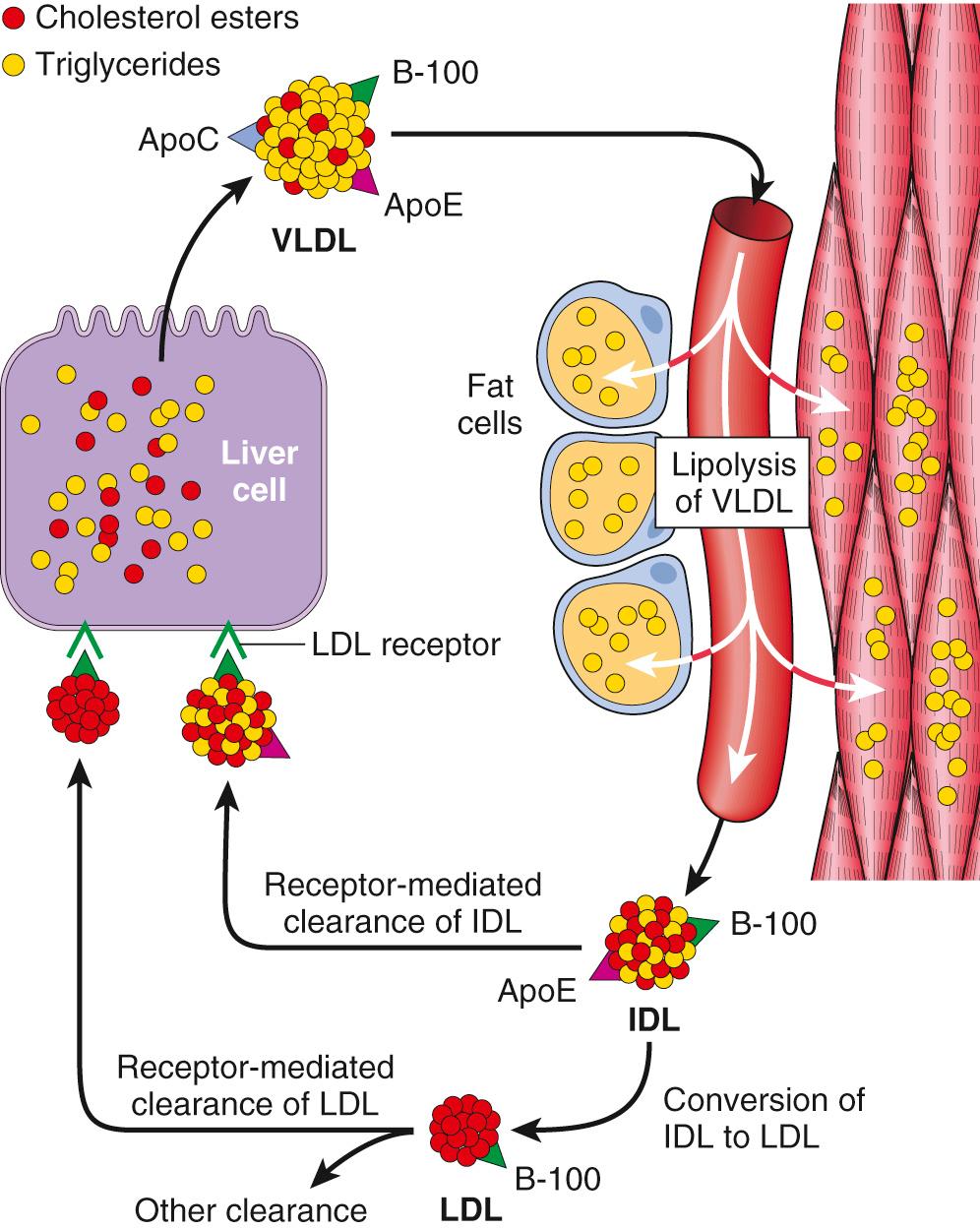
Cholesterol may be derived from the diet or from endogenous synthesis. Dietary triglycerides and cholesterol are incorporated into chylomicrons in the intestinal mucosa and travel by way of the gut lymphatics to the blood. These chylomicrons are hydrolyzed by an endothelial lipoprotein lipase in the capillaries of muscle and fat. The chylomicron remnants, rich in cholesterol, are then delivered to the liver. Some of the cholesterol enters the metabolic pool (to be described), and some is excreted as free cholesterol or as bile acids into the biliary tract. The endogenous synthesis of cholesterol and LDL begins in the liver ( Fig. 5.6 ). The first step in this process is the secretion of very-low-density lipoprotein (VLDL) from the liver into the blood. VLDL particles are rich in triglycerides, but they contain lesser amounts of cholesteryl esters. In addition, they carry apolipoproteins ApoB, ApoC, and ApoE on their surface. In the capillaries of adipose tissue and muscle, the VLDL particle undergoes lipolysis and is converted to VLDL remnant, also called intermediate-density lipoprotein (IDL). Compared with VLDL, IDL particles have reduced content of triglycerides and an increase in cholesteryl esters. ApoC is lost, but ApoB and ApoE are retained. After release from the capillary endothelium, the IDL particles have one of two fates. Approximately 50% of newly formed IDL is rapidly taken up by the liver by receptor-mediated transport. The receptor responsible for the binding of IDL to the liver cell membrane recognizes both ApoB and ApoE. It is called ApoB/E, or more commonly the LDL receptor, because it is also involved in the hepatic clearance of LDL (described later). In the liver cells, IDL is recycled to generate VLDL. The IDL particles not taken up by the liver are subjected to further metabolic processing that removes most of the remaining triglycerides and ApoE, yielding ApoB carrying cholesterol-rich LDL particles.
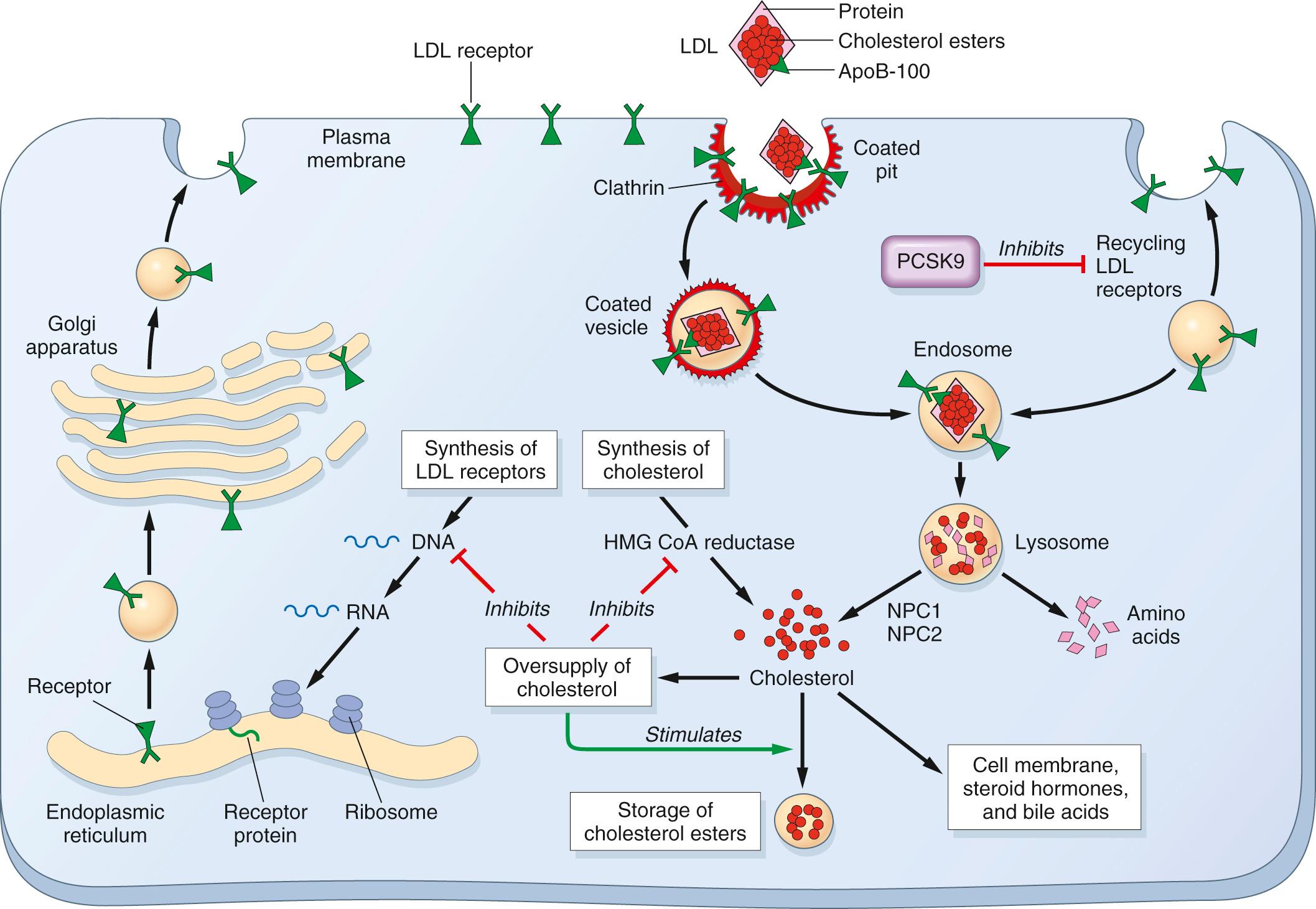
Although many cell types, including fibroblasts, lymphocytes, smooth muscle cells, hepatocytes, and adrenocortical cells, possess high-affinity LDL receptors, approximately 70% of the plasma LDL is cleared by the liver, using a quite sophisticated transport process ( Fig. 5.7 ). The first step involves binding of LDL to cell-surface receptors, which are clustered in specialized regions of the plasma membrane called coated pits ( Chapter 1 ). After binding, the coated pits containing the receptor-bound LDL are internalized by invagination to form coated vesicles, after which they migrate within the cell to fuse with the lysosomes. Here the LDL dissociates from the receptor, which is recycled to the surface. The recycling of LDL receptors is regulated by PCSK9, which binds to LDL receptors on the surface of hepatocytes and causes their degradation after endocytosis. In the lysosomes the LDL molecule is enzymatically degraded; the apoprotein part is hydrolyzed to amino acids, whereas the cholesteryl esters are broken down to free cholesterol. This free cholesterol, in turn, crosses the lysosomal membrane to enter the cytoplasm, where it is used for membrane synthesis and as a regulator of cholesterol homeostasis. The exit of cholesterol from the lysosomes requires the action of two proteins, called NPC1 and NPC2 (see “Niemann-Pick Disease Type C”). Four separate processes are affected by the released intracellular cholesterol, as follows (see Fig. 5.7 ):
Cholesterol suppresses cholesterol synthesis within the cell by inhibiting the activity of the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase, which is the rate-limiting enzyme in the synthetic pathway.
Cholesterol activates the enzyme acyl-coenzyme A:cholesterol acyltransferase, favoring esterification and storage of excess cholesterol.
Cholesterol suppresses the synthesis of LDL receptors, thus protecting the cells from excessive accumulation of cholesterol.
Cholesterol upregulates the expression of PCSK9, which reduces recycling of LDL receptors and causes degradation of endocytosed LDL receptors. This provides an additional mechanism of protecting the cells from excessive accumulation of cholesterol.
As mentioned earlier, FH results from mutations in the gene encoding the receptor for LDL or the two genes that compromise its function. The impact of mutations in these genes is as follows:
Mutations in LDLR gene . Heterozygotes with FH due to mutation in the LDLR gene possess only 50% of the normal number of high-affinity LDL receptors because they have only one normal gene. As a result of this defect in transport, the catabolism of LDL by the receptor-dependent pathways is impaired, and the plasma level of LDL increases two- to three-fold. Homozygotes have virtually no normal LDL receptors in their cells and have much higher levels of circulating LDL. In addition to defective LDL clearance, both the homozygotes and the heterozygotes have increased synthesis of LDL. The increased synthesis that contributes to hypercholesterolemia also results from a lack of LDL receptors (see Fig. 5.6 ). As mentioned above, IDL, the immediate precursor of plasma LDL, also uses hepatic LDL receptors (apoB/E receptors) for its transport into the liver. In FH, impaired IDL transport into the liver secondarily diverts a greater proportion of plasma IDL into the precursor pool for plasma LDL.
Mutations in gene encoding ApoB. Since ApoB on the surface of LDL particles is the ligand for LDL receptors, mutant ApoB reduces the binding of LDL molecules with LDL receptors. This compromise in the binding of LDL particles to its receptors increases serum LDL cholesterol.
Activating mutation in the PCSK9 gene. This mutation greatly reduces the number of LDL receptors on the cell surface because of their increased degradation during the recycling process.
The transport of LDL via the scavenger receptor seems to occur at least in part into the cells of the mononuclear phagocyte system. Monocytes and macrophages have receptors for chemically altered (e.g., acetylated or oxidized) LDL. Normally the amount of LDL transported along this scavenger receptor pathway is less than that mediated by the LDL receptor–dependent mechanisms. In the face of hypercholesterolemia, however, there is a marked increase in the scavenger receptor–mediated traffic of LDL cholesterol into the cells of the mononuclear phagocyte system and possibly the vascular walls ( Chapter 11 ). This increase is responsible for the appearance of xanthomas and contributes to the pathogenesis of premature atherosclerosis.
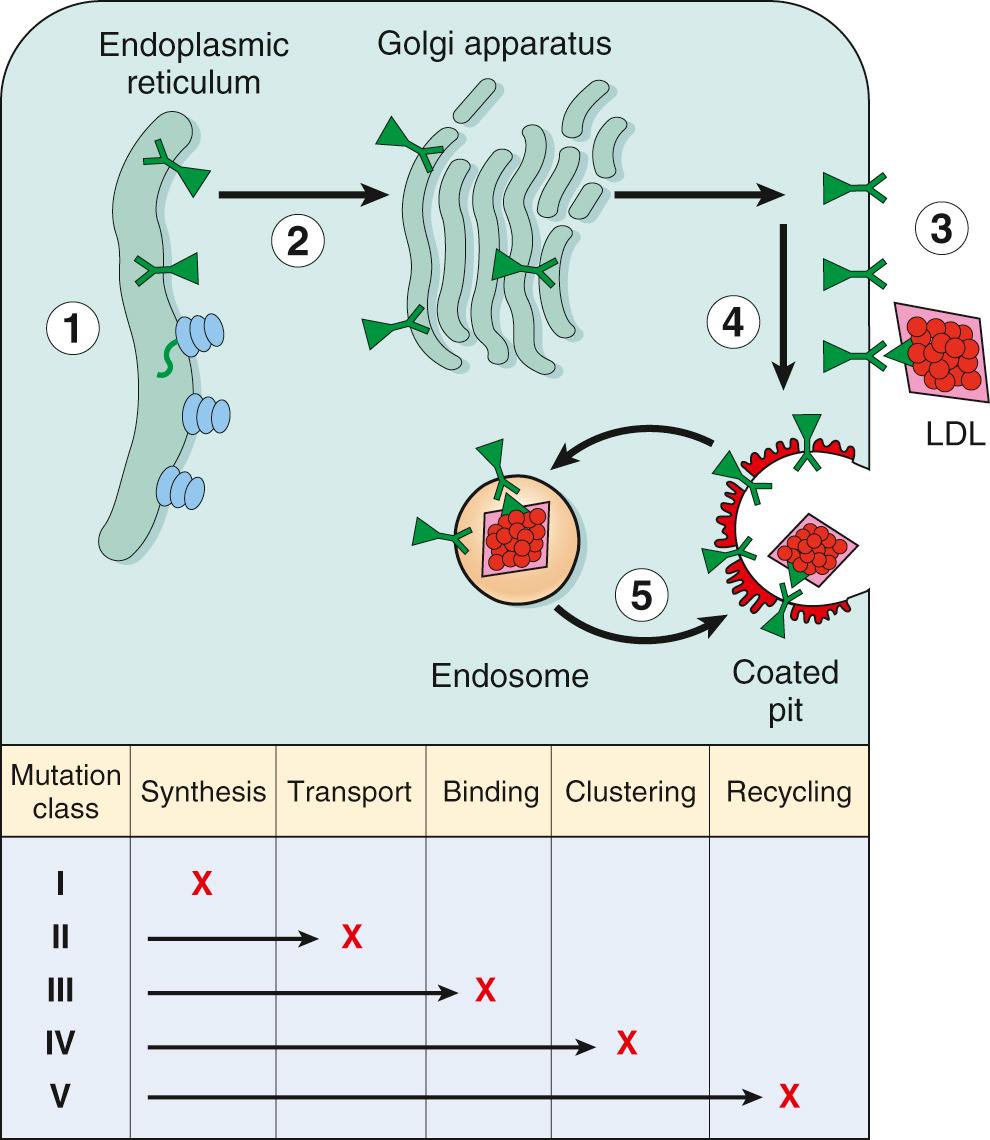
The molecular genetics of FH is extremely complex. More than 2000 mutations involving the LDL receptor gene, including DNA copy number variations, insertions, deletions, and missense and nonsense mutations, have been identified. These can be classified into six groups ( Fig. 5.8 ). Class I mutations are relatively uncommon and lead to a complete failure of synthesis of the LDL receptor protein (null allele). Class II mutations are fairly common; they encode LDL receptor proteins that accumulate in the endoplasmic reticulum because their folding defects make it impossible for them to be transported to the Golgi complex. Class III mutations affect the ApoB binding site of the receptor; the mutant LDL receptors reach the cell surface but fail to bind LDL or do so poorly. Class IV mutations encode LDL receptors that are synthesized and transported to the cell surface efficiently. They bind LDL normally, but they fail to localize in coated pits, and hence the bound LDL is not internalized. Class V mutations encode LDL receptors that are expressed on the cell surface, can bind LDL, and can be internalized; however, the pH-dependent dissociation of the receptor and the bound LDL fails to occur. Such receptors are trapped in the endosome, where they are degraded, and hence they fail to recycle to the cell surface. Class VI mutations result in the failure of initial targeting of the LDL receptor to the basolateral membrane.
The discovery of the critical role of LDL receptors in cholesterol homeostasis has led to the rational design of drugs that lower plasma cholesterol by increasing the number of LDL receptors (see Fig. 5.8 ). One strategy is based on the ability of certain drugs (statins) to suppress intracellular cholesterol synthesis by inhibiting the enzyme HMG CoA reductase. The reduction in intracellular cholesterol allows greater synthesis of LDL receptors by removing the braking action of cholesterol on LDL receptor synthesis. In another strategy, antibodies that inhibit PCSK9 function reduce the degradation of LDL receptors, thereby increasing their abundance on the cell membrane and consequent increased clearance of LDL cholesterol from the blood. These agents have profound cholesterol-lowering effects, and large clinical trials have shown the benefits of using this class of drugs in the treatment of patients who do not respond adequately to statins alone. Interestingly, PCSK9 was discovered accidentally in individuals with exceedingly low serum cholesterol who were found to have loss-of-function variants of the PCSK9 gene.
FH is an autosomal dominant disorder caused by mutations in the genes encoding the (1) LDL receptor (85% cases), (2) ApoB protein (5% to 10% cases), or (3) activating mutations of PCSK9 (1% to 2% cases).
Patients develop hypercholesterolemia as a consequence of impaired transport of LDL into the cells.
In heterozygotes for mutations in the LDLR gene, elevated serum cholesterol greatly increases the risk of atherosclerosis and resultant coronary artery disease; homozygotes have an even greater increase in serum cholesterol and a higher frequency of ischemic heart disease. Cholesterol also deposits along tendon sheaths to produce xanthomas.
Lysosomes are key components of the intracellular digestive system. They contain a battery of hydrolytic enzymes, which have two special properties. First, they function in the acidic milieu of the lysosomes. Second, these enzymes constitute a special category of secretory proteins that are destined not for the extracellular fluids but for intracellular organelles. This latter characteristic requires special processing within the Golgi apparatus, which merits brief discussion.
Similar to all other secretory proteins, lysosomal enzymes (or acid hydrolases, as they are sometimes called) are synthesized in the endoplasmic reticulum and transported to the Golgi apparatus. Within the Golgi complex they undergo a variety of posttranslational modifications including the attachment of terminal mannose-6-phosphate groups to some of the oligosaccharide side chains. The phosphorylated mannose residues serve as an “address label” that is recognized by specific receptors found on the inner surface of the Golgi membrane. Lysosomal enzymes bind these receptors and are thereby segregated from the numerous other secretory proteins within the Golgi. Subsequently, small transport vesicles containing the receptor-bound enzymes are pinched off from the Golgi and proceed to fuse with the lysosomes. Thus the enzymes are targeted to their intracellular abode, and the vesicles are shuttled back to the Golgi ( Fig. 5.9 ). As indicated later, genetically determined errors in this remarkable sorting mechanism may give rise to one form of lysosomal storage disease. Recent studies have established a close link between lysosomal storage diseases and several neurodegenerative disorders. The cellular and molecular mechanisms of this linkage will be discussed below.
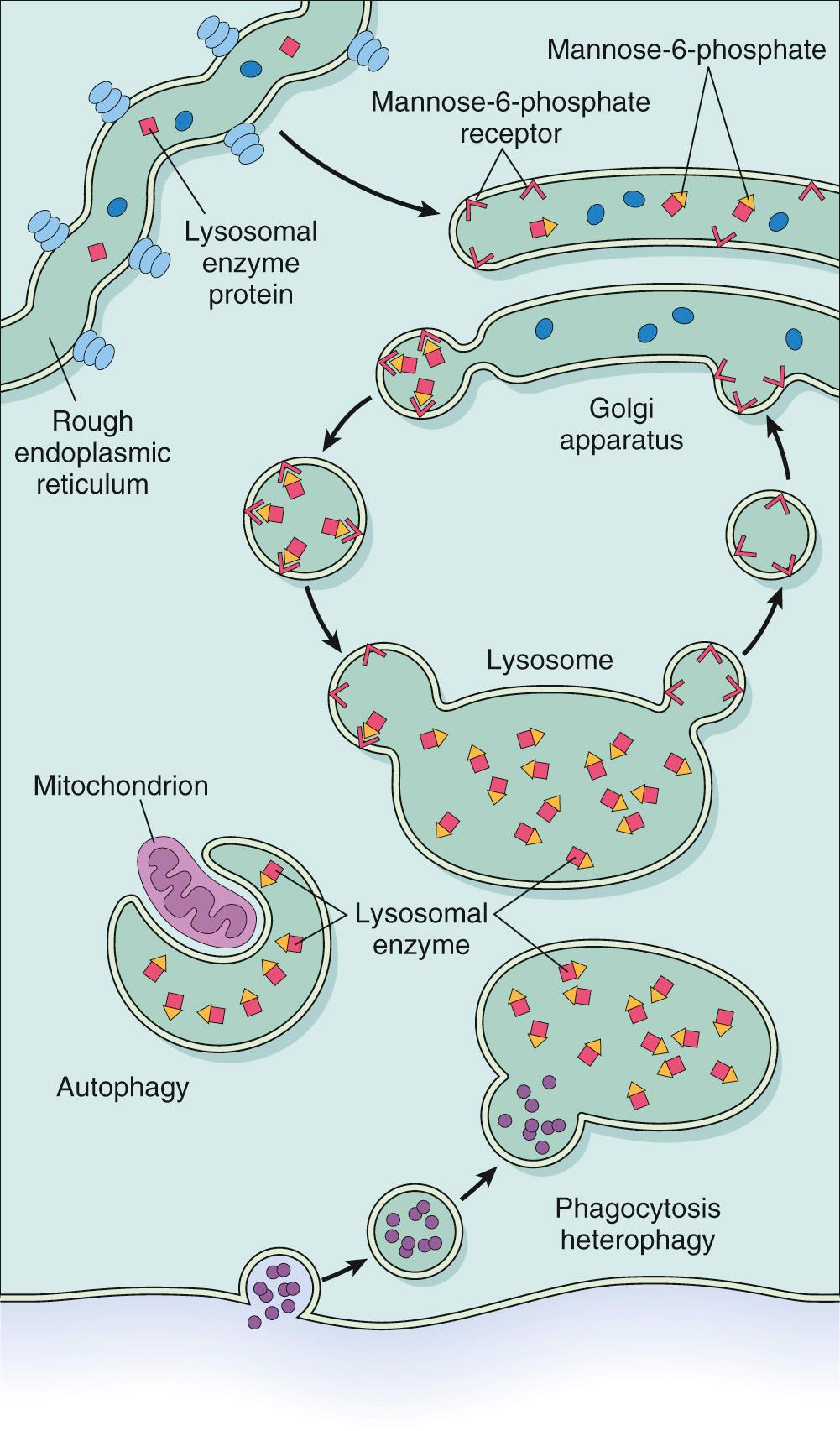
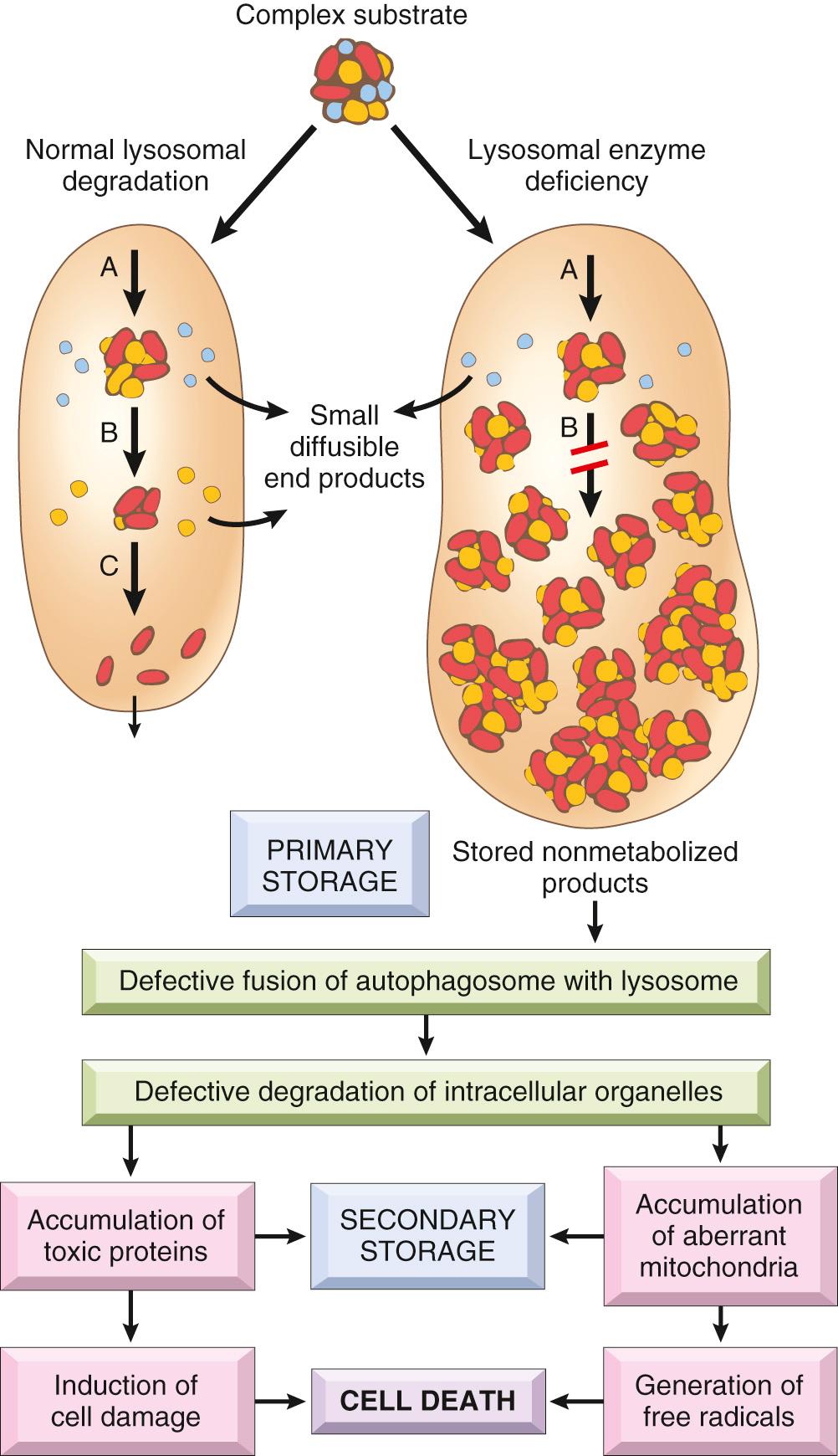
The lysosomal enzymes catalyze the breakdown of a variety of complex macromolecules. These large molecules may be derived from the metabolic turnover of intracellular organelles (autophagy), or they may be acquired from outside the cells by phagocytosis (heterophagy). An inherited deficiency of a functional lysosomal enzyme gives rise to two pathologic consequences ( Fig. 5.10 ).
Catabolism of the substrate of the missing enzyme remains incomplete, leading to the accumulation of the partially degraded insoluble metabolite within the lysosomes. This is called “primary accumulation.” Stuffed with incompletely digested macromolecules, lysosomes become large and numerous enough to interfere with normal cell functions.
There is a tight linkage between autophagy, mitochondrial functions, and lysosomes . As discussed in Chapter 2 , a diverse range of cellular organelles and molecules are degraded by autophagy including complex lipids, polyubiquitinated proteins, mitochondria, and fragments of the endoplasmic reticulum. In particular, autophagy is essential for turnover of mitochondria by a process termed mitophagy. This serves as a quality control system whereby dysfunctional mitochondria are degraded. Because of the accumulation of undigested macromolecules in the lysosomes, the rate at which lysosomes process organelles delivered by autophagocytic vacuoles is markedly reduced. This leads to persistence of dysfunctional and leaky mitochondria with poor calcium-buffering capacity and altered membrane potentials in the lysosomes. Damaged mitochondria generate free radicals and release molecules that trigger the intrinsic pathway of apoptosis. Impaired autophagy gives rise to secondary accumulation of autophagic substrates including ubiquitinated and aggregate-prone polypeptides such as α-synuclein and Huntingtin protein. This provides a molecular link between neurodegenerative disorders and lysosomal storage diseases such as Gaucher disease (discussed below).
There are three general approaches to the treatment of lysosomal storage diseases. The most obvious is enzyme replacement therapy, currently in use for several of these diseases. Another approach, substrate reduction therapy, is based on the premise that if the substrate to be degraded by the lysosomal enzyme can be reduced, the residual enzyme activity may be sufficient to catabolize it and prevent accumulation. A more recent strategy is based on the understanding of the molecular basis of enzyme deficiency. In many disorders, exemplified by Gaucher disease, the enzyme activity is low because the mutant proteins are unstable and prone to misfolding and hence degraded in the endoplasmic reticulum. In such diseases an exogenous competitive inhibitor of the enzyme can, paradoxically, bind to the mutant enzyme and act as the folding template that assists proper folding of the enzyme and thus prevents its degradation. Such molecular chaperone therapy is under active investigation. In addition to the aforementioned, hematopoietic stem cell transplants and gene therapy are also being evaluated in specific cases.
Although the combined frequency of lysosomal storage disorders is about 1 in 5000 live births, lysosomal dysfunction may be involved in the etiology of several more common diseases. For example, an important genetic risk factor for developing Parkinson disease is the carrier state for Gaucher disease, and virtually all patients with Gaucher disease develop Parkinson disease. Niemann-Pick type C disease is another lysosomal storage disorder that increases the risk for Alzheimer disease. Such interconnectedness stems from the multifunctionality of the lysosome. For example, lysosomes play critical roles in (1) autophagy, resulting from fusion with the autophagosome; (2) immunity, because they fuse with phagosomes; and (3) membrane repair, through fusion with the plasma membrane.
Approximately 70 lysosomal storage diseases have been identified. These may result from abnormalities of lysosomal enzymes or proteins involved in substrate degradation, endosomal sorting, or lysosomal membrane integrity. Lysosomal storage disorders are divided into categories based on the biochemical nature of the substrates and the accumulated metabolites ( Table 5.6 ). Within each group are several entities, each resulting from the deficiency of a specific enzyme.
| Disease | Enzyme Deficiency | Major Accumulating Metabolites |
|---|---|---|
| Glycogenosis | Type 2—Pompe disease | Glycogen |
| α-1,4-Glucosidase (lysosomal glucosidase) | ||
| Sphingolipidoses | ||
| G M1 gangliosidosis | G M1 ganglioside β-galactosidase | G M1 ganglioside, galactose-containing oligosaccharides |
| Type 1—infantile, generalized | ||
| Type 2—juvenile | ||
| G M2 gangliosidosis | ||
| Tay-Sachs disease | Hexosaminidase A | G M2 ganglioside |
| Sandhoff disease | Hexosaminidase A and B | G M2 ganglioside, globoside |
| G M2 gangliosidosis variant AB | Ganglioside activator protein | G M2 ganglioside |
| Sulfatidoses | ||
| Metachromatic leukodystrophy | Arylsulfatase A | Sulfatide |
| Multiple sulfatase deficiency | Arylsulfatase A, B, C; steroid sulfatase; iduronate sulfatase; heparan N -sulfatase | Sulfatide, steroid sulfate, heparan sulfate, dermatan sulfate |
| Krabbe disease | Galactosylceramidase | Galactocerebroside |
| Fabry disease | α-Galactosidase A | Ceramide trihexoside |
| Gaucher disease | Glucocerebrosidase | Glucocerebroside |
| Niemann-Pick disease: types A and B | Sphingomyelinase | Sphingomyelin |
| Mucopolysaccharidoses (MPSs) | ||
| MPS I-H (Hurler) | α- l -Iduronidase | Dermatan sulfate, heparan sulfate |
| MPS II (Hunter) | Iduronate 2-sulphatase | |
| Mucolipidoses (MLs) | ||
| I-cell disease (ML II) and pseudo-Hurler polydystrophy | Deficiency of phosphorylating enzymes essential for the formation of mannose-6-phosphate recognition marker; acid hydrolases lacking the recognition marker cannot be targeted to the lysosomes, but are secreted extracellularly | Mucopolysaccharide, glycolipid |
| Other diseases of complex carbohydrates | ||
| Fucosidosis | α-Fucosidase | Fucose-containing sphingolipids and glycoprotein fragments |
| Mannosidosis | α-Mannosidase | Mannose-containing oligosaccharides |
| Aspartylglycosaminuria | Aspartylglycosamine amide hydrolase | Aspartyl-2-deoxy-2-acetamido-glycosylamine |
| Other lysosomal storage diseases | ||
| Wolman disease | Acid lipase | Cholesterol esters, triglycerides |
In general, the distribution of the stored material, and hence the organs affected, is determined by two interrelated factors: (1) the tissue where most of the material to be degraded is found and (2) the location where most of the degradation normally occurs. For example, the brain is rich in gangliosides, and hence defective hydrolysis of gangliosides, as occurs in G M1 and G M2 gangliosidoses, results primarily in accumulation within neurons and consequent neurologic symptoms. Defects in degradation of mucopolysaccharides affect virtually every organ because mucopolysaccharides are widely distributed in the body. Because cells of the mononuclear phagocyte system are especially rich in lysosomes and are involved in the degradation of a variety of substrates, organs rich in phagocytic cells, such as the spleen and liver, are frequently enlarged in several forms of lysosomal storage disorders. The ever-expanding number of lysosomal storage diseases can be divided into rational categories based on the biochemical nature of the accumulated metabolite, thus creating such subgroups as glycogenoses, sphingolipidoses (lipidoses), mucopolysaccharidoses (MPSs), and mucolipidoses (see Table 5.6 ).
Most of these conditions are very rare, and their detailed description is better relegated to specialized texts and reviews. Only a few of the more common conditions are considered here.
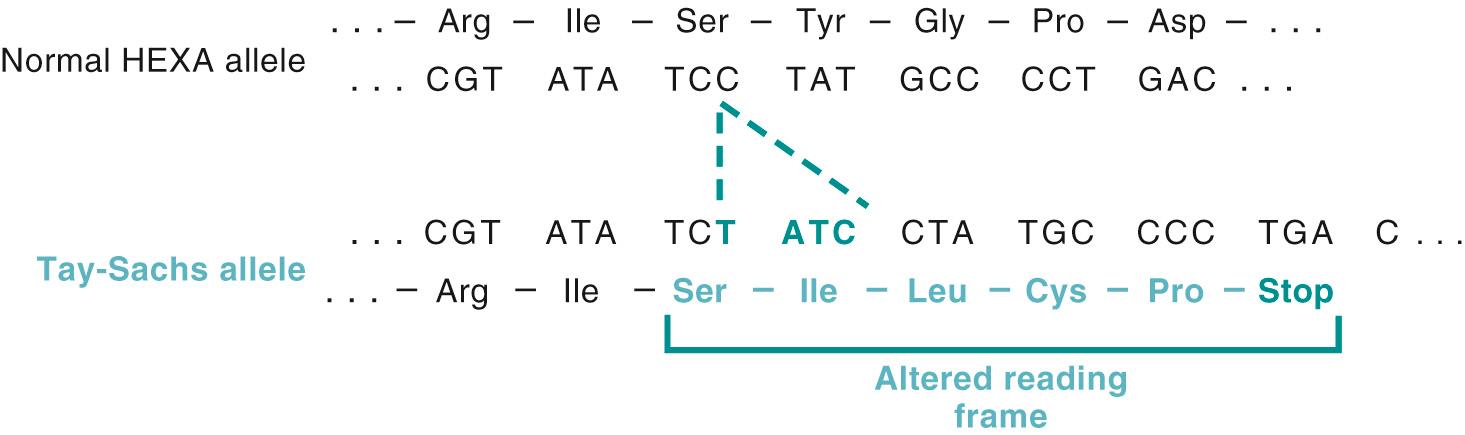
G M2 gangliosidoses are a group of three lysosomal storage diseases caused by deficiency of the enzyme β-hexosaminidase resulting in an inability to catabolize G M2 gangliosides. There are two isoenzymes of β-hexosaminidase: Hex A, consisting of two subunits, α and β, and Hex B, a homodimer of β-subunits. Degradation of G M2 gangliosides requires three polypeptides encoded by three distinct genes— HEXA (on chromosome 15), which encodes the α-subunit of Hex A; HEXB (on chromosome 5), which encodes the β-subunit of Hex A and Hex B; and GM2A (on chromosome 5), which encodes the activator of hexosaminidase. The phenotypic effects of mutations affecting these genes are fairly similar because they result from accumulation of G M2 gangliosides. The underlying enzyme defect, however, is different for each. Tay-Sachs disease, the most common form of G M2 gangliosidosis, results from mutations in the α-subunit locus on chromosome 15 that cause a severe deficiency of hexosaminidase A. This disease is especially prevalent among Jews, particularly among those of Eastern European (Ashkenazic) origin, in whom a carrier rate of 1 in 30 has been reported.
The molecular basis for neuronal injury resulting from hexosaminidase deficiency is not fully understood. More than 100 mutations have been described in the HEXA α-subunit gene; most affect protein folding. Because the mutant protein is misfolded, it induces the so-called unfolded protein response ( Chapter 2 ). If such misfolded enzymes are not stabilized by chaperones, they undergo proteasomal degradation, leading to accumulation of toxic substrates and intermediates within neurons. These findings have spurred clinical trials of molecular chaperone therapy for some variants of later-onset Tay-Sachs and other selected lysosomal storage diseases. Such therapy involves the use of synthetic chaperones that can cross the blood–brain barrier, bind to the mutated protein, and enable its proper folding. Sufficient functional enzyme can then be rescued to ameliorate the effects of the inborn error.
Hexosaminidase A is absent from virtually all the tissues, so G M2 ganglioside accumulates in many tissues (e.g., heart, liver, spleen, nervous system), but the involvement of neurons in the central and autonomic nervous systems and retina dominates the clinical picture. On histologic examination, the neurons are ballooned with cytoplasmic vacuoles, each representing a markedly distended lysosome filled with gangliosides ( Fig. 5.11A ). With the electron microscope, several types of cytoplasmic inclusions can be visualized, the most prominent being whorled configurations within lysosomes composed of onion-skin layers of membranes ( Fig. 5.11B ). In time there is progressive destruction of neurons, proliferation of microglia, and accumulation of complex lipids in phagocytes within the brain substance. The ganglion cells in the retina are similarly swollen with G M2 ganglioside, particularly at the margins of the macula. A cherry-red spot thus appears in the macula, representing accentuation of the normal color of the macular choroid contrasted with the pallor produced by the swollen ganglion cells in the remainder of the retina ( Chapter 29 ). This finding is characteristic of Tay-Sachs disease and other storage disorders affecting the neurons.
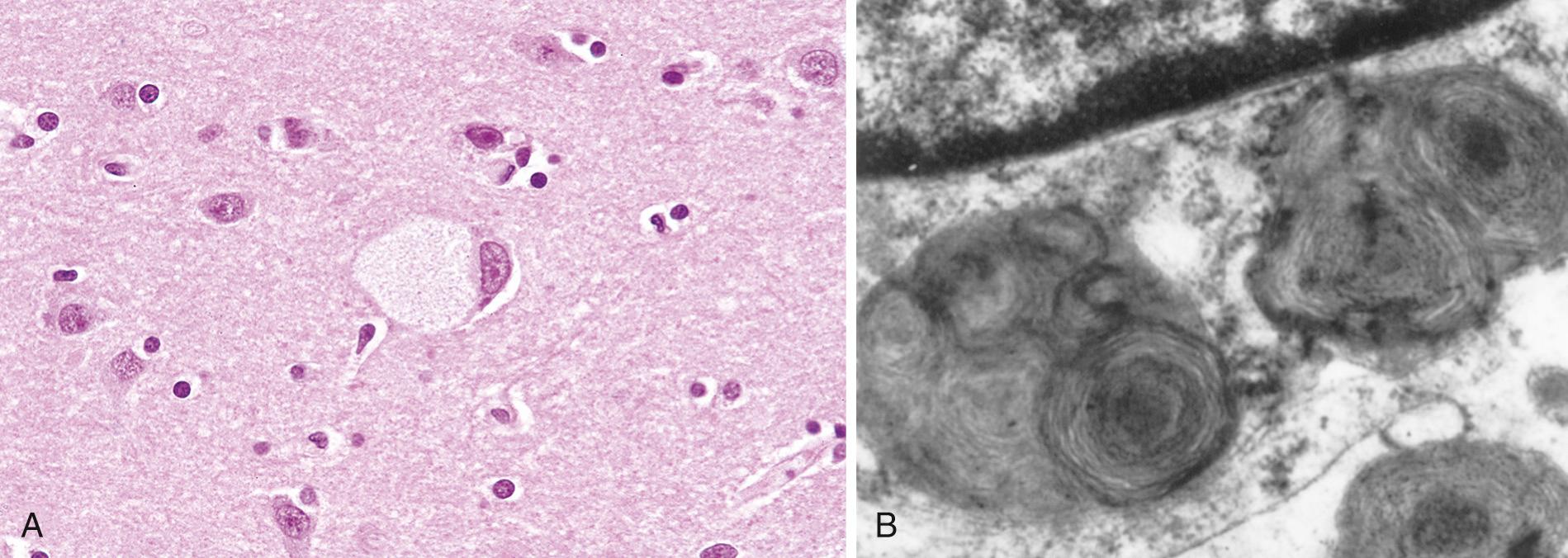
Affected infants appear normal at birth but begin to manifest signs and symptoms at about age 6 months. There is relentless motor and mental deterioration, resulting in motor incoordination and intellectual disability leading eventually to muscular flaccidity, blindness, and increasing dementia. Sometime during the early course of the disease, the characteristic, but not pathognomonic, cherry-red spot appears in the macula of the eye in almost all patients. Over the span of 1 or 2 years a complete vegetative state is reached, followed by death at age 2 to 3 years. Antenatal diagnosis and carrier detection are possible by enzyme assays and DNA-based analysis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here