Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In this chapter we discuss genetic and pediatric diseases together, because many disorders of childhood are of genetic origin. However, it must be borne in mind that not all genetic disorders manifest in infancy and childhood, and, conversely, many pediatric diseases are not of genetic origin. To the latter category belong diseases resulting from immaturity of organ systems.
The contributions of Dr. Anirban Maitra, Department of Translational Molecular Pathology, University of Texas, MD Anderson Cancer Center, Houston, Texas, to this chapter in several previous editions of this book are gratefully acknowledged.
To facilitate an understanding of the molecular basis of genetic disorders, we begin this chapter with an overview of the architecture of the human genome.
Remarkable advances have been made in our understanding of the genetic basis of both inherited and acquired human diseases in the past 50 years. This has been brought about by a deeper understanding of the structure of the human genome and the factors that regulate gene expression.
The sequencing of the human genome at the beginning of the 21st century was a landmark achievement of biomedical science. Since then, the rapidly dropping cost of sequencing and the computational capacity to analyze vast amounts of data have revolutionized our understanding of health and disease. At the same time, the emerging information has also revealed a breathtaking level of complexity far beyond the linear sequence of the genome. The potential for these new powerful tools to expand our understanding of pathogenesis and drive therapeutic innovation excites and inspires scientists and the lay public alike. While early studies were focused on discovery of genes that encode proteins, more recent investigations have led to important insights into the role of noncoding DNA in regulating gene expression. We discuss this next.
The human genome contains about 3.3 billion DNA base pairs. Yet, within the genome there are only slightly more than 19,000 protein-encoding genes, making up just 1.5% of the genome. The proteins encoded by these genes are the fundamental constituents of cells, functioning as enzymes, structural elements, and signaling molecules. Although 19,000 underestimates the actual number of proteins encoded (many genes produce multiple RNA transcripts that produce distinct protein isoforms), it is nevertheless startling that worms composed of fewer than 1000 cells—and with genomes 30-fold smaller—are also assembled from roughly 20,000 protein-encoding genes. Perhaps even more surprising is that many of these proteins are recognizable homologs of molecules expressed in humans. What then separates humans from worms? The answer is not completely known, but evidence supports the assertion that the difference lies in the 98.5% of the human genome that does not encode proteins. We now know that more than 85% of the human genome is transcribed, with almost 80% being devoted to the regulation of gene expression. It follows that, whereas proteins provide the building blocks and machinery required for assembling cells, tissues, and organisms, it is the noncoding regions of the genome that provide the critical “architectural planning.”
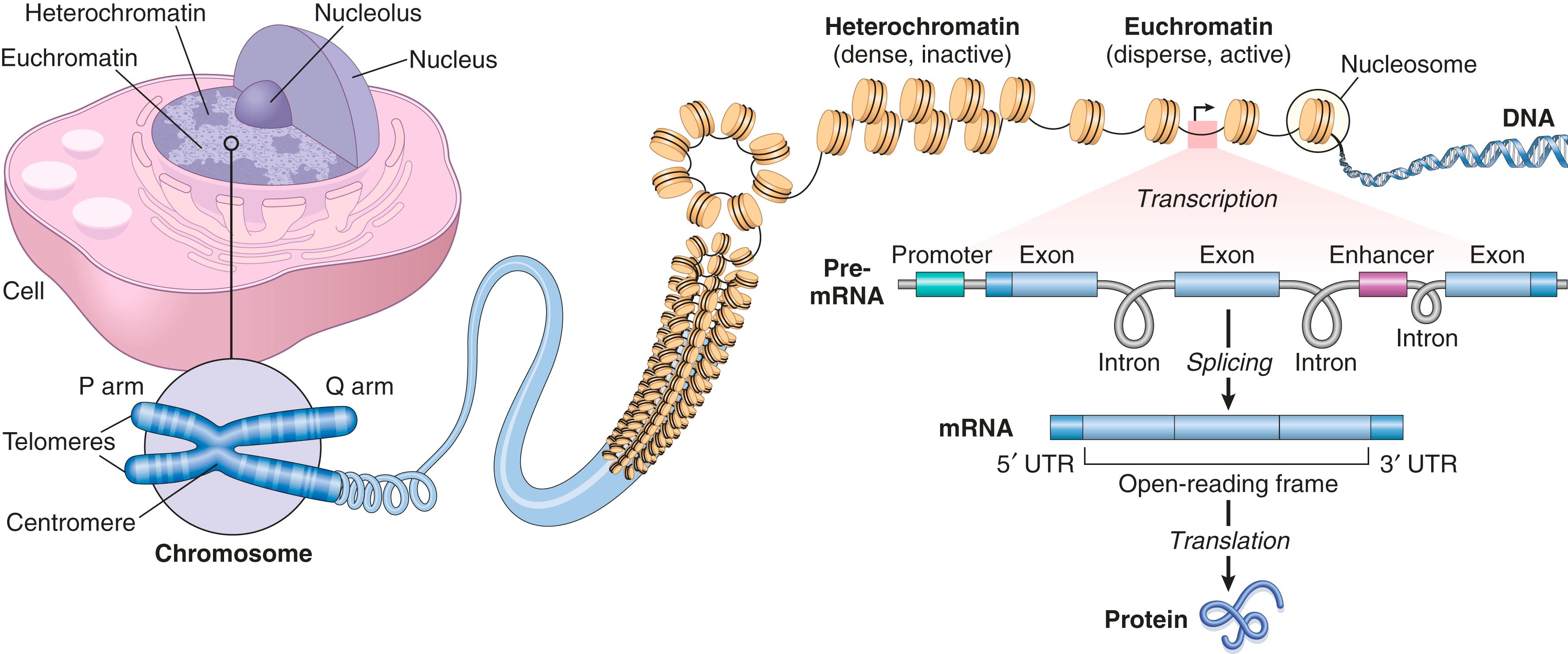
The major classes of functional non–protein-coding DNA sequences found in the human genome include ( Fig. 4.1 ):
Promoter and enhancer regions that bind transcription factors
Binding sites for proteins that organize and maintain higher-order chromatin structures
Noncoding regulatory RNAs. Of the 80% of the genome dedicated to regulatory functions, the vast majority is transcribed into RNAs—micro-RNAs and long noncoding RNAs (described later)—that are never translated into protein but can regulate gene expression
Mobile genetic elements (e.g., transposons ). Remarkably, more than one-third of the human genome is composed of these “jumping genes,” which can move around to various sites in the genome and are implicated in gene regulation and chromatin organization.
Special structural regions of DNA, including telomeres (chromosome ends) and centromeres (chromosome “tethers”)
Importantly, many genetic variations (polymorphisms) associated with diseases are located in non–protein-coding regions of the genome. Thus, variation in gene regulation may prove to be more important in disease causation than structural changes in specific proteins. Genome sequencing has shown that any two humans are typically >99.5% DNA identical. Thus, individual variation, including differential susceptibility to diseases and environmental exposures, is encoded in <0.5% of our DNA.
The two most common forms of DNA variation in the human genome are single-nucleotide polymorphisms (SNPs) and copy number variations (CNVs) .
SNPs are variants at single nucleotide positions and are almost always biallelic (only two choices exist at a given site within the population, such as A or T). More than 6 million human SNPs have been identified, with many showing wide variation in frequency in different populations. The following features are worthy of note:
SNPs occur across the genome—within genes and noncoding regions.
Roughly 1% of SNPs occur in coding regions, which is about what would be expected by chance, because coding regions constitute about 1.5% of the genome.
SNPs located in noncoding regions can occur in regulatory elements in the genome, thereby altering gene expression; in such instances the SNP may have a direct influence on disease susceptibility.
SNPs can also be “neutral” variants with no effect on gene function or phenotype.
Even “neutral” SNPs may be useful markers if they happen to be coinherited with a disease-associated gene as a result of physical proximity. In other words, the SNP and the causative genetic factor are in linkage disequilibrium. Linkage disequilibrium refers to two genetic markers that are found together at frequencies not predicted by their binomial probability. It can result from either positive natural selection favoring one genetic marker, increasing the frequency of all nearby genetic variants, or genetic drift (random association of two alleles due to chance events).
The effect of individual SNPs on disease susceptibility is weak, particularly for complex diseases such as diabetes, heart disease, or cancer. This is because many genetic systems are at play and the contributions of individual SNPs are small. It remains to be seen if the identification of such variants, alone or in combination, can be used to better understand pathogenesis or to develop effective strategies for disease prediction or prevention.
CNVs are a form of genetic variation consisting of different numbers of large contiguous stretches of DNA. These can range from thousands to millions of base pairs. In some instances, these loci are, like SNPs, biallelic and simply duplicated or deleted in a subset of the population. In other instances there are complex rearrangements (inversions) of genomic material, with multiple alleles in the human population. CNVs are responsible for several million base pairs of sequence difference between any two individuals. Approximately 50% of CNVs involve gene-coding sequences; thus, CNVs may underlie a large portion of human phenotypic diversity.
It is important to note that alterations in DNA sequence cannot by themselves explain the diversity of phenotypes in human populations. All phenotypes result from a complex interaction between genes, environment, and chance. Moreover, phenotypic variation may be observed even in genetically identical monozygotic twins. In addition, posttranslational modifications such as DNA methylation and histones, have profound impacts on gene expression. We discuss this next.
Even though virtually all cells in the body have the same genetic composition, differentiated cells have distinct structures and functions arising through lineage-specific programs of gene expression. Such cell type–specific differences in DNA transcription and translation are regulated by epigenetic modifications of chromatin that profoundly influence gene expression.
One central mechanism of epigenetic regulation is methylation of cytosine residues in gene promoters—heavily methylated promoters become inaccessible to RNA polymerase, leading to transcriptional silencing. Promoter methylation and silencing of tumor suppressor genes ( Chapter 6 ) are observed in many human cancers, leading to unchecked cell growth. Another major player in epigenetic regulation of transcription involves the family of histone proteins, which are components of structures called nucleosomes, around which DNA is coiled. Histone proteins undergo a variety of reversible modifications such as methylation and acetylation that affect secondary and tertiary DNA structure, and hence gene transcription. As expected, abnormalities in histone modification are observed in many acquired diseases such as cancer, leading to transcriptional dysregulation. Histone deacetylases and inhibitors of DNA methylation are being used in treatment of certain cancers. Physiologic epigenetic silencing during development is called imprinting, and disorders of imprinting are discussed later in this chapter.
Another mechanism of gene regulation depends on the functions of noncoding RNAs. As the name implies, these are encoded by genes that are transcribed but not translated. Although many distinct families of noncoding RNAs exist, only two examples are discussed here: small RNA molecules called microRNAs and long noncoding RNAs >200 nucleotides in length.
Micro-RNAs (miRNAs) are relatively short RNAs (22 nucleotides on average) that function primarily to modulate the translation of target mRNAs into their corresponding proteins. Posttranscriptional silencing of gene expression by miRNA is a fundamental and evolutionarily conserved mechanism of gene regulation present in all eukaryotes. Even bacteria have their own version of the same general machinery that they use to protect themselves against foreign DNA (e.g., from phages and viruses).
The human genome contains almost 6000 miRNA genes, only 3.5-fold less than the number of protein-coding genes. Moreover, individual miRNAs appear to regulate multiple protein-coding genes, allowing each miRNA to coregulate entire programs of gene expression. Transcription of miRNA genes produces a primary transcript (pri-miRNA) that is processed into progressively smaller segments, including trimming by the enzyme Dicer , an endonuclease. This generates mature single-stranded miRNAs of 21 to 30 nucleotides that associate with a multiprotein aggregate called RNA-induced silencing complex (RISC) ( Fig. 4.2 ). Subsequent base pairing between the miRNA strand and its target mRNA directs the RISC to either induce mRNA cleavage or repress its translation. In this way, the target mRNA is posttranscriptionally silenced.
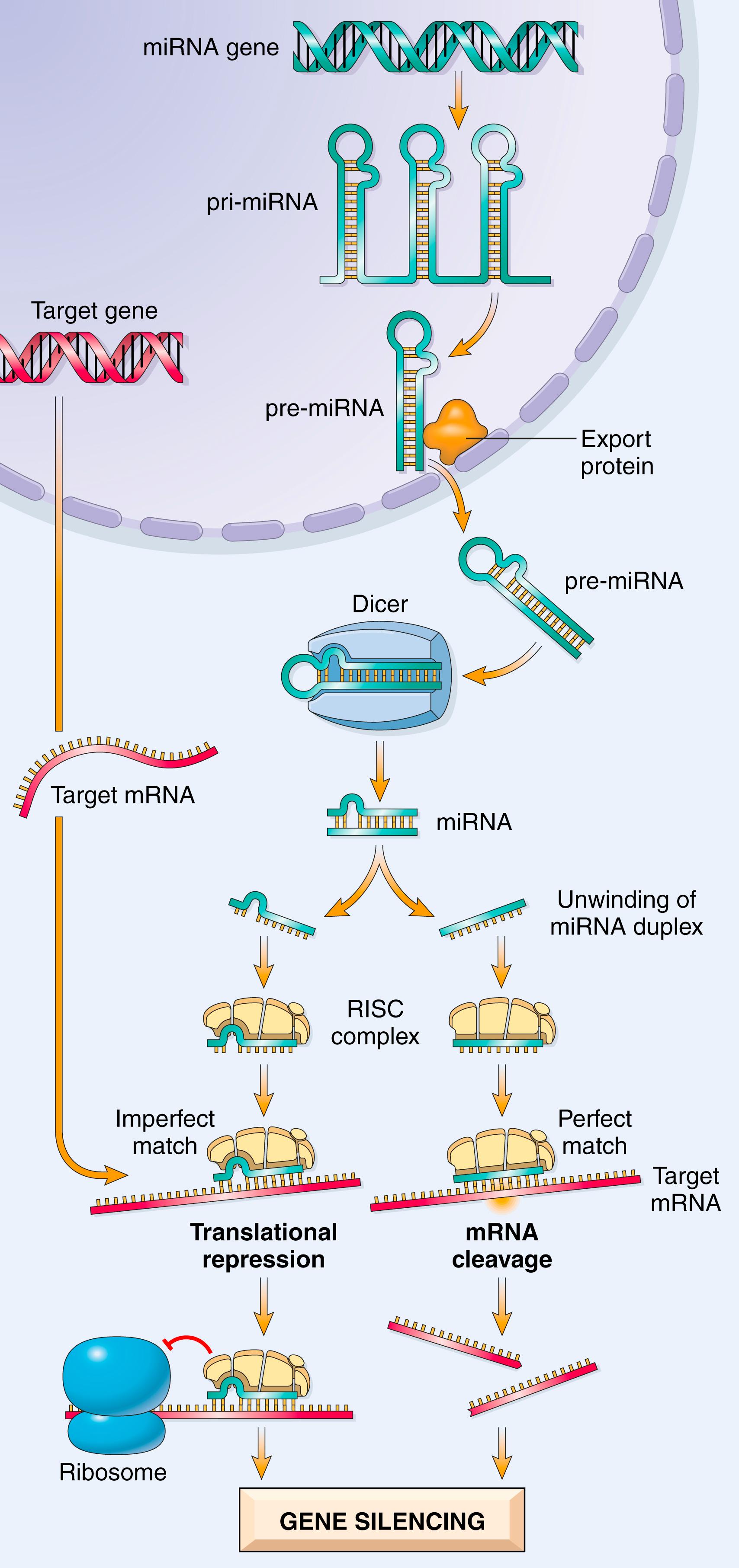
Taking advantage of the same pathway, small interfering RNAs (siRNAs) are short RNA sequences that can be introduced into cells. These serve as substrates for Dicer and interact with the RISC complex in a manner analogous to endogenous miRNAs. Synthetic siRNAs that can target specific mRNA species are therefore powerful laboratory tools to study gene function (so-called knockdown technology). Several siRNAs are also now in use for treatment of disorders such as macular degeneration.
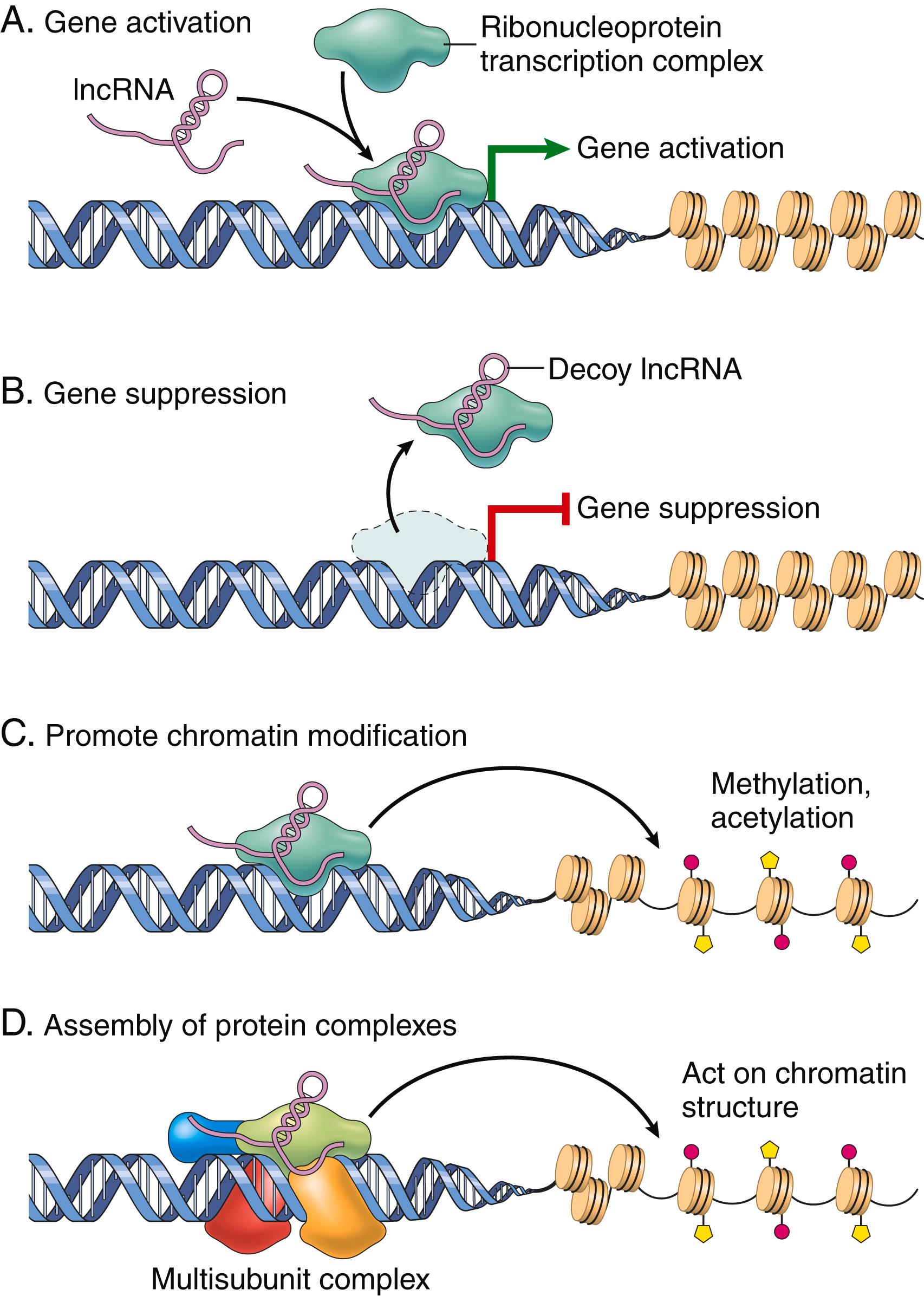
Long noncoding RNA (lncRNA). lncRNAs modulate gene expression in many ways ( Fig. 4.3 ); for example, they can bind to regions of chromatin, restricting RNA polymerase access to coding genes within the region. The best-known example of a repressive function involves XIST, which is transcribed from the X chromosome and plays an essential role in physiologic X chromosome inactivation. XIST itself escapes X inactivation but forms a repressive “cloak” on the X chromosome from which it is transcribed, resulting in gene silencing. Conversely, it has been appreciated that many enhancers are sites of lncRNA synthesis, with the lncRNAs enhancing transcription from spatially associated gene promoters through a variety of mechanisms (see Fig. 4.3 ). Ongoing studies are exploring the role of lncRNAs in diseases such as atherosclerosis and cancer.
Exciting new developments that enable exquisitely specific genome editing are ushering in a biomedical revolution. These advances come from a wholly unexpected source: the discovery of clustered regularly interspaced short palindromic repeats (CRISPRs) and Cas (or CRISPR-associated genes), such as the Cas9 nuclease. These are linked genetic elements that endow prokaryotes with a form of acquired immunity to phages and plasmids. Bacteria use this system to sample the DNA of infecting agents, incorporating it into the host genome as CRISPRs. CRISPRs are transcribed and processed into an RNA sequence that binds and directs the nuclease Cas9 to a sequence (e.g., a phage), leading to its cleavage and the destruction of the phage. Gene editing repurposes this process by using artificial guide RNAs (gRNAs) that bind Cas9 and are complementary to a DNA sequence of interest. Once directed to the target sequence by the gRNA, Cas9 induces double-stranded DNA breaks ( Fig. 4.4 ).
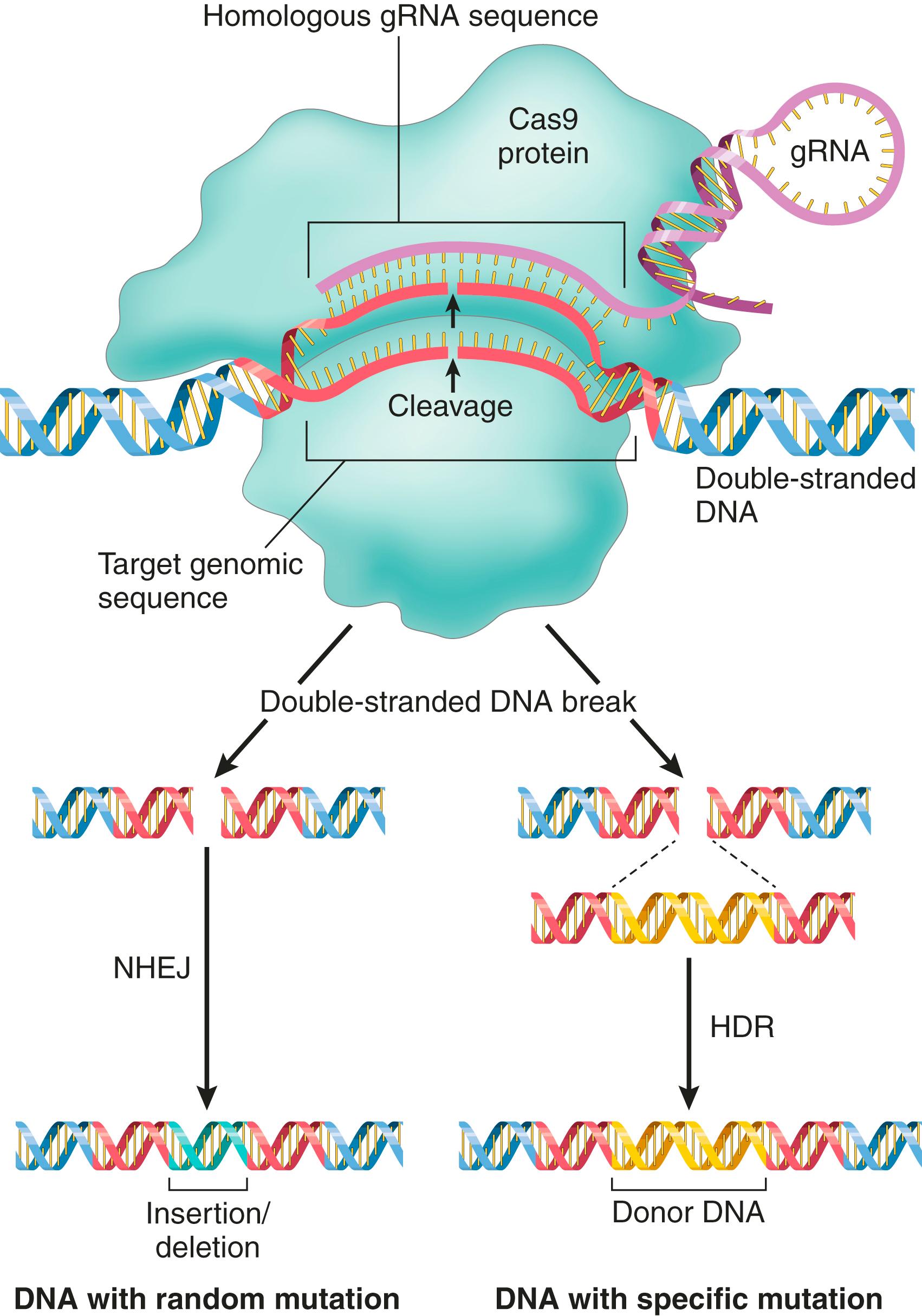
Repair of the resulting highly specific cleavage sites can lead to somewhat random disruptive mutations in the targeted sequences (through nonhomologous end joining [NHEJ]) or the precise introduction of new sequences of interest (by homologous recombination). Both the gRNAs and the Cas9 enzyme can be delivered to cells with a single easy-to-build plasmid. However, the real beauty of the system (and the excitement about its potential for genetic engineering) comes from its impressive flexibility and specificity, which is substantially better than other previous editing systems. Applications include inserting specific mutations into the genomes of cells to model cancers and other diseases and rapidly generating transgenic animals from edited embryonic stem cells. On the flip side, it now is feasible to selectively “correct” mutations that cause hereditable disease, or—perhaps more worrisome—to just eliminate less “desirable” traits. Predictably, the technology has inspired a vigorous debate regarding its application.
Before we start a discussion of genetic diseases it is helpful to clarify three commonly used terms: hereditary, familial, and congenital:
Hereditary disorders are transmitted in the parents’ gametes and therefore are familial.
Congenital simply implies “present at birth.” Congenital diseases need not be genetic (e.g., congenital syphilis), and not all genetic diseases are congenital: the symptoms of Huntington disease, for example, begin after the third or fourth decade of life.
There are several types of genetic changes that affect the structure and function of proteins, disrupting cellular homeostasis and contributing to disease.
The term mutation refers to permanent changes in the DNA . Those that affect germ cells are transmitted to progeny and may give rise to inherited diseases. Mutations in somatic cells are not transmitted to progeny but are important in the causation of cancers and some congenital disorders.
Details of specific mutations and their effects are discussed along with the relevant disorders throughout this book. Cited here are some common examples of gene mutations and their effects:
Point mutations result from the substitution of a single nucleotide base by a different base, which may result in the replacement of one amino acid by another in the protein product. For example, a point mutation in the β-globin chain of hemoglobin gives rise to hemoglobin S (HbS), instead of hemoglobin A. The altered properties of HbS produces sickle cell disease. Such mutations are sometimes called missense mutations. By contrast, certain point mutations may create a stop codon, resulting in a truncated protein or complete failure of mRNA translation and protein synthesis. Such mutations are referred to as nonsense mutations.
Frameshift mutations occur when the insertion or deletion of one or two base pairs alters the reading frame of the DNA strand.
Trinucleotide repeat mutations belong to a special category, because they are characterized by amplification of a sequence of three nucleotides. The specific nucleotide sequence that undergoes amplification varies with different disorders. For example, in fragile X syndrome, prototypical of this category of disorders, there are 200 to 4000 tandem repeats of the sequence CGG within the familial mental retardation-1 gene (FMR1) . In unaffected populations, the number of repeats is small, averaging 29. The expansions of the trinucleotide sequences may prevent appropriate expression of the FMR1 gene, which gives rise to intellectual disability. Another distinguishing feature of trinucleotide repeat mutations is that they are dynamic (i.e., the degree of amplification increases during gametogenesis). These features, discussed in greater detail later in this chapter, influence the pattern of inheritance and the phenotypic manifestations of the diseases caused by this class of mutations.
In addition to alterations in DNA sequence, coding genes also can undergo structural variations, such as copy number changes— amplifications or deletions —or translocations that result in aberrant gain or loss of protein function. As with mutations, structural changes may occur in the germline or be acquired in somatic tissues. In many instances, pathogenic germline alterations involve a contiguous portion of a chromosome rather than a single gene, such as in the 22q microdeletion syndrome, discussed later. With the widespread availability of next-generation sequencing technology for assessing DNA copy number variation at very high resolution genome wide, copy number variants have been linked to a higher risk of developing several disorders, including autism. Cancers often contain somatically acquired structural alterations, including amplifications, deletions, and translocations. The so-called “Philadelphia chromosome”—translocation t(9;22) between the BCR and ABL genes in chronic myeloid leukemia ( Chapter 10 )—is a classic example.
With this brief review of the nature of the changes that contribute to the pathogenesis of human diseases, we can turn our attention to the four major categories of genetic disorders:
Mendelian disorders resulting from mutations in single genes. These mutations show high penetrance, meaning that most individuals who inherit the anomaly show phenotypic effects. Mendelian disorders are hereditary and familial and include relatively uncommon conditions, such as storage diseases caused by enzyme defects and other inborn errors of metabolism.
Complex disorders involving multiple genes as well as environmental influences. These are called complex, or multifactorial, diseases. They include some of the most common disorders of mankind, including hypertension, diabetes, and allergic and autoimmune diseases.
Diseases arising from changes in chromosomal number or structure. Several developmental diseases such as Down syndrome are attributable to this type of chromosomal alterations.
Other genetic diseases, which involve single gene mutations but do not follow simple mendelian rules of inheritance. These single-gene disorders with nonclassic inheritance patterns include those resulting from triplet repeat mutations or from mutations in mitochondrial DNA, and those in which the transmission is influenced by an epigenetic phenomenon called genomic imprinting. Each of these four categories is discussed separately.
Single-gene mutations follow the well-known mendelian patterns of inheritance ( Tables 4.1 and 4.2 ). Although individually rare, together they are responsible for a significant disease burden. The Online Mendelian Inheritance in Man (OMIM) database ( https://www.omim.org/ ) of the National Center for Biotechnology Information is a useful site for information concerning human genetic diseases. Listed next are a few important tenets and caveats when considering mendelian disorders:
Mutations involving single genes follow one of three patterns of inheritance: autosomal dominant, autosomal recessive, or X-linked.
A single-gene mutation may have many phenotypic effects (pleiotropy) and, conversely, mutations at several genetic loci may produce the same trait (genetic heterogeneity). Marfan syndrome, which results from a structural defect in connective tissue, is associated with widespread effects involving the skeleton, eyes, and cardiovascular system, all of which stem from a mutation in the gene encoding fibrillin, a component of connective tissues. On the other hand, several different types of mutations can cause retinitis pigmentosa, an inherited disorder associated with abnormal retinal pigmentation and consequent visual impairment. Recognition of genetic heterogeneity not only is important in genetic counseling but also feasibility of molecular diagnosis of disorders such as phenylketonuria, discussed later. Although mendelian diseases are rare, elucidation of the genes responsible has been very helpful in understanding normal pathways and the consequences of their disruption in acquired diseases.
The phenotypic manifestations of mutations affecting a known single gene are influenced by other genetic loci, which are called modifier genes. As discussed later in the section on cystic fibrosis, these modifier loci can affect the severity or extent of disease.
The use of proactive prenatal genetic screening in high-risk populations (e.g., persons with Ashkenazi Jewish ancestry) has significantly reduced the incidence (see Table 4.1 ) of certain genetic disorders such as Tay-Sachs disease.
| Disorder | Estimated Prevalence |
|---|---|
| Autosomal Dominant Inheritance | |
| Familial hypercholesterolemia | 1 in 500 |
| Polycystic kidney disease | 1 in 1000 |
| Hereditary spherocytosis | 1 in 5000 (U.S. European descent) |
| Marfan syndrome | 1 in 5000 |
| Huntington disease | 1 in 10,000 |
| Autosomal Recessive Inheritance | |
| Sickle cell anemia | 1 in 500 (U.S. African descent) a |
| Cystic fibrosis | 1 in 3200 (U.S. North European descent) |
| Tay-Sachs disease | 1 in 3500 (U.S. Ashkenazi Jewish ancestry; French Canadians) |
| Phenylketonuria | 1 in 10,000 |
| MPSs—all types | 1 in 25,000 |
| Glycogen storage diseases—all types | 1 in 50,000 |
| Galactosemia | 1 in 60,000 |
| X-Linked Inheritance | |
| Duchenne muscular dystrophy | 1 in 3500 (U.S. males) |
| Hemophilia | 1 in 5000 (U.S. males) |
a The prevalence of heterozygous sickle cell trait is 1 in 12 for U.S. African descent. Prevalence of sickle cell anemia is increased in areas with evolutionary pressure for malaria including parts of sub-Saharan Africa, southern Europe, the middle East, and India ( Chapter 10 ).
| Disease | Abnormal Protein | Protein Type/Function |
|---|---|---|
| Autosomal Dominant Inheritance | ||
| Familial hypercholesterolemia | LDL receptor | Receptor transport |
| Marfan syndrome | Fibrillin | Structural support: extracellular matrix |
| Ehler-Danlos syndrome a | Collagen | Structural support: extracellular matrix |
| Hereditary spherocytosis | Spectrin, ankyrin, or protein 4.1 | Structural support: red cell membrane |
| Neurofibromatosis, type 1 | Neurofibromin-1 (NF-1) | Growth regulation |
| Adult polycystic kidney disease | Polycystin-1 (PKD-1) | Cell–cell and cell–matrix interactions |
| Autosomal Recessive Inheritance | ||
| Cystic fibrosis | Cystic fibrosis transmembrane regulator | Ion channel |
| Phenylketonuria | Phenylalanine hydroxylase | Enzyme |
| Tay-Sachs disease | Hexosaminidase | Enzyme |
| Severe combined immunodeficiency c | Adenosine deaminase | Enzyme |
| α- and β-thalassemias b | Hemoglobin | Oxygen transport |
| Sickle cell anemia b | Hemoglobin | Oxygen transport |
| X-Linked Recessive Inheritance | ||
| Hemophilia A | Factor VIII | Coagulation |
| Duchenne/Becker muscular dystrophy | Dystrophin | Structural support: cell membrane |
| Fragile X syndrome | FMRP | RNA translation |
a Some variants of Ehler-Danlos syndrome have an autosomal recessive inheritance pattern.
b Although full-blown symptoms require biallelic mutations, heterozygotes for thalassemia and sickle cell anemia may present with mild clinical disease. Thus, these disorders sometimes are categorized as “autosomal codominant.”
c Some cases of severe combined immunodeficiency are X-linked.
Disorders of autosomal dominant inheritance are manifested in the heterozygous state, so at least one parent in an index case is usually affected. Both males and females are affected, and both sexes can transmit the condition. When an affected person has a child with an unaffected one, each child has one chance in two of having the disease. The following features also pertain to autosomal dominant diseases:
Not all patients have affected parents. Such patients owe their disorder to new mutations involving either the egg or the sperm from which they were derived. The siblings of these individuals are unaffected.
Clinical features can be modified by reduced penetrance and variable expressivity. Some persons inherit the mutant gene but are phenotypically normal, a phenomenon referred to as reduced penetrance. The variables that determine penetrance are not clearly understood. In contrast to penetrance, if a trait is consistently associated with a mutant gene but is expressed differently among persons carrying the gene, the phenomenon is called variable expressivity. For example, manifestations of neurofibromatosis 1 range from brownish spots on the skin to multiple tumors and skeletal deformities.
In many conditions, the age at onset is delayed, and symptoms and signs do not appear until adulthood . Examples include Huntington disease and several germline mutations that lead to increased risk of adult-onset cancers.
In autosomal dominant disorders, a 50% reduction in the normal gene product is associated with clinical signs and symptoms. Because a 50% loss of enzyme activity can be compensated for, involved genes in autosomal dominant disorders usually do not encode enzyme proteins but instead fall into several other categories of proteins:
Proteins involved in the regulation of complex metabolic pathways, often subject to feedback control (e.g., membrane receptors, transport proteins). An example of this pathogenic mechanism is found in familial hypercholesterolemia, which results from mutation in the low-density lipoprotein (LDL) receptor gene (discussed later).
Key structural proteins, such as collagen and components of the red cell membrane skeleton (e.g., spectrin, mutations of which result in hereditary spherocytosis).
The biochemical mechanisms by which a 50% reduction in the levels of structural proteins results in a disease phenotype are not fully understood. In some instances, especially when the gene encodes one subunit of a multimeric protein, the product of the mutant allele is expressed at normal levels but interferes with the assembly of a functionally normal multimer. For example, the collagen molecule is a trimer in which the three collagen chains are arranged in a helical configuration. The presence of mutated collagen chains reduces the assembly of the remaining normal chains, producing a marked deficiency of collagen. In this instance, the mutant allele is called dominant negative, because it impairs the function of a wild-type allele. This effect is illustrated in some forms of osteogenesis imperfecta ( Chapter 19 ).
Disorders of autosomal recessive inheritance are manifested in the homozygous state. They occur when both alleles at a given gene locus are mutated. Therefore, such disorders are characterized by the following features: (1) the trait does not usually affect the parents, who each carry one mutant allele, but multiple siblings may show the disease; (2) siblings have one chance in four of being affected (i.e., the recurrence risk is 25% for each birth); and (3) if the mutant gene occurs with a low frequency in the population, there is a strong likelihood that the affected patient (the proband) has consanguineous parents. Autosomal recessive disorders make up the largest group of mendelian disorders.
In contrast with the features of autosomal dominant diseases, the following features generally apply to most autosomal recessive disorders:
The expression of the defect tends to be more uniform than in autosomal dominant disorders.
Complete penetrance is common.
Onset is frequently early in life.
Although new mutations for recessive disorders do occur, they are rarely detected clinically. Because an individual carrying the mutation is an asymptomatic heterozygote, several generations may pass before the descendant of such a person mates with another heterozygote and produces affected offspring.
In many cases, the affected gene encodes an enzyme. In heterozygotes, equal amounts of wild-type and affected enzyme are synthesized. Usually the natural “margin of safety” ensures that cells with half of their complement of the enzyme function normally.
For the most part, sex-linked disorders are X linked. The Y chromosome is home to the testes-determining gene SRY as well as several other genes that control spermatogenesis and map to the male specific Y-region (MSY), which directs male sexual differentiation. Males with mutations affecting the Y chromosome are infertile, so no Y chromosome–linked mendelian disorders have been reported. Most X-linked disorders are X-linked recessive and are characterized by the following features:
Heterozygous female carriers transmit the trait only to sons, who are hemizygous for the X chromosome. Sons of heterozygous women have one chance in two of receiving the mutant gene.
Heterozygous females rarely express the full phenotypic change, because they have a wild-type allele. Although one of the X chromosomes in females is inactivated (see further text), this process of inactivation is random, which typically allows sufficient numbers of cells with expression of the wild-type X to emerge.
An affected male does not transmit the disorder to sons, but all daughters are carriers.
Marfan syndrome is an autosomal dominant disorder of connective tissues, manifested principally by changes in the skeleton, eyes, and cardiovascular system. It is caused by an inherited defect in an extracellular glycoprotein called fibrillin .
Fibrillin is secreted by fibroblasts and it is the major component of microfibrils found in the extracellular matrix. Microfibrils serve as scaffolds for the deposition of tropoelastin, an integral component of elastic fibers. Although microfibrils are widely distributed in the body, they are particularly abundant in the aorta, ligaments, and the ciliary zonules that support the ocular lens, precisely the tissues that are affected in Marfan syndrome.
Fibrillin is encoded by the FBN1 gene, which maps to chromosomal locus 15q21. Mutations in FBN1 are found in all patients with Marfan syndrome. More than 1000 distinct causative mutations in the very large FBN1 gene have been found, which complicates diagnosis by DNA sequencing. Therefore the diagnosis is mainly based on clinical findings. Because heterozygotes have clinical symptoms, it is thought that the mutant fibrillin protein acts as a dominant negative, preventing the assembly of normal microfibrils. The prevalence of Marfan syndrome is estimated to be 1 in 5000 worldwide. Approximately 70% to 85% of cases are familial, and the rest are sporadic, arising from de novo FBN1 mutations in the germ cells of parents.
Although many of the findings in Marfan syndrome can be explained on the basis of the structural defect of connective tissues, some, such as overgrowth of bones, are difficult to relate to simple loss of fibrillin. It is now evident that loss of microfibrils leads to excessive activation of transforming growth factor-β (TGF-β), because normal microfibrils sequester TGF-β, thereby limiting the bioavailability of this cytokine. Excessive TGF-β signaling has deleterious effects on vascular smooth muscle development and the integrity of the extracellular matrix. In support of this hypothesis, mutations in the TGF-β type II receptor give rise to a related syndrome, called Marfan syndrome type 2 (MFS2). Of note, certain angiotensin receptor type II blockers that inhibit the activity of TGF-β are now in clinical use for prevention of cardiovascular disease along with β-adrenergic blocking agents, which lower blood pressure to reduce the risk of cardiovascular catastrophe.
Skeletal changes are the most evident feature of Marfan syndrome. Patients have a slender, elongated habitus with extremely long legs, arms, and fingers (arachnodactyly); a high-arched palate; and hyperextensibility of joints. A variety of spinal deformities, such as severe kyphoscoliosis, may be present. The chest may exhibit either pectus excavatum (i.e., deeply depressed sternum) or a pigeon-breast deformity. The most characteristic ocular change is bilateral dislocation, or subluxation, of the lens secondary to weakness of its suspensory ligaments (ectopia lentis). Ectopia lentis, particularly if bilateral, is highly specific for Marfan syndrome and strongly suggests the diagnosis. Most serious, however, is the involvement of the cardiovascular system. Fragmentation of the elastic fibers in the tunica media of the aorta predisposes affected patients to aneurysmal dilation and aortic dissection ( Chapter 8 ). These changes, called cystic medionecrosis, are not specific for Marfan syndrome; similar lesions occur in hypertension and with aging. Loss of medial support causes dilation of the aortic valve ring, giving rise to aortic incompetence. The cardiac valves, especially the mitral valve, may be excessively distensible and regurgitant (floppy valve syndrome), giving rise to mitral valve prolapse and congestive cardiac failure ( Chapter 9 ). Aortic rupture is the most common cause of death and may occur at any age. Less frequently, cardiac failure is the terminal event.
Although the lesions described are typical of Marfan syndrome, they are not seen in all cases. There is much variation in clinical expression, and some patients may exhibit predominantly cardiovascular lesions with minimal skeletal and ocular changes. It is believed that the variable expressivity is related to different mutations in the FBN1 gene.
Ehlers-Danlos syndromes (EDSs) are a group of diseases characterized by defects in collagen synthesis or structure. EDSs are caused by mutations in a number of different genes; the most commonly affected genes encode various collagens, and all affected genes, through one mechanism or another, lead to a defect in collagens. All are single-gene disorders, but the mode of inheritance encompasses both autosomal dominant and recessive patterns. There are approximately 30 distinct types of collagen; all have characteristic tissue distributions and are the products of different genes. To some extent, the clinical heterogeneity of EDS can be explained by mutations in different collagen genes.
At least thirteen clinical and genetic variants of EDS are recognized. Although individually rare, the collective frequency of all cases of EDS is 1 in 5000 births worldwide. Since defective collagen is the basis for these disorders, certain clinical features are common to all variants.
Tissues rich in collagen, such as skin, ligaments, and joints, are frequently affected in most variants of EDS. Because the abnormal collagen fibers lack adequate tensile strength, joints are hypermobile. These features permit remarkable hyperflexibility, such as bending the thumb backward to touch the forearm or bending the knee upward to create almost a right angle. Indeed, it is believed that most contortionists have some form of EDS; however, a predisposition to joint dislocation is one of the prices paid for this virtuosity.
Skin fragility. The skin is extraordinarily stretchable, extremely fragile, and vulnerable to trauma. Minor injuries produce gaping defects, and surgical repair or any surgical intervention is accomplished only with great difficulty because of the lack of normal tensile strength.
Structural failure of organ or tissues. The structural defect in connective tissue may lead to serious internal complications, including rupture of the colon and large arteries (vascular EDS); ocular fragility, with rupture of the cornea and retinal detachment (kyphoscoliotic EDS); and diaphragmatic hernia (classical EDS), among others.
The molecular bases for three of the more common variants are as follows:
Deficient synthesis of type III collagen resulting from mutations affecting the COL3A1 gene . This variant, vascular EDS, is inherited as an autosomal dominant disorder and is characterized by weakness of tissues rich in type III collagen (e.g., blood vessels, bowel wall), predisposing them to rupture.
Deficiency of the enzyme lysyl hydroxylase. Decreased hydroxylation of lysyl residues in types I and III collagen interferes with the formation of crosslinks among collagen molecules. As might be expected, this variant (kyphoscoliotic EDS), resulting from an enzyme deficiency, is inherited as an autosomal recessive disorder. Patients typically manifest with congenital scoliosis and ocular fragility.
Deficient synthesis of type V collagen resulting from mutations in COL5A1 and COL5A2 is inherited as an autosomal dominant disorder (classical EDS).
Familial hypercholesterolemia (FH) is a “receptor disease” caused most commonly (in 80%–85% cases) by loss-of-function mutations in the gene encoding the LDL receptor, which is involved in the transport and metabolism of cholesterol. As a consequence of receptor abnormalities, there is a loss of feedback control that normally holds cholesterol synthesis in check. The resulting elevated levels of cholesterol induce premature atherosclerosis and greatly increase the risk of myocardial infarction. Familial hypercholesterolemia is among the most common of mendelian disorders; the frequency of the heterozygous condition is 1 in 500 in the general population worldwide. The prevalence in those with atherosclerotic cardiovascular disease is 20-fold higher.
Approximately 7% of the body’s cholesterol circulates in the plasma, predominantly in the form of LDL. As might be expected, the amount of plasma cholesterol is influenced by its synthesis and catabolism, and the liver plays a crucial role in both these processes, as described later. Cholesterol may be derived from the diet or from endogenous synthesis. Dietary triglycerides and cholesterol are incorporated into chylomicrons in the intestinal mucosa and travel by way of gut lymphatics to the blood. These chylomicrons are hydrolyzed by an endothelial lipoprotein lipase in the capillaries of muscle and fat. The chylomicron remnants, rich in cholesterol, are then delivered to the liver. Some of the cholesterol enters the metabolic pool (to be described), and some is excreted as free cholesterol or as bile acids into the biliary tract.
The endogenous synthesis of cholesterol and LDL begins in the liver ( Fig. 4.5 ). The first step is the secretion of triglyceride-rich very-low-density lipoprotein (VLDL) by the liver into the blood. In the capillary endothelium of adipose tissue and muscle, the VLDL particle undergoes lipolysis and is converted to intermediate-density lipoprotein (IDL). In comparison with VLDL, the content of triglyceride is reduced and that of cholesteryl esters is enriched in IDL, but IDL retains on its surface the VLDL-associated apolipoproteins B-100 and E. Further metabolism of IDL occurs along two pathways: Most of the IDL particles are taken up by the liver through the LDL receptor (described later); others are converted to cholesterol-rich LDL by a further loss of triglycerides and the loss of apolipoprotein E. In the liver cells, IDL is recycled to generate VLDL.
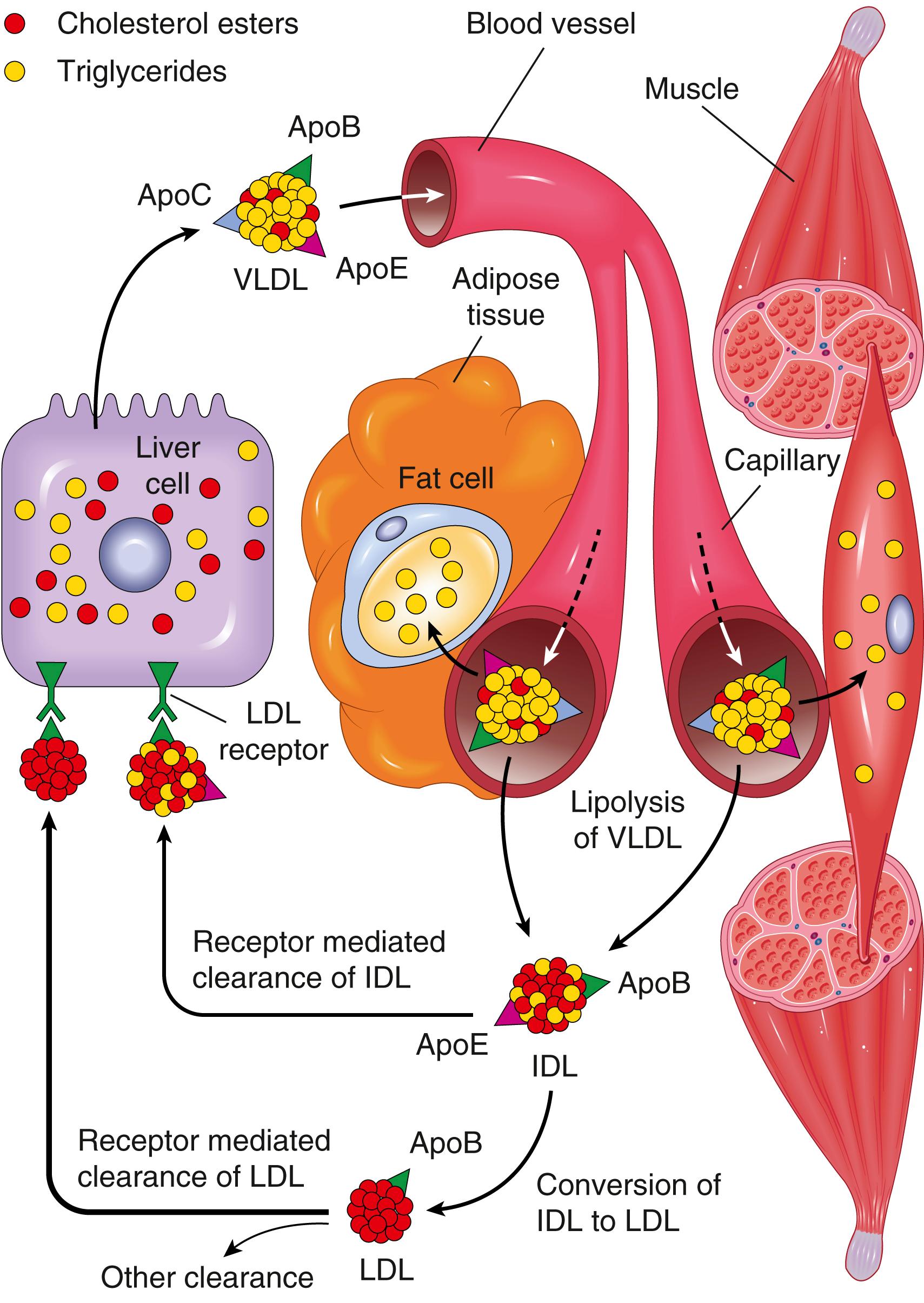
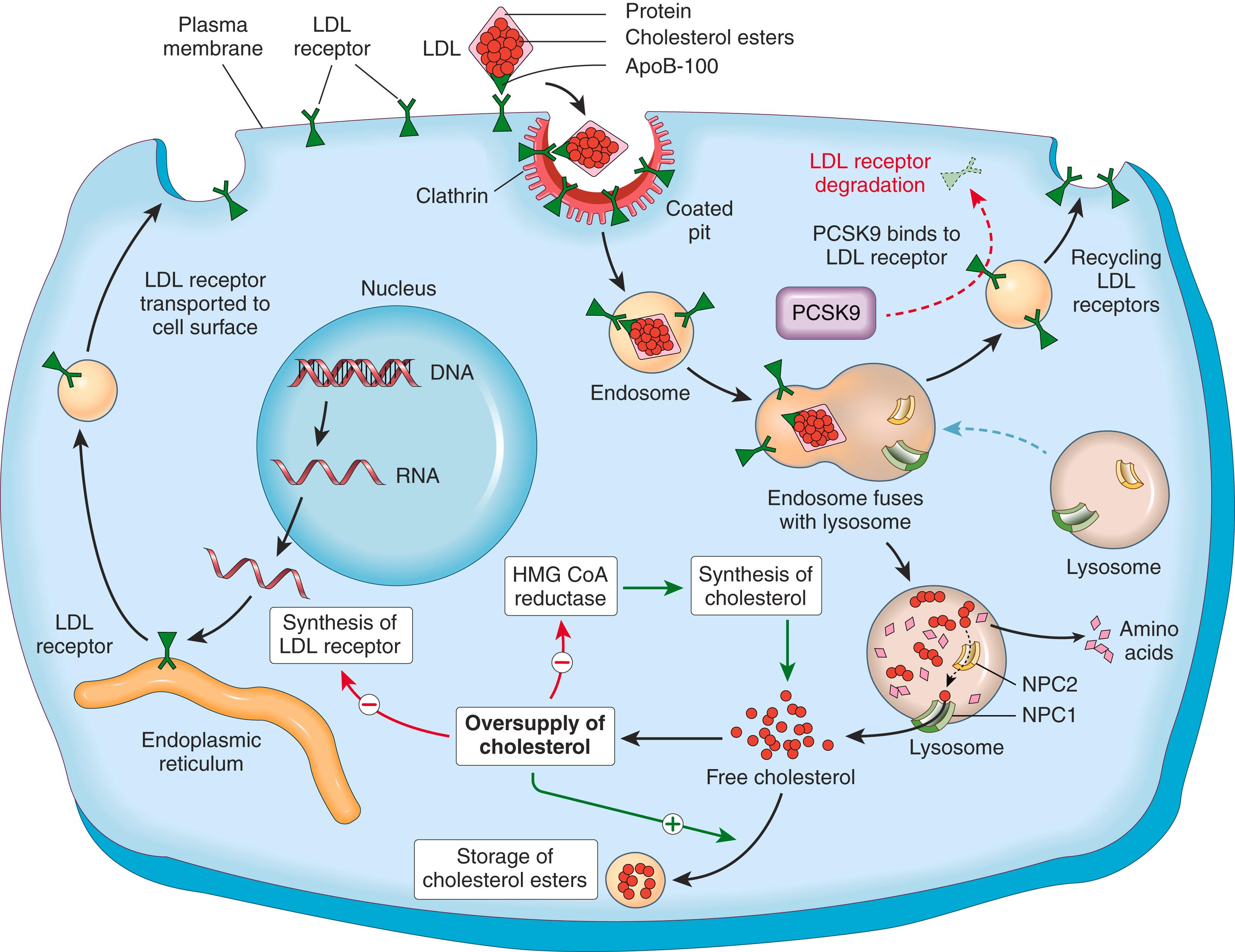
The LDL receptor pathway metabolizes two-thirds of the resultant LDL particles, and the rest is metabolized by a receptor for oxidized LDL (scavenger receptor), to be described later. The LDL receptor binds to apolipoproteins B-100 and E and thus is involved in the transport of both LDL and IDL. Although the LDL receptors are widely distributed, approximately 75% are located on hepatocytes, so the liver plays an extremely important role in LDL metabolism. The first step in the receptor-mediated transport of LDL involves binding to the cell surface receptor, followed by internalization inside so-called clathrin-coated pits and then into endosomes ( Fig. 4.6 ). Within the cell, the endocytic vesicles fuse with the lysosomes. Here the LDL dissociates from the receptor, which is recycled to the surface. The recycling of LDL receptors is negatively regulated by an enzyme, better known as PCSK9, which degrades the LDL receptor. In the lysosomes LDL molecule is enzymatically degraded, resulting ultimately in the release of free cholesterol into the cytoplasm. The exit of cholesterol from the lysosomes requires the action of two proteins, called NPC1 and NPC2 (described later in the context of Niemann-Pick disease type C). Cholesterol not only is used by the cell for membrane synthesis but also takes part in intracellular cholesterol homeostasis by a sophisticated system of feedback control:
It suppresses cholesterol synthesis by inhibiting the activity of the enzyme 3-hydroxy-3-methylglutaryl–coenzyme A reductase (HMG-CoA reductase), which is the rate-limiting enzyme in the synthetic pathway.
It stimulates the formation of cholesterol esters, the storage form of cholesterol.
It downregulates the synthesis of cell surface LDL receptors, thus preventing excessive accumulation of cholesterol within cells.
Cholesterol upregulates the expression of the PCSK9, which reduces recycling of LDL receptors by degradation of endocytosed LDL receptors. This provides an additional mechanism of protecting the cells from excessive accumulation of cholesterol.
The transport of LDL by the scavenger receptor, alluded to earlier, seems to occur in cells of the mononuclear phagocyte system and possibly in other cells as well. Monocytes and macrophages have receptors for chemically modified (e.g., acetylated or oxidized) LDLs. The amount catabolized by this scavenger receptor pathway is directly related to the plasma cholesterol level.
In familial hypercholesterolemia, mutations in the LDL receptor protein impair the surface expression and endocytosis of LDL receptors, resulting in the accumulation of LDL cholesterol in the blood. In addition, the absence of LDL receptors on liver cells impairs the transport of IDL into the liver, so a greater proportion of plasma IDL is converted into LDL. Thus, patients with familial hypercholesterolemia develop excessive levels of blood cholesterol as a result of the combined effects of reduced catabolism and excessive biosynthesis (see Fig. 4.5 ). This leads to a marked increase of cholesterol uptake by monocytes and macrophages and vascular walls through the scavenger receptor, accounting for the appearance of skin xanthomas and premature atherosclerosis. As mentioned earlier, mutations in the LDL receptor account for 80% to 85% of cases of FH. Less commonly, FH is caused by mutations in two other genes involved in clearance of plasma LDL. Specifically, these mutations consist of (1) loss of function mutations in B-100 (ApoB), the ligand for the LDL receptor on the LDL particle (5% to 10% of cases), and (2) gain of function mutations in the enzyme PCSK9 (1% to 2% of cases), which normally reduces the level of LDL receptor on hepatocytes by dampening their recycling, leading to their degradation in lysosomes. As with mutations in the LDL receptor, these additional types of mutations impair hepatic clearance of LDL and give rise to clinically indistinguishable disease. More than 2000 mutations involving the LDL receptor gene have been identified. One of the most common mutant forms encodes LDL receptor protein that has a folding defect, preventing its expression on the cell surface.
Familial hypercholesterolemia is an autosomal dominant disease. Heterozygotes have a 2-fold to 3-fold elevation of plasma cholesterol levels, whereas homozygotes may have in excess of a 5-fold elevation. Although their cholesterol levels are elevated from birth, heterozygotes remain asymptomatic until adult life, when they may develop cholesterol deposits (xanthomas) along tendon sheaths and premature atherosclerosis resulting in coronary artery disease. Homozygotes are much more severely affected, developing cutaneous xanthomas in childhood and often dying of myocardial infarction before the age of 20 years.
The discovery of the critical role of LDL receptors in cholesterol homeostasis has led to the rational design of the statin family of drugs that are now widely used to lower plasma cholesterol. They inhibit the activity of HMG-CoA reductase and thus promote greater synthesis of LDL receptors (see Fig. 4.6 ). However, the upregulation of LDL receptors is accompanied by a compensatory increase in PCSK9 levels, which dampens the effects of statins. Therefore, agents, such as antibodies that antagonize PCSK9 enzymatic function and siRNAs that inhibit transcription of PCSK9, have been developed for treatment of patients with refractory hypercholesterolemia.
Cystic fibrosis (CF) is an inherited disorder of epithelial ion transport affecting fluid secretion in exocrine glands and the epithelial linings of the respiratory, gastrointestinal, and reproductive tracts. The ion transport defects lead to abnormally viscid mucous secretions that block the airways and the pancreatic ducts, which in turn are responsible for the two most important clinical manifestations: (1) recurrent and chronic pulmonary infections and (2) pancreatic insufficiency. In addition, although the exocrine sweat glands are structurally normal (and remain so throughout the course of this disease), a high level of sodium chloride in the sweat is a consistent, characteristic biochemical finding in CF . At the same time, it must be remembered that CF can present with a bewilderingly variable set of clinical findings. This variation in phenotype results from diverse mutations in CFTR, the gene encoding the CF transmembrane conductance regulator, and the influence of disease modifier genes.
With an incidence of 1 in 2500 live births in the United States, CF is the most common life-limiting genetic disease that affects individuals of European descent. The carrier frequency in the United States is 1 in 20 among individuals of European descent but significantly lower among individuals of other ancestral origins. CF follows simple autosomal recessive transmission, although even heterozygous carriers have a predisposition toward pulmonary and pancreatic disease at higher rates than the general population.
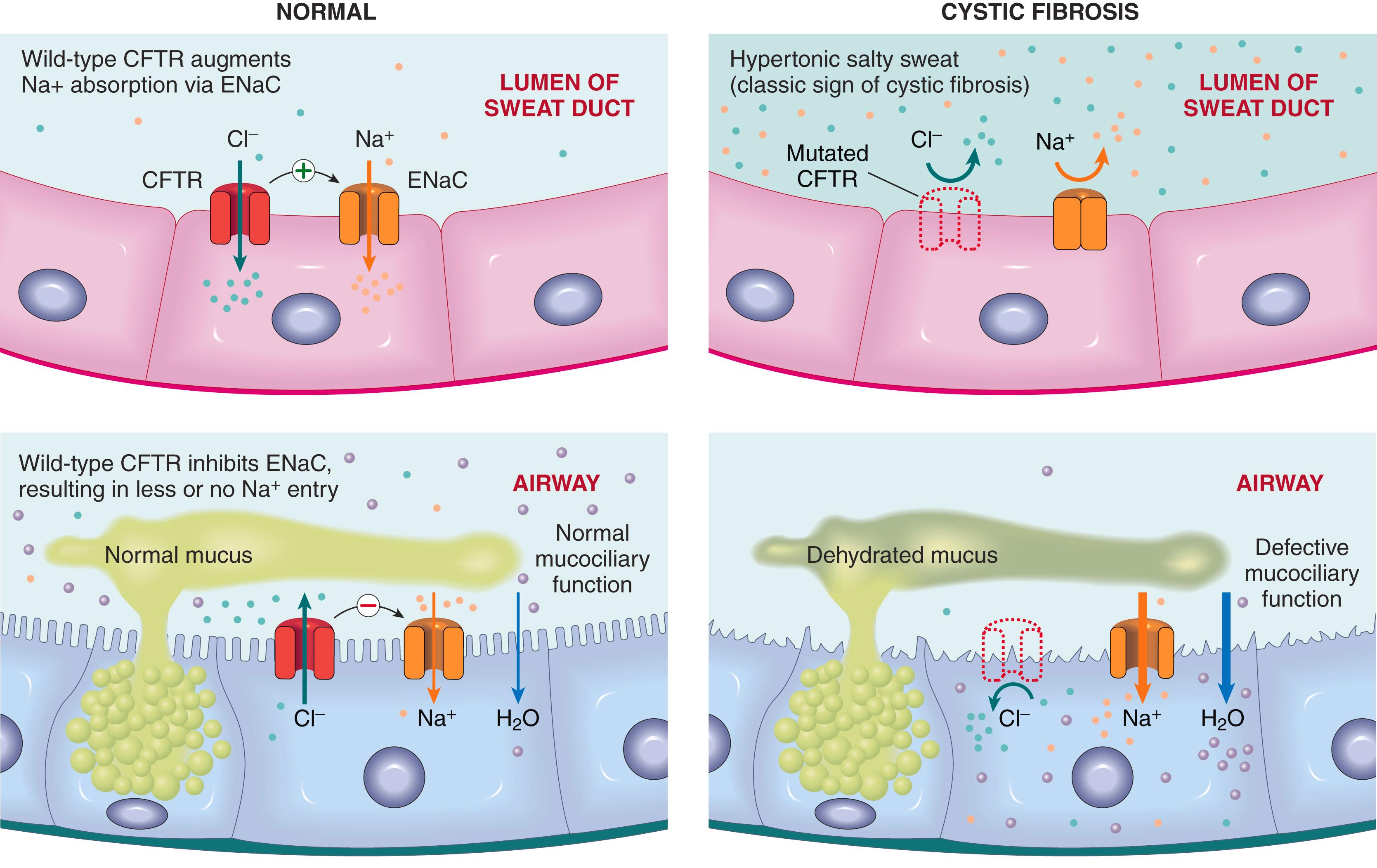
The primary defect in CF is reduced production or abnormal function of CFTR, an epithelial chloride and bicarbonate channel protein. Disruptive mutations in CFTR render the epithelial membranes relatively impermeable to chloride ions ( Fig. 4.7 ). The impact of this defect on transport function is tissue specific.
The major function of the CFTR protein in the sweat gland ducts is to reabsorb luminal chloride ions and augment sodium reabsorption through the epithelial sodium channel (ENaC). Therefore, in the sweat ducts, loss of CFTR function leads to decreased reabsorption of sodium chloride and production of hypertonic (“salty”) sweat (see Fig. 4.7 , top ).
In contrast to sweat glands, CFTR in the respiratory and intestinal epithelium is one of the most important avenues for active luminal secretion of chloride. At these sites, CFTR mutations result in loss or reduction of chloride secretion into the lumen (see Fig. 4.7 , bottom ). Because of loss of inhibition of the ENaC activity, active luminal sodium absorption through ENaCs is increased, and both of these ion changes increase passive water reabsorption from the lumen, lowering the water content of the surface fluid layer coating mucosal cells. Thus, unlike the sweat ducts, there is no difference in the salt concentration of the surface fluid layer coating the respiratory and intestinal mucosal cells in unaffected individuals and in those with CF. Instead, respiratory and intestinal complications in CF seem to stem from dehydration of the surface fluid layer. In the lungs, this dehydration leads to impaired mucociliary action and the accumulation of concentrated, viscid secretions that obstruct the air passages and predispose to recurrent pulmonary infections. Viscid secretions also may obstruct pancreatic ducts and the vas deferens, leading to pancreatic insufficiency and male infertility, respectively.
CFTR also regulates the transport of bicarbonate ions in the epithelial cells of the exocrine pancreas, and defective CFTR function therefore leads to reduced bicarbonate secretion and the acidification of pancreatic secretions. This results in precipitation of mucin and diminished activity of digestive enzymes such as trypsin that function best under alkaline conditions, both of which exacerbate pancreatic insufficiency.
Since CFTR was cloned in 1989, more than 2000 disease-causing mutations have been identified. They can be classified on the basis of the clinical features or the nature of the underlying defect. Mechanistically, they may produce disease by reducing the transport of CFTR to the cell surface or by impairing CFTR function. CFTR mutations can also be classified as severe or mild, depending on the clinical phenotype. Severe mutations are associated with complete loss of CFTR protein function, whereas mild mutations allow some residual function. The most common CFTR mutation is a deletion of three nucleotides coding for phenylalanine at amino acid position 508 (ΔF508) that causes misfolding of CFTR, leading to its degradation within the cell. The small amount of mutated ΔF508 protein that reaches the cell surface is also dysfunctional. Worldwide, the ΔF508 mutation is found in approximately 70% of patients with CF. Although CF remains one of the best-known examples of the “one gene–one disease” axiom, there is increasing evidence that other genes modify the frequency and severity of organ-specific manifestations. Two of these modifier genes encode mannose-binding lectin 2 (MBL2) and transforming growth factor-β1 (TGF-β1). It is postulated that polymorphisms in these genes influence the ability of the lungs to tolerate infections with virulent microbes (see later), thus modifying the natural history of CF.
Patients with CF can present with a multitude of manifestations ( Fig. 4.8 ). Pancreatic abnormalities are present in 85% to 90% of patients. In milder cases, there may be only accumulations of mucus in the small ducts, with some dilation of the exocrine glands. In more advanced cases, usually seen in older children or adolescents, the ducts are totally plugged, causing atrophy of the exocrine glands and progressive fibrosis ( Fig. 4.9 ). The total loss of pancreatic exocrine secretion impairs fat absorption and may lead to vitamin A deficiency. This may contribute to squamous metaplasia of the lining epithelium of the pancreatic ducts, which may exacerbate injury caused by the inspissated mucus secretions. Thick viscid plugs of mucus may also be found in the small intestine of infants. These may cause small bowel obstruction, known as meconium ileus.
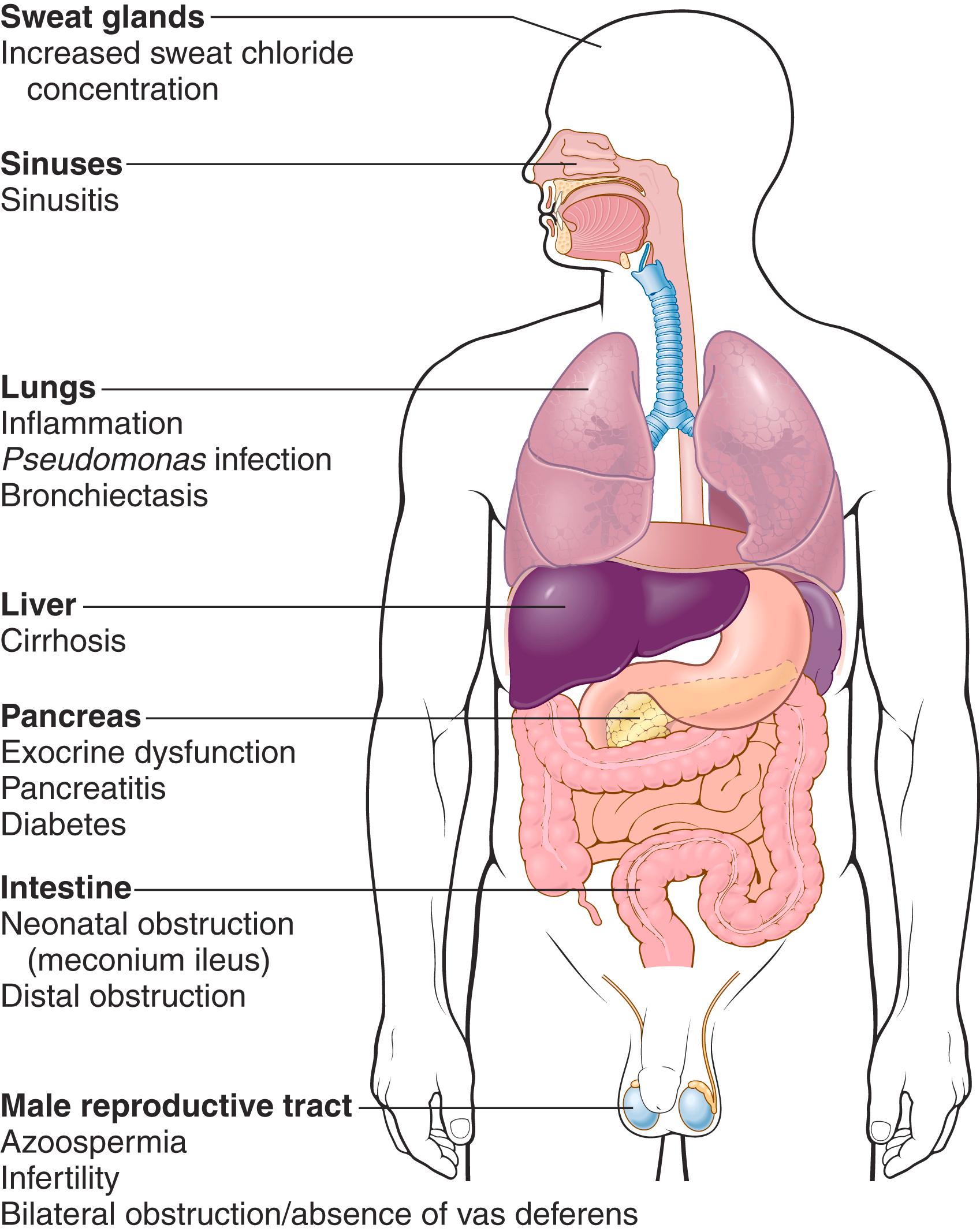
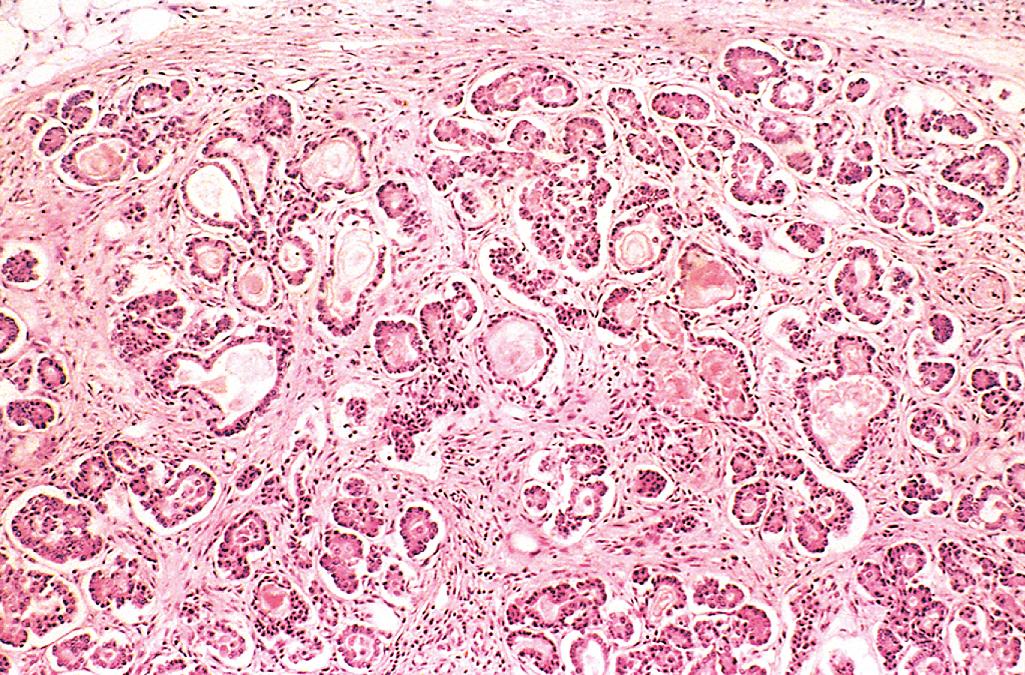
The pulmonary changes are the most serious complications of CF ( Fig. 4.10 ). These changes stem from obstruction of the air passages by viscous mucus secretions of submucosal glands and superimposed infections. The bronchioles are often distended with thick mucus, associated with marked hyperplasia and hypertrophy of the mucus-secreting cells. Superimposed infections give rise to severe chronic bronchitis and bronchiectasis. Development of lung abscesses is common. Staphylococcus aureus (including methicillin resistant variants), Pseudomonas aeruginosa , and nontuberculous mycobacteria are the three most common organisms responsible for lung infections. Even more sinister is the increasing frequency of infection with another pseudomonad, Burkholderia cepacia . B. cepacia , originally thought to be a single species, is now known to consist of multiple separate species, collectively known as B. cepacia complex. This opportunistic bacterium is particularly hardy, and infection with this organism has been associated with fulminant illness (“cepacia syndrome”). The liver involvement follows the same basic pattern. Bile canaliculi are plugged by mucinous material, accompanied by ductular proliferation and portal inflammation. Hepatic steatosis (“fatty liver”) is a common finding in liver biopsies. With time, cirrhosis develops, resulting in diffuse hepatic nodularity. Such severe hepatic involvement is encountered in less than 10% of patients. Azoospermia and infertility are found in 95% of the affected males who survive to adulthood. CF often causes atrophy of the vas deferens during the development of the embryo leading to bilateral absence of the vas deferens . In some males, this may be the only feature suggesting an underlying CFTR mutation.
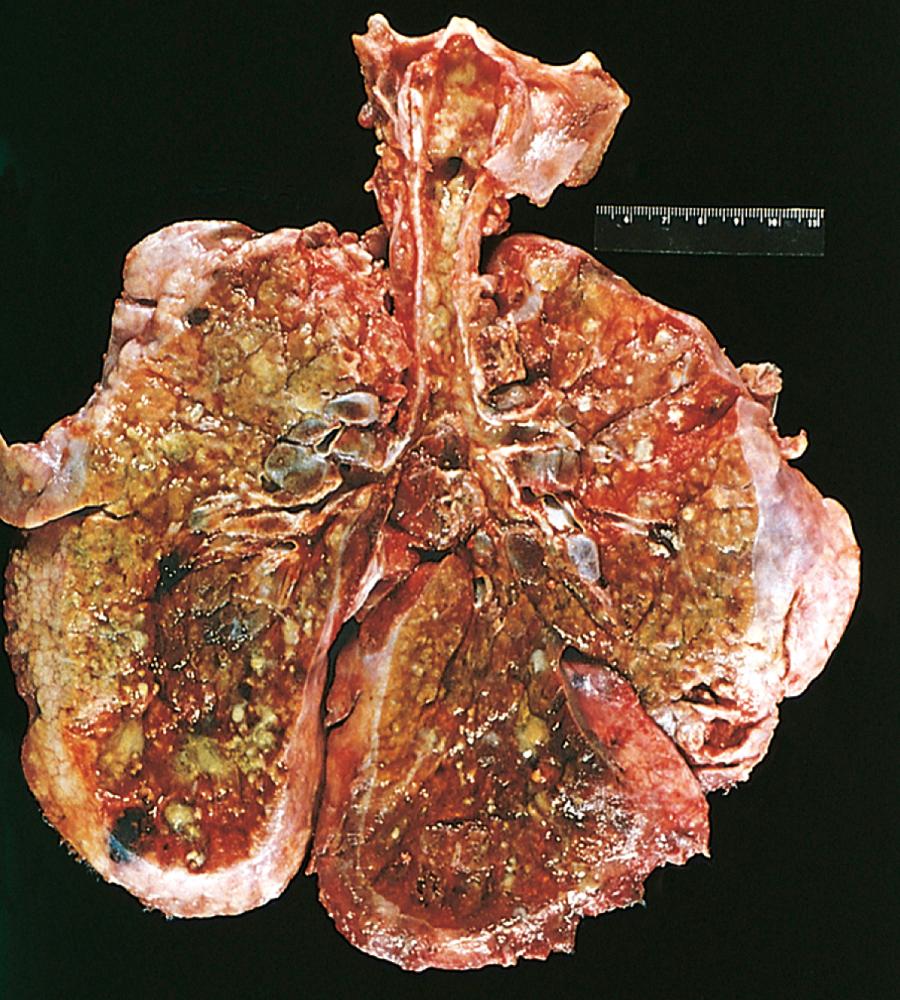
Few childhood diseases display clinical manifestations as protean as CF (see Fig. 4.8 ). Approximately 5% to 10% of the cases come to clinical attention at birth or soon after because of meconium ileus. Exocrine pancreatic insufficiency occurs in 85% to 90% of patients and is associated with “severe” CFTR mutations of both alleles (e.g., ΔF508/ΔF508). By contrast, 10% to 15% of patients who have one “severe” and one “mild” CFTR mutation or two “mild” CFTR mutations have sufficient pancreatic exocrine function to avoid the need for enzyme supplementation (pancreas-sufficient phenotype). Pancreatic insufficiency is associated with malabsorption and increased fecal loss of protein and fat. Manifestations of malabsorption (e.g., large, foul-smelling stools; abdominal distention; poor weight gain) appear during the first year of life. Faulty fat absorption may induce deficiency states of fat-soluble vitamins, resulting in manifestations of vitamin A, D, or K deficiency ( Chapter 7 ). Protein malnutrition may be sufficiently severe to cause hypoproteinemia and generalized edema. Persistent diarrhea may result in rectal prolapse in as many as 10% of children with CF. The pancreas-sufficient phenotype is usually not associated with other gastrointestinal complications, and, in general, these patients demonstrate excellent growth and development. In contrast to exocrine insufficiency, endocrine insufficiency (i.e., diabetes) is uncommon in CF and occurs late in the course of the disease.
In the United States, in patients who receive follow-up care in CF centers, cardiorespiratory complications, such as chronic cough, persistent lung infections, obstructive pulmonary disease, and cor pulmonale, constitute the most common cause of death (accounting for approximately 80% of fatalities). By 18 years of age, 80% of patients with severe CF harbor Pseudomonas aeruginosa , and a subset also harbor Burkholderia cepacia . With the indiscriminate use of antibiotic prophylaxis, there has been an unfortunate resurgence of resistant strains of Pseudomonas in many patients. Significant liver disease occurs late in the natural history of CF and is foreshadowed by pulmonary and pancreatic involvement; with increasing life expectancy, liver disease is now the third most common cause of death in patients with CF (after cardiopulmonary and transplant-related complications).
The clinical spectrum of CF is broader than the “classic” multisystem disease described earlier. For example, some patients suffering from recurrent bouts of abdominal pain and pancreatitis since childhood who were previously classified as having “idiopathic” chronic pancreatitis are now known to harbor biallelic CFTR variants that are distinct from those seen in “classic” CF. CF carriers were initially thought to be asymptomatic, but studies suggest that they have an increased lifetime risk for chronic lung disease (especially bronchiectasis) and recurrent sinonasal polyps. In most cases, the diagnosis of CF is based on persistently elevated sweat electrolyte concentrations (the mother says her infant “tastes salty”), characteristic clinical findings (sinopulmonary disease and gastrointestinal manifestations), or a family history. Sequencing the CFTR gene is the gold standard for diagnosis of CF.
There have been major improvements in the management of acute and chronic complications of CF, including more potent anti-microbial therapies, pancreatic enzyme replacement, and bilateral lung transplantation. These advances have extended the median life expectancy to 40 years, changing a lethal disease of childhood into a chronic disease of adults. Drug therapies are also now available that improve the folding, membrane expression, and function of mutated CFTR molecules. It is too early to determine the impact of these emerging molecular therapies on prognosis and survival.
PKU results from mutations that cause a severe lack of the enzyme phenylalanine hydroxylase (PAH) leading to hyperphenylalaninemia. It affects 1 in 10,000 live-born infants of European descent, and there are several variants of this disease. The most common form is referred to as classic phenylketonuria; its incidence is higher in European populations and less common in individuals from other geographic regions.
Homozygotes with this autosomal recessive disorder classically have a severe lack of PAH, leading to hyperphenylalaninemia and PKU. They are unaffected at birth but within a few weeks exhibit a rising plasma phenylalanine level, which impairs brain development. Usually, by 6 months of life severe mental disability becomes evident. The vast majority has intelligence quotients (IQs) less than 60. About one-third of these children never walk, and two-thirds cannot talk. Seizures, other neurologic abnormalities, decreased pigmentation of hair and skin, and eczema often accompany the mental disability in untreated children. Hyperphenylalaninemia and the resultant mental disability can be avoided by restricting phenylalanine intake early in life. Hence, several screening procedures are routinely performed to detect PKU in the immediate postnatal period. Dietary treatment is recommended for life.
Female patients with PKU who discontinue dietary treatment in adulthood may appear to be healthy but have marked hyperphenylalaninemia. Between 75% and 90% of children born to such women have severe mental disability and are microcephalic, and 15% have congenital heart disease, even though the infants themselves are heterozygotes. This syndrome, termed maternal PKU, results from the teratogenic effects of phenylalanine or its metabolites that cross the placenta and affect specific fetal organs during development.
The biochemical abnormality in PKU is the inability to convert phenylalanine into tyrosine. In children with normal PAH activity, less than 50% of the dietary intake of phenylalanine is necessary for protein synthesis. The remainder is converted to tyrosine by the phenylalanine hydroxylase system ( Fig. 4.11 ). When phenylalanine metabolism is blocked because of a lack of PAH enzyme, shunt pathways are activated, yielding several intermediates that are excreted in large amounts in the urine and in the sweat. These impart a strong musty or mousy odor to affected infants. It is believed that excess phenylalanine or its metabolites contribute to the brain damage in PKU. Concomitant lack of tyrosine (see Fig. 4.11 ), a precursor of melanin, is responsible for the light color of hair and skin.
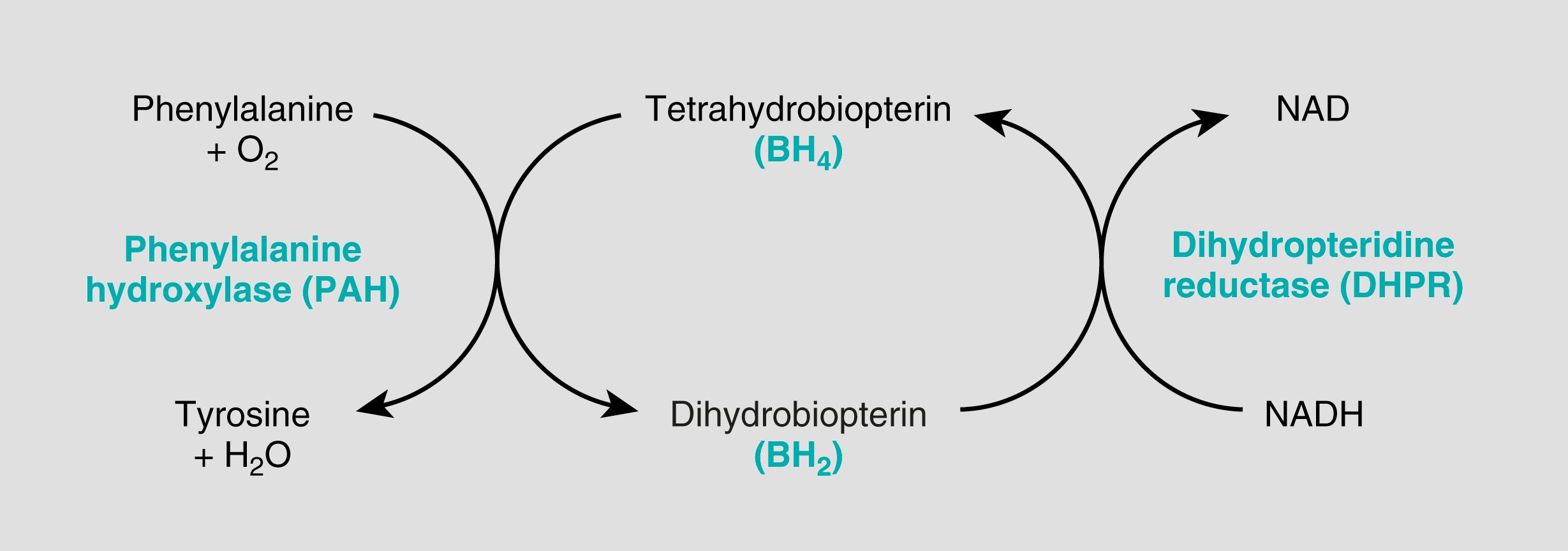
Approximately 1000 mutant alleles of PAH have been identified, only some of which cause a severe deficiency of the enzyme. Infants with mutations resulting in severe PAH deficiency develop classic features of PKU, whereas those with some PAH activity may develop milder disease or may be asymptomatic, a condition referred to as benign hyperphenylalaninemia. Because the numerous disease-causing alleles of the PAH gene complicate molecular diagnosis, measurement of serum phenylalanine levels is used to differentiate benign hyperphenylalaninemia from PKU; the levels in the latter disorder typically are ≥5 times higher than normal. After a biochemical diagnosis is established, the specific mutation causing PKU can be determined. With this information, carrier testing of at-risk family members can be performed. Currently, enzyme infusion therapy is being tested in patients with classic PKU. The infused enzyme, known as phenylalanine ammonia lyase (or PAL), converts excess phenylalanine to ammonia and other metabolites, thereby alleviating the toxic effects of phenylalanine.
Galactosemia is an autosomal recessive disorder of galactose metabolism resulting from a mutation in the gene encoding the enzyme galactose-1-phosphate uridyltransferase (GALT). It affects 1 in 53,000 live-born infants in the United States. Lactase splits lactose, the major carbohydrate of mammalian milk, into glucose and galactose in the intestinal microvilli. Galactose is then converted to glucose in several steps, one of which requires the enzyme GALT. Due to this transferase deficiency, galactose-1-phosphate and other metabolites, including galactitol, accumulate in many tissues, including the liver, spleen, lens of the eye, kidney, cerebral cortex, and red cells.
The liver, eyes, and brain bear the brunt of the damage. The early-onset hepatomegaly results largely from fatty change, but in time widespread scarring that resembles the cirrhosis of excess alcohol use may supervene ( Chapter 14 ). Opacification of the lens (cataract) develops, probably because the lens absorbs water and swells as galactitol (produced by alternative metabolic pathways) accumulates, increasing tonicity. Nonspecific alterations appear in the central nervous system, including loss of nerve cells, gliosis, and edema. There is still no clear understanding of the mechanism of injury to the brain, although elevated galactitol levels in neuronal tissues suggest that this may contribute to the damage.
Almost from birth, affected infants fail to thrive. Vomiting and diarrhea appear within a few days of milk ingestion. Jaundice and hepatomegaly usually become evident during the first week of life. Accumulation of galactose and galactose-1-phosphate in the kidney impairs amino acid transport, resulting in aminoaciduria. Fulminant Escherichia coli septicemia occurs with increased frequency. Newborn screening tests are widely utilized in the United States. They depend on fluorometric assay of GALT enzyme activity on a dried blood spot. A positive screening test must be confirmed by quantitative assay of GALT levels in red cells.
Many of the clinical and morphologic changes of galactosemia can be prevented or ameliorated by removal of galactose from the diet for at least the first 2 years of life. If instituted soon after birth, this diet prevents cataracts and liver damage and development is only mildly impaired. Even with dietary restrictions, however, older patients are frequently affected by a speech disorder and gonadal failure (especially premature ovarian failure) and, less commonly, by ataxia.
Lysosomes, the digestive system of cells, contain a variety of hydrolytic enzymes that are involved in the breakdown, and subsequent recycling, of complex substrates, such as sphingolipids and mucopolysaccharides, into soluble end products. These substrates may be derived from the turnover of intracellular organelles that enter the lysosomes by autophagy, or they may be acquired from outside the cell by endocytosis or phagocytosis. With an inherited lack of a lysosomal enzyme, catabolism of its substrate remains incomplete, leading to accumulation of partially degraded insoluble metabolites within the lysosomes ( Fig. 4.12 ). Stuffed with incompletely digested macromolecules, lysosomes become large and numerous enough to interfere with normal cell functions. Because lysosomal function is also essential for autophagy, impaired autophagy gives rise to additional storage of autophagic substrates such as polyubiquitinated proteins and dysfunctional mitochondria. The absence of this quality-control mechanism causes accumulation of defective mitochondria, which can trigger the generation of free radicals and apoptosis.
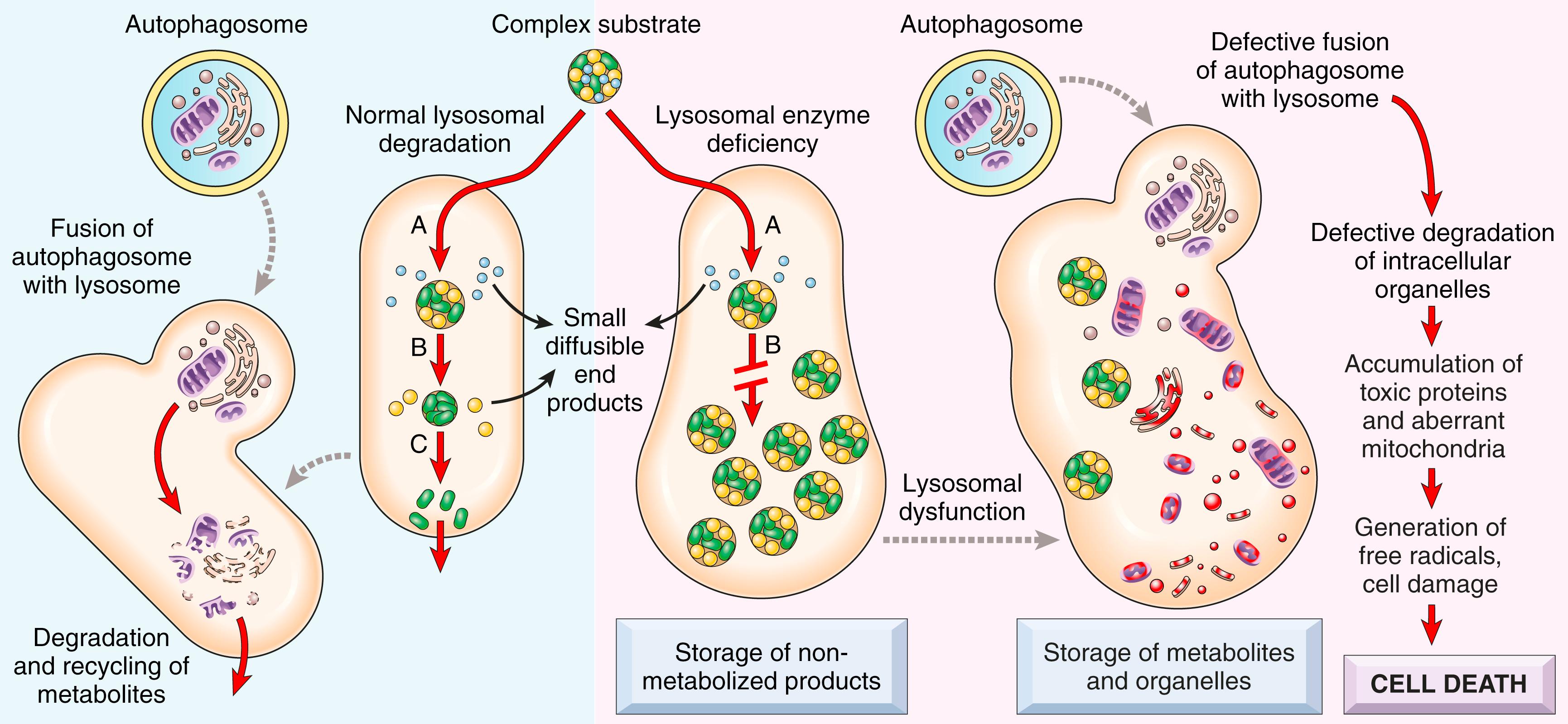
Approximately 70 lysosomal storage diseases have been identified. These may result from abnormalities of lysosomal enzymes or proteins involved in substrate degradation, endosomal sorting, or lysosomal membrane integrity. Lysosomal storage disorders are divided into categories based on the biochemical nature of the substrates and the accumulated metabolites ( Table 4.3 ). Within each group are several entities, each resulting from the deficiency of a specific enzyme.
| Disease | Enzyme Deficiency | Major Accumulating Metabolites |
|---|---|---|
| Glycogenosis, type 2-Pompe disease | α-1,4-Glucosidase (lysosomal glucosidase) | Glycogen |
| Sphingolipidoses | ||
| GM1 gangliosidosis | GM1 ganglioside β-galactosidase | GM1 ganglioside, galactose-containing oligosaccharides |
| GM2 gangliosidosis | ||
|
Hexosaminidase A Hexosaminidase A and B |
GM2 ganglioside GM2 ganglioside, globoside |
| Sulfatidoses | ||
| Metachromatic leukodystrophy | Arylsulfatase A | Sulfatide |
| Krabbe disease | Galactosylceramidase | Galactocerebroside |
| Fabry disease | α-Galactosidase A | Ceramide trihexoside |
| Gaucher disease | Glucocerebrosidase | Glucocerebroside |
| Niemann-Pick disease: types A and B | Sphingomyelinase | Sphingomyelin |
| Mucopolysaccharidoses (MPSs) | ||
| MPS I H (Hurler) MPS II (Hunter) |
α-L-Iduronidase l-Iduronosulfate sulfatase |
Dermatan sulfate, heparan sulfate |
| Mucolipidoses (MLs) | ||
| I-cell disease (ML II) and pseudo-Hurler polydystrophy | Deficiency of phosphorylating enzymes essential for the formation of mannose-6-phosphate recognition marker; acid hydrolases lacking the recognition marker cannot be targeted to the lysosomes but are secreted extracellularly | Mucopolysaccharide, glycolipid |
| Other lysosomal storage diseases | ||
| Wolman disease Acid phosphate deficiency |
Acid lipase Lysosomal acid phosphatase |
Cholesterol esters, triglycerides Phosphate esters |
Although the combined frequency of lysosomal storage disorders (LSDs) is about 1 in 2500 live births, lysosomal dysfunction may be involved in the etiology of several more-common diseases. For example, an important genetic risk factor for developing Parkinson disease is the carrier state for Gaucher disease, and virtually all Gaucher disease patients develop Parkinson disease. Niemann Pick C is another LSD connected to risk for Alzheimer disease. These associations stem from the multifunctionality of the lysosome. Lysosomes play critical roles in (1) autophagy, resulting from fusion with the autophagosome; (2) immunity, as they fuse with phagosomes; and (3) membrane repair, through fusion with the plasma membrane.
Despite this complexity, certain features are common to most diseases in this group:
Autosomal recessive transmission
Patient population consisting of infants and young children
Storage of insoluble intermediates in the mononuclear phagocyte system, giving rise to hepatosplenomegaly
Frequent CNS involvement with associated neuronal damage
Cellular dysfunction caused not only by storage of undigested material but also by a cascade of secondary events, for example, macrophage activation and release of cytokines
Most of these conditions are very rare, and their detailed description is better relegated to specialized texts and reviews. Only a few of the more common conditions are considered here.
Gangliosidoses are characterized by accumulation of gangliosides, principally in the brain, as a result of a deficiency of one of the lysosomal enzymes that catabolize these glycolipids. Depending on the ganglioside involved, these disorders are subclassified into GM1 and GM2 categories. Tay-Sachs disease, by far the most common of all gangliosidoses, is caused by loss-of-function mutations of the alpha subunit of the enzyme hexosaminidase A, which is necessary for the degradation of GM2. More than 100 mutations have been described; most disrupt protein folding or intracellular transport. Due to founder effects, Tay-Sachs disease, similar to other lipid storage disorders, has an increased prevalence among individuals of Ashkenazi Jewish ancestry, among whom the frequency of heterozygous carriers is estimated to be 1 in 30. The Ashkenazim originated in Eastern and Central Europe and constitute more than 90% of the Jewish population in the United States. Heterozygote carriers can be detected by measuring the level of hexosaminidase in serum or by DNA sequencing.
In the absence of hexosaminidase A, GM2 ganglioside accumulates in many tissues (e.g., heart, liver, spleen, nervous system), but the involvement of neurons in the central and autonomic nervous systems and retina dominates the clinical picture. The accumulation of GM2 occurs within neurons, axon cylinders of nerves, and glial cells throughout the CNS. Affected cells appear swollen and sometimes foamy ( Fig. 4.13A ). Electron microscopy shows whorled onionskin-like configurations within lysosomes composed of layers of membranes ( Fig. 4.13B ). These pathologic changes are found throughout the CNS (including the spinal cord), peripheral nerves, and autonomic nervous system. The retina is usually involved as well, where the pallor produced by swollen ganglion cells in the peripheral retina results in a contrasting “cherry red” spot in the relatively unaffected central macula.
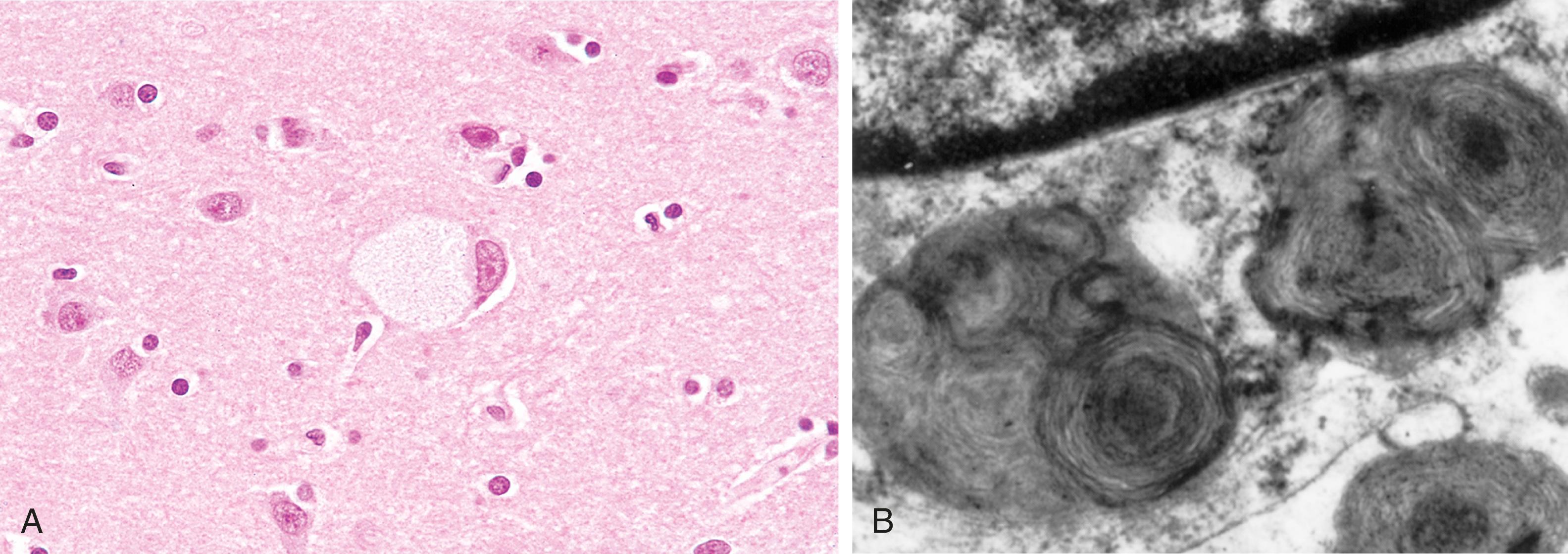
The molecular basis for neuronal injury is not fully understood. Because in many cases the mutant protein is misfolded, it induces the so-called “unfolded protein” response ( Chapter 1 ). If such misfolded enzymes are not stabilized by chaperones, they undergo proteasomal degradation, leading to accumulation of toxic substrates and intermediates within neurons. These findings have spurred clinical trials of molecular chaperone therapy for some variants of later-onset Tay-Sachs and other selected lysosomal storage diseases. Such therapy involves the use of synthetic chaperones that can cross the blood–brain barrier, bind to the mutated protein, and enable its proper folding, thereby generating sufficient enzyme activity to restore cellular health.
In the most common acute infantile variant of Tay-Sachs disease, motor weakness begins at 3 to 6 months of age, followed by neurologic impairment, onset of blindness, and progressively more severe neurologic dysfunctions. Death occurs within 2 to 3 years.
Type A and type B Niemann-Pick diseases are related entities characterized by a primary deficiency of acid sphingomyelinase and the resultant accumulation of sphingomyelin. As with Tay-Sachs disease, there is an increased incidence of Niemann-Pick disease types A and B in persons of Ashkenazi Jewish ancestry. The gene for acid sphingomyelinase is one of the imprinted genes that is preferentially expressed from the maternal chromosome as a result of epigenetic silencing of the paternal gene (discussed later).
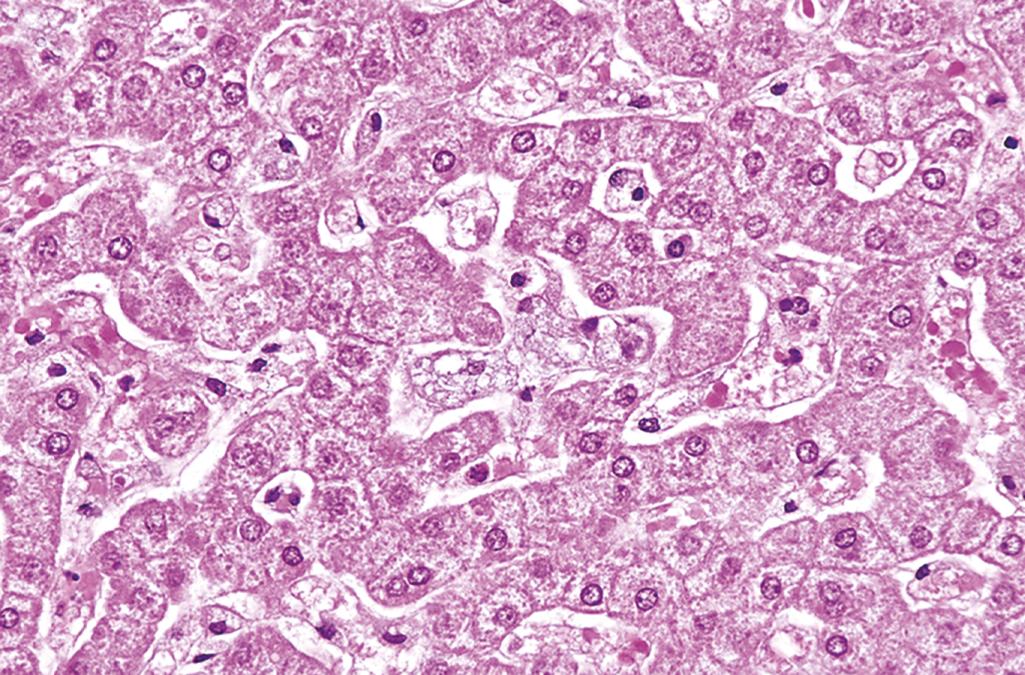
In type A, characterized by a severe deficiency of sphingomyelinase, the breakdown of sphingomyelin into ceramide and phosphorylcholine is impaired, and excess sphingomyelin accumulates in phagocytic cells and in neurons. Macrophages become stuffed with droplets or particles of the complex lipid, imparting a fine vacuolation or foaminess to the cytoplasm ( Fig. 4.14 ). Electron microscopy shows engorged secondary lysosomes that often contain membranous cytoplasmic bodies resembling concentric lamellated myelin figures, sometimes called “zebra” bodies. Because of their high content of phagocytic cells, the organs most severely affected are the spleen, liver, bone marrow, lymph nodes, and lungs. Splenic enlargement may be striking. In addition, the entire CNS, including the spinal cord and ganglia, is involved in this inexorable process. The affected neurons are enlarged and vacuolated as a result of the accumulation of lipids. This variant manifests in infancy with massive organomegaly and severe neurologic deterioration. Death usually occurs within the first 3 years of life. By comparison, patients with the type B variant, which is associated with mutant sphingomyelinase with some residual activity, have organomegaly but no neurologic manifestations. Estimation of sphingomyelinase activity in the leukocytes can be used for diagnosis of suspected cases, as well as for detection of carriers. Molecular genetic tests are also available for diagnosis at specialized centers.
Although previously considered to be related to type A and type B, Niemann-Pick disease type C (NPC) is molecularly and biochemically distinct and is more common than types A and B combined. Mutations in two related genes, NPC1 and NPC2 , give rise to this disorder, with NPC1 being responsible for a majority of cases. Unlike most other lysosomal storage diseases, NPC results from a primary defect in lipid transport. Both NPC1 and NPC2 are involved in the transport of free cholesterol from the lysosomes to the cytoplasm (see Fig. 4.6 ). Affected cells accumulate cholesterol as well as gangliosides such as GM1 and GM2. NPC is clinically heterogeneous. The most common form manifests in childhood and is marked by ataxia, vertical supranuclear gaze palsy, dystonia, dysarthria, and psychomotor regression.
Gaucher disease results from mutations in the gene that encodes glucocerebrosidase, and the resultant deficiency of this enzyme leads to an accumulation of glucocerebroside, an intermediate in glycolipid metabolism, in mononuclear phagocytic cells. There are three autosomal recessive variants of Gaucher disease resulting from distinct allelic mutations. Common to all is deficient activity of glucocerebrosidase, which catalyzes the cleavage of a glucose residue from ceramide. Normally, macrophages, particularly in the liver, spleen, and bone marrow, sequentially degrade glycolipids derived from the breakdown of senescent blood cells. In Gaucher disease, the degradation stops at the level of glucocerebrosides, which accumulate in macrophages. These cells— so-called “Gaucher cells”—become enlarged, with some reaching a diameter as great as 100 μm, because of the presence of distended lysosomes, and they acquire a pathognomonic cytoplasmic appearance resembling “wrinkled tissue paper” ( Fig. 4.15 ). It is now evident that Gaucher disease is caused not just by the burden of storage material but also by activation of macrophages. High levels of macrophage-derived cytokines, such as interleukins (IL-1, IL-6) and tumor necrosis factor (TNF), are found in affected tissues.
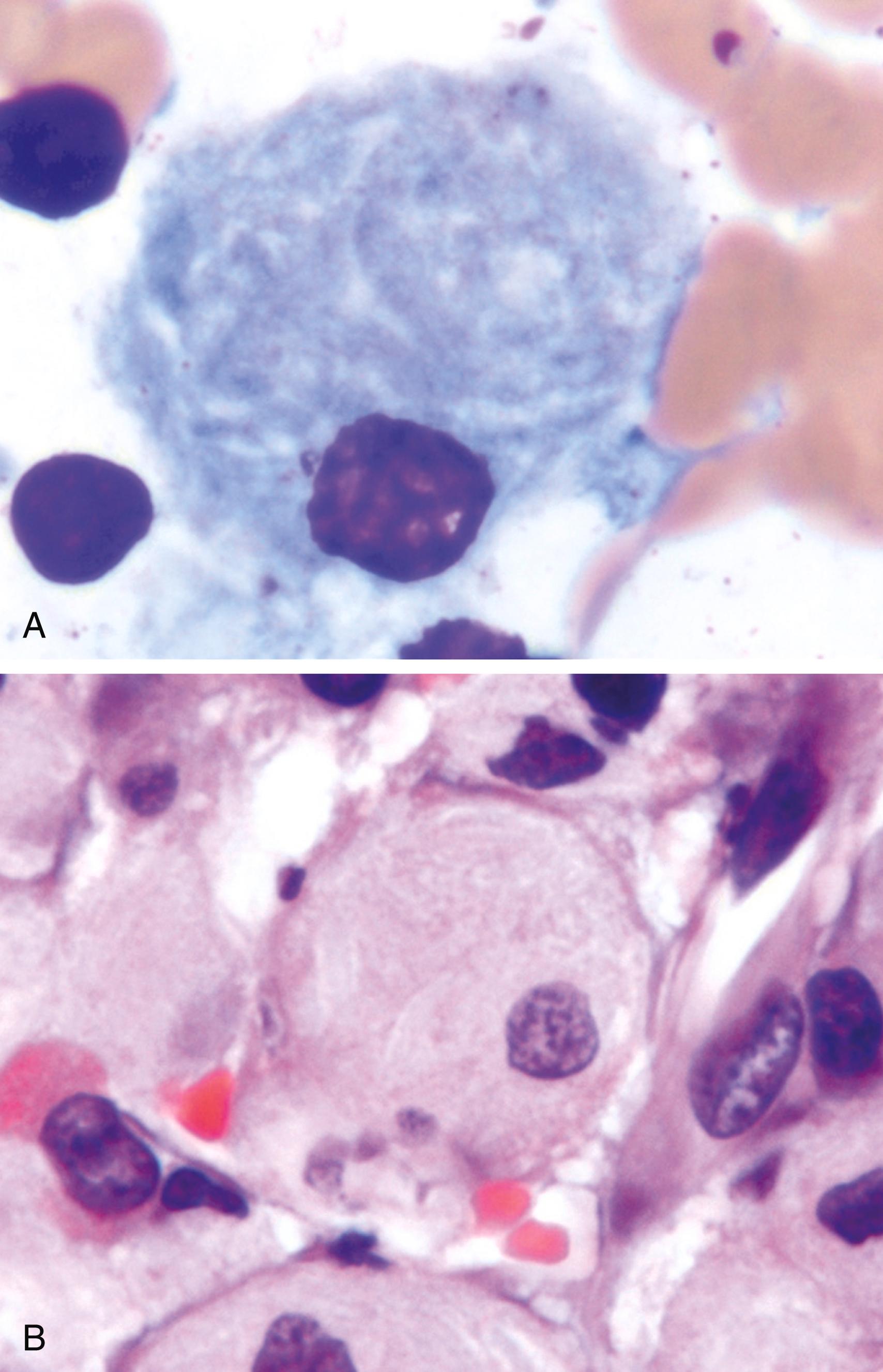
One variant, type 1, also called the chronic nonneuronopathic form, accounts for 99% of cases of Gaucher disease. It is characterized by clinical or radiographic bone involvement (osteopenia, focal lytic lesions, and osteonecrosis) in 70% to 100% of cases. Additional features are hepatosplenomegaly and the absence of CNS involvement. The spleen often enlarges to massive proportions, filling the entire abdomen. Gaucher cells are found in the liver, spleen, lymph nodes, and bone marrow. Marrow replacement and cortical erosion may produce radiographically visible skeletal lesions and peripheral blood cytopenias. It is believed that bone changes are caused by the aforementioned macrophage-derived cytokines. Unlike other variants, type 1 is compatible with long life. The carrier frequency of the type 1 in Ashkenazi Jewish population is quite high being close to 1 in 12. By comparison the carrier frequency in non-Jewish population is 1 in 40,000.
Neurologic signs and symptoms characterize types 2 and 3 variants. In type 2, these manifestations appear during infancy (acute infantile neuronopathic form) and are more severe, whereas in type 3, they emerge later and are milder (chronic neuronopathic form) . Although the liver and spleen also are involved, the clinical features in types 2 and 3 are dominated by neurologic disturbances, including convulsions and progressive mental deterioration.
As mentioned earlier, mutation of the glucocerebroside gene is a strong risk factor for Parkinson disease. Patients with Gaucher disease have a 20-fold higher risk of developing Parkinson disease (compared to controls), and 5% to 10% of patients with Parkinson disease have mutations in the gene encoding glucocerebrosidase. The level of glucocerebrosides in leukocytes or cultured fibroblasts is helpful in diagnosis and in the detection of heterozygote carriers. DNA testing is also available.
Currently, there are two approved therapies for type I Gaucher disease. The first is lifelong enzyme replacement therapy via infusion of recombinant glucocerebrosidase. The second, known as substrate reduction therapy, involves oral intake of an inhibitor of the enzyme glucosylceramide synthase. This leads to reduced systemic levels of glucocerebroside, the substrate for the defective enzyme in Gaucher disease. Substrate reduction therapy leads to reduced spleen and liver sizes, improved blood counts, and enhanced skeletal function. Other emerging treatments include gene therapy through transplant of hematopoietic stem cells engineered to express normal glucocerebrosidase.
Mucopolysaccharidoses (MPSs) are characterized by defective degradation and excessive storage of mucopolysaccharides in various tissues. Recall that mucopolysaccharides are part of the extracellular matrix and are synthesized by connective tissue fibroblasts. Most mucopolysaccharide is secreted, but a fraction is degraded within lysosomes through a catabolic pathway involving multiple enzymes. Several clinical variants of MPS, classified numerically as MPS I to MPS VII, have been described, each resulting from the deficiency of one specific enzyme in this pathway. The mucopolysaccharides that accumulate within the tissues include dermatan sulfate, heparan sulfate, keratan sulfate, and (in some cases) chondroitin sulfate.
Hepatosplenomegaly; skeletal deformities; lesions of heart valves; subendothelial arterial deposits, particularly in the coronary arteries, and lesions in the brain are features that are seen in all MPSs. Coronary subendothelial lesions lead to myocardial infarction and cardiac decompensation. Most cases are associated with coarse facial features, clouding of the cornea, joint stiffness, and intellectual disability. Urinary excretion of the accumulated mucopolysaccharides is often increased. With all MPSs except one, the mode of inheritance is autosomal recessive; the exception, Hunter syndrome, is an X-linked recessive disease. Of the eleven recognized variants, only two well-characterized syndromes are discussed briefly here.
MPS type I, also known as Hurler syndrome, is caused by a deficiency of α-L-iduronidase. In Hurler syndrome, affected children have a life expectancy of 6 to 10 years, and death is often a result of cardiac complications. Accumulation of mucopolysaccharides is seen in cells of the mononuclear phagocyte system, in fibroblasts, and within endothelium and smooth muscle cells of the vascular wall. The affected cells are swollen and have clear cytoplasm, resulting from the accumulation of stored material within engorged, vacuolated lysosomes. Lysosomal inclusions also are found in neurons, accounting for the intellectual disability.
MPS type II, or Hunter syndrome, is caused by a deficiency of L-iduronate sulfatase. It differs from Hurler syndrome in its mode of inheritance (X-linked), the absence of corneal clouding, and its often milder clinical course. Diagnosis is made by measuring the level of alpha-L-iduronidase in leukocytes. DNA diagnosis is not routinely employed because of the large number of distinct causative mutations.
An inherited deficiency of enzymes involved in glycogen synthesis or degradation can result in excessive accumulation of glycogen or some abnormal form of glycogen in various tissues. The type of glycogen stored, its intracellular location, and the tissue distribution of the affected cells vary depending on the specific enzyme deficiency. Regardless of the tissue or cells affected, the glycogen is most often stored within the cytoplasm. One variant, Pompe disease, is also a form of lysosomal storage disease, because the missing enzyme is localized to lysosomes. Most glycogenoses are autosomal recessive diseases, as is common with “missing enzyme” syndromes.
Approximately a dozen forms of glycogenoses have been described in association with specific enzyme deficiencies. Based on pathophysiologic findings, they can be grouped into three categories ( Table 4.4 ):
Hepatic type. The liver contains several enzymes that synthesize glycogen for storage and break it down into free glucose. Hence, a deficiency of the hepatic enzymes involved in glycogen metabolism is associated with two major clinical effects: enlargement of the liver due to storage of glycogen and hypoglycemia due to a failure of glucose production ( Fig. 4.16 ). Von Gierke disease (type I glycogenosis), resulting from a lack of glucose-6-phosphatase, is the most important example of the hepatic form of glycogenosis (see Table 4.4 ).
Myopathic type. In striated muscle, glycogen is an important source of energy, and most forms of glycogen storage disease affect muscles. When enzymes that are involved in glycolysis are deficient, glycogen storage occurs in muscles and there is an associated muscle weakness due to impaired energy production. Typically, the myopathic forms of glycogen storage diseases are marked by muscle cramps after exercise, myoglobinuria, and failure of exercise to induce an elevation in blood lactate levels because of a block in glycolysis. In this category are included McArdle disease (type V glycogenosis), resulting from a deficiency of muscle phosphorylase, and type VII glycogenosis, resulting from a deficiency of muscle phosphofructokinase.
Type II glycogenosis (Pompe disease) is caused by a deficiency of acid alpha glucosidase (lysosomal acid maltase) and is associated with deposition of glycogen in virtually every organ, but cardiomegaly is most prominent ( eFig. 4.1 ). Most affected patients die within 2 years of onset of cardiorespiratory failure. Replacement therapy with glucosidase can reverse cardiac muscle damage and modestly increase longevity.
| Clinicopathologic Category | Specific Type | Enzyme Deficiency | Morphologic Changes | Clinical Features |
|---|---|---|---|---|
| Hepatic type | Hepatorenal (von Gierke disease, type I) | Glucose-6-phosphatase | Hepatomegaly: intracytoplasmic accumulations of glycogen and small amounts of lipid; intranuclear glycogen Renomegaly: intracytoplasmic accumulations of glycogen in cortical tubular epithelial cells |
In untreated patients, failure to thrive, stunted growth, hepatomegaly, and renomegaly Hypoglycemia resulting from failure of glucose mobilization, often leading to convulsions Hyperlipidemia and hyperuricemia resulting from deranged glucose metabolism; many patients develop gout and skin xanthomas Bleeding tendency caused by platelet dysfunction With treatment (providing continuous source of glucose), most patients survive and develop late complications (e.g., hepatic adenomas) |
| Myopathic type | McArdle disease (type V) | Muscle phosphorylase | Skeletal muscle only: accumulations of glycogen predominant in subsarcolemmal location | Painful cramps associated with strenuous exercise Myoglobinuria occurs in 50% of cases Onset in adulthood (>20 years) Muscular exercise fails to raise lactate level in venous blood Compatible with normal longevity |
| Miscellaneous type | Generalized glycogenosis (Pompe disease, type II) | Lysosomal acid alpha glucosidase (acid maltase) | Mild hepatomegaly: ballooning of lysosomes with glycogen creating lacy cytoplasmic pattern Cardiomegaly: glycogen within sarcoplasm as well as membrane bound Skeletal muscle: similar to heart |
Massive cardiomegaly, muscle hypotonia, and cardiorespiratory failure before age 2 Milder adult form with only skeletal muscle involvement manifests with chronic myopathy |
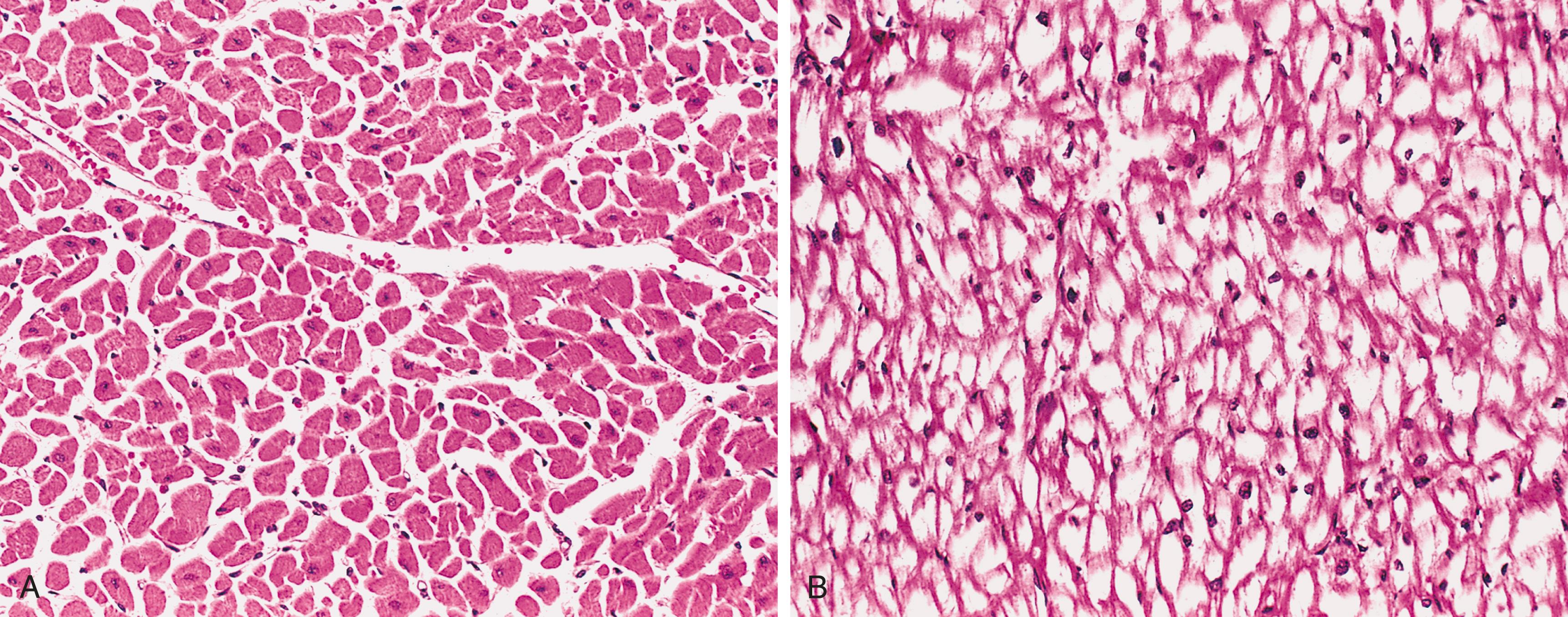
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here