Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In this chapter, concepts are identified and described that are intrinsic to musculoskeletal trauma, such as the purpose of the imaging report, vector forces causing musculoskeletal injury, musculoskeletal injury terminology, and fractures and dislocations in which the associated soft tissue component—not routinely delineated by conventional radiography—is the principal element of the injury. Because most of the musculoskeletal injuries described in this chapter are discussed and illustrated in greater detail in the chapters on specific anatomic regions, this chapter can also serve as a quick synoptic reference.
The imaging report is not only the formal transmittal of the findings of the examination but also serves as the basis of patient management, the source of comparison with subsequent examinations, a historic record, and a source of possible research data. For all these reasons, all musculoskeletal injury imaging reports must be as accurate and thorough as possible. The report must be dictated after critical observation, analysis, and synthesis of the data present on the examination.
Timely transmission of information derived from the musculoskeletal injury imaging examination to the referring health care provider is essential for proper patient management. The initial examination (conventional radiography, CT, or MRI) may be requested in acute or emergent circumstances, in which event the radiologist should communicate the findings directly to the referring health care provider or designated representative. In less acute or urgent circumstances, the information may be transmitted in the form of a written report. When the referring health care provider does not request a “stat” report, the radiologist should determine whether the injury should be considered emergent or urgent based on the findings of the examination and reasonable medical judgment.
Follow-up musculoskeletal injury examination information may be considered as “routine” and the information transmitted by written report. Should the follow-up study show related but unexpected findings, such as infection or change in position of fragments, the radiologist should transmit the findings directly to the referring health care provider or designee.
In every instance, all deviations from normal, including artifacts, recorded on the examination must be included in the report. All pertinent negative observations must be included as well.
With respect to CT and MRI, the technical factors and imaging sequences, respectively, must be appropriate to the indications for the examination and should be included in the body of the report.
Digital imaging and electronic communication should preclude informal, “curbside” verbal communication between the radiologist and referring health care provider. Informal verbal communication may lead to misinterpretation, misunderstanding of the transmitted information, or even misdiagnosis, with resultant patient mismanagement and the potential for medical liability. When the radiologist and referring health care provider do consult directly, the consultation must be supported by a formal written report.
The Practice Guidelines of the American College of Radiology recommend that the radiologist's initial report consist of two parts: a heading called “Impression,” “Diagnosis,” or “Conclusion” and a descriptive portion (body) frequently designated as “Comment.” The body of the report should describe the findings that prompted the impression, diagnosis, or conclusion.
If a single diagnosis is not possible, the impression, diagnosis, or conclusion should be expanded to include a reasonable differential diagnosis with the possibilities listed in the order of likely probability.
The body or “comment” portion of the study should indicate the part or anatomic region examined and the views (projections) obtained (e.g., “anteroposterior, lateral, and open-mouth projections of the cervical spine were obtained”).
Some practices have started using standard reports (“lexicons”) for all imaging reports. This has the advantage of being a checklist of the important anatomic areas to be examined and provides standard terminology between radiologists. Referring health care providers often prefer this approach as they become accustomed to the standard report outline and do not have to deal with individual variations in dictation methods.
Follow-up examinations need not contain a “Diagnosis” or “Impression;” however, they should indicate the views and projections constituting the examination and always include the phrase, “In comparison with the previous examination of the (part or anatomic area) dated _____, etc.” All changes between the previous and current examination must be carefully and accurately described.
The different types of bone include the following :
Long: length greater than width (e.g., femur, phalanx)
Short: cuboid shape (e.g., carpal and tarsal bones)
Flat: two layers of compact bone separated by a thin marrow space; usually preformed in a membrane (e.g., sternum, ribs, scapula)
Irregular: mixed shape (e.g., vertebra, pelvis)
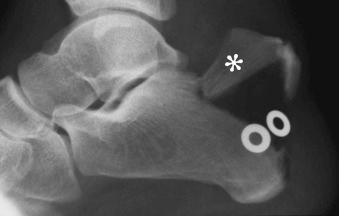
Sesamoid: small, usually round or oval bones that develop in tendons. Sesamoid bones are usually flat on the side adjacent to the neighboring bone (see eFig. 2-1 ).
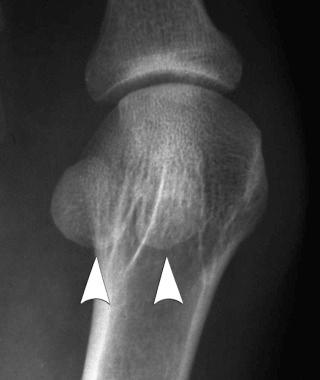
Accessory (supernumerary): arise from separate centers of ossification located adjacent to a parent bone and found most frequently in the foot (e.g., os intermetatarseum (see eFig. 2-2 ).

Long and short bones have their origin from primary and secondary ossification centers (see eFig. 2-3 ). The primary ossification originates in the midshaft of the bone, with ossification progressing proximally and distally from that center. Secondary ossification centers develop in the proximal and distal epiphyses, which unite with the metaphysis of the shaft at the physis to provide longitudinal growth of the bone (see eFig. 2-4 ). Other secondary centers arise in cartilage and form apophyses, which develop into tuberosities or exostoses from which tendons and ligaments arise or to which they insert (see eFig. 2-4 ).
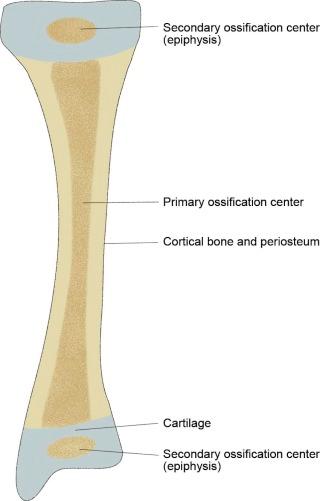
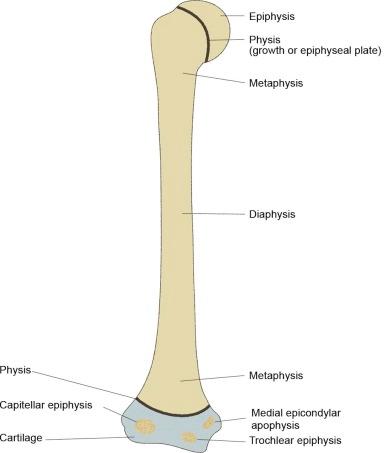
Portions of long and short bones are designated as “diaphysis” (shaft), “metaphysis” (transitional area between the diaphysis and the epiphysis), and “epiphysis,” which is the proximal and distal fused growth center (see eFig. 2-4 ).
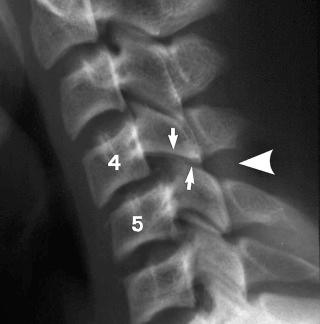
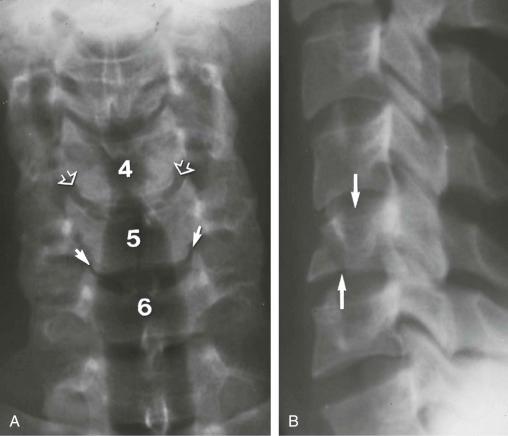
Anterior subluxation (hyperflexion sprain) of the cervical spine. This is caused by a primary hyperflexion mechanism of injury of the cervical spine resulting in the disruption of the posterior ligament complex (see eFig. 2-5 ).
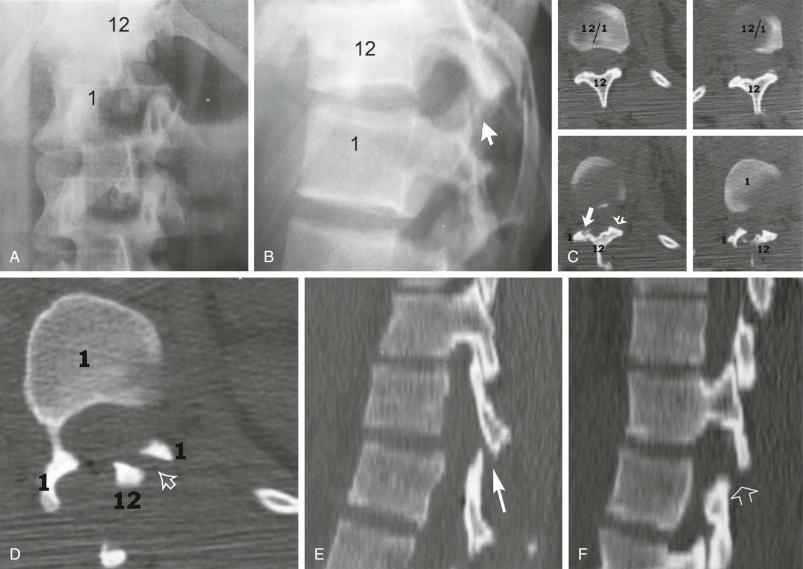
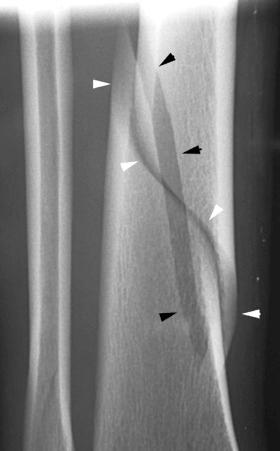
Soft tissue Chance injury. This is a hyperflexion injury of the thoracolumbar (T10–L2) spine in which the vertebra at the level of the injury remains intact but all ligamentous structures including the intervertebral disk and the anterior and posterior longitudinal ligaments are disrupted ( Fig. 2-1 ). This injury, like the Chance fracture, is associated with an approximate 20% incidence of intra-abdominal injury, such as to the pancreas.
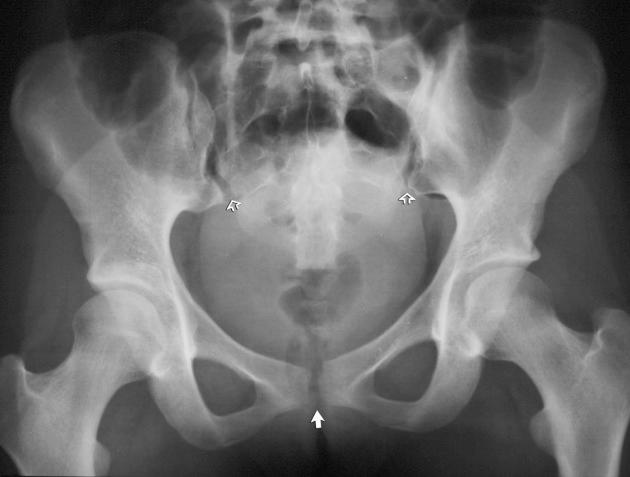
Pelvic ring disruption. The pubic symphysis and one or both of the sacroiliac joints are disrupted without associated fracture (see eFig. 2-6 ).
Rupture of the Achilles tendon.
Subluxation: partial disruption of a joint (see eFig. 2-7 ).
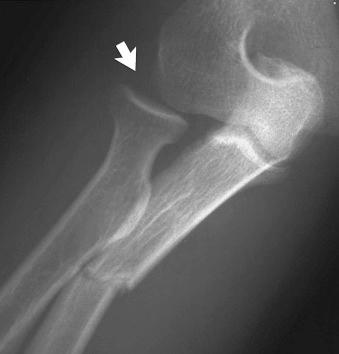
Dislocation (luxation): complete disruption of a joint (see eFig. 2-8 ).
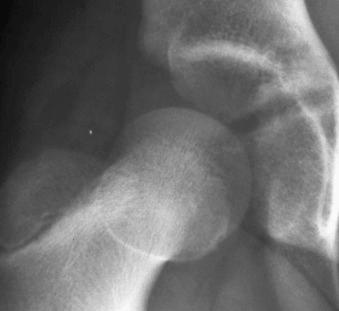
Fracture: break in the continuity of a bone (see eFig. 2-9 ).
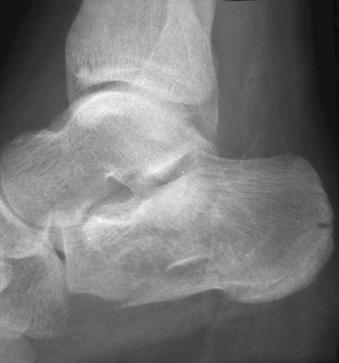
Fracture-dislocation: a musculoskeletal injury in which both disruption of a bone and complete dislocation of a joint occur simultaneously (see eFig. 2-10 ).
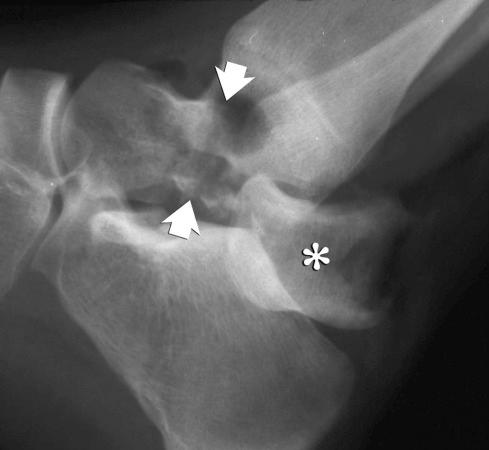
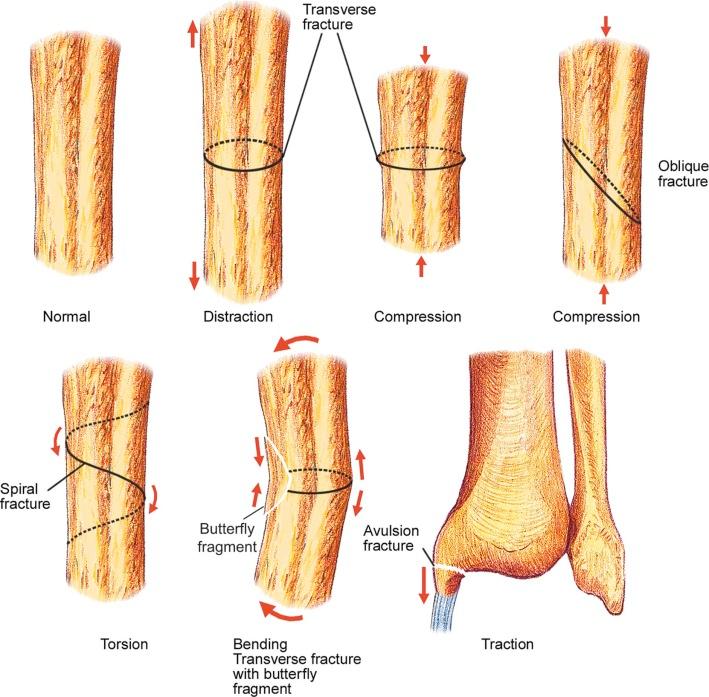
Skeletal injuries are the result of the effect of the major injury vector force on the involved bone. The major injury vectors include axial load (compression), distraction, bending, torsion, and traction (avulsion). The effect of each major injury vector on a long or short bone is schematically illustrated in Figure 2-2 .
The same vector forces may be applied to the pediatric skeleton. Injuries peculiar to the pediatric age group involve the physis (growth plate, epiphyseal plate), the epiphysis, and the metaphysis (see eFig. 2-4 ) alone or in combination. These injuries are defined by the Salter-Harris classification and are described and illustrated in Section 3, Pediatric Injuries. Three injuries involving the distal end of the pediatric tibia—the biplanar fracture of Tillaux, the triplanar fracture, and the Rang type VI physeal injury—are not included in the Salter-Harris classification.
The biplanar fracture of Tillaux (see eFig. 2-11 ), sometimes erroneously called a lateral Salter-Harris type III injury, is actually an avulsion fracture of the lateral aspect of the distal tibial epiphysis pulled off by the intact distal tibiofibular ligaments. The biplanar fracture occurs in the 10- to 14-year age group and involves the lateral aspect of the epiphysis because the distal tibial physis fuses from medial to lateral, allowing the separate fragment to be laterally retracted. In contradistinction, the Salter-Harris type III injury is caused by axial loading, with the vertical fracture typically in the midsagittal plane of the epiphysis (see eFig. 2-12 ).
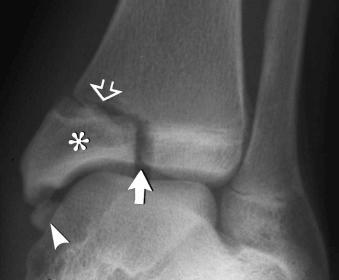
The triplanar fracture consists of a sagittally oriented fracture of the distal tibial epiphysis, a horizontal (axial) fracture through the adjacent physis, and a coronally oriented fracture of the posterior aspect of the adjacent metaphysis. The triplanar fracture may be either two part (see eFig. 2-13 ), in which the component parts constitute a single fragment, or three part, in which each component is a separate fragment.
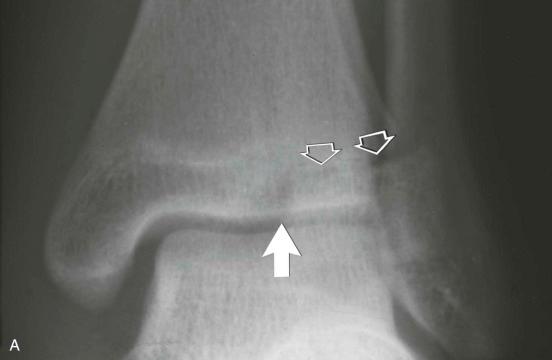
The Rang type VI physeal injury is rare and reportedly caused by a direct blow to the periosteum or perichondral ring. It is characterized by a tiny fragment arising from either the epiphysis or the metaphysis and located in the periphery of the physis. The presence of the tiny fragment distinguishes the Rang type VI injury from the Salter-Harris type I physeal injury.
Fractures may be classified by the causative force as either “direct” or “indirect.” Direct forces include gunshot, explosion, stabbing, and crushing injuries. Direct fractures occur in random patterns, whereas most indirect fractures occur in reasonably predictable patterns.
Fractures may also be classified as being “closed” (intact skin of the involved body part) or “open,” in which the skin of the involved body part is disrupted. The “open” fracture may be caused from inside out, as when the overlying skin is lacerated by the jagged edge of a displaced fracture fragment (see eFig. 2-14 ). An “open” fracture also may be the result of a penetrating or crushing injury (outside in) (see eFig. 2-15 ).
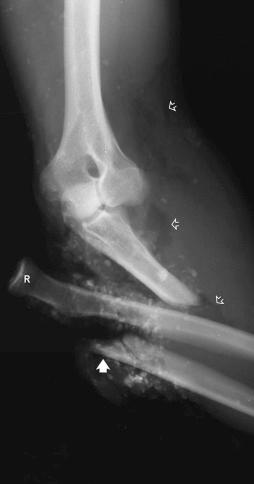
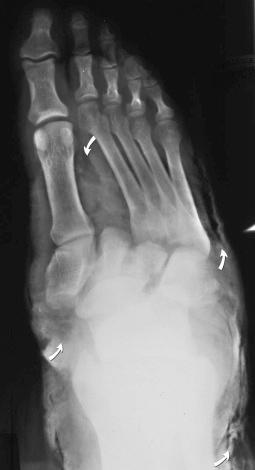
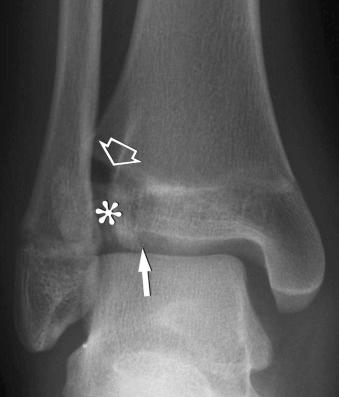
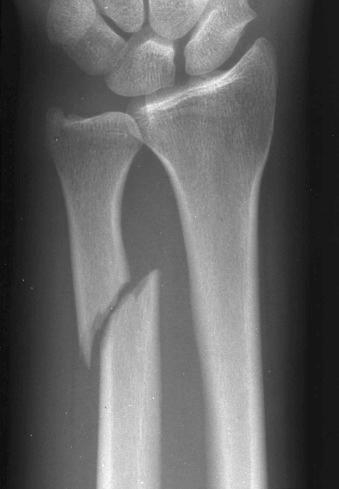
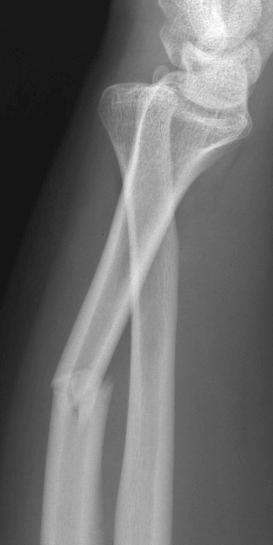
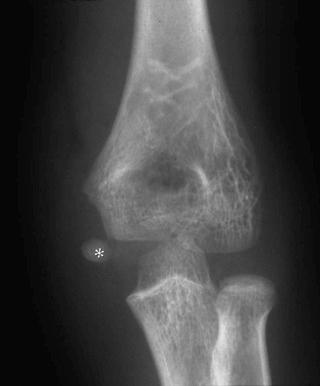
Fractures may be classified by the orientation of the fracture line, such as transverse (see eFig. 2-16 ), distracted (see eFig. 2-17 ), oblique (see eFig. 2-18 ), spiral (see eFig. 2-19 ), or impacted (see eFig. 2-20 ); by the orientation of the fracture fragments, such as nondisplaced ( Fig. 2-3 ) (stress fracture), displaced (see eFig. 2-21 ), angulated (see eFig. 2-22 ), displaced and angulated (see eFig. 2-23 ), and bayonet (overlapped) (see eFig. 2-24 ); or as “simple” (two fragments) (see eFig. 2-21 ), “comminuted” (more than two fragments) (see eFig. 2-9 ), “segmental” (two fracture sites in the same bone resulting in three separate fragments) (see eFig. 2-25 ), and “complete” (circumferential cortical disruption) (see eFig. 2-16 ) and “incomplete” or “greenstick” (partial cortical disruption) (see eFig. 2-26 ).
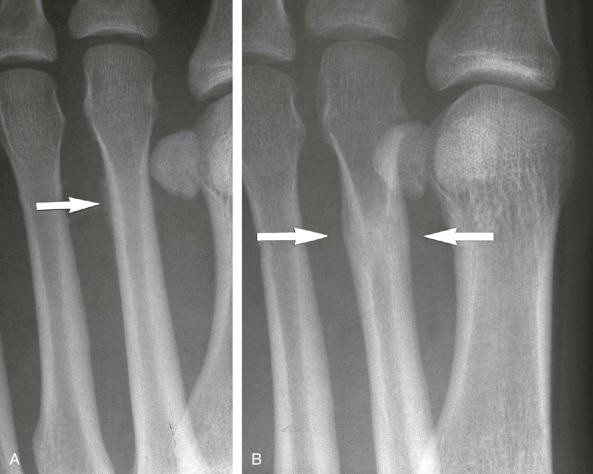
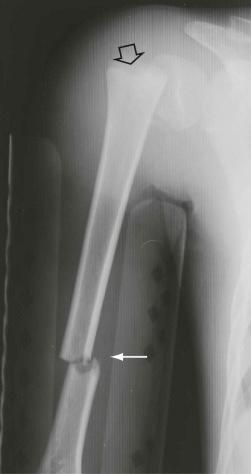
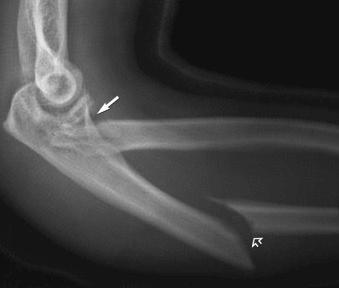
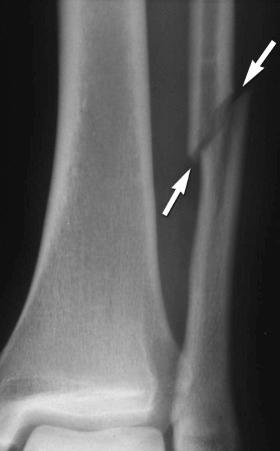
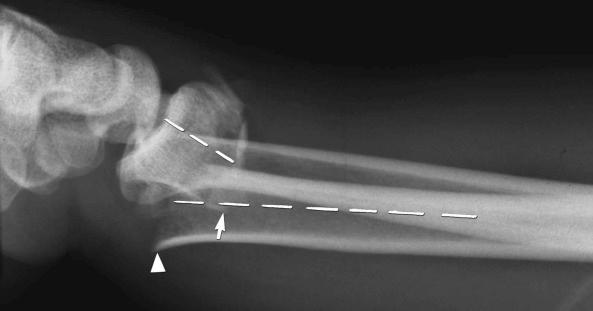
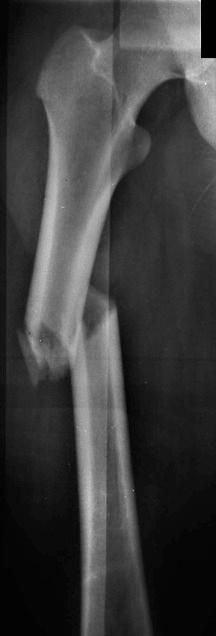
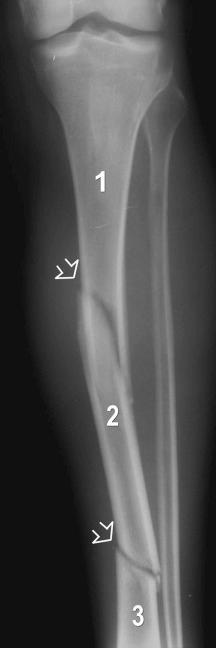
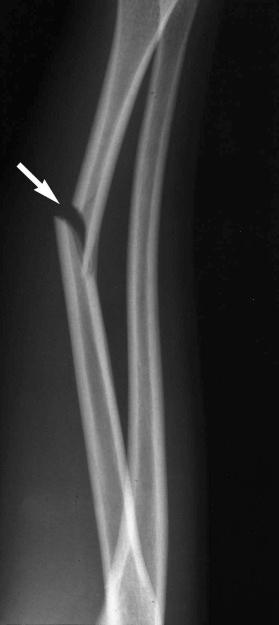
Insufficiency fractures may occur in areas of bone demineralization (osteoporosis) (see eFig. 2-27 ) or areas of skeletal neoplasia (pathologic fracture) (see eFig. 2-28 ).
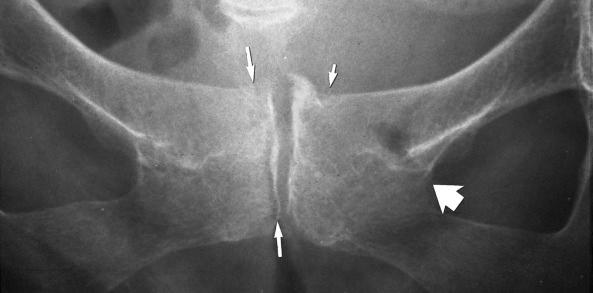
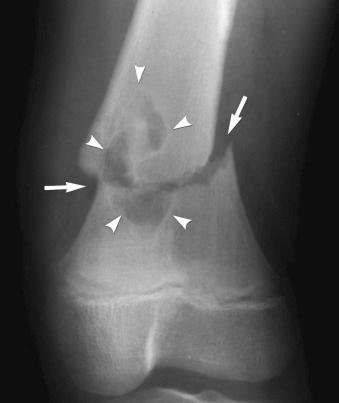
Axial loading may cause impaction (see eFig. 2-29 ) or separation of fragments (see eFig. 2-20 ).
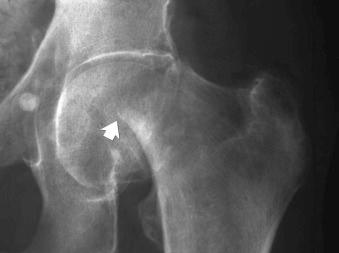
Fractures may be caused by forceful abrupt (see eFig. 2-30 ) or repetitive less forceful (see eFig. 2-31 ) ligamentous traction (“Little Leaguer's elbow”).
After fracture, the fracture repair pattern depends on the biomechanical environment. True osseous material can only be accomplished after achieving mechanical stability. Healing fractures first form an intermediate callus before actual bone formation. There are three phases of bone healing, each overlapping to some degree: inflammation, repair, and remodeling ( Fig. 2-4 ).
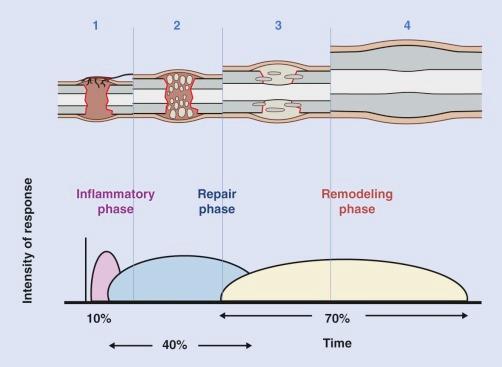
The inflammatory phase begins immediately after disruption of bone and surrounding soft tissues and will persist for 3 to 4 days or longer, depending on the degree of force that caused the injury, until cartilage or bone formation is initiated. Clinically, the inflammatory phase is associated with pain, and the conclusion of this phase usually results in a decrease in soft tissue swelling and pain. Fractures result in disruption of medullary vessels and extravasation of blood. A fibrin-rich clot/hematoma forms at the fracture site, and spontaneous healing is initiated. The hematoma may act as a scaffolding for cells and a spacer that determines the size and shape of the callus. It is also thought that the hematoma sets the stage for the repair phase of healing by releasing growth factors that stimulate angiogenesis and bone formation. Within hours, an extraosseous blood supply emerges in the soft tissues, thus revascularizing the hypoxic fracture site. Mononuclear phagocytes are delivered to the fracture site by the neovascularity and assist in the removal of necrotic bone, thus aiding in the development of callus. The bone fragment ends are then resorbed, widening the fracture gap, lowering interfragmentary strain, and minimizing local tissue deformation. As the medullary blood flow returns, the neovascularity of the extraosseous blood supply subsides gradually.
The repair phase of bone repair results in a slight gain in mechanical strength, as granulation tissue can withstand a tensile force of up to 0.1 Nm/mm 2 . The granulation tissue matures into connective tissue and collagen fibers become more abundant. Types I, II, and III collagen are deposited initially, with type I collagen fibers predominating. Mesenchymal cells within the cambium layer of periosteum, endosteum, bone marrow, and periosteal soft tissues that proliferated during the inflammatory phase of healing now differentiate into chondrocytes or osteoblasts, a process orchestrated by numerous growth factors including transforming growth factor β (TGF-β) and bone morphogenetic proteins (BMPs). The periosteum at the fracture site thickens and then undergoes chondrogenic transformation, resulting in an external callus vascularized by extraosseous vessels. This “soft callus” usually develops by 3 weeks and is stronger than granulation tissue, having a tensile strength of 4 to 19 Nm/mm 2 , which is similar to that of fibrous tissue. Mineralization of this “soft callus” proceeds from the fracture fragment toward the center of the fracture site, thus forming “hard callus.” The mechanism of this transformation is unknown but thought to be secondary to mitochondrial accumulation of calcium-containing granules that are released into the hypoxic environment created by anaerobic metabolism. These intramitochondrial deposits are released into the extracellular matrix and become seeds for the growth of apatite microcrystallites. The subsequent bony substitution at the site of injury closely resembles endochondral ossification from this stage on. Vascular invasion of fibrocartilage is coupled with the degradation of nonmineralized matrix by macrophages and blood vessels, and osteoprogenitor cells form new trabeculae, assuming that there is adequate vascularity. Bone union is usually achieved by the end of the repair phase of healing. The bone structure at this time is, however, different from that of the original bone. At the end of this phase, the injured bone has regained enough strength and rigidity to allow very low-impact exercise and stress.
The final phase of fracture repair, the “remodeling phase,” is characterized by adaptation of the bone to regain normal function and strength and may take up to 6 to 9 years to occur. During this time there is a balanced action of osteoclastic resorption and osteoblastic deposition that is governed by Wolff's law, modulated by piezoelectricity, the phenomenon of creation of electrical polarity by pressure exerted in a crystalline environment. In this process, axial loading of long bones creates an electropositive convex environment in which the osteoclastic activity predominates. On the concave surface, electronegativity predominates with increased osteoblastic activity. In the ideal setting, there will be remodeling of internal callus, thus allowing the renewal of a continuous medullary cavity in the diaphysis of the bone. Of course, vascular compromise or excessive instability will permit only the formation of fibrous tissue and atrophic nonunion. If the fracture gap is well vascularized, there may be uncontrolled interfragmentary motion with the progression to cartilaginous callus. In this setting, hypertrophic nonunion or pseudoarthrosis may develop.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here