Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter describes the methods used to evaluate dynamic changes in protein metabolism in the newborn infant. Throughout life, proteins not only form the key structural components of cells but also have key physiologic roles as, for example, transporters, immune mediators, receptor proteins, enzymes, and hormones. In a consideration of the relevant kinetics, the fundamental point of interest is that amino acids—the building blocks of protein—differ from carbohydrates and fats only by the inclusion of a nitrogen (N) atom in its molecule. Alanine without its single N atom is pyruvate, which is half a glucose molecule. Similar to carbohydrates, amino acids yield 4 kcal/g if not used for protein synthesis but are oxidized to ammonia and CO 2 instead.
Proteins consist of polymers of 20 different available amino acids that vary in length from two (dipeptide) to thousands of amino acids. Proteins are synthesized after transcription and translation from DNA and RNA, respectively. Amino acids, in turn, are classified as nonessential (dispensable), essential (indispensable), or conditionally essential ( Table 40.1 ). This distinction depends on whether they can be synthesized de novo (nonessential amino acids) or whether they can only be derived from dietary sources (essential amino acids), or whether their enzymatic synthesis capacity is suboptimal to meet total requirements during stress, disease, or otherwise increased demands (conditionally or semi-essential amino acids). If the dietary intake of any essential amino acid is too low, protein synthesis is limited by the availability of that amino acid, giving an excess of all other amino acids that would normally be integrated into protein. Those excess amino acids that are not incorporated into new protein synthesis are then oxidized.
| Essential | Semi-essential | Nonessential |
|---|---|---|
| Histidine | Arginine | Alanine |
| Isoleucine | Cysteine | Asparagine |
| Leucine | Glutamine | Aspartic acid (aspartate) |
| Lysine | Glycine | Glutamic acid (glutamate) |
| Methionine | Proline | Ornithine |
| Phenylalanine | Taurine a | Serine |
| Threonine | Tyrosine | |
| Tryptophan | ||
| Valine |
Besides considering total protein intake, it is therefore important to acknowledge dietary protein quality as well. This relates to the amount of each essential amino acid from dietary intake (parenteral or enteral). Parenteral amino acid solutions for infants have for example higher relative amounts of (conditionally) essential amino acids as compared with solutions for adults, because of their higher growth rate and immature enzymatic systems. Regarding enteral nutrition, the highest-quality source of proteins for term infants is from breast milk.
All proteins undergo a continuous process of synthesis and subsequent degradation into separate amino acids. This process can serve to replace damaged or defective proteins or to regulate the amount of a specific protein (e.g., an active enzyme). Proteins have a range of life spans from only minutes (enzymes), to slow turnover rates of up to weeks or months (e.g., muscle structural proteins and collagen), or to practically never (protein in neurons and the lens of the eye). The total amount of protein that is broken down and reconstituted every day is several times higher than the amount of protein that is derived from the diet. The net difference between protein degradation and synthesis determines whether an individual is catabolic or anabolic, leading to cachexia or growth, respectively.
Box 40.1 lists the methods used to measure protein and N metabolism in humans. Most of them will be elaborated upon later.
Growth assessment
Nitrogen balance method
End-product method
Turnover of individual components
Essential amino acids (index of protein breakdown)
Nonessential amino acids (de novo synthesis and gluconeogenesis)
Urea (protein oxidation)
Arteriovenous measurement of amino acid concentrations across a tissue bed
Protein synthesis of a specific protein by following tracer incorporation
Protein degradation of a specific protein by following disappearance of tracer from the protein
Early nutritional studies investigated anthropometric changes (growth) to determine efficacy of the level and quality of protein in the diet of infants. However, these methods require prolonged periods of study diet and may be unethical and inaccurate.
The most widely used method to follow changes in body protein is the N balance method. This method defines the difference of N between that is going in and that coming out of the body and for long has been the standard modality for defining minimum levels of dietary protein and essential amino acid intake in humans of all ages. This determination is done by carefully recording all food consumed and collecting all material excreted: urine, feces, sputum, and so on during at least 12 to 24 hours in neonates but preferably longer. The N from aliquots of each food, urine, and fecal collection is tediously converted to ammonia by boiling the specimens in concentrated acid. The ammonia content is determined, and N intake and excretion are calculated. In practice, N losses are routinely underestimated because of incomplete collections of urine and feces and insensible losses through skin, sweat, and so on, and N intake is routinely overestimated because of variable content or availability of protein in foods consumed, inaccurate recording of ingested amounts, and so forth.
Although measures of anthropometry or N balance often have been used to study infant nutrition, , both methods require weeks or days, respectively, to measure effects. Neither method provides any information concerning the turnover of N within the body. For example, an infant may be receiving adequate protein but insufficient energy intake for growth. Owing to this restricted intake, the child will not grow and has a N balance of zero. When more calories are provided, the infant starts growing and shows a positive N balance. How does the child’s body respond to produce this effect? N balance and growth measurements do not provide this information.
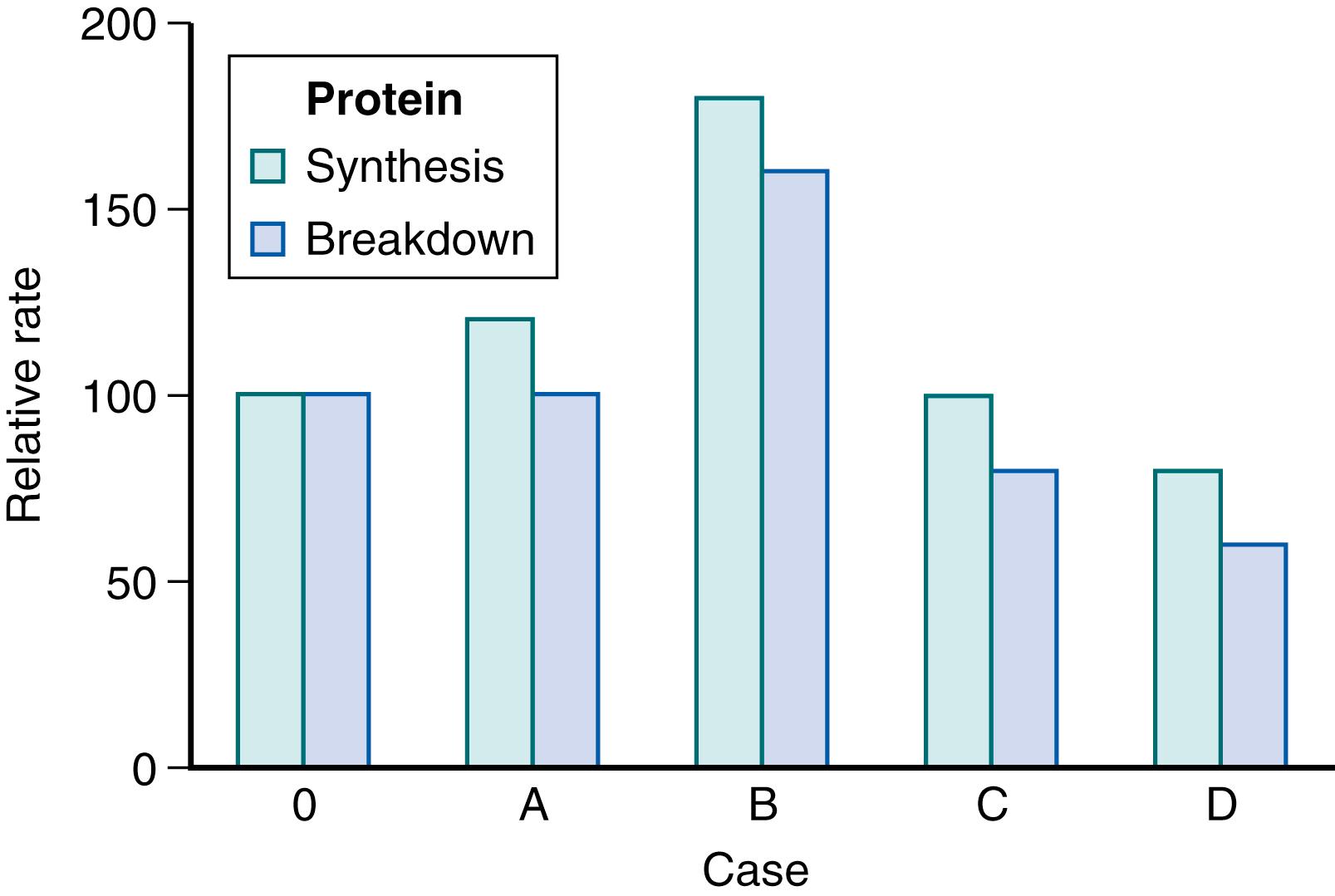
Fig. 40.1 shows four possible responses to the original clinical situation. Case 0 is the starting zero N balance. Positive N balance could be obtained by increasing protein synthesis ( case A in the figure), decreasing protein breakdown (case C), increasing both protein synthesis and breakdown but with a greater increase in protein synthesis (case B) , or decreasing both but with a greater decrement in protein breakdown (case D). The effect is a positive N balance for all four scenarios, but the energy implications are considerably different. Because protein synthesis costs energy, cases A and B are more “expensive,” whereas cases C and D require less energy than in the starting scenario (case 0). Investigation of these possible mechanisms requires direct assessment of rates of protein breakdown, protein synthesis, and amino acid turnover within the body. Such assessment can be accomplished with the use of stable isotope or radiolabeled tracers, although the latter technique is not used for studying the pediatric population. Underneath we will explain several of the stable isotope techniques that can be used to study neonatal nutrition and metabolism. These methods have also been elaborated on more in detail elsewhere.
The simplest approach to the study of amino acid and protein kinetics is to hypothesize a single, free pool of amino acid N, with two inflows —amino acid from dietary protein and amino acid released from protein breakdown—and two outflows —amino acid oxidation to end products (CO 2 and ammonia, which is subsequently metabolized into urea) and amino acid uptake for protein synthesis ( Fig. 40.2 ). This model can be considered either for whole-body protein turnover, in which the pool is the total free amino acid pool (see Fig. 40.2 ), or for the kinetics of a single amino acid, in which the pools are for a particular amino acid (illustrated for a 13 C essential amino acid stable isotope tracer in Fig. 40.3 ). The difference between the two approaches is related to how the system is viewed: on the one hand, looking at protein turnover versus, on the other hand, looking at the kinetics of a specific amino acid from which inferences to whole-body protein turnover are drawn.
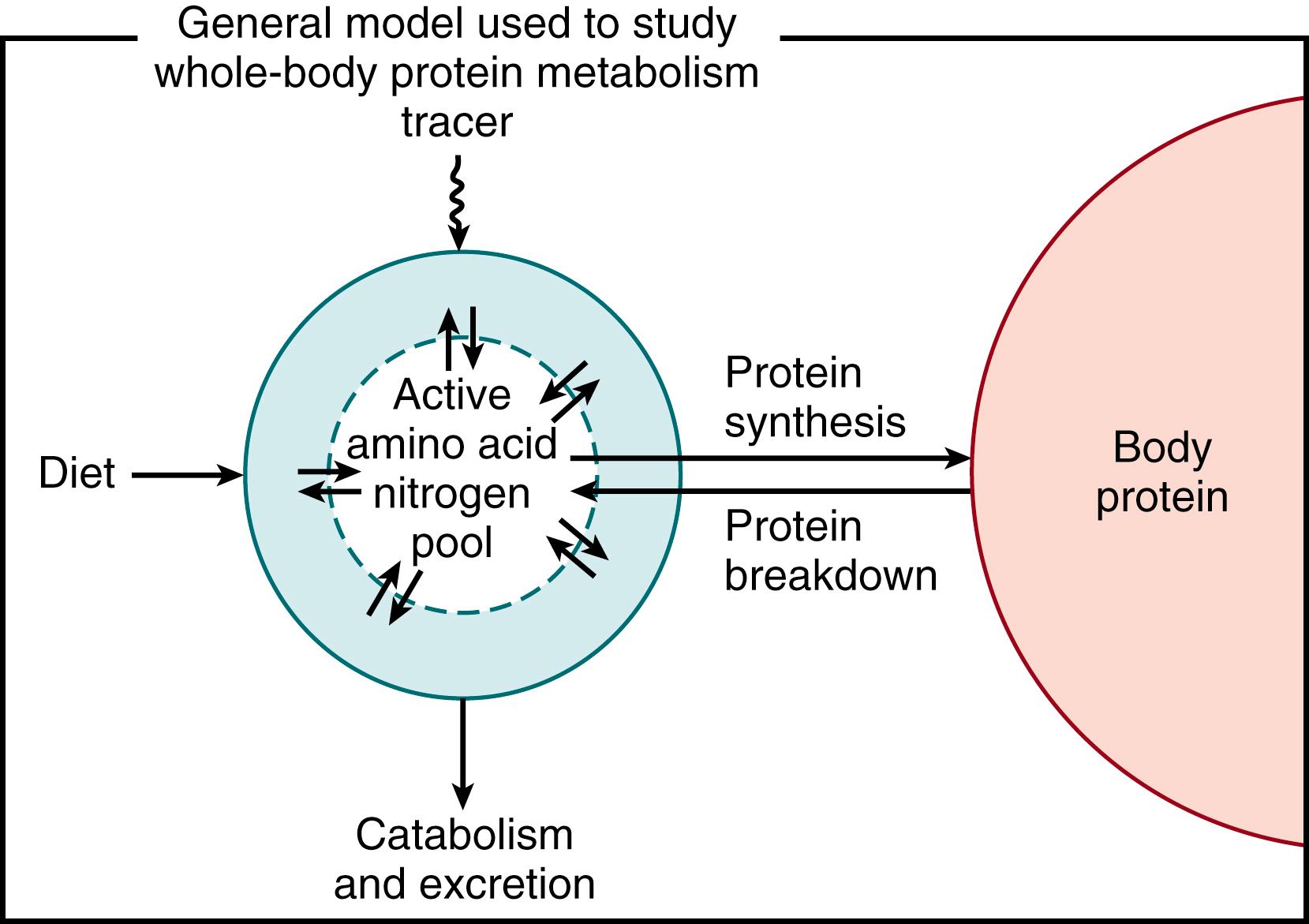
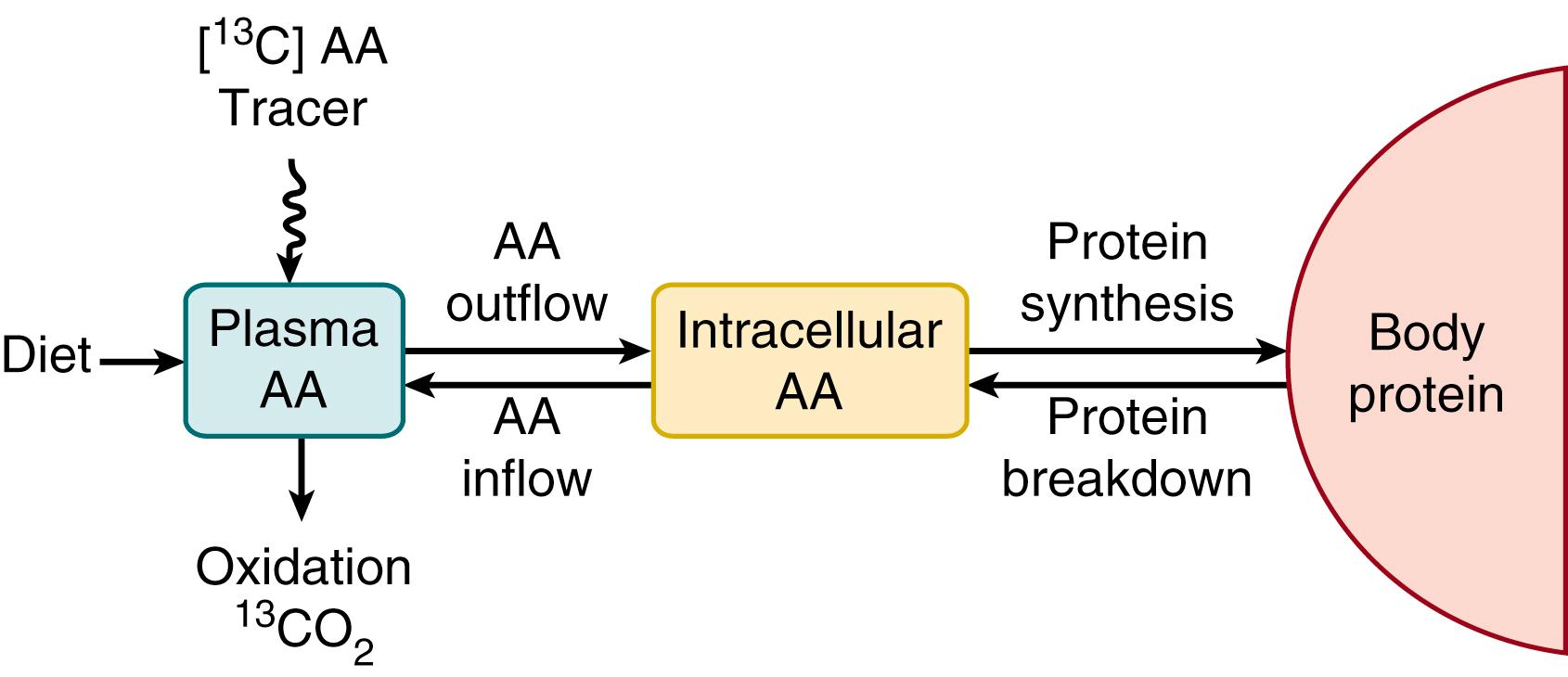
However, lumping all body protein into a single entity is of course a gross oversimplification. Each of the many different tissues of the body is made up of a wide range of proteins with different turnover rates. Obviously, following the individual rates of hundreds of proteins would be impracticable. However, because most of the important stores of N in the body turnover at similarly slow rates, it is possible to simplify the system into a conceptual model of clinical and experimental utility.
Changes in amino acid metabolism for a specific tissue can be inferred by measuring the difference between the amino acids delivered to the tissue (arterial blood levels) and the amino acids released from the tissue (venous blood levels). In animals and adults, this technique has been applied to studies of forearm, leg, liver, kidney, and brain metabolism. In pregnant animals and women, this model has been important in dissecting fetal and placental metabolism. However, measuring an arteriovenous substrate concentration difference across a tissue bed is similar to the N balance technique—it provides information about the balance but tells nothing about the mechanism within the tissue affecting the balance. Most exciting is the combination of tracer infusion with the measurement of amino acid balance across the tissue bed. This technique allows for a complete solution of the various pathways operating in the tissue for each amino acid tracer used and can measure tissue-specific rates of protein synthesis and balance. However, the use of arterial and venous catheters restricts the applicability of this method primarily to animal models. , Yet, a limited number of investigations have addressed human fetal amino acid metabolism by infusing amino acid tracers into the mother before delivery and obtaining cord blood at delivery to determine fetal metabolism. Alternatively, specific tissue amino acid metabolism in human neonates across the splanchnic tissues may be assessed with the simultaneous use of different enteral and intravenous tracers. First-pass amino acid metabolism of the small intestine and liver is measured by comparison of the tracer enrichments of both tracers in plasma. The plasma enrichment of the enteral administered tracer will be lower than that of the intravenously infused tracer by the amount of tracer sequestered by the gut and liver on the first pass during enteral absorption of the tracer. The ratio between the enrichment of the intravenously administered tracer and that of the enterally administered tracer is used to calculate the first-pass uptake.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here