Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
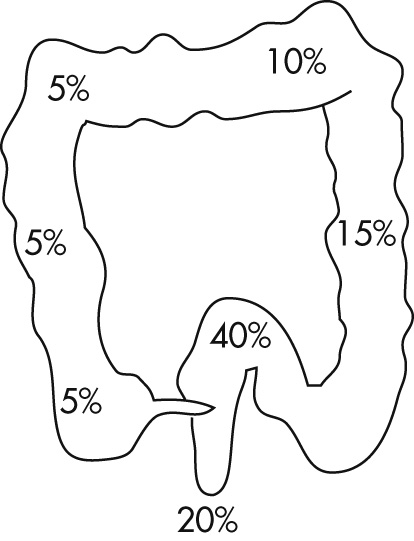
Cricopharyngeus
Postcricoid impressions (mucosal fold over vein)
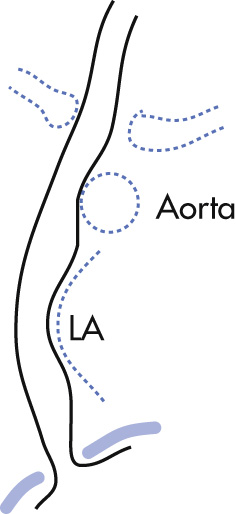
Aortic impression
Left mainstem bronchus (LMB)
Left atrium (LA)
Diaphragm
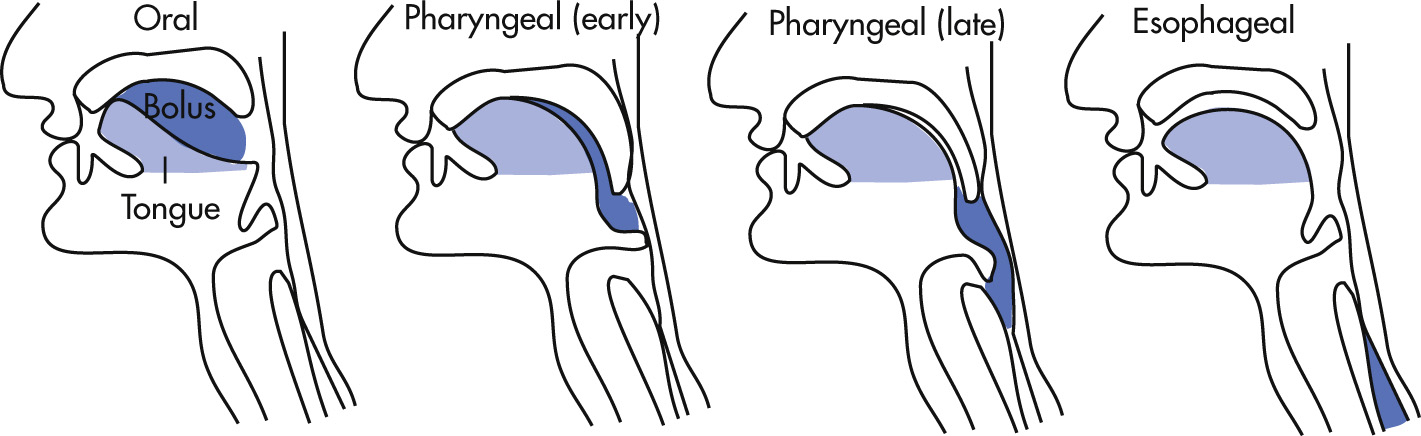
Peristaltic waves
Mucosa: thin transient transverse folds: feline esophagus (vs. thick folds in chronic reflux esophagitis); tiny nodules in older adults: glycogenic acanthosis
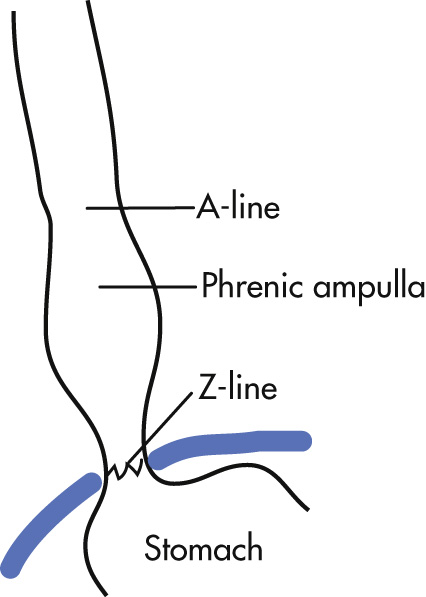
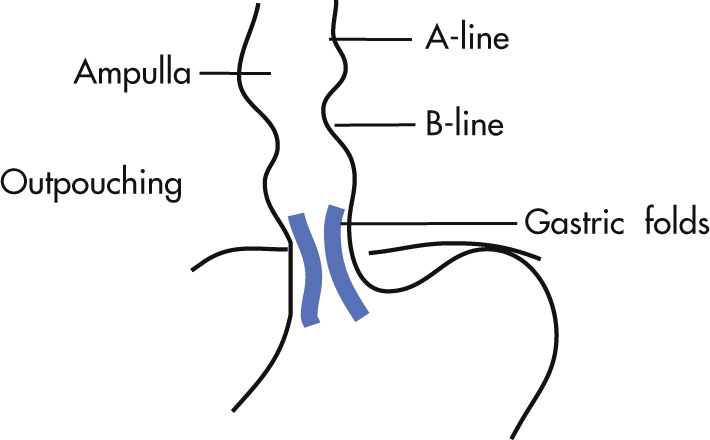
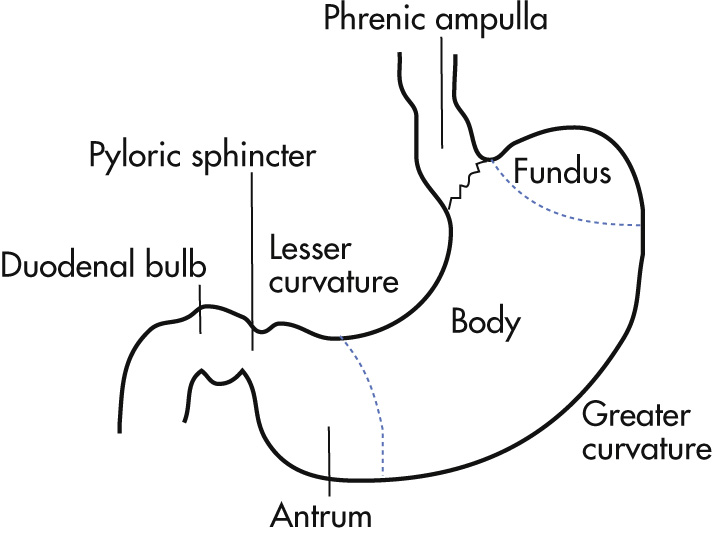
Phrenic ampulla: normal expansion of the distal esophagus; does not contain gastric mucosa
A-ring (for a bove; Wolf ring): indentation at upper boundary of the phrenic ampulla
B-ring (for b elow): indentation at lower boundary of the phrenic ampulla; normally not seen radiologically unless there is a hiatal hernia
Z-line ( z igzag line): squamocolumnar mucosal junction between esophagus and stomach; not visible radiologically
C-ring: diaphragmatic impression
The esophagus lacks a serosa. Upper one-third has striated muscle; lower two-thirds has smooth muscle.
Primary contractions: initiated by swallowing; distally progressive contraction waves strip the esophagus of its contents; propulsive wave
Secondary contractions: anything not cleared from the esophagus by a primary wave may be cleared by a locally initiated wave; propulsive wave
Tertiary contractions: nonpropulsive, uncoordinated contractions; these random contractions increase with age and are rarely of clinical significance in absence of symptoms of dysphagia; nonpropulsive wave; only peristaltic activity in achalasia
Peristalsis should always be evaluated fluoroscopically with the patient in a horizontal position. In the erect position the esophagus empties by gravity.
| Swallowing Phase | Tongue | Palate | Larynx | Pharyngeal Constrictors |
|---|---|---|---|---|
|
Dorsum controls bolus; base assumes vertical position | Resting | Resting | Resting |
|
Strips palate and moves dorsally | Velopharynx closure | Epiglottis deflects, larynx moves anterosuperiorly | Middle constrictors |
|
Meets relaxing palate | Begins descent | Vocal folds close, epiglottis retroflexes | Inferior constrictors |
|
Returns to resting | Resting | Returns to resting | Completion of constriction, resting |
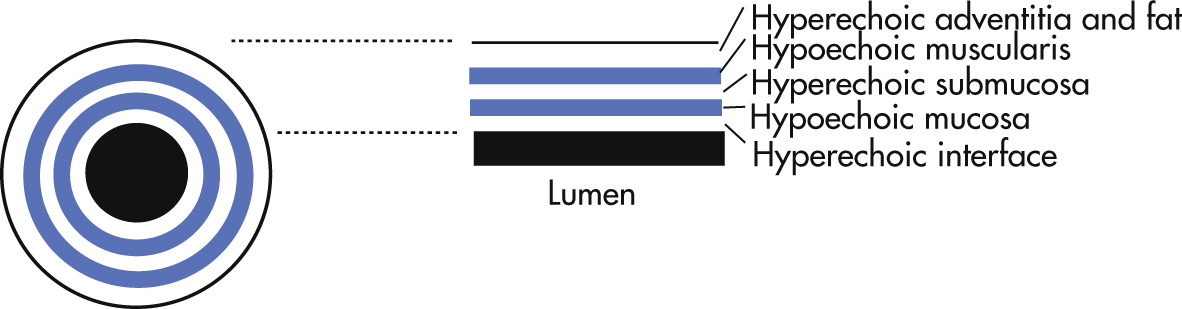
Endoscopic esophageal transabdominal or gastric US is performed mainly for staging of cancer or detection of early cancer. Most mass lesions and lymph nodes (LNs) appear as hypoechoic structures disrupting the normal US “gut signature,” consisting of different layers of hyperechogenic and hypoechogenic lines.
Thin annular symmetric narrowing at the junction of esophagus with the stomach (B-ring level). Present in 10% of population, 30% of whom are symptomatic. Symptoms (dysphagia, heartburn) usually occur if rings cause esophageal narrowing of ≤12 mm. Now considered a consequence of reflux.
Mucosal structures (web = asymmetric, ring = symmetric) may occur anywhere in the esophagus.
Iron-deficiency anemia (cervical webs): Plummer–Vinson syndrome
Hypopharyngeal carcinoma
There are two types:
Sliding hernia (axial type), 95%
GEJ is above the diaphragm.
Reflux is more likely with larger hernias.
“Mixed” variant when hernia and esophagus are not in straight axis.
Paraesophageal hernia, 5%
GEJ is in its normal position (i.e., below diaphragm).
Part of the fundus is herniated above the diaphragm through esophageal hiatus and lies to the side of the esophagus.
Reflux is not necessarily associated.
More prone to mechanical complications; prophylactic surgery a consideration
Usually nonreducible
Criteria for diagnosing sliding hernia:
Gastric folds above diaphragm
Concentric indentation (B-line) above diaphragm
Schatzki ring above diaphragm
Esophagitis, 25%
Duodenal ulcers, 20%
Maximally distend distal esophagus in horizontal position; distention can be achieved by sustained inspiratory effort
Determine the type of hernia
Determine if there is reflux by Valsalva maneuver or Crummy water-siphon test (patient in supine right posterior oblique (RPO) position continually drinks water to see if barium refluxes into midesophagus or above)
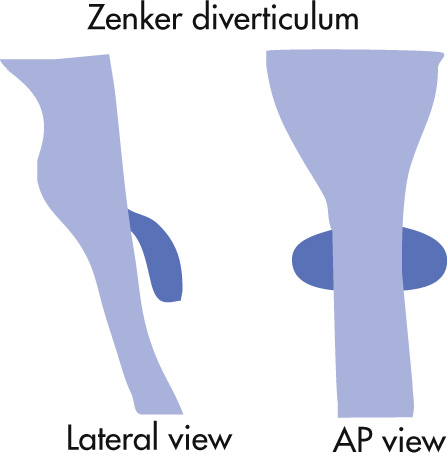
AP esophagram at level of pharynx demonstrates lateral outpouchings through weakness in thyrohyoid membrane. Large in glassblowers and wind instrument players.
Pulsion diverticulum originates in the midline of the posterior wall of the hypopharynx at an anatomic weak point known as Killian dehiscence (above cricopharyngeus at fiber divergence with inferior pharyngeal constrictor). During swallowing, increased intraluminal pressure forces mucosa to herniate through the wall. The cause of Zenker diverticulum is not firmly established, but premature contraction and/or motor incoordination of the cricopharyngeus muscle are thought to play a major role. Complications include:
Aspiration
Ulceration
Carcinoma
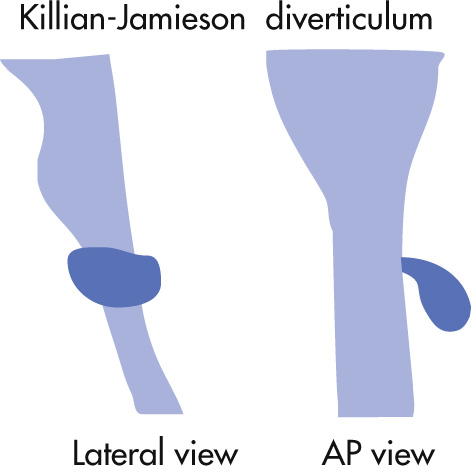
Below cricopharyngeus
Off midline
Lateral to cervical esophagus
May occasionally be recognized on chest radiographs (CXRs) by presence of soft tissue mass (often with air-fluid level) that mimics a hiatal hernia.
Large diverticulum can compress the true esophageal lumen, causing dysphagia.
Outpouching of midesophagus as a result of adjacent inflammatory process (e.g., tuberculosis [TB]).
Calcified mediastinal LNs
Numerous small esophageal outpouchings representing dilated glands interior to the muscularis. Usually occur at >50 years of age. Dysphagia is the presenting symptom. Underlying diseases include candidiasis, alcoholism, and diabetes.
Esophageal stricture may occur above and/or below stricture.
Esophagitis
Thin flask-shaped structures in longitudinal rows parallel to the long axis of the esophagus
Diffuse distribution or localized clusters near peptic strictures
Much smaller than true diverticula
When viewed en face, the pseudodiverticula can sometimes be mistaken for ulcers. When viewed in profile, however, they often seem to be “floating” outside the esophageal wall with barely perceptible channel to the lumen; esophageal ulcers almost always visibly communicate with the lumen.
Esophagitis may present with erosions, ulcers, and strictures and rarely with perforations and fistulas.
Infectious (common in debilitated patients)
Herpes
Candidiasis
Cytomegalovirus (CMV)
Chemical
Reflux esophagitis
Corrosives (lye)
Iatrogenic
Radiotherapy
Extended use of nasogastric (NG) tubes
Drugs: tetracycline, antiinflammatory drugs, potassium, iron
Other
HIV
Scleroderma
Crohn disease (rare)
Dermatologic manifestations (pemphigoid, dermatomyositis bullosa)
Thickening, nodularity of esophageal folds
Irregularity of mucosa: granularity, ulcerations
Retraction, smooth, tapered luminal narrowing, stricture just above GE junction
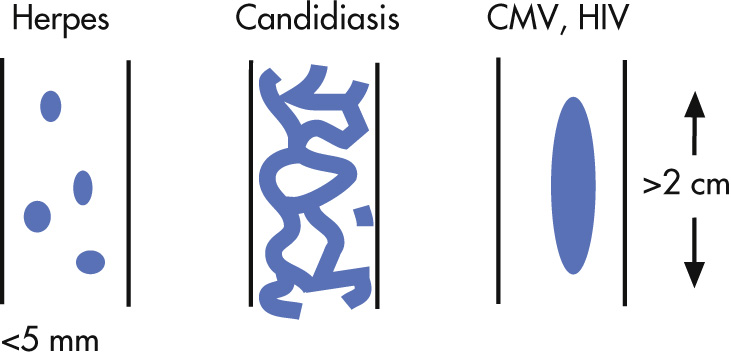
Herpes simplex
Small ulcers, <5 mm
Normal mucosa between ulcers
More diffuse than reflux ulcers
Candidiasis
Plaque like, reticular
Shaggy margins
Often involve entire esophagus
CMV and HIV
Typically, elliptical large ulcers but may be tiny ulcers such as herpes
Etiologic distinction between CMV and HIV ulcers is important because therapies are different.
Behçet disease may have a similar appearance.
Mycobacterial
Ulcers, sinus tracts
Dysphagia may be chronic, history of allergies, eosinophilia
Segmental proximal or midesophageal mild narrowing
May involve entire esophagus
Increased risk of iatrogenic tear
Responds to steroids
Esophagus is abnormally lined with columnar, metaplastic acid-secreting gastric mucosa. It is usually due to chronic reflux esophagitis. Because there is an increased risk of esophageal cancer, close follow-up and repeated biopsies are recommended.
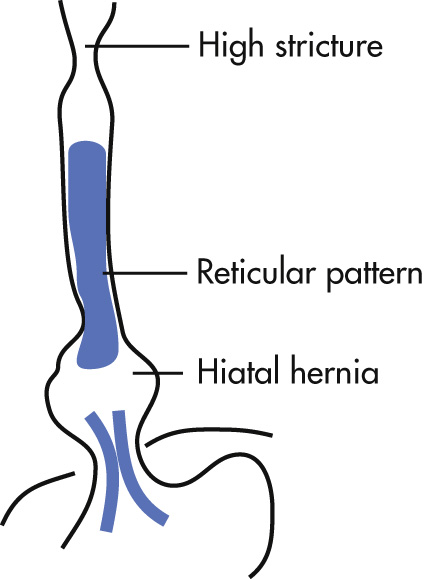
A reticular mucosal pattern, which may be discontinuous in the distal esophagus (short segment), is the most sensitive finding.
Suspect the diagnosis if there is:
Upper or midesophageal stricture accompanied by reticular mucosal pattern below transition or ulcer
Low strictures: the majority cannot be differentiated from simple reflux esophagitis strictures, and biopsies are required
Spontaneous perforation of the esophagus as a result of a sudden increase in intraluminal esophageal pressure. Severe epigastric pain. Treatment is with immediate thoracotomy. Mortality, 25%.
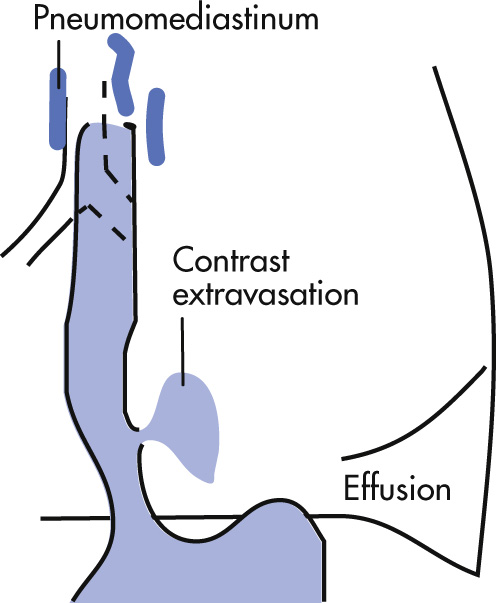
Pneumomediastinum
Pleural effusion (left > right)
Mediastinal hematoma
Rupture immediately above diaphragm, usually on left posterolateral side (90%)
Mucosal tear in proximal stomach, across GEJ, or in distal esophagus (10%), usually caused by prolonged vomiting (alcoholics) or increased intraluminal pressure. Because the tear is not transmural, there is no pneumomediastinum.
Radiographs are usually normal.
Intravasation rather than extravasation
There may be subtle mucosal irregularity.
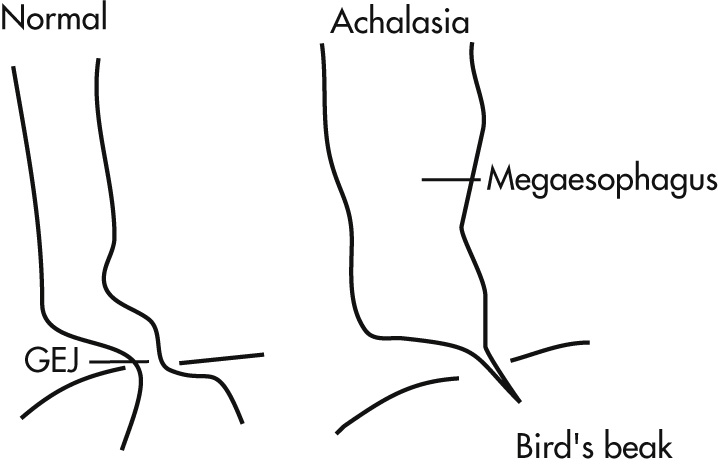
The gastroesophageal (GE) sphincter fails to relax because of degeneration of Auerbach plexus. The sphincter relaxes only when the hydrostatic pressure of the column of liquid or food exceeds that of the sphincter; emptying occurs more in the upright than in the horizontal position.
Primary (idiopathic)
Secondary (destruction of myenteric plexus by tumor cells)
Metastases
Adenocarcinoma invasion from cardia
Infectious: Chagas disease
Primary occurs predominantly in young patients (in contradistinction to esophageal tumors); onset: 20–40 years
Dysphagia, 100% to both liquids and solids when symptoms begin
Weight loss, 90%
Need to exclude malignancy (fundal carcinoma and lymphoma destroying Auerbach plexus), particularly in older adults
Need to exclude esophageal spasm
Manometry is the most sensitive method to diagnose elevated lower esophageal sphincter (LES) pressure and incomplete relaxation.
Two diagnostic criteria must be met:
Primary and secondary peristalsis absent throughout esophagus
LES fails to relax in response to swallowing
Dilated esophagus typically curves to right and then back to left when passing through diaphragm.
There may be minimal esophageal dilation in the early stage of disease.
Beaked tapering at GEJ
Tertiary waves
Air-fluid level in esophagus on plain radiograph
| Parameter | Esophageal Spasm | Achalasia |
|---|---|---|
| Symptoms | ||
| Dysphagia | Substernal | Xiphoid or suprasternal notch |
| Pain | Common | Rare |
| Weight loss | Rare | Common |
| Emotional | Common | Common |
| Motility | ||
| Waves | Simultaneous | Tertiary |
| LES relaxation | Present | Absent |
| Imaging Features | ||
| Esophageal contraction | Vigorous | Discoordinated |
| Esophageal emptying | Efficient | Poor |
| Response to Therapy | ||
| Pneumostatic dilation | Not indicated | Good |
| Surgery | Long myotomy | Low cardioesophageal myotomy |
Recurrent aspiration and pneumonias, 10%
Increased incidence of esophageal cancer
Drugs: nitrates, β-adrenergic agonists, calcium blockers (effective in <50%)
Balloon dilatation (effective in 70%)
Myotomy: procedure of choice
Collagen vascular disease that involves the smooth muscle of esophagus, stomach, and small bowel (SB).
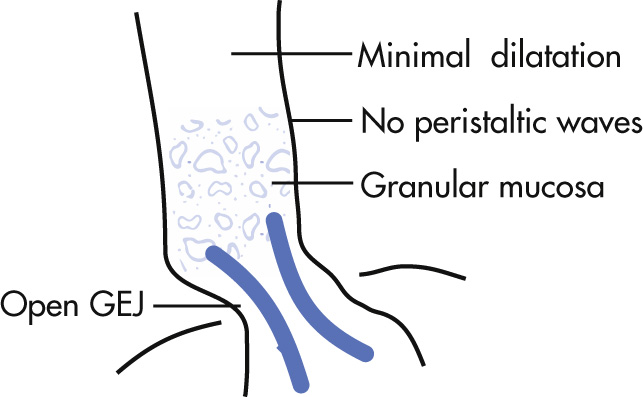
Lack of primary waves in distal two-thirds
GEJ patulous unless stricture supervenes
Reflux esophagitis (common)
Strictures occur late in disease
Esophagus dilates most when stricture supervenes
| Achalasia | Scleroderma | |
|---|---|---|
| Esophagus | Massively dilated | Mildly dilated |
| GEJ | Closed, tapers to beak shape | Open; stricture late |
| Horizontal swallow | Tertiary contractions | Primary in proximal third, tertiary contraction in distal two-thirds |
| Reflux | No | Yes |
| Complications | Aspiration pneumonia | Early: esophagitis Late: stricture, interstitial lung disease |
Characterized by intermittent chest pain, dysphagia, and forceful contractions. Diagnosis is diffuse esophageal spasm with manometry.
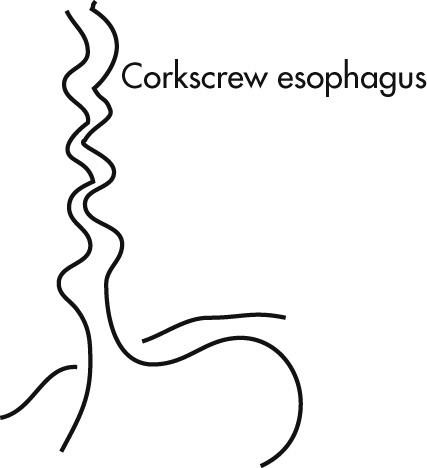
Primary neurogenic abnormality (vagus)
Secondary reflux esophagitis
Nutcracker corkscrew esophagus
Nonspecific esophageal dysmotility disorders
Caused by Trypanosoma cruzi, which multiply in reticuloendothelial system (RES), muscle, and glia cells. When these cells rupture and organisms are destroyed, a neurotoxin is released that destroys ganglion cells in the myenteric plexus. Mortality, 5% (myocarditis, encephalitis).
Esophagus
Early findings: hypercontractility, distal muscular spasm; normal caliber
Classic late findings (denervation): megaesophagus, aperistalsis, bird's beak appearance at GEJ (achalasia lookalike)
Esophageal complications:
Ulcers, hemorrhage
Perforation into mediastinum, abscess formation; carcinoma, 7%
Colon
Megacolon (anal sphincter neuropathy)
Sigmoid volvulus, 10%
Heart
Cardiomyopathy (cardiomegaly)
Clear lungs, no pericardial effusions
Central nervous system (CNS)
Encephalitis
Leiomyoma (may calcify) 50%
Fibrovascular polyp (may be large and mobile attached to upper esophagus and may contain fat on computed tomography [CT]), 25%
Cysts, 10%
Papilloma, 3%
Fibroma, 3%
Hemangioma, 2%
Squamous cell carcinoma (SCC; most common worldwide)
Adenocarcinoma, usually in distal esophagus at GEJ (in the United States, the incidence is now higher than SCC)
Lymphoma
Leiomyosarcoma
Metastasis
SCCs are associated with:
Head and neck cancers
Smoking
Alcohol
Achalasia
Lye ingestion
Adenocarcinoma is associated with:
Barrett esophagus
Gastroesophageal reflux disease (GERD)
Obesity (probably because of the link with GERD)
Staging (CT)
Invasion into mediastinum, aorta
Local LN enlargement
Metastases: liver, lung, lymphadenopathy, gastrohepatic ligament
Staging (endoscopic US)
Extension through wall
LN metastases
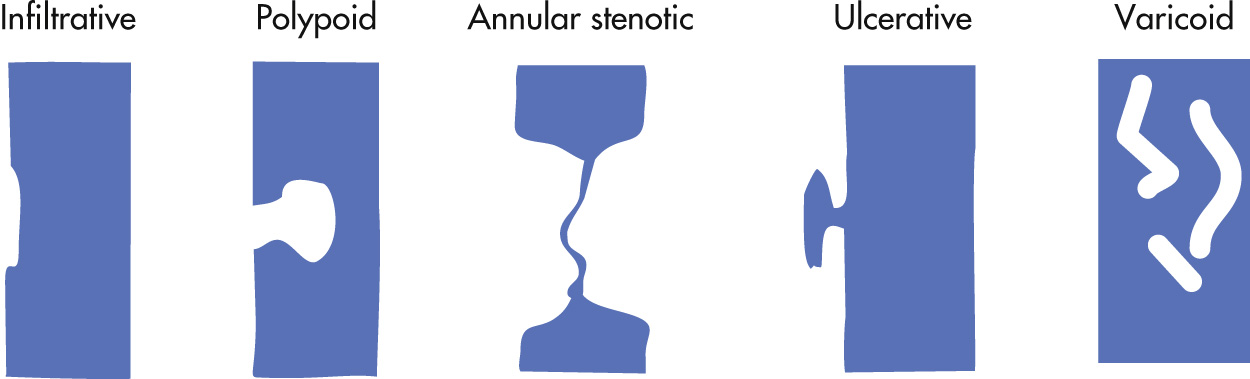
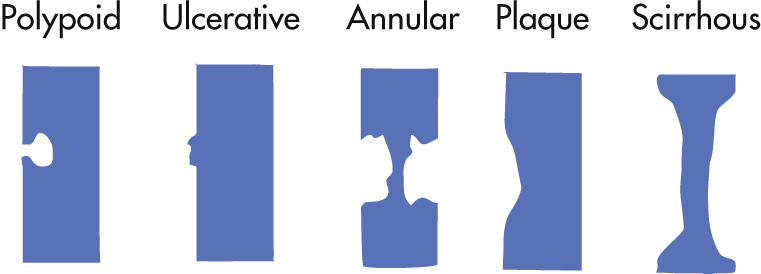
Spectrum of appearance
Infiltrative, shelf-like margins
Annular, constricting
Polypoid
Ulcerative
Varicoid: does not change in configuration during fluoroscopy in contrast to esophageal varices
Unusual bulky forms: carcinosarcoma, fibrovascular polyp, leiomyosarcoma, metastases
Because the esophagus and stomach do not normally have lymphocytes, primary lymphoma is rare unless present from inflammation. Secondary metastatic lymphoma is more common. Secondary esophageal lymphoma accounts for <2% of all gastrointestinal (GI) tract lymphomas (stomach > SB). Four radiographic presentations are infiltrative, ulcerating, polypoid, and endoexophytic.
Foreign body usually lodges in coronal orientation.
It is important to exclude underlying Schatzki ring or esophageal carcinoma once the foreign body is removed.
| Single Contrast | Double Contrast | |
|---|---|---|
| Contrast agent | Thin barium (40% w/w) | Thick barium (85% w/w) |
| Effervescent granules | ||
| Differences | Opaque distention Compression is necessary to allow penetration of beam |
Translucent distention (“see-through”) Compression less important |
| Fluoroscopy emphasized | Filming emphasized | |
| Indication | Acute setting, uncooperative patient, obstruction | All elective barium studies |
Nothing by mouth for 8 hours before examination
If a BE has been performed within the past 48 hours, give 4 tablespoons of Milk of Magnesia 12 hours before examination (cathartic).
Patient is in upright position and drinks thin barium. Spot GEJ.
Prone position to observe esophageal motility. Spot GEJ, antrum, and bulb.
Turn supine under fluoroscopic control.
Turn left posterior oblique (LPO) for air contrast of antrum, bulb. Evaluate duodenum and proximal SB.
Overhead radiographs: LPO, right anterior oblique (RAO) of stomach, PA of abdomen

Patient upright in slight LPO position. Administer effervescent granules with 20 mL of water. Start patient drinking thick barium (120 mL) and obtain air-contrast spot radiographs of esophagus.
Table down with patient in prone position (compression view may be obtained here). Patient rolls to supine position through the left side. Check mucosal coating: if not adequate, turn patient to prone again and back to supine position, roll to keep left side dependent (so that emptying of barium into the duodenum is delayed).
Obtain views of the stomach. This is generally the most important part of the study. The patient is turned to get air into different regions of the stomach.
Patient supine (for body of stomach)
Patient LPO (for antrum)
Patient RPO (for Schatzki view for lesser curvature)
Patient RAO (for fundus)
Views of bulb in contrast and gas relief. First leave the patient in RAO view and then turn to LPO view. Include some C-loop.
Study of esophagus. Patient RAO drinking regular “thin” barium. Observe entire esophagus and evaluate motility. Take spot radiographs (routinely of distended GEJ).
Overhead radiographs (optional):
RAO, drinking esophagus
AP of abdomen
LPO and RAO stomach
Water-siphon test to exclude GE reflux, unless small-bowel follow-through (SBFT) is simultaneously scheduled then done at end after swallowing additional barium
Success rate 95%, minor complications 1%–2%, and major complications 2%–4%.
Decompression in terminally ill patients with gastric outlet or small bowel obstruction (SBO): gastrostomy is sufficient.
Feeding: gastrojejunostomy preferred
Half cup of barium the night before to opacify colon. NG tube placement.
Mark liver edge under US.
Distend stomach by insufflating air through NG tube; mark entry high in midbody pointing toward pylorus, avoid marked colon and opacified bowel.
Anesthetic through four 25-gauge needles 1–2 cm away from insertion point. Leave needles in.
Gastropexy with T-tacks. Deploy them with 0.35-in ring wire under fluoroscopic guidance. Crimp T-tacks in place.
Place needle with guidewire into stomach through central insertion point.
Dilators 8, 10, 12, 14 Fr; place 15-Fr peel-away sheath.
Place 14-Fr gastrostomy catheter (e.g., Cook). Gastric decompression can be achieved with smaller-bore catheters (>10 Fr). Secure catheter in place.
Organs overlying stomach: liver, colon, ribs (high position of stomach)
Massive ascites (perform therapeutic paracentesis before gastrostomy)
Abnormal gastric wall (ulcer, tumor): hemorrhage is common.
Elevated bleeding time
Fundus
Body
Antrum
Pylorus
Curvatures: lesser, greater
Gastric rugae (prominent in body, proximal antrum): in double-contrast studies, rugae are more often effaced by gaseous distention.
Area gastricae (normal gastric mucosal pattern) is most prominent in antrum and body; ectopic duodenal area gastricae is present in 20%.
There are three morphologic types of lesions:
Ulcer: abnormal accumulation of contrast media
Polypoid lesion (masses): filling defect
Coexistent pattern: ulcerated mass
The above lesions have different appearances depending on whether they are imaged with single- or double-contrast techniques, whether they exist on dependent or nondependent walls, and whether they are imaged in profile or en face.
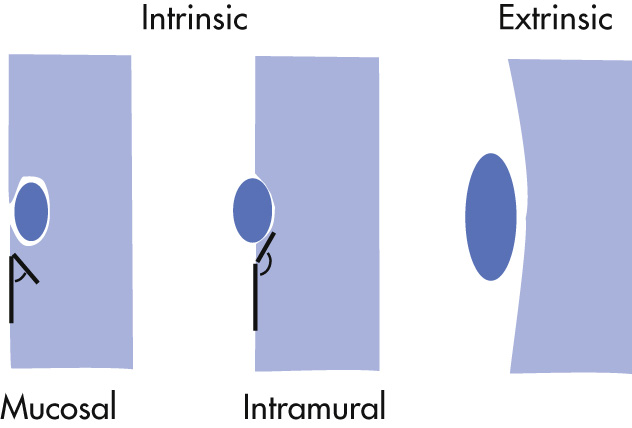
The location of a lesion can be evaluated by observing the angle the lesion forms with the wall:
Acute angle (looks like an a ): mucosal (polyp, cancer)
Obtuse angle (looks like an o ): extramucosal (intramural or extramural)
Preservation of mucosal pattern is also a hint to location of lesions:
Disruption of normal pattern: mucosal
Presence of normal pattern: intramural or extramural location
Distinction of outline:
Smooth, distinct: extramucosal
Irregular, fuzzy: mucosal
Helicobacter pylori (gram negative) plays a major role in the development of peptic ulcer.
Not all individuals with H. pylori will develop ulcers. Prevalence of H. pylori: 10% of population <30 years, 60% of population >60 years.
Prevalence of H. pylori in duodenal and gastric ulcers: 80%–90%; risk factor for adenocarcinoma and lymphoma
Approach:
Precaution against infection should be taken by all GI personnel.
H. pylori serology may become useful for diagnosis of PUD.
PUD heals faster with antibiotics and antacids than with antacids alone.
Incidence markedly decreased
Detection rate of ulcers by double-contrast barium is 60%–80%.
Ulcer crater seen en face: distinct collection of barium that persists on different views; the collection is most often round but can be linear.
Ulcer crater seen in profile: barium collection extends outside the projected margin of the gastric or duodenal wall.
Double-contrast studies: the crater has a white center with surrounding black “collar.”
Greater curvature ulcers are commonly because of malignancy or nonsteroidal antiinflammatory drug (NSAID) ingestion. (Aspirin-induced ulcers are also called sump ulcers because of their typical location on greater curvature.)
Multiple ulcers are usually due to NSAID ingestion.
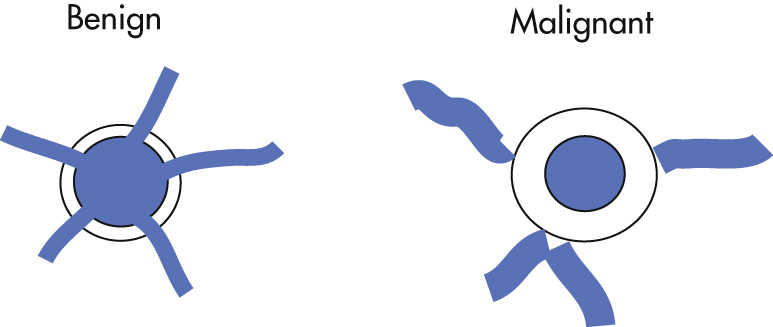
Signs of benign and malignant ulcers
| Parameter | Benign Ulcer | Malignant Ulcer |
|---|---|---|
| Mucosal folds | Thin, regular, extend up to crater edge | Thick, irregular, do not extend through collar |
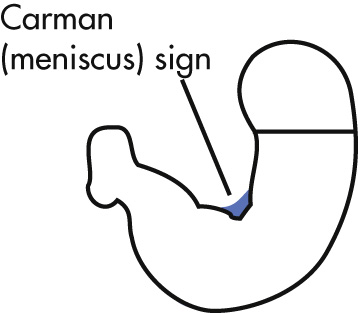
| Penetration | Margin of ulcer crater extends beyond projected luminal surface | Ulcers project within (projected) luminal surface; Carman (meniscus) sign a |
| Location | Centrally within mound of edema | Eccentrically in tumor mound |
| Collar | Hampton line: 1–2-mm lucent line around the ulcer b | Thick, nodular, irregular |
| Other | Normal peristalsis | Limited peristalsis |
| Incisura: invagination of opposite wall | Limited distensibility | |
| Gastrohepatic lymph nodes | Occasional | Common |
a Results from the fluoroscopically induced apposition of rolled halves of the tumor margin forming the periphery of the ulcerated carcinoma; meniscus refers to meniscoid shape of ulcer.
b This line is caused by thin mucosa overhanging the crater mouth seen in tangent; it is a reliable sign of a benign ulcer, but present in very few patients.
Causes
NSAIDs
H. pylori
Alcohol
Multiple tiny, aphthoid-like erosions throughout antrum, body
Occurs on rugal folds
Prominent area gastricae
Identify and treat causal agent
H2 blockers
Carcinoma, 90%
Lymphoma, 5%
Rare malignancies (sarcoma, carcinoid, metastases)
Obstruction
Posterior penetration of ulcer into pancreas
Perforation
Bleeding: filling defect in the ulcer crater may represent blood clots.
Gastroduodenal fistulas: double-channel pylorus
Categorize all gastric ulcers as definitely/probably benign or malignant.
All patients, except for those with de novo definitely benign gastric ulcers, should proceed to endoscopy with biopsy.
Benign ulcers decrease 50% in size within 3 weeks and show complete healing within 6 weeks with successful medical treatment.
Benign ulcers may heal with local scarring.
Ectopic pancreatic rest may contain a central umbilication that represents a rudimentary duct, not ulcer. Commonly located in antrum.
Gastric diverticulum: commonly in posterior fundus; contains mucosal folds, neck, changes shape during fluoroscopy
Large gastric rugal folds (hypertrophic gastritis) with protein-losing enteropathy. Clinical triad: achlorhydria, hypoproteinemia, edema. Typically occurs in middle-aged men. Complication: gastric carcinoma, 10%.
Giant gastric rugal folds, usually proximal half of stomach
Hypersecretion: poor coating, dilution of barium
Gastric wall thickening
Small intestinal fold thickening because of hypoproteinemia
Peptic ulcers are uncommon
Inflammatory disease of unknown cause characterized by focal or diffuse eosinophilic infiltration of the GI tract. An allergic or immunologic disorder is suspected because 50% of patients have another allergic disease (asthma, allergic rhinitis, hay fever). Only 300 cases have been reported to date. Treatment is with steroids.
Abdominal pain, 90%
Diarrhea, 40%
Eosinophilia
Stomach, 50%
Tapered antral stenosis (common)
Pyloric stenosis (common)
Gastric fold thickening
SB, 50%
Fold thickening (common)
Dilatation
Luminal narrowing
Aphthous ulcers, usually in antrum and duodenum
Stricture: Pseudo-Billroth I appearance on barium studies
Fistulization
Syndrome caused by excessive gastrin production.
Diarrhea
Recurrent PUD
Pain
Gastrinoma, 90%
Islet cell tumor in pancreas or duodenal wall, 90%
50% of tumors are malignant
10% of tumors are associated with multiple endocrine neoplasia (MEN) type I
Antral G-cell hyperplasia, 10%
Ulcers
Location: duodenal bulb > stomach > postbulbar duodenum
Multiple ulcers, 10%
Thickened gastric and duodenal folds
Increased gastric secretions
Reflux esophagitis
Gastric polyps are far less common than colonic polyps (2% of all patients with polyps).
Hyperplastic polyps (80% of all gastric polyps; <1 cm, sessile; not premalignant)
Associated with chronic atrophic gastritis
Familial adenomatous polyposis (hyperplastic polyps in stomach, adenomatous polyps in colon)
Typically, similar size, multiple, and clustered in the fundus and body
Synchronous gastric carcinoma in 5%–25% of patients
Adenomatous polyps; infrequent, malignant degeneration very rare
Solitary
Malignant transformation in 50%
Villous polyps (uncommon; cauliflower-like, sessile); strong malignant potential
Hamartomatous polyps rare; Peutz-Jeghers; Cronkhite-Canada syndrome, juvenile polyposis
Fourth most common GI malignancy (colon > pancreas > liver/biliary > stomach)
Pernicious anemia
Adenomatous polyps
Chronic atrophic gastritis
Billroth II > Billroth I
Fundus/cardia, 40%
Antrum, 30%
Body, 30%
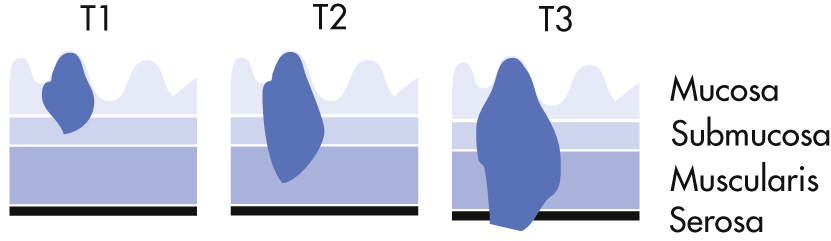
T1: Limited to mucosa, submucosa (5-year survival, 85%)
T2: Muscle, serosa involved (5-year survival, 50%)
T3: Penetration through serosa
T4: Adjacent organs invaded
Features of early gastric cancers:
Polypoid lesions (type 1)
>0.5 cm (normal peristalsis does not pass through lesion)
Difficult to detect radiographically
Superficial lesions (type 2)
2A: <0.5 cm
2B: most difficult to diagnose (mucosal irregularity only)
2C: 75% of all gastric carcinoma (folds tend to stop abruptly at lesion)
Excavated lesion (type 3) = malignant ulcer
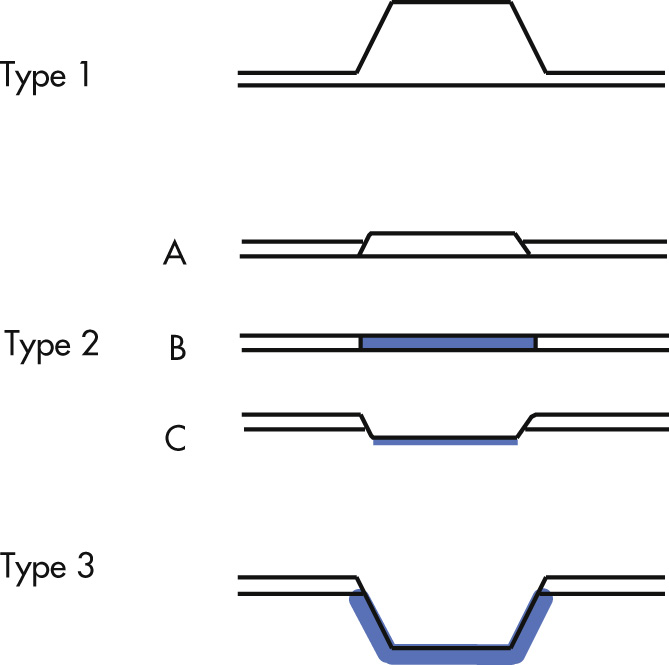
Features of advanced gastric cancer:
Malignant ulcer: folds short of collar
Ulcerated luminal mass
Rigidity, diffuse narrowing: linitis plastica
Thickened wall >1 cm by CT
Lymphadenopathy
Gastrohepatic ligament
Gastrocolic ligament
Perigastric nodes
Hepatic metastases
Three percent of all gastric malignancies. Non-Hodgkin lymphoma (NHL) (common) > Hodgkin lymphoma (uncommon)
Primary gastric lymphoma (arises from lymphatic tissue in lamina propria mucosae), 10%. Usually originates from mucosa associated lymphoid tissue (MALT lymphoma)
Secondary (gastric involvement in generalized lymphoma), 90%
H. pylori infection
Inflammatory bowel disease (IBD)
Celiac disease
Diffuse infiltrating disease
Normal gastric wall 2–5 mm (distended stomach) ≥6 mm is abnormal except at GEJ
Thick folds
Ulcerating mass
More often than carcinoma, lymphomas spread across the pylorus into the duodenum.
Hodgkin lymphoma of the stomach mimics scirrhous carcinoma (strong desmoplastic reaction).
GIST is the most common mesenchymal neoplasm of the GI tract and is defined by its expression of KIT (CD117), a tyrosine kinase growth factor receptor. The expression of KIT is important, as it distinguishes GIST from other mesenchymal neoplasms such as leiomyomas, leiomyosarcomas, schwannomas, and neurofibromas. Pharmacologically targeting this receptor with a KIT tyrosine kinase inhibitor (STI-571, imatinib, Gleevec) has been shown to be of clinical use. In the stomach, small intestine, colon, and anorectum, GIST accounts for almost all mesenchymal tumors, because leiomyomas and leiomyosarcomas in these sites are very rare. GIST most frequently occurs in the stomach (70% of cases), followed by the small intestine (20%–30%), anorectum (7%), colon, and esophagus. Patients with neurofibromatosis type 1 (NF1) have an increased prevalence of GIST, often multiple small GISTs.
Exophytic masses of stomach or SB that may ulcerate; obstruction rare despite size
Heterogeneous contrast enhancement
Crescent-shaped necrosis (Torricelli-Bernoulli sign) in large GIST
30% show aneurysmal dilation of enteric bowel.
Liver is the most common site of metastases, followed by mesentery.
Mesenteric metastases are smooth and multiple.
Lymphadenopathy not common; if lymphadenopathy is present, consider alternate diagnosis of lymphoma.
Good response to Gleevec (competitively binds to ATP binding site of tyrosine kinase, leading to cell death by apoptosis; areas of apoptosis appear as cystic spaces on CT or magnetic resonance imaging [MRI])
positron emission tomography (PET) is a sensitive modality for follow-up of patients on therapy.
From colon (gastrocolic, gastrosplenic ligament)
From liver (gastrohepatic ligament)
From pancreas: direct invasion
Melanoma (most common)
Breast
Lung
Diffuse uniform thickening, nondistensible, absent gastric folds, linitis plastica
Multiple lesions with bull's eye appearance: sharply demarcated with central ulcer (much bigger than aphthoid ulcer)
Gastric leiomyosarcoma
Functioning extraadrenal paraganglioma
Pulmonary chondroma
Benign tumors are usually submucosal.
Leiomyoma: most common benign tumor; may ulcerate, 10% malignant
Lipoma, fibroma, schwannoma, hemangioma, lymphangioma
Carcinoid (malignant transformation in 20%)
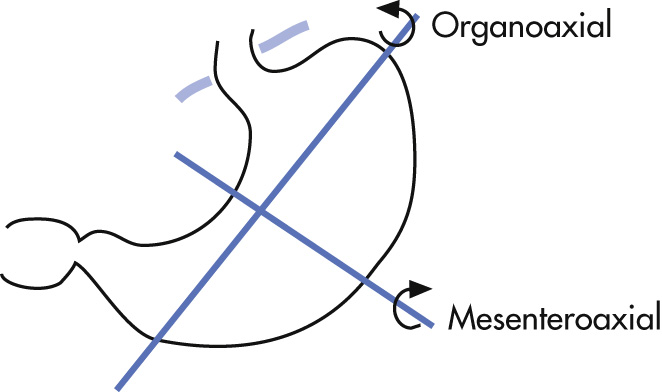
Abnormal rotation of stomach. Two types ( Fig. 3.21 ):
Organoaxial
Rotation around the long axis of stomach
Stomach rotates 180 degrees so that greater curvature is cranially located; upside-down stomach
Observed in adults with large hiatal hernia
Complications rare
Mesenteroaxial
Stomach rotates around its short axis (perpendicular to long axis)
Fundus is caudal to antrum
More common when large portions of stomach are above diaphragm (traumatic diaphragmatic rupture in children)
Obstruction, ischemia likely
Gastric varices represent dilated peripheral branches of short gastric and left gastric veins and appear as serpentine, nodular folds in body or fundus or as polypoid filling defects in the fundus.
Gastric varices are commonly associated with esophageal varices; the combination is often due to portal hypertension.
Gastric varices without esophageal varices are often caused by splenic vein obstruction and are most commonly secondary to pancreatitis or pancreatic carcinoma.
Gas in wall of stomach is usually due to:
Trauma from endoscopy, infection, ischemia, increased intraluminal pressure, vomiting, spontaneous or traumatic rupture of a pulmonary bulla into areolar tissue surrounding the esophagus
No discernible underlying disease
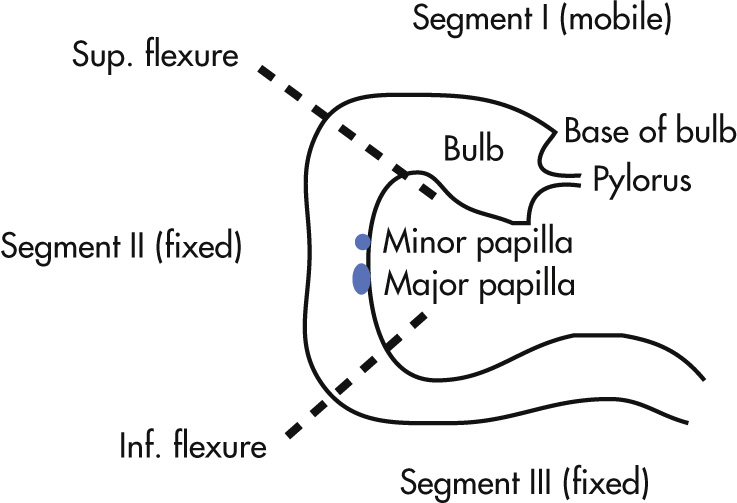
The duodenum has four segments ( Fig. 3.22 ):
Segment I
Begins at pylorus and extends to the superior duodenal flexure
Contains the duodenal bulb
Intraperitoneal position: freely mobile
Segment II
Begins at superior duodenal flexure and extends to inferior duodenal flexure
Contains the major and minor papilla and the promontory
Fixed retroperitoneal position
Segment III
Extends from inferior duodenal flexure transversely, crossing the midline
Fixed retroperitoneal position
Segment IV
Extends superiorly to the ligament of Treitz
Fixed retroperitoneal position
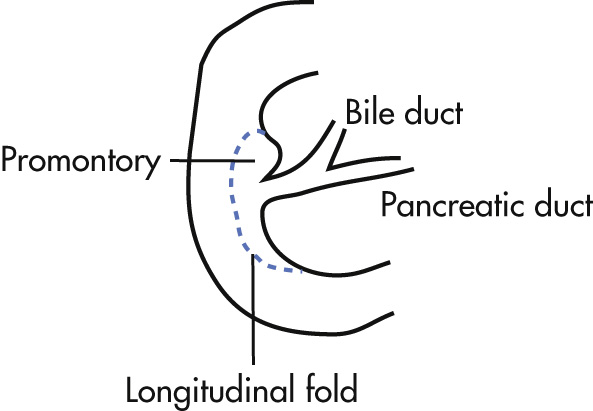
Folds in the duodenal bulb are longitudinally oriented.
In the descending portion of the duodenum, Kerkring folds are transversely oriented.
These folds are usually visible despite complete duodenal distention.
Major papilla (Vater papilla): orifice for ducts
Appears as round filling defect
Located below the promontory
8–10 mm in length
Abnormal if >15 mm
Minor papilla (accessory papilla, Santorini papilla)
Located superiorly and ventral to major papilla
Mean distance from major papilla 20 mm
Not usually visualized
Promontory:
Shoulder-like luminal projection along medial aspect of the second portion of the duodenum
Begins superior to major papilla
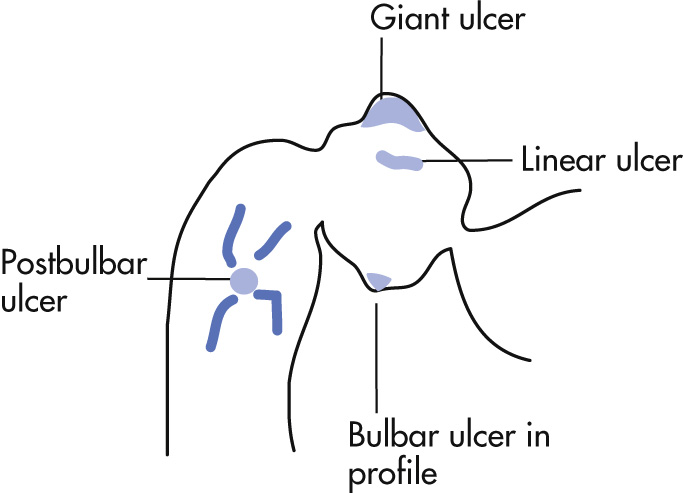
Duodenal ulcers are two to three times more common than gastric ulcers. All bulbar duodenal ulcers are considered benign. Postbulbar or multiple ulcers raise the suspicion for Zollinger-Ellison syndrome.
Bulbar, 95%
Anterior wall: most common site, perforate
Posterior wall: penetration into pancreas
Postbulbar, 5%
Chronic obstructive pulmonary disease (COPD)
Severe stress: injury, surgery, burn
Steroids
Persistent round or elliptical collection; radiating folds, spasm
Linear ulcers, 25%
Kissing ulcers: 2 or more ulcers located opposite each other
Giant ulcers
Crater is >2 cm.
Ulcer largely replaces the duodenal bulb.
A large ulcer crater may be mistaken for a deformed bulb but does not change shape during fluoroscopy.
Duodenal ulcers often heal with a scar; this can lead to deformity and contraction of the duodenal bulb: cloverleaf deformity, or hourglass deformity.
Postbulbar ulcers: any ulcer distal to the first portion of the duodenum should be considered to have underlying malignancy until proved otherwise (only 5% are benign ulcers, mostly secondary to Zollinger-Ellison syndrome).
Duodenal injuries are due either to penetrating (stab, gunshot) wounds or to blunt trauma (motor vehicle accident). Because the duodenum is immobile in retroperitoneum, most perforations occur there. Mortality of untreated duodenal rupture is 65%.
Duodenum/proximal jejunum, 95%
Colon, 5%
Perforation (requires surgery)
Transection (requires surgery)
Hematoma (nonsurgical treatment)
| Blunt Trauma (%) | Penetrating Trauma (%) | |
|---|---|---|
| Liver | 30 | 55 |
| Pancreas | 45 | 35 |
| Spleen | 25 | 2 |
| Colon | 15 | 10 |
| Small bowel | 10 | 25 |
| Kidney | 10 | 20 |
Perforation:
Extraluminal retroperitoneal gas
Extravasation of oral contrast material
Perforation or hematoma:
Thickening of duodenal wall or high-density mass (clotted blood) can narrow the lumen
Fluid in right anterior pararenal space or in the peritoneum
Duodenal diverticula commonly project into head or uncinate process of pancreas and rarely present coming from lateral wall.
Simple repair
Pyloric exclusion for complex injuries
Whipple procedure is rarely necessary.
Surgical complications:
Intraabdominal abscess, 15%
Duodenal fistula, 4%
Duodenal dehiscence, 4%
Pancreatic fistula, 1%
More common than malignant duodenal tumors.
Lipoma, leiomyoma (most common)
Villous adenoma (cauliflower-like), adenomatous polyp
Lymphoid hyperplasia
Heterotopic gastric mucosa: small angular filling defects in bulb, larger than nodules of lymphoid hyperplasia and smaller than Brunner gland hyperplasia
Brunner gland hyperplasia
Ectopic pancreas
Anatomic variant characterized by movement of gastric mucosa bulging into the base of the duodenal bulb. No pathophysiologic significance.
Lobulated stellate filling defect in the duodenal bulb
Filling defect in contiguity with antral rugal folds
Infrequent. The most common locations of malignant tumors are in the periampullary and infraampullary areas.
Adenocarcinoma (most common)
Leiomyosarcoma
Lymphoma
Metastases
Benign tumors with malignant potential: villous and adenomatous polyps, carcinoid
Immediate complications:
Anastomotic leak
Abscess
Gastric outlet obstruction (edema)
Bile reflux gastritis
Ileus
Late complications:
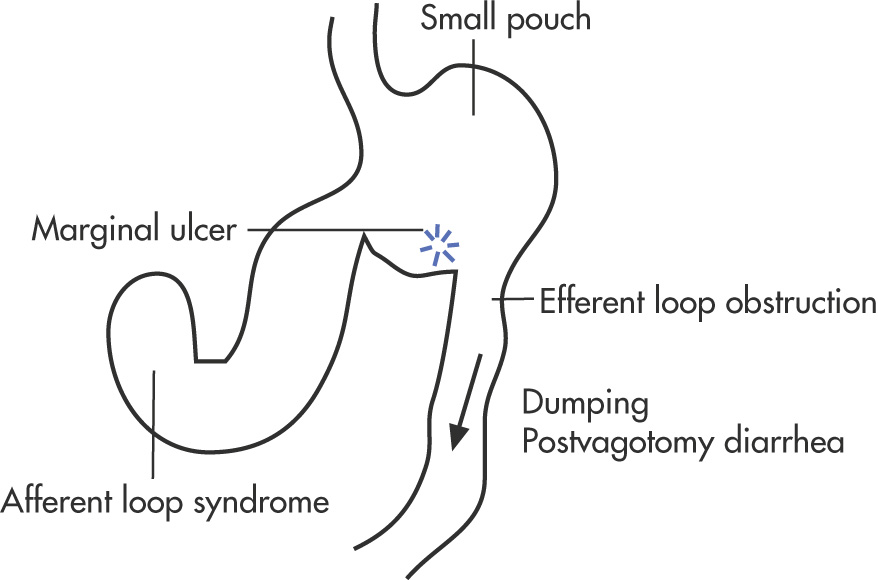
Bowel dysmotility: dumping, postvagotomy hypotonia
Ulcer
Bowel obstruction: outlet obstruction, adhesions, stricture
Prolapse, intussusception
Gastric carcinoma (in 5% of patients 15 years after surgery), Billroth II > Billroth I
Metabolic effects: malabsorption
Afferent loop syndrome
Small pouch syndrome
| Type | Anastomosis/Surgery | Common Indication |
|---|---|---|
| Antireflux | Fundoplication (Nissen, Toupet, Belsey Mark IV)
|
Prevention of gastroesophageal reflux |
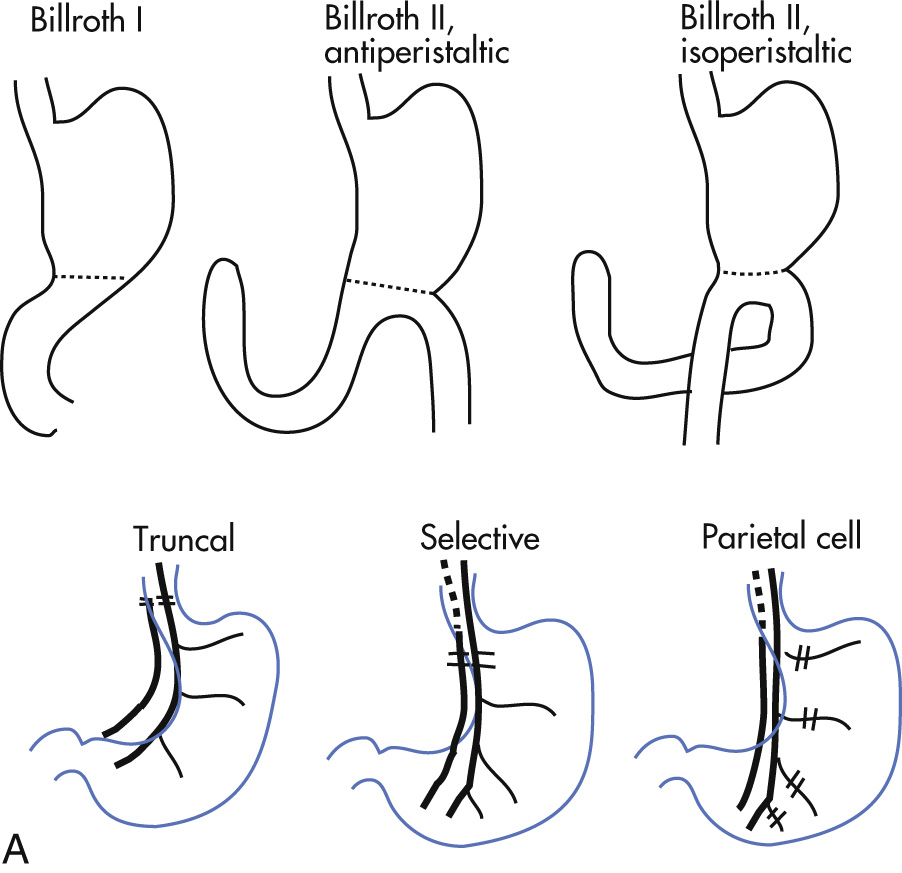
| Gastrectomy ( Fig. 3.26A ) | Gastroduodenostomy (Billroth I) | Gastroduodenal ulcer |
| Gastrojejunostomy (Billroth II) | Gastroduodenal ulcer | |
| Total gastrectomy | Gastric cancer | |
| Vagotomy ( Fig. 3.26B ) | Truncal vagotomy Selective vagotomy |
|
| Parietal cell vagotomy | ||
| Drainage procedures | Facilitate gastric emptying after vagotomy | |
| Gastroenterostomy | ||
| Palliative curative | Pancreaticoduodenectomy (Whipple)
|
Pancreatic cancer |
Numerous bariatric surgical procedures exist, the most common is now the sleeve gastrectomy. This procedure recently supplanted the Roux-en-Y gastric bypass as the most popular bariatric surgery. Roux-en-Y gastric bypass is still the preferred method to treat obesity in patients with diabetes.
In gastric bypass surgery, a small gastric pouch (<30 mL) and a small gastrojejunostomy (<12 mm) are constructed (Roux-en-Y gastric bypass procedure). The remaining stomach is intact but functionally separate from food pathway. Currently, the Roux-en-Y gastric bypass combines restrictive and malabsorptive properties by creating a small gastric pouch and a Roux limb.
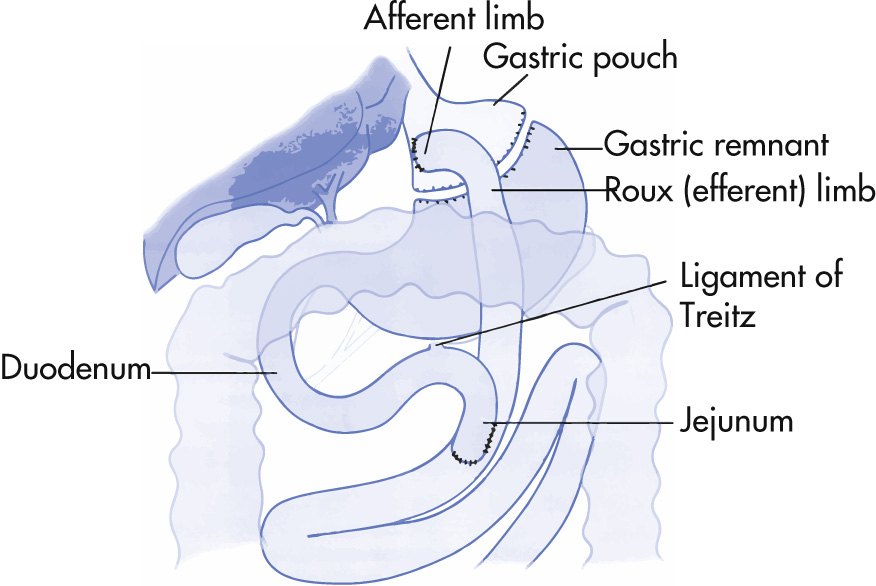
Narrowing/stenosis
Immediate postsurgical narrowing is common and often subsides
Stenosis (lumen <6 mm) at >6 weeks after surgery is rare. Weight loss can be dramatic.
Anastomotic leak
Incidence of leaks after Roux-en-Y bypass surgery is 1%–6%. More common after laparoscopic than open surgery.
The leak is commonly at the gastrojejunal anastomosis or at the enteroenteric anastomosis (both are life threatening).
Contrast material outside the confines of the gastric pouch and anastomosis indicates a leak. Although leaks from the enteroenteric anastomosis are rapidly clinically evident and severe, they are usually not diagnosed radiographically.
Immediate surgical exploration is needed.
Fistula
During surgery, the gastric pouch and remnant are surgically separated. Oral contrast should not directly enter the gastric remnant. The presence of oral contrast in the remnant thus indicates a gastro-gastric fistula.
Contrast may be seen in excluded stomach on SBFT by retrograde filling through the duodenum.
Internal hernia: The most common herniation is the transmesenteric (or transmesocolonic) type, which occurs through the defect in the transverse mesocolon. The herniated bowel is usually the Roux limb itself with a varying amount of additional SB loops.
CT findings:
Abnormal position of distended SB loops anterior to the pancreas and above the transverse colon; obstruction
Inferior displacement of the transverse colon
Crowding, engorgement, and deviation of mesenteric vessels
The transition point is proximal to the jejunojejunostomy.
Hernias may also occur in the SB mesenteric defect at the jejunojejunostomy site and in the space posterior to the Roux limb (Peterson type).
Weight gain
Degradation of pouch restriction: very rapid passage of contrast through a patulous anastomosis degrades the restrictive properties of the laparoscopic Roux-en-Y gastric bypass, resulting in weight gain.
Gastrogastric fistula: although uncommon, a fistulous tract arising from the pouch may opacify the excluded stomach and is thought to be a result of patient overeating.
An adjustable band is laparoscopically placed around the proximal stomach, creating a small upper pouch 2 cm distal to the GEJ. The cuff is connected to a reservoir placed in the anterior rectus sheath or subcutaneous tissues. The reservoir allows adjustment of band diameter percutaneously. The angle of the cuff with the spinal column (Phi angle) should be 4–58 degrees on plain radiographs.
Band placed too low in stomach
Band not placed around stomach
Band placed around esophagus, which is undesirable because sensation of satiety lacking and risk of perforation
Slippage of the band with upward herniation of stomach (late complication).
Left side (greater curvature and fundus) of stomach is surgically removed laparoscopically after staples are placed from the angle of His (angle formed as lateral border of esophagus meets the medial border of fundus) and the pylorus.
The SB can be examined by conventional SBFT, enteroclysis, CT, CT enteroclysis (the former two procedures are most common), MR, or MR enteroclysis. The specific indications for enteroclysis are:
Occult bleeding
Recurrent obstructive symptoms
Malabsorption
To determine extent of Crohn disease
Luminal diameter
>3 cm is abnormal
Fold thickness
Valvulae conniventes measure 1–2 mm; more prominent in jejunum than ileum
>3 mm is abnormal
Wall thickness
Normal is 1–1.5 mm
Secretions
There should normally be no appreciable fluid in SB.
Excess secretions cause dilution of barium column.
Normal wall thickness: 1–1.5 mm
Incomplete distention or luminal fluid may mimic abnormally thick wall; look for antidependent luminal gas collections to better assess wall thickness.
Jejunal and ileal outpouchings may predispose to bacterial overgrowth, vitamin B 12 deficiency, and megoblastic anemia.
Syndrome develops after bypassing SB by an enteroanastomosis with subsequent stagnation of bowel contents. Malabsorption in large diverticula may cause similar dynamics.
Abnormal absorption of fat, water, protein, and carbohydrates from SB.
Dilatation of bowel loops
Diluted barium (mixes with watery bowel content)
Flocculated barium: barium aggregates into particles (mainly seen with older barium suspensions)
Slow transit
Segmentation of barium (lack of continuous column) rarely occurs with new agents
Moulage pattern: featureless barium collection (rarely occurs with new agents)
Hidebound pattern: valvulae thinner, closer together, wrinkled look
Many of these features may no longer be seen with newer barium products.
Three entities:
Tropical sprue (unknown cause; responds to antibiotics)
Nontropical (adults; intolerance to gluten in wheat and other grains; HLA-DR3, IgA, IgM antibodies)
Celiac disease (children)
Dilatation of SB is the most typical finding (caliber increases with severity of disease).
Nodular changes in duodenum (bubbly duodenum)
Reversal of jejunal and ileal fold patterns: “The jejunum looks like the ileum, the ileum looks like the jejunum, and the duodenum looks like hell.”
Segmentation
Hypersecretion and mucosal atrophy cause the moulage sign (rare).
Transient intussusception pattern (coiled spring) is typical.
Increased secretions: flocculation with older barium suspensions
Increased incidence of malignancy, aggressive lymphoma, carcinoma
Dermatitis herpetiformis
Selective IgA deficiency
Hyposplenism
Adenopathy
Cavitary mesenteric LN syndrome
Ulcerative jejunoileitis: several segments of bowel wall thickening with irregularity and ulceration strictures may follow
Enteropathy: associated T-cell lymphoma
Increased incidence of cancers of esophagus, pharynx, duodenum, and rectum
Sprue, SBO, scleroderma (SOS): dilated, prolonged motility, normal folds
| Disease | Primary Pattern | Comment |
|---|---|---|
| Scleroderma | AM + D, hidebound folds | Muscularis replaced by fibrosis |
| Whipple | DFTN, adenopathy may occur | Intestinal lipodystrophy |
| Amyloidosis | DFTN | Tiny nodule filling defects |
| Lymphangiectasia | DFTN, MA | Dilated lymphatics in wall |
| Ig deficiencies | DFTN | Nodular lymphoid hyperplasia |
| Mastocytosis | DFTN | Hepatomegaly, PUD, dense bones |
| Eosinophilic gastroenteritis | Very thick folds (polypoid) | Food allergy, 70% |
| Graft-versus-host disease | Effaced folds (ribbon like) | Bone marrow transplants |
| MAI infection | DFTN, MA, pseudo-Whipple | Immunocompromised host |
a See also Infectious Enteritis .
Systemic mast cell proliferation in reticuloendothelial system (RES) (SB, liver, spleen, LNs, bone marrow) and skin (95%) with histamine release.
Diarrhea
Steatorrhea
Histamine effects (flushing, tachycardia, pruritus, PUD)
SB
Irregular fold thickening
Diffuse small nodules
Other
Sclerotic bone lesions
Hepatosplenomegaly
Peptic ulcers (increased hydrochloric acid [HCl] secretion)
Heterogeneous group of disorders characterized by abnormal extracellular deposition of insoluble fibrillar protein material. Diagnosis is established by biopsy of affected organs (birefringence, staining with Congo red). Clinical amyloidosis syndromes include:
Systemic amyloidosis
Immunocyte dyscrasia (myeloma, monoclonal gammopathy)
Chronic/active disease (see below)
Hereditary syndromes
Neuropathic form
Nephropathic form
Cardiomyopathic form
Chronic hemodialysis
Senile form
Localized amyloidosis
Cerebral amyloid angiopathy (Alzheimer disease, senile dementia)
Cutaneous form
Ocular form
Others
Common chronic/active diseases that are associated with systemic amyloidosis (there are many less common causes):
Infections (recurrent and chronic)
Tuberculosis
Chronic osteomyelitis
Decubitus ulcers
Bronchiectasis
Chronic pyelonephritis
Chronic inflammatory disease
Rheumatoid arthritis (5%–20% of cases)
Ankylosing spondylitis
Crohn disease
Reiter syndrome
Psoriasis
Neoplasm
Hodgkin disease (4% of cases)
Renal cell carcinoma (RCC; 3% of cases)
Kidneys
Nephrotic syndrome
Renal insufficiency
Renal tubular acidosis
Renal vein thrombosis
GI tract
Diffuse thickening of SB folds
Jejunization of ileum
SB dilatation
Multiple nodular filling defects, >2 mm
Hepatosplenomegaly
Macroglossia
Colonic pseudodiverticulosis (may be unilateral and large)
Heart
Cardiomyopathy (restrictive)
Rhythm abnormalities
Nervous system
Signs of dementia
Carpal tunnel syndrome
Peripheral neuropathy
Spectrum of lymphatic abnormality (dilated lymphatics in lamina propria of SB) that clinically results in protein-losing enteropathy.
Congenital (infantile) form presents with:
Generalized lymphedema
Chylous pleural effusions
Diarrhea, steatorrhea
Lymphocytopenia
Acquired (adult) form as a result of:
Obstruction of thoracic duct (radiation, tumors, retroperitoneal fibrosis)
SB lymphoma
Pancreatitis
Diffuse nodular thickening of folds in jejunum and ileum caused by dilated lymphatics and hypoalbuminemic edema; mesenteric adenopathy on CT
Dilution of contrast material as a result of hypersecretion
Lymphographic studies
Hypoplastic lymphatics of lower extremity
Tortuous thoracic duct
Hypoplastic LNs
Distinct subgroup of lymphoma that primarily arises in lymphoid tissue of the bowel rather than in LNs.
GI lymphoma in otherwise healthy patients:
Gastric lymphoma arising from mucosa-associated lymphoid tissue (MALT)
Usually low-grade malignancy
Represent 20% of malignant SB tumors; usual age: 5th to 6th decade
Imaging features
Mass, nodule, fold thickening (focal or diffuse) confined to GI tract in 50%
Adenopathy, 30%
Extraabdominal findings, 30%
Large ulcerated mass presenting as endoenteric or exoenteric tumor (differential diagnosis [DDX]: GI stromal tumor, metastatic melanoma, jejunal diverticulitis with abscess, ectopic pancreas)
Aneurysmal dilatation: localized dilated, thick-walled, noncontractile lumen because of mural tumor; Auerbach plexus neuropathy
GI lymphoma in HIV-positive or immunosuppressed patients:
Usually aggressive NHL with rapid spread, poor response to chemotherapy, short survival
Widespread extraintestinal involvement, 80%
Imaging features
GI abnormalities: nodules, fold thickening, mass
Splenomegaly, 30%
Adenopathy, 30%
Ascites, 20%
Donor lymphocytes react against organs (GI tract, skin, liver) of the recipient after bone marrow transplant. Pathology: granular necrosis of crypt epithelium.
Classic finding: SB loops, which are too narrow, and featureless margins (ribbon bowel)
Luminal narrowing is due to edema of the bowel wall.
Flattening of mucosal folds (edema)
Prolonged coating of barium for days
Scleroderma or progressive systemic sclerosis (PSS) is a systemic disease that involves primarily skin, joints, and the GI tract (esophagus > SB > colon > stomach). Age: 30–50 years; female > male.
SB
Dilation of bowel loops with hypomotility is a key feature.
Mucosal folds are tight and closer together (fibrosis): hidebound appearance.
Pseudosacculations along antimesenteric border, may involve both small and large bowel
Segmentation, fragmentation, hypersecretion are absent.
Other
Dilated dysmotile esophagus, esophagitis, incompetent LES, reflux, stricture
Dilated duodenum and colon (pseudoobstruction)
Pneumatosis cystoids coli (steroid therapy)
Pulmonary interstitial fibrosis
Acroosteolysis
Soft tissue calcification
Rare multisystem disease caused by Tropheryma whippelii . Structures primarily involved include SI joints, joint capsule, heart valves, CNS, and jejunum.
Middle-aged men, United States, Northern Europe
Diarrhea, steatorrhea
Immune defects
1–2-mm diffuse micronodules in jejunum
No dilatation or increased secretions
Nodal masses in mesentery (echogenic by US); nodes have low CT density
Sacroiliitis
Fistulas of SB with adjacent structures can be seen with Crohn disease, colorectal cancer, after surgery, and in diverticular disease.
Enteroenteric: SB → SB
Enterocolonic: SB → colon
Enterocutaneous: SB → skin
Enterovesical: SB → bladder
Enterovaginal: SB → vagina
Fistulogram (enterocutaneous fistula): injection of water-soluble contrast material through small catheter inserted into fistula
UGI and SBFT
Barium enema
Total parenteral nutrition to achieve “bowel rest”
Postoperative fistulas usually heal spontaneously with conservative measures.
Fistulas in active Crohn disease usually require excision of diseased bowel.
Cyclosporine and other immunosuppressants have been used to heal fistulas in Crohn disease; more recently monoclonal antibody (infliximab) has been used.
Chemotherapeutic agents can induce spontaneous GI edema, necrosis, and even perforation.
Most common in long-term immunosuppressive treatment to prevent homograft rejection or in those receiving long-term chemotherapy for leukemia or lymphoma.
CT findings can be seen in diseased or disease-free intestinal segments.
Chemotherapy-induced enteropathy appears as nonspecific focal or diffuse bowel wall thickening with or without the target sign or as regional mesenteric vascular engorgement and haziness, more often in distal SB.
Angiotensin-converting enzyme (ACE) inhibitors may cause angioedema resulting in reversible wall thickening.
Cryptosporidium species are protozoa that frequently cause enteritis in AIDS patients but rarely in immunocompetent patients. Diagnosis is made by examination of stool or duodenal aspirate.
| Infection | Common Radiographic Patterns |
|---|---|
| Parasites | |
| Hookworms (Necator, Ancylostoma) | TFN |
| Tapeworms | FD |
| Ascaris | FD, intestinal obstruction |
| Infectious ( Fig. 3.28 ) | |
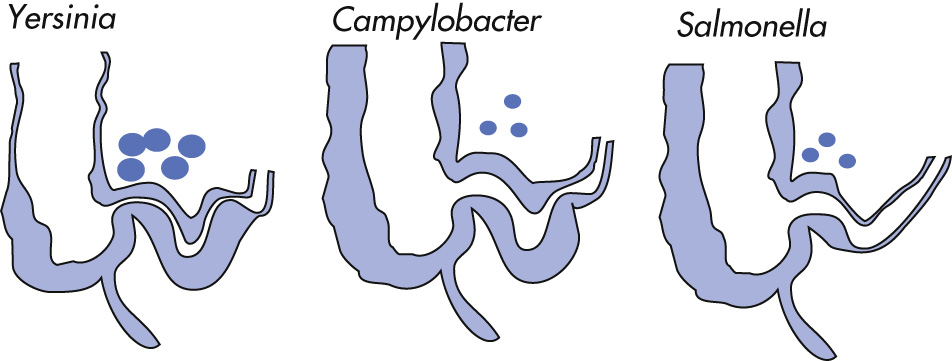
| Yersinia enterocolitica | TFN, ulcers, TI |
| TB | Stricture → obstruction, TI |
| Histoplasmosis | TFN |
| Salmonellosis | TFN, TI |
| Campylobacter | TFN, loss of haustration, TI |
| Common in AIDS | |
| Cytomegalovirus | TF in cecum, pancolitis |
| Tuberculosis | TF in cecum; adenopathy (low central attenuation), TI |
| MAI | TFN, adenopathy (homogeneous) |
| Cryptosporidiosis | TFN |
| Giardiasis | TFN, largely jejunal, jejunal spasm |
Thickened SB folds
Dilatation of SB
| Organism (Treatment) | Route | Clinical Findings |
|---|---|---|
| Nematode (Mebendazole) | ||
| Ascaris lumbricoides a | Fecal-oral | Intestinal, biliary obstruction, PIE |
| Ancylostoma duodenale | Skin penetration | Iron-deficiency anemia, PIE |
| Necator americanus | Skin penetration | Iron-deficiency anemia, PIE |
| Strongyloides stercoralis | Skin penetration | Malabsorption, PIE |
| Nematode (Mebendazole) | ||
| Trichuris trichiura | Fecal-oral | Rectal prolapse |
| Enterobius vermicularis | Fecal-oral | |
| Cestode (Praziquantel) | ||
| Beef tapeworm ( Taenia saginata ) | Raw beef | |
| Pork tapeworm ( Taenia solium ) | Raw pork | Cysticercosis: CNS |
| Fish tapeworm | Raw fish | Vitamin B 12 deficiency |
| Dwarf tapeworm | Fecal-oral | Diarrhea |
| Trematode (Praziquantel) | ||
| Heterophyes heterophyes | Raw fish | Diarrhea |
| Metagonimus yokogawai | Raw fish | Diarrhea |
Infection with Ascaris lumbricoides (roundworm, 15–35 cm long) is the most common parasitic infection worldwide.
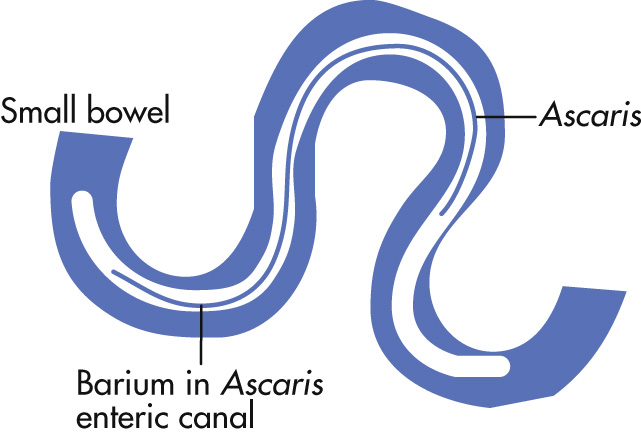
GI tract
Jejunum > ileum, duodenum, stomach
Worms visible on SBFT as longitudinal filling defects
Enteric canal of worm is filled with barium
Worms may cluster: “bolus of worm”
Mechanical SBO
Other complications: perforation, volvulus
Biliary tract
Intermittent biliary obstruction
Granulomatous stricture of bile duct (rare)
Oriental cholangiohepatitis
Carcinoid tumors arise from enterochromaffin cells.
Location:
GI tract, 85%
Location: appendix, 50% > SB (33%), gastric, colon and rectum (2%); virtually never occur in esophagus
90% of SB carcinoids arise in distal ileum
30% of SB carcinoids are multiple; 40%–80% of GI tract carcinoids spread to mesentery
Bronchial tree, 15%
90% central, 10% peripheral
Other rare locations
Thyroid
Teratomas (ovarian, testicular)
Symptoms of GI carcinoids:
Asymptomatic, 70%; obstruction, 20%; weight loss, 15%; palpable mass, 15%
Ninety percent of patients with carcinoid syndrome have liver metastases. The tumor produces ACTH, histamine, bradykinin, kallikrein, serotonin (excreted as 5-HIAA in urine), causing:
Recurrent diarrhea, 70%
Right-sided endocardial fibroelastosis → tricuspid insufficiency, pulmonary valvular stenosis (left side of heart is spared because of metabolism by monoamine oxidase inhibitor [MAOI] in lung)
Wheezing, bronchospasm, 15%
Flushing of face and neck
Mass lesion in SB: filling defect
Strong desmoplastic reaction causes angulation, kinking of bowel loops (tethered appearance), mesenteric venous congestion
Mesenteric mass on CT with spokewheel pattern is virtually pathognomonic; the only other disease that causes this appearance is retractile mesenteritis (very rare).
Stippled calcification in mesenteric mass
Obstruction secondary to desmoplastic reaction
Very vascular tumors (tumor blush at angiography, very hyperintense on T2-weighted [T2W] images)
Liver metastases (arterial phase indicated)
Ischemia with mesenteric venous compromise
Hemorrhage
Malignant degeneration: gastric and appendiceal tumors rarely metastasize; SB tumors metastasize commonly.
Damage of SB mucosa and wall as a result of therapeutic radiation
Highest to lowest tolerance: duodenum > jejunum, ileum > transverse colon, sigmoid colon > esophagus and rectum
Tolerance dose (TD 5/5) is the total dose that produces radiation damage in 5% of patients within 5 years; TD 5/5 is 4500 cGy in SB and colon and 5000 cGy in rectum.
Findings: mural thickening and luminal narrowing, usually in pelvic bowel loops, after treatment of gynecologic or urinary bladder cancers
Long-term sequelae: narrowing or stenosis of affected segment; adhesions with angulation between adjacent loops; reduced or absent peristalsis
Acute onset or recurrent acute abdominal pain
Segmental area of circumferential bowel wall thickening
Decreased submucosal attenuation
Mesenteric edema
Ascites
Engorgement of the vasa recta
Clear liquid diet day before examination
Magnesium citrate, 300 mL, afternoon before examination
50 mL castor oil evening before examination
Cleansing enema morning of examination
Insert tube with patient in lateral position.
Decide if need to inflate balloon for retention; if so, inflate under fluoroscopic control, being sure that balloon is in the rectum.
Fluoroscopy as the barium goes in.
Patient in supine position (as opposed to double-contrast study). Instill barium just beyond sigmoid colon. Take AP and two oblique spot radiographs of sigmoid.
Try to follow head of column fluoroscopically.
Take spot views of both splenic flexure and hepatic flexure.
Spot cecum and terminal ileum. If a filling defect is encountered, palpate to see if it is sessile or floating.
Obtain overhead views and postevacuation radiographs.
Patient is in lateral position. Insert tube.
Patient supine: administer glucagon IV. Turn patient prone: this helps barium flow to the descending colon, decreases pooling in the rectum and sigmoid, and thereby is less uncomfortable. Instill barium beyond the splenic flexure. Stand patient up, bag to the floor, and let barium drain.
Place patient in horizontal prone position. Start slow inflation with air and rotate patient toward you into a supine position. Take spot views of the sigmoid in different obliquities (take spots of any air-filled loop). When patient is supine, check to see if barium has already coated the ascending colon.
Stand patient up to facilitate coating of ascending colon. Drain as much barium as possible through rectal tube. Insufflate more air.
Spot views of splenic and hepatic flexure in upright position. Take spots in slightly different obliquities of both flexures.
Patient prone; put table down. Take spot views of the cecum and sigmoid.
Obtain overhead views:
AP, PA
Prone, cross-table lateral
Decubitus
Postevacuation radiographs
Suspected colonic perforation (use water-soluble iodinated contrast)
Patients at risk for intraperitoneal leakage (use Gastrografin):
Severe colitis
Toxic megacolon (TMC)
Recent deep biopsy
If colonoscopy needs to follow enema; use water-soluble iodinated contrast
Severe recent disease: myocardial infarction, cerebrovascular accident (CVA)
Perforation (incidence 1 : 5000), typically caused by overinflation or traumatic insertion of balloon or fragile colonic walls
Appearance of gas in portal venous system in patients with IBD (no significant ill effects)
Allergy to latex tips
Glucagon is a 29–amino acid peptide produced in A cells of the pancreas.
Physiologically, the main stimulus for glucagon release is hunger (hypoglycemia).
Effects:
Antagonist to insulin (increases blood glucose)
Relaxation of smooth muscle cells
Relaxation of gallbladder (GB) sphincter and sphincter of Oddi; increased bile flow
Glucagon is a useful adjunct (0.1–1 mg IV) to barium enema (BE) or whenever smooth muscle spasm is suspected of producing a “pseudostenotic lesion.” Thus it can be used in evaluation of the esophagus, stomach, duodenum, small intestine, common bile duct (CBD), and colon.
Contraindications include:
Pheochromocytoma
Insulinoma
Glaucoma
Replacing DCBE for detecting and screening of colonic neoplasms.
Helically acquired axial images of the gas-distended colon are obtained during breath holding in both prone and supine positions.
Images are combined into a detailed model of the colon subsequently viewed using either 2D multiplanar reconstructions or primary 3D endoluminal display.
Standard examination does not require IV contrast and uses extremely low dose x-ray technique, typically 20% radiation of standard diagnostic CT, and approximately 10% less than double-contrast barium enema.
Detection of large polyps (>10 mm) is comparable with optical colonoscopy (OC); detection of polyps 6–9 mm approximately equal to OC; detection of polyps <6 mm, OC is superior.
Studies using latest techniques (primary 3D visualization, fecal tagging) demonstrate sensitivity of 92% for polyps >10 mm; per patient specificity 96%
Currently requires cathartic bowel preparation similar to that for fiberoptic colonoscopy
Performed to evaluate the small and large bowel in patients with IBD
MRE has the advantage of depicting extraluminal abnormalities, the ability to distinguish active from fibrotic strictures, and better delineates fistulas. There is no ionizing radiation.
Despite the advantages, there are some limitations, foremost is the relatively long acquisition times. It may be difficult to identify early mucosal lesions.
Optimal amount of oral contrast for MRE is approximately 1300 mL
Types of oral contrast for MRE:
Positive: increased signal of bowel lumen on T1 and T2; solutions containing carbohydrate sugar alcohols (VoLumen) or gadolinium (Gd)-based agents
Negative: decreased signal of bowel lumen on T1 and T2; iron oxide containing oral contrast (Gastromark)
Typical patient preparation for MRE
Fasting for 6 hours before procedure
Sequences for MRE:
Half-Fourier-acquired single-shot turbo spin echo (HASTE) or SSFSE; insensitive to motion. Provides high contrast between lumen and bowel wall
High-resolution ultrafast balanced GRE (fast imaging employing steady-state acquisition [FIESTA], True FISP). Insensitive to motion and provides uniform luminal opacification. Ideal for detection, mesenteric findings in patients with Crohn disease
T2W (fat saturated) or STIR. Ideal for detection of fistulas and for correlating inflammatory changes with Gd enhanced images.
T1-weighted (T1W) 3D gradient echo sequence before and after gadolinium (Gd) administration. Primarily used to assess bowel wall enhancement, extraluminal findings
Fibrosis findings
Low T2 signal in a thickened loop
Delayed enhancement
Acute inflammation findings
Wall thickening
Early mucosal enhancement
Engorgement of the vasa recta
Diffusion restriction in the wall
A wide variety of colonic polyps exist. Nonneoplastic, hyperplastic polyps have been cited as the most frequent type for a long time.
| Single Polyp | Multiple Polyps | |
|---|---|---|
| Neoplastic | ||
| Epithelial | Tubular adenoma | Familial polyposis |
| Tubulovillous adenoma | Gardner syndrome | |
| Villous adenoma | ||
| Turcot syndrome | ||
| Nonepithelial | Carcinoid | |
| Leiomyoma | ||
| Lipoma | ||
| Fibroma | ||
| Nonneoplastic | ||
| Hamartomas | Hamartoma | Cronkhite–Canada syndrome |
| Juvenile polyposis Peutz-Jeghers syndrome |
||
| Inflammatory | Benign lymphoid polyp | Juvenile polyp, benign lymphoid polyp |
| Fibroid granulation polyp | Granulomatous colitis | |
| Unclassified | Hyperplastic polyp | Hyperplastic polyposis |
Most common true colonic tumor (in up to 10% of the population by the seventh decade). Up to 50% of polyps are multiple.
Asymptomatic
Diarrhea
Pain
Hemorrhage
| Tubular | Tubulovillous | Villous | |
|---|---|---|---|
| Frequency | 64% | 27% | 9% |
| Malignancy potential | |||
| 0.5–0.9 cm | 0.3% | 1% | 2% |
| 1.0–1.9 cm | 4% | 7% | 6% |
| 2.0–2.9 cm | 7% | 11% | 17% |
Rectum and sigmoid, 60%
Descending colon, 15%
Transverse colon, 15%
Ascending colon, 10%
Histologic differentiation of polyps is invariably difficult radiographically, so the majority of lesions require endoscopic sampling.
| Feature | Benign | Malignant |
|---|---|---|
| Size a | <1 cm | >2 cm |
| Stalk | Present (pedunculated, thin) | Absent (sessile) |
| Contour | Smooth | Irregular, lobulated |
| Number | Single | Multiple |
| Underlying colonic wall | Smooth | Indented, retracted |
a The larger the size of any polyp, the more likely it is malignant: <1 cm: 0.4%, 1–2 cm: 4%, >2 cm: >10%.
Polyp ≥1 cm, high-grade dysplasia
Of all polyps <1 cm, the likelihood that a lesion is an advanced adenoma is 3% (the majority are hyperplastic and the rest are benign adenomas).
Focal proliferation of normal mucosa, hence not an adenoma. Hyperplastic polyps have no malignant potential. Hyperplastic polyps are due to excessive cellular proliferation in Lieberkühn crypts. Seventy-five percent of polyps occur distal to the splenic flexure, mostly in the rectosigmoid area.
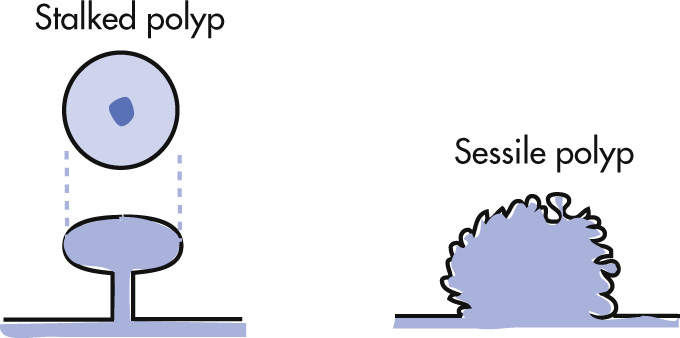
Stalked polyp
Sessile but <5 mm
Postinflammatory polyposis (pseudopolyposis, filiform polyps) represents a benign condition, not associated with cancer. PIPs occur most commonly in Crohn disease and ulcerative colitis (UC) as a result of regeneration of nonulcerated tissue.
Filiform polyps: thin, short, branching structures
| Polyp | Pseudopolyp | |
|---|---|---|
| Size | Uniform in size | Uniform in size |
| Form | Round, stalked, sessile | Y-shaped, filiform, irregular |
| Margins | Well delineated | Fuzzy (inflammation) |
| Colonic haustra | Preserved | Distorted (inflammation) |
| Type | Trait | Gastric | SB | Colon | Histology | GI Malignancy | Extraintestinal |
|---|---|---|---|---|---|---|---|
| Familial polyposis | AD | <5% | <5% | 100% | Adenoma | 100% | — |
| Gardner | AD | 5% | 5% | 100% | Adenoma | 100% | Osteoma, others a |
| Peutz-Jeghers | AD | 25% | 95% | 30% | Hamartoma | Rare | Perioral pigmentation |
| Juvenile polyposis | AD | — | — | 100% | Inflammatory | ? | — |
| Turcot | AR | — | — | 100% | Adenoma | 100% | Glioma |
| Cronkhite-Canada | NH | 100% | 50% | 100% | Inflammatory | None | Ectodermal changes |
| Cowden b | AD | — | — | — | Hamartoma | None | Oral papilloma c |
| Ruvalcaba-Myhre b | AD | Yes | Yes | Yes | Hamartoma | None | Macrocephaly, penile macules, mental retardation, SC lipomas |
a Soft tissue tumors, sarcomas, ampullary carcinoma, ovarian carcinoma.
Most common intestinal polyposis syndrome (incidence 1 : 8000)
Screening of family members of familial polyposis patients should start at puberty: malignant degeneration by 40 years (treatment: prophylactic total proctocolectomy)
Usually >100 polyps
Polyps may be sessile or stalked.
Familial adenomatous polyposis syndrome is a common umbrella name under which Gardner and familial polyposis syndromes are included.
Polyposis: colon 100%, duodenum 90%, other bowel segments <10%
Hamartomas of stomach
Soft tissue tumors: inclusion cysts, desmoids (30%), fibrosis
Osteoma in calvarium, mandible, sinuses
Entrapment of cranial nerves
Malignant transformation in 100% if untreated
SB and pancreaticoduodenal malignancies
Total colectomy recommended
Second most common intestinal polyposis
Autosomal dominant
Hamartomas throughout GI tract except the esophagus
Mucocutaneous pigmentations (buccal mucosa, palm, sole)
Polyps have virtually no malignant potential.
Slightly increased risk of stomach, duodenal, and ovarian cancer; benign neoplasm of testes, thyroid, and GU system
Usually large polyps in rectum
Usually single; 80% are located in the rectosigmoid.
Present in children with bleeding, prolapse, or obstruction
Multiple hamartoma syndrome
Mucocutaneous pigmentations (buccal mucosa, palm, sole)
Most common in rectosigmoid with lesions also present in esophagus
Mucocutaneous lesions → facial papules, oral mucosal papillomatosis, acral keratoses, sclerotic fibromas
Thyroid gland abnormalities: goiter, adenomas
Breast cancer (ductal), bilateral in 30%
Uterine and cervical carcinomas
Transitional cell carcinoma (TCC) of bladder and ureter
Glioma polyposis syndrome
Colonic adenomas with CNS gliomas and medulloblastomas
Third most common cancer in both men and women. Risk factor: being overweight or obese, being inactive, and diets high in red meat and/or low in fruits and vegetables. Synchronous lesions, 5%; metachronous lesions, 3%.
Being overweight or obese
Inactivity
Diets high in red meat
Diets low in fruits and vegetables
Smoking
Heavy alcohol use
Personal history of colorectal cancer or adenomatous polyps
Personal history of chronic IBD
Strong family history of colorectal cancer or polyps (cancer or polyps in a first-degree relative <60 years old or in two first-degree relatives of any age).
Older age
African American or Ashkenazi Jewish descent
Note: A first-degree relative is defined as a parent, sibling, or child.
Rectum, 35%
Sigmoid, 25%
Descending colon, 10%
Ascending colon, 10%
Transverse colon, 10%
Cecum, 10%
Polypoid
Ulcerative
Annular constricting (apple core); <5 cm long
Plaque-like (e.g., cloacogenic tumor at anorectal junction)
Scirrhous carcinoma (rare): long (>5 cm) circumferential spread
Obstruction
Intussusception (in polypoid lesions), rare
Local perforation (simulates diverticulitis)
Local tumor recurrence
Peritoneal spread
Dukes staging system
Dukes A: Limited to bowel wall, 15%; 93% 5-year survival
Dukes B: Extension into serosa or mesenteric fat, 35%; 77% 5-year survival
Dukes C: LN metastases, 35%; 48% 5-year survival
Dukes D: Distant metastases (15%): liver, hydronephrosis, peritoneal cavity, adrenal; 7% 5-year survival
Tx: No description of the tumor's extent is possible.
Tis: The cancer is in the earliest stage. It has not grown beyond the mucosa (inner layer) of the colon or rectum. This stage is also known as carcinoma in situ or intramucosal carcinoma.
T1: Cancer has grown through the mucosa and extends into the submucosa.
T2: Cancer has grown through the submucosa, and extends into the muscularis propria.
T3: Cancer has grown completely through the muscularis propria into the subserosa but not to any neighboring organs or tissues.
T4: Cancer has spread completely through the wall of the colon or rectum into nearby tissues or organs.
Nx: No description of LN involvement is possible because of incomplete information.
N0: No LN involvement
N1: Cancer cells found in 1–3 nearby LNs.
N2: Cancer cells found in 4 or more nearby LNs.
Mx: No description of distant spread is possible because of incomplete information.
M0: No distant spread is seen.
M1: Distant spread is present.
Detection of LN metastases
CT: 50%–70%
MRI currently < CT
Detection of liver metastases
Contrast-enhanced CT: 60%–70%
MRI: 70%–80%
Primarily used to stage and differentiate T2 from T3 disease since T3 disease requires neoadjuvant chemoradiation before surgery
T2W is the most reliable sequence for local staging.
Can have desmoplastic reaction with linear T2 hypointensity in T2 disease
Need nodular extension of T2 intermediate tumor outside of the lamina propria to claim T3 diseaseNeed nodular extension of T2 intermediate tumor outside of the muscalaris propria to claim T3 disease
Closely evaluate external iliac LNs as these denote M1 (distant metastatic disease) in rectal cancer.
Idiopathic IBD
UC
Crohn disease
Behçet disease
Infectious colitis
Ischemic colitis
Iatrogenic
Radiation
Chemotherapy
The imaging features of bowel inflammation (any cause) depend mainly on the location of the process:
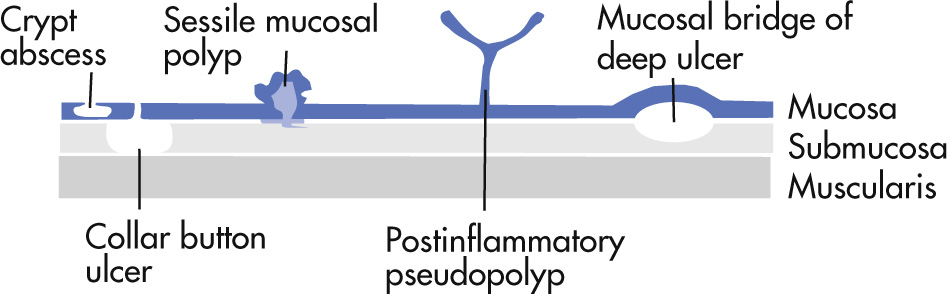
Mucosal inflammation
Ulceration
Shallow: granularity of mucosa, aphthoid
Deeper: flask like collections of barium: collar button
Edema
Displacement of barium: translucent halo around central ulcer
Spasm
Localized persistent contraction
Narrowing of bowel lumen
Submucosal inflammation
Ulcers (deeper linear than in mucosal inflammation): cobblestone appearance
Bowel wall thickening
CT: wall thickened (>3 mm with lumen distended)
Halo: fatty (chronic), gray (subacute or chronic), white (acute) with enhancement
Subserosal mesenteric inflammation
Stranding in surrounding fat
Inflammatory mass
Fistula
Creeping fat: excessive fat deposited around serosal surface (Crohn disease)
Recurrent inflammatory condition of bowel because of altered immunity to intestinal flora. Lesions may occur in the entire GI tract but are most common in:
SB (especially terminal ileum), 80%
30% only SB involved
50% SB and colon involved
Colon, 70%
25% of patients with colonic disease have pancolitis (entire colon affected)
Duodenum, 20%
Pathologic development of lesions: initial event → hyperplasia of lymphoid tissue in submucosa → lymphedema → aphthoid ulcerations → deeper ulcers → fistulas, abscesses → strictures
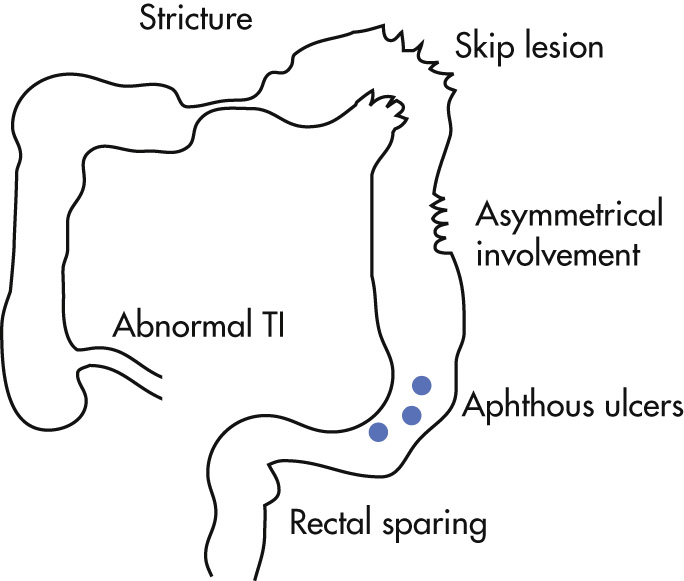
Types of lesions
Thickening of folds (edema)
Nodular pattern (submucosal edema and inflammation)
String sign: tubular narrowing of intestinal lumen (edema, spasm, scarring depending on chronicity)
Omega sign: asymmetric wall involvement results in contracture and C-shaped loop on SB series
Ram horn sign: loss of antral fornices with progressive narrowing from antrum to pylorus
Ulcerations:
Aphthoid ulcers are early lesions and correspond to mucosal erosions (pinpoint ulcer in edematous mound up to 3 mm in diameter and 2 mm in depth).
Ulcers are irregularly scattered throughout GI tract and are interspersed with normal mucosa.
Ulcerations grow and fuse with each other in linear fashion and, with intervening edematous mucosa, produce an ulceronodular pattern (“cobblestone” appearance).
Filiform polyposis: thin, elongated, branching mucosal lesions; represents proliferative sequelae of mucosa adjacent to denuded surface
Sinus tracts and fistulas originate in fissures or deep ulcers; characteristic of Crohn disease at advanced stages.
Fatty thickening along mesenteric border (creeping fat) and paracolonic lymphadenopathy separate bowel loops.
Fibrosis and scarring may result in:
Pseudodiverticula (fibrosis develops eccentrically)
Rigid, featureless bowel
Strictures and obstruction
Foreshortening of bowel
Spatial arrangement of lesions
Transitional zones are typical; they occur between involved bowel loops and normal bowel.
Skip lesions are typical: discontinuous involvement of bowel
Lesions appear preferentially at mesenteric side of intestine.
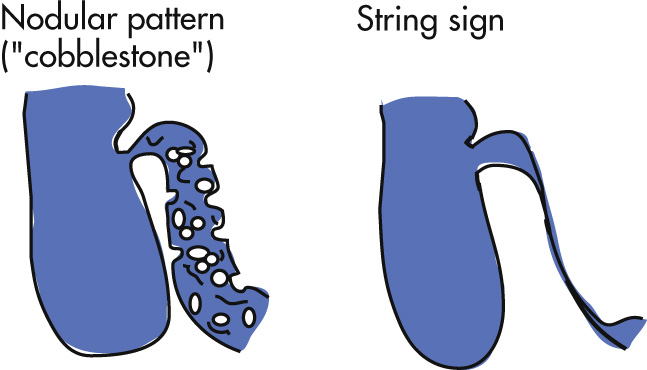
Primary intestinal findings
Bowel wall thickening
Normally <3 mm if well distended
Mean diameter in Crohn disease: 10 mm
If >10 mm, include pseudomembranous, ischemic, or CMV colitis in DDX.
Circumferential submucosal low attenuation surrounded by higher outer attenuation: halo sign
Inner and outer layers surrounding low-attenuation middle layer: target sign; middle layer of fat density; chronic, middle layer of water density
Peribowel inflammation (“dirty fat,” “ground glass”)
Mesentery
Fat accumulates on serosal surfaces (“creeping fat”)
Inflamed mesentery fat (stranding, “misty”)
Adenopathy in SB mesentery common
Comb sign: vasa recta stretched out along one wall of colon
Extraintestinal findings
Gallstones
Osseous complications (may cause pain)
Spondylitis
Sacroiliitis
Steroid complications: osteomyelitis, osteoporosis, avascular necrosis
Renal stones, 7%
Recurrence of disease after surgery (in contrast to UC, in which proctocolectomy is curative)
Increased incidence of malignancies:
GI tract tumors
Lymphoma
TMC
Fistulas
Abscess formation
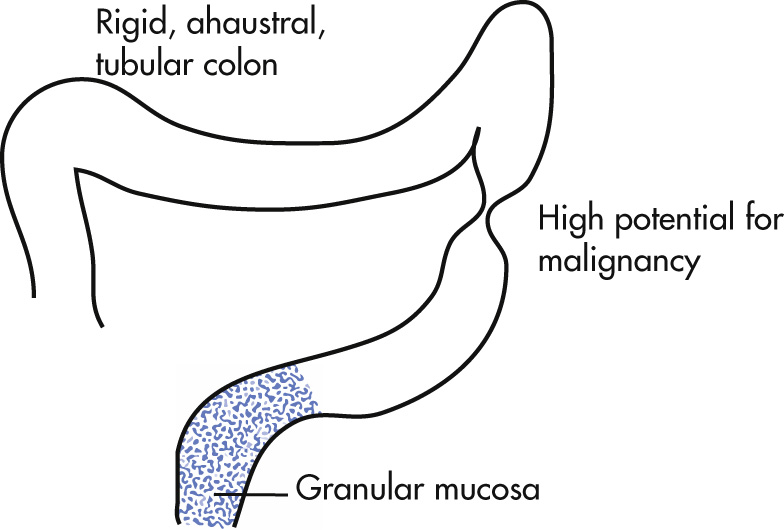
Unknown causes. Clinical findings include diarrhea and rectal bleeding. Disease affects primarily mucosa (crypt abscesses) and typically starts in rectum.
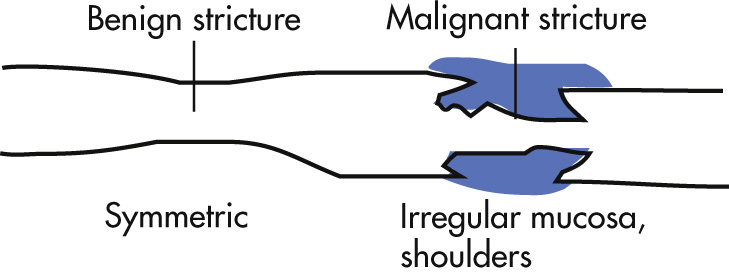
Joints, 25%: arthritis, arthralgia, ankylosing spondylitis
Liver, 10%: sclerosing cholangitis, chronic active hepatitis, cholangiocarcinoma
Skin: pyoderma gangrenosum, erythema nodosum
Uveitis, episcleritis
Ahaustral, foreshortened colon (lead pipe)
Granular mucosa, shallow confluent ulcerations
Polyps:
Pseudopolyps
Filiform polyps (regenerative mucosa, small thin, branching polyps)
Spread:
Disease first and worst in rectum
Continuous spread from distal to proximal colon
Circumferential bowel involvement
Backwash ileitis: only short segment of terminal ileum unlike Crohn disease, gaping ileocecal valve
Atypical imaging features:
Atypical pattern of distribution: sparing of rectum (which may be healing), 5%
Crohn-like findings: discontinuous disease in 5%–10% of patients
Mural and extramural changes (CT findings):
Bowel wall thickening less than Crohn disease
Distribution from rectum continuously proximal
If pancolitis, terminal ileum may be affected for a very short length.
Superimposed carcinoma: mass lesion or thick wall stricture
Excessive fat surrounding rectosigmoid (creeping fat)
Local LN enlargement; common in SB mesentery, infrequently the retroperitoneum
TMC: ahaustral transverse colon: >6 cm with pseudopolyps on kidney, urethra, bladder (KUB; can complicate most colitides)
Strictures, obstruction: benign or malignant. Any stricture is suggestive of malignancy.
Malignancy (5–30-fold higher risk than general population)
Annual incidence of 10% after the first decade
Multiple in 25% of cases
Often flat and scirrhous
| Crohn Disease | Ulcerative Colitis | |
|---|---|---|
| General Features | ||
| Distribution | Skip lesions, entire GI tract | Confined to colon, distal terminal ileum |
| Symmetry | Eccentric | Concentric |
| Ulcers | Early: aphthoid ulcers (mucosa) | Superficial |
| Late: deep ulcers (cobblestone) | ||
| Fistula | Common | Rare |
| Pseudopolyps | Yes | 20% |
| Toxic dilatation | Uncommon | Common |
| Strictures | Yes | Yes |
| Involvement | ||
| Rectum | 50% | 95% |
| Anus | Perianal fistula and fissures | Normal |
| Terminal ileum | Narrowed, inflamed, fissured (cobblestone) | Dilated and wide open (backwash ileitis), gaping valve |
| Other | ||
| Surgery | May exacerbate disease | Curative |
| Cancer risk | Uncommon | High |
| Recurrence | Common | Not after colectomy |
| Clinical Findings | ||
| Diarrhea | ++++ | ++++ |
| Rectal bleeding | +++ | ++++ |
| Pain | ++++ | +++ |
| Weight loss | ++ | ++ |
| Fever | +++ | + |
| Abdominal mass | ++++ | – |
| Malnutrition | +++ | + |
Abnormal connection between epithelialized surface of the anal canal and skin.
Primary; obstruction of anal gland, stasis, infection, fistula
Secondary; iatrogenic (surgery), Crohn, infection, malignancy
Intersphincteric (common)
Transsphincteric (common)
Suprasphincteric
Extrasphincteric (uncommon and seen in patients with multiple operations)
MRI is best technique for assessment
Describe and classify type of fistula
Distance of mucosal defect to perianal skin
Secondary findings; abscesses, bowel wall abnormalities, reactive nodes
Fistulotomy
Fistulectomy
Sphincter-saving fistulectomy
Seton fistulotomy
Crohn mimic. HLA-B51-associated vasculitis.
Ulcers
Strictures
Fistulas
Oral, genital, skin ulcers
Uveitis
Aneurysms
Arthritis
Some agents more commonly cause superficial lesions (similar to UC), and some are more likely to cause transmural inflammation (similar to Crohn disease). Infectious colitis is common in immunocompromised hosts.
| Pathogen | Pattern | Comments |
|---|---|---|
| Campylobacter | SU, DU | Usually in distal colon |
| Shigella | SU | Most severe in rectosigmoid |
| Salmonella | SU, TI | Confined to colon |
| Gonococcus | SU | Rectum |
| Amebiasis | SU, DU | Diffuse but most severe in right colon (ameboma); small bowel rarely affected |
| Tuberculosis | DU | Loss of demarcation between cecum and terminal ileum (Stierlin sign); lymph nodes |
| Lymphogranuloma venereum | DU | Rectal strictures typical |
| Yersinia | DU | Typically, terminal ileum, cecum |
| Strongyloides | SU | Duodenum, jejunum > colon |
| Trichuris trichiura (whipworm) | Rectal prolapse | |
| Schistosomiasis | Hepatosplenomegaly | |
| Chagas disease | Megacolon, megaesophagus |
Occurs mainly in immunocompromised hosts.
Superficial (aphthoid) or deep ulcers
Thick wall: >10 mm
Localized distribution in cecum, terminal ileum, or universal colitis
Radiographically may be indistinguishable from pseudomembranous colitis
Acute necrotizing colitis involving the cecum, terminal ileum, and/or ascending colon in patients with leukemia undergoing therapy and/or immunosuppression. Incidence in autopsy series of young leukemia patients is 10%–25%. Unknown pathogenesis (leukemic bowel infiltration, intramural hemorrhage, necrosis, local ischemia). Indications for surgery include failed medical therapy, perforation, hemorrhage, pericecal abscess, and uncontrolled sepsis.
RLQ pain, 50%
Diarrhea, 40%
Peritoneal signs in perforation
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here