Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The intestinal tract secretes a number of hormones that coordinate local, peripheral, and central responses to food intake. Hormones produced in the stomach are regulated rapidly after food ingestion and are largely involved in the control of acid and enzyme secretion. As food reaches the small intestine, it triggers the secretion of a range of hormones that serve to match the release of digestive enzymes, electrolytes, and bile acids to the composition of the ingested food and to regulate the rate of delivery of nutrients into the duodenum. When nutrients are subsequently absorbed into the bloodstream, the parallel release of gut hormones reflects the rate of nutrient absorption and facilitates downstream hormonal responses such as insulin release, as well as sending signals to the brain to control appetitive behaviors.
Gut hormones are produced from specialized enteroendocrine cells (EECs) located in the epithelium of the gastrointestinal (GI) tract from the stomach through to the rectum. Like other cell types of the intestinal epithelium, EECs are continuously replaced by new cells formed from crypt stem cells. Approximately 1% of newly formed epithelial cells differentiate into EECs, and they share with neighboring enterocytes a similar life span of ~ 3–5 days in the small intestine, and up to a few weeks in the stomach and colon. Many EECs have an apical surface facing into the intestinal lumen and a basolateral surface facing the interstitium, and are known as open-type cells because they make contact with luminal contents. The exception is the stomach, where except in the antrum, most EECs are closed type and do not have a surface opening into the lumen. Whereas open-type EECs are believed to respond primarily to nutritional stimuli arriving in the local vicinity after food ingestion, closed-type cells are regulated by paracrine, circulating, or neural signals, although nutrients might directly regulate these cells if concentrations rise in their vicinity postabsorption.
EECs have traditionally been classified and named according to the principal hormones they produce as determined by immunostaining ( Table 2.1 ) with each hormone and cell type exhibiting a characteristic distribution along the length of the GI tract. Gastric epithelium, for example, contains a large number of EECs-producing gastrin, somatostatin (SST), ghrelin, or histamine. Small intestine preferentially generates EECs-producing cholecystokinin (CCK), secretin, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptides 1 and 2 (GLP-1, GLP-2), peptide YY (PYY), neurotensin (NT), and serotonin (5-HT). In the colon and rectum, EECs have been shown to secrete serotonin, GLP-1, GLP-2, PYY, NT, SST, and insulin-like peptide-5 (INSL5).
| Peptide | Cell of Origin | Locations of GI Tract Secreted From | Function |
|---|---|---|---|
| Gastrin | G cells | Gastric antrum | Stimulates gastric acid secretion. Differentiation and integrity of gastric mucosa. |
| Somatostatin (SST) | D cells | Whole GI tract | Delays gastric emptying and gastrointestinal motility. Inhibits secretion of all other gastrointestinal hormones. Reduces colonic fluid secretion. Reduces bile flow and pancreatic exocrine secretion. Reduces splanchnic blood flow. |
| Ghrelin | X/A like cells | Stomach | Stimulates hunger. Protective during fasting induced hypoglycaemia. |
| Cholecystokinin (CCK) | I cells | Duodenum | Stimulates gallbladder contraction and pancreatic exocrine secretion. Inhibits gastric emptying and acid secretion. Signals satiety. |
| Secretin | S cells | Duodenum, jejunum | Stimulates pancreatic exocrine secretion. Inhibits gastric emptying and acid production. |
| Motilin | M cells | Duodenum, jejunum | Stimulates gastrointestinal motility. |
| Neurotensin (NT) | N cells | Ileum | Delays gastrointestinal motility. Stimulates pancreatic exocrine secretion. |
| Glucose-dependent insulinotropic polypeptide (GIP) | K cells | Duodenum, jejunum | Enhances glucose-stimulated insulin secretion (incretin effect). Promotes fat deposition. Reduces bone turnover. |
| Glucagon-like peptide 1 (GLP-1) | L cells | Jejunum, ileum, colon | Enhances glucose-stimulated insulin secretion (incretin effect), inhibits glucagon secretion. Delays gastric emptying. Signals satiety, reduces food intake. |
| Glucagon-like peptide 2 (GLP-2) | L cells | Jejunum, ileum, colon | Adaptation and recovery of intestinal mucosa in response to injury. |
| Oxyntomodulin | L cells | Jejunum, ileum, colon | Body weight homeostasis. |
| Peptide YY (PYY) | L cells | Jejunum, ileum, colon, | Signals satiety. Inhibits gastric emptying and acid secretion. Maintenance of salt/water homeostasis. |
| Insulin-like peptide 5 (INSL5) | L cells | Colon | Stimulates hunger. |
Recent molecular techniques examining EEC subpopulations, labeled with fluorescent reporters driven by hormone specific promoters in transgenic mice, have yielded transcriptomic data at odds with the simple EEC classification suggested by the early immunostaining studies that used only one or two antibodies at a time. At the messenger RNA (mRNA) level, there is a high degree of overlap between different EEC types that were originally thought to be distinct, and many single EECs produce mRNA for a number of different gut hormones. Coproduction of several different hormones in the same EECs has been confirmed by immunostaining. It is now thought that intestinal EECs-producing CCK, GIP, secretin, GLP-1, PYY, and 5-HT form a continuum, with individual cells producing a mix of hormones dependent on their position along the GI tract. Within individual cells, there are conflicting views about whether coexpressed hormones are localized in the same or distinct vesicles, but no convincing evidence has yet been presented to show separate mobilization of different hormones from individual cells.
Peptide hormones are biosynthesized as prepropeptides containing N-terminal signal sequences that direct the growing peptide chain into the lumen of the endoplasmic reticulum during translation. Propeptides transit through the Golgi and are packaged into secretory vesicles where they are cleaved by prohormone convertases (PCs) and further posttranslationally modified by, for example, amidation, sulfation, or acylation. The predominant PC identified in most intestinal EECs is PC1/3, which cleaves propeptides at dibasic residues and is likely responsible for the majority of peptide hormone processing in the small intestine and colon. By contrast, PC2 plays a more prominent role in the stomach. EECs are often identified by immunolabeling with antibodies against chromogranins and secretogranins. These large granin proteins are believed to play a functional role in vesicular packaging but are also subject to PC-mediated cleavage, resulting in the generation of smaller peptides that might themselves play signaling roles.
It has long been recognized that a wide variety of nutritional and nonnutritional signals trigger gut hormone secretion, with some stimuli preferentially linked to the release of certain hormones. Comparisons between plasma gut hormone concentrations following matched nutrient loads administered orally versus intravenously in humans have revealed that most gut hormones are preferentially released after oral nutrient ingestion. Many additional studies have demonstrated that polymeric macronutrients must be digested into monomers (monosaccharides, free fatty acids, and monoacylglycerides or di/tripeptides and amino acids) before they are capable of triggering gut hormone release.
Transcriptomic analysis and single-cell characterization of fluorescently tagged murine EECs have revealed that they express a range of receptors and transporters capable of detecting a wide variety of stimuli. Even at the single cell level, individual EECs produce machinery capable of detecting multiple stimuli. Unlike taste cells in the tongue, therefore, individual EECs seem to be multimodal rather than tuned to respond to single stimuli. There are two major molecular pathways by which EECs detect ingested nutrients—one involving nutrient transporters and a second involving G-protein-coupled receptors (GPCRs).
Enterocytes typically employ ion-coupled transporters to absorb nutrients across the brush border, using inwardly directed gradients for Na + or H + ions to drive the uphill absorption of nutrients. A large body of evidence supports the idea that many EECs have hijacked sodium-coupled glucose transporters (SGLT1) on the apical membrane to act as glucose sensors, as the coupled uptake of Na + ions with glucose molecules generates an inward current capable of triggering electrical activity, leading to Ca 2+ entry through voltage-gated Ca 2+ channels and activation of vesicular exocytotic pathways. There is some, albeit weaker, evidence that certain amino acids and di/tripeptides might similarly trigger gut hormone release via their Na + - and H + -coupled uptake.
Many small molecules are detected by members of the GPCR superfamily, which include receptors specifically responsive to small molecules including long- and short-chain fatty acids, monoacylglycerides, amino acids, bile acids, and bitter tastants. Nutrient and bile acid responsive GPCRs are highly and specifically expressed in EECs within the intestinal epithelium and likely underlie gut hormone responses to ingested fats and protein, as well as bile acids. GPCRs linked to the stimulation of EECs are mostly G s and G q coupled, linked, respectively, to the elevation of cytoplasmic cAMP and Ca 2+ concentrations. An increasing body of evidence suggests that coincident activation of different signaling pathways in EECs results in synergistic enhancement of gut hormone secretion.
Rather than merely “tasting” the luminal contents, it is increasingly apparent that EECs respond to the local rates of nutrient absorption. In the case of glucose, the rate of SGLT1-mediated glucose uptake by EECs, and hence the degree of glucose-dependent membrane depolarization, will mirror rates of glucose influx by neighboring enterocytes, being determined by the local concentrations of glucose and Na + ions. Results from perfused intestinal preparations and Ussing chambers have now shown that EEC receptors for long chain fatty acids and bile acids are functionally located on the basolateral rather than the apical surface of EECs, requiring local absorption across the epithelium prior to receptor activation. Linking gut hormone secretion to local nutrient absorption might ensure that the circulating hormonal signal reflects the rate of nutrient entry into the bloodstream, rather than the mass of unabsorbed nutrients in the lumen that do not yet require the activation of a peripheral homeostatic response.
In the sections below, we will describe pathologies primarily affecting specific gut hormones, but there are a few conditions that have more generalized effects on the enteroendocrine system. There have been rare case reports of humans born with an almost complete lack of EECs due to mutations in the transcription factor NeuroG3, which is required for cell differentiation down the EEC pathway. Affected neonates presented with severe malabsorptive diarrhea. Rare human cases have also been reported with homozygous loss of PC1/3 due to mutations in the PCSK1 gene, resulting in a variable presentation that can include malabsorptive diarrhea, impaired glucose homeostasis, and obesity, as well as other endocrinopathies attributable to the global deficiency of many active hormones and peptide neurotransmitters in the gut, pancreas, and central nervous system. Secondary EEC deficiency associated with gastrointestinal symptoms has been described in the autoimmune-polyendocrine-candidiasis-ectodermal-dystrophy (APECED) syndrome, associated with a mutation in the AIRE gene.
Neuroendocrine tumors (NETs) of the GI tract can produce a range of unprocessed, partially processed, and fully processed peptide hormones, with the consequence that clinical presentations vary markedly between cases. In most cases, the exact pattern of active peptides produced by an individual tumor is not currently measurable, because of the lack of suitable methodology for the identification and quantification of partially processed peptides.
Some of the most dramatic gut hormone changes in humans have been observed after upper GI surgical procedures such as Roux-en-Y gastric bypass (RYGB) surgery, gastrectomy, or esophagectomy. RYGB and sleeve gastrectomy are performed routinely as a treatment for morbid obesity, but have dramatic metabolic consequences that result in the resolution of the majority of cases of type 2 diabetes. As discussed in some of the sections below, dramatic postprandial elevations of gut hormones such as GLP-1 and PYY in these patients are likely caused by increased nutrient delivery to and absorption in the more distal small intestine, and almost certainly contribute to observed improvements in glucose tolerance and reduced appetite. Similar hormonal changes have been observed in lean subjects, for example, following resection for gastric cancer, and may contribute to some of the symptoms encompassed under the umbrella of “dumping syndrome.”
In the sections below, we provide details of the major identified gut hormones produced by EECs, focusing particularly on hormones that have known cognate receptors and functional roles. Peptide sequence nomenclature is based on human sequences, as published in the Uniprot/Swissprot database. The list is not exhaustive and does not include the large number of additional signaling peptides produced by non-EECs types in the gut, such as enteric nerves [e.g., vasoactive intestinal peptide (VIP), gastrin-releasing peptide, galanin], Paneth cells (e.g., defensins), enterocytes (e.g., FGF 15/19), immune cells (e.g., interleukins), and as yet unidentified cell types (e.g., guanylin/uroguanylin).
It was first observed in 1905 that mucosal extracts from the gastric antrum stimulated gastric acid secretion when injected intravenously in cats, but it was not until 1942 that this effect was demonstrated to be due to a peptide, gastrin, rather than contamination with histamine. The main physiological actions of gastrin are regulation of gastric acid secretion and control of gastric epithelial cell growth and differentiation.
Gastrin is primarily secreted from gastric antral G cells, but has also been identified in the pituitary gland, developing pancreas and sperm. The gastrin gene encodes a 101 amino acid prepropeptide, containing a 21 amino acid N-terminal signal peptide and 80 amino acid progastrin peptide. All subsequent amino acid position nomenclature refers to the position in the preprogastrin peptide. Following cleavage of the signal peptide in the endoplasmic reticulum, progastrin is sulfated at tyrosine 86 and phosphorylated at serine 96. Further processing in the trans-golgi network and secretory vesicles results in the two mature, C-terminal amidated forms—Gastrin34 (G34) and Gastrin17 (G17). Progastrin is cleaved by PC 1/3 (PC1/3) and carboxypeptidase E (CPE) at amino acid positions 58-59 and 92-93 (the latter removing a C-terminal flanking peptide). The resulting 34 amino acid peptide (G34-Gly) is amidated by peptidyl-glycine α-amidating monooxygenase (PAM), with the glycine group acting as an amide donor. G34 is then cleaved by PC2 to G17, with the two forms present in human G cell vesicles at a G34:G17 ratio of 1:9 ( Fig. 2.1 ).
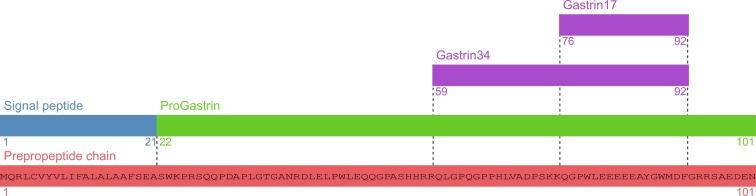
Gastrin shares a significant degree of sequence and structural homology with CCK, and G34 and G17 both act through the CCK2 receptor. Whereas G17 and G34 undergo regulated exocytosis, progastrin, and nonamidated forms of G17 and G34 are secreted via the constitutive pathway. They have no known receptor and have previously been regarded as inactive metabolites, although recent evidence suggests that they may play a role in colonic mucosal proliferation and have a complementary role to that of the amidated gastrins.
Gastrin secretion is regulated by neuronal-, hormonal-, and nutrient-responsive factors. Gastrin is secreted in response to luminal amino acids detected via apical calcium-sensing receptors (CaSRs), sympathetic and parasympathetic nervous activity, and gastrin-releasing peptide derived from local neurons. Gastrin secretion is inhibited by SST, when the gastric luminal pH is below 3. Chronic use of proton pump inhibitors results in hypergastrinaemia.
Gastrin’s key role in gastric acid secretion has been demonstrated through gastrin infusion and CCK2R antagonist experiments in man, immunoneutralization in dogs, and in gastrin gene knockout in mice. Gastrin acts on enterochromaffin-like (ECL) cells to stimulate histamine secretion, which then acts in a paracrine fashion via H2 receptors on parietal cells to stimulate acid secretion. Interestingly, in gastrin-deficient mice, the coinfusion of G17 and the nonamidated G17-Gly more potently restored gastric acid secretion than G17 alone. In addition to stimulating acute histamine secretion, the gastrin upregulated the expression of histidine decarboxylase, the enzyme responsible for conversion of histidine to histamine, in ECL cells. Although CCK2 receptors are also present on parietal cells, these appear to be only of limited role for gastrin stimulation of parietal cell acid secretion, with the majority of the effect of gastrin on acid secretion arising due to histamine from ECLs.
Gastrin is not essential for the development and maintenance of the gastric mucosa, but gastrin gene knockout mice had reduced numbers of parietal and ECL cells, which could be restored by infusion of gastrin. It therefore appears that gastrin plays a key role in the differentiation and integrity of the gastric mucosa, although the underlying pathways remain subject to ongoing investigation. One pathway of note involves the urokinase plasminogen activator (uPA) family, including uPA and plasminogen activator inhibitors 1 and 2 (PAI1, PAI2), which localize to gastric parietal and ECL cells.
Zollinger-Ellison syndrome, hypergastrinemia secondary to gastrin secreting NETs, is a cause of gastric acid hypersecretion, multiple peptic ulcers, and secretory diarrhea. This is associated with multiple endocrine neoplasia type 1 (MEN1) in up to 20% of cases.
The role of gastrin in gastric mucosal proliferation is of interest in the pathogenesis and treatment of gastric cancer. Specifically, it has been demonstrated that gastrin stimulates the growth of gastric cancer cell lines in vitro by stimulation of CCK2 receptors, and nonendocrine gastric cancer cell lines can secrete gastrin, which may act in an autocrine fashion. Despite this, any link between hypergastrinemia secondary to proton pump inhibitor therapy and an increased prevalence of gastric adenocarcinoma remains controversial. However, there is a more established link between hypergastrinemia and ECL cell NETs of the stomach, evidence arising from potent H2 receptor blockade in rats using loxtidine and transgenic Men1 / Sst knockout mice treated with omeprazole. Gastric carcinoids in man can be associated with hypergastrinemia due to Zollinger-Ellison syndrome (principally in the presence of multiple endocrine neoplasia type 1 [MEN1]) or atrophic gastritis, but not PPI therapy.
SST was originally described in 1973 as a 14 amino acid peptide inhibitor of hypothalamic growth hormone secretion. A 28 amino acid N-terminal extended form was subsequently identified from the GI tract, and the two SST forms are now considered together as a global counterregulatory hormone, with inhibitory effects in multiple target tissues.
Both SST-14 and SST-28 are products of a single 116 amino acid prepropeptide translated from the SST gene. The prepropeptide consists of a 24 amino acid N-terminal signal peptide and a 92 amino acid propeptide, of which the terminal 14 and 28 amino acids correspond to the active SST peptides ( Fig. 2.2 ).
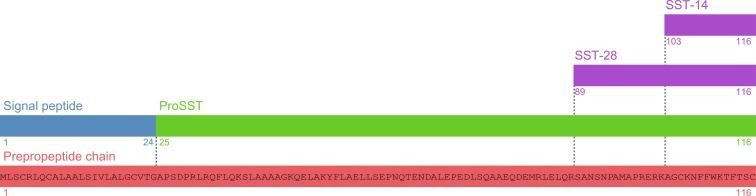
Both 14 and 28 amino acid forms of SST are secreted from gastric and intestinal D cells and pancreatic δ cells, with SST-28 predominating in the small intestine, and SST-14 predominating in the rest of the GI tract and pancreas. Gastric D cells differ between the proximal and distal stomach, with oxyntic D cells exhibiting a closed-type morphology and those in the antrum an open-type morphology. Closed-type oxyntic D cells are inhibited by the vagus nerve soon after food ingestion, thereby reducing the tonic inhibitory control by SST of gastrin and histamine secretion that predominates between meals. SST release from the distal antrum is stimulated by nutrient ingestion, reduced gastric pH, CCK, GIP, GLP-1, acetylcholine, VIP, CGRP, and secretin, resulting in a delayed feedback inhibition of gastric secretions that restores acid secretion to basal levels.
There are five G-protein-coupled SST receptors, labeled numerically from 1 to 5, with SSTR2 having two isoforms, SSTR2A and SSTR2B. All SSTRs act through pertussis toxin-sensitive pathways (G i ) to inhibit adenylate cyclase, activate inwardly rectifying potassium channels, and prevent cellular depolarization, calcium influx, and subsequent vesicle exocytosis. SSTRs also activate other downstream pathways that reduce cellular proliferation through the action of protein tyrosine phosphatases on MAPKs. SST-14 and SST-28 bind with equal affinity to SSTRs 1–4, but SST-28 has a 10–30 fold higher affinity for SSTR5 than other SSTRs, whereas SST-14 has reduced affinity at SSTR5.
SST acts to inhibit gastrin-mediated acid secretion from gastric parietal cells, acting in a paracrine, endocrine, and neurocrine fashion. SST receptor knockout mouse experiments suggest this is mediated by SSTR2, although a detailed discussion of gastric acid secretion is the topic of a further chapter of this book.
SST delays intestinal transit by slowing gastric emptying and prolonging migrating motor complexes (MMCs), as well as inhibiting the relaxation of the lower oesophageal sphincter. It however remains a topic of some debate as to whether these are global effects, or if SST has differential effects on stomach, small intestine, and colon. Experiments to elucidate the underlying mechanisms by which SST has this effect have focused on ex vivo intestines or intestinal smooth muscle. SST has been shown to inhibit VIP-induced relaxation or acetylcholine- and CCK-induced contraction independent of the intestinal section and species investigated; in isolated human colonic smooth muscle cells, removing thereby indirect effects through the modulation of the release of myenteric plexus-derived transmitters, a combination of SSTR1 and SSTR2 activity relaxed smooth muscle cells directly, although high concentrations in the absence of other contracting agents resulted in SST-induced contraction. In rodent small intestine examined ex vivo, SST prolonged MMCs in a SSTR2 and nitric oxide-dependent fashion.
In keeping with its global counterregulatory role, SST inhibits the secretion of multiple gut peptides, including gastrin, GLP-1, motilin, secretin, ghrelin, PYY, 5-HT, and GIP.
SST, acting directly on colonocytes, reduces colonic fluid secretion.
A series of in vivo and in vitro experiments in dogs, rodents, and humans have used gastroduodenal perfusion and sampling, bile duct ligation, and endoscopic sphincter of Oddi cannulation to examine the role of SST in bilio-pancreatic secretion. SST has been demonstrated to reduce bile flow by inhibiting secretion and enhancing resorption of fluid by cholangiocytes. SST appears to inhibit secretin-mediated pancreatic bicarbonate secretion, but had limited effects on basal pancreatic secretion, with the net result of reduced sphincter of Oddi flow in human infusion experiments, albeit with conflicting evidence on whether it induces sphincter contraction.
Exogenous administration of SST or its analogues has been shown to reduce splanchnic blood flow and pressure in dogs and man, although there is little information on the underlying mechanism of action. It has been proposed as a treatment for bleeding oesophageal varices, although there is evidence that its pressure lowering effects are less potent in the cirrhotic patient and a recent Cochrane review concluded that it had no mortality benefit and only a modest reduction in transfusion requirements.
SST analogues are of considerable utility in the diagnosis and treatment of gastroentero-pancreatic NETs. As many moderately and well-differentiated NETs express receptors to SST, radio-nucleotide labeled SST analogues can be used in the diagnosis and staging of disease and for targeted radiotherapy. Palliative treatment with SST analogues, in the presence of symptomatic NETs, can control hormone-mediated symptoms including diarrhea, tachycardia, and flushing and has recently been shown to delay tumor progression.
Other GI uses of SST analogues are based on limited case series or expert opinion and utilize their counterregulatory and antisecretory effects. The evidence is at present equivocal on the benefits of SST analogues in the prevention of postpancreatectomy cutaneous fistula, or the treatment of enterocutaneous fistula. Long- and short-acting SST analogues have also been used for the management of congenital hyperinsulinemia and reactive hypoglycemia and accelerated intestinal transit after upper GI surgery, with mixed success.
Ghrelin was first identified in 1999 as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R). While primarily described as an orexigenic hormone through its hypothalamic actions, it has also diverse roles including as a GHS, promoter of adipogenesis, and suppressor of pancreatic insulin secretion ( Fig. 2.3 ).
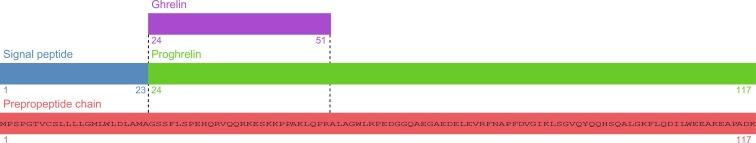
Ghrelin is primarily secreted by X/A-like cells of the stomach, but has also been identified in other tissues including duodenum, pancreas, lymphocytes, and the central nervous system. Following total gastrectomy, circulating total and acyl ghrelin concentrations are undetectable, suggesting that the extra-gastric sources do not contribute significantly to circulating levels. It is encoded by the GHRL gene, located on chromosome 3p25-26. Translation of Ghrl mRNA produces a 117 amino acid preprohormone (preproghrelin), which is cleaved to the active 28 amino acid ghrelin by PC1/3. Ghrelin is modified by the addition of an octanoyl moiety to the hydroxyl group of the serine at position 3 of proghrelin catalyzed by ghrelin O -acyltransferase (GOAT, also membrane bound O -acyltransferase, MBOAT4), although it is unclear whether this step precedes or follows cleavage of proghrelin to ghrelin. Acylation of serine 3 is essential for activity at the GHSR1a receptor, and acyl-ghrelin has historically been regarded as the active, and des-acyl-ghrelin the inactive, form of the peptide, although independent functions have been considered for the latter. The fatty acid chain used for ghrelin acylation appears to derive from the diet.
Circulating concentrations of ghrelin are highest in the fasting state, with secretion suppressed by glucose and fat ingestion, and exercise, but less so by protein intake or gastric distension. In vitro experimental evidence exists for direct sensing of fatty acids, glucose, and glutamate by X/A-like cells, for suppression of ghrelin secretion by insulin, leptin, and GLP-1 and for stimulation of ghrelin secretion by glucagon. However, X/A cells are predominantly closed-type EECs making no contact with the gastric lumen, so they are likely regulated primarily by internal factors. Pharmacological experiments in rats demonstrated increased ghrelin secretion in response to muscarinic and beta-adrenergic activity and decreased secretion in response to alpha-adrenergic activity. Plasma ghrelin concentrations increased in healthy humans in response to a cholinergic agonist and were suppressed by a muscarinic antagonist. Vagotomy initially suppressed ghrelin secretion in rats, but seven days postvagotomy plasma ghrelin concentrations were elevated. GLP-1 and PYY, independently and synergistically, suppressed ghrelin secretion in a study of 25 overweight men. Investigation of FACS purified ghrelin secreting cells from mice demonstrated G-protein-coupled actions of α-CGRP, long- and short-chain fatty acids, lactate, SST, GIP, and α-MSH, but interestingly not PYY or GLP-1 on ghrelin secretion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here