Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Proton beam radiation (PBR) has the potential to improve the therapeutic ratio in the treatment of several gastrointestinal malignancies by decreasing the dose to nontarget critical structures. In general, radiotherapy to the abdomen and pelvis is challenging because of the inherent sensitivity of gastrointestinal organs to radiation toxicity.
Postoperative radiotherapy for gastric cancer was once the established standard of care on the basis of the Intergroup 0116 study showing the survival benefit of adjuvant fluorouracil-based chemoradiation (CRT) compared with surgery alone. When CRT is administered, the prescribed dose is 45 to 50.4 Gray (Gy) in 1.8-Gy fractions, with higher doses sometimes considered for positive margins or gross residual disease. However, patients treated at most centers in Western countries are more likely to receive perioperative chemotherapy, which has also been shown to improve survival over surgery alone. In Eastern countries, the practice is to routinely perform more extensive surgery, including D2 lymph node dissection, and chemotherapy is the preferred adjuvant treatment. Patients with node-positive disease and intestinal-type histology did seem to benefit from adjuvant CRT when compared with adjuvant chemotherapy, and this subset of patients is the subject of an ongoing trial.
Completion rates for adjuvant CRT and chemotherapy are historically poor, with 15% and 10% of patients not able to complete adjuvant CRT and chemotherapy, respectively, as a result of toxicity. , For this reason, a neoadjuvant therapy approach is currently favored at the University of Texas MD Anderson Cancer Center. In a cooperative group phase II trial, the pathologic complete response (pCR) rate and margin-negative resection (R0) rates were 26% and 77%, respectively, and 98% of patients were able to complete all therapy per protocol.
Other efforts to decrease acute toxicity and improve the tolerability of CRT for gastric cancer have centered on the use of more conformal radiation techniques to minimize the normal tissue irradiated. Current US guidelines state that intensity-modulated radiation therapy (IMRT) may be used to reduce dose to organs at risk. Studies have shown IMRT to be feasible and well tolerated in both the neoadjuvant and adjuvant settings. , However, IMRT leads to a larger volume of normal tissue receiving low radiation doses, which raises the concern for increased secondary malignancy rates. PBR has the potential to improve nontarget tissue sparing and further improve the tolerability and therapeutic ratio for gastric cancer. Furthermore, PBR has the potential to reduce the secondary malignancy rates that have been reported in higher numbers after adjuvant CRT when compared with surgery alone on long-term follow-up.
In the postoperative setting, PBR has been shown to reduce the volume of several normal organs receiving low to moderate doses. One treatment planning study showed the median volume of small bowel receiving 15 Gy (V15) was 133 cc with IMRT and 82 cc with 2- to 3-field double-scattered-uniform scanning technique proton therapy. Mean liver and kidney doses were also lower with PBR compared with IMRT. Perhaps more importantly, a significantly lower mean heart dose was achieved with PBR compared with IMRT (7.4 Gy relative biologic effectiveness [RBE], assuming an RBE of 1.1 for protons compared with photons, vs. 9.5 Gy). With data from long-term breast cancer survivors suggesting that the rates of major coronary events increase linearly with mean heart dose at a rate of 7.4% per Gy with no apparent threshold, this suggests a meaningful reduction in late toxicity may be afforded by PBR in this population. Dionisi et al. also showed the robustness of PBR in the postoperative treatment of gastric cancer by reporting that target coverage on repeat verification computed tomography scans was within ±2% of the initial simulation scan. This is important because one of the challenges of using PBR in the treatment of gastric cancer is the variability of gastric volume, contents, and the presence of gas. Appropriate volumes to account for uncertainties in day-to-day target volumes, as well as dietary management, such as following a low-residue diet and fasting for a defined period before treatment, are essential for the accurate and effective treatment of gastric cancer.
The potential for both feasibility and clinically meaningful toxicity in the preoperative or inoperable settings can be extrapolated from work done in esophageal and gastroesophageal junction cancers. pCR rates and near-pCR rates were high with few severe toxicities in a prospective study performed at MD Anderson evaluating proton-based preoperative CRT to a dose of 50.4 Gy equivalents (GyE) for esophageal cancer. A retrospective study of definitive proton-based CRT to 60 GyE performed at the University of Tsukuba likewise showed good control rates and very low rates of serious acute toxicities. Two case reports in the 1990s demonstrate the feasibility of using definitive PBR for inoperable advanced gastric cancer , and showed clinical CRs after 61 GyE in 35 fractions and 83 to 86 GyE, respectively. The potential dosimetric and clinical benefits of PBR for gastric cancer certainly merit further study. The specific location, size, and extent of tumor will likely influence the magnitude of benefit ( Fig. 10.1 ).
Pancreatic adenocarcinoma is a particularly aggressive disease with surgical resection as the only current curative option, but median overall survival (OS) time after surgery alone is only 20 months. Systemic therapy has been shown to improve disease-free survival, but the use of radiotherapy in general for pancreatic malignancies is somewhat controversial. , A currently open randomized trial is evaluating whether concurrent fluoropyrimidine-based CRT improves survival for patients who have received 5 months of adjuvant chemotherapy after surgical resection of pancreatic adenocarcinoma. Because completion rates of adjuvant therapy are low, interest is growing in a neoadjuvant approach. , This neoadjuvant treatment approach is currently favored at MD Anderson. Neoadjuvant CRT can help convert borderline-resectable cancer to potentially operative disease, improve the margin-negative (R0) resection rates, , and potentially reduce costs associated with care. Stereotactic body radiotherapy (SBRT) has emerged as an alternative to standard fractionated radiotherapy. A currently accruing trial by the Alliance for Clinical Trials in Oncology (A021501) is comparing neoadjuvant therapy consisting of either chemotherapy alone or chemotherapy followed by either SBRT (33–40 Gy in 5 fractions) or hypofractionated image-guided radiation (25 Gy in 5 fractions). Finally, despite the lack of an OS benefit demonstrated in the randomized trial evaluating consolidative CRT given after 4 months of induction chemotherapy, CRT did result in improved local control (LC) with no increased rates of grade 3 to 4 toxicity except for nausea. Studies have shown that local progression is the leading cause of death for patients with locally advanced pancreatic cancer after 15 months. As systemic therapy improves distant control, local treatment modalities, such as CRT, may have the chance to prove their value.
The pancreas is located in an anatomically challenging part of the body surrounded by radiosensitive organs, such as the stomach, duodenum, jejunum, kidneys, and liver. Radiation to the pancreas can cause acute side effects, such as fatigue, nausea, vomiting, diarrhea, and abdominal cramping. Radiation is only one part of a multidisciplinary approach, so it is important to minimize the side effects of this component to improve the completion rates for all therapy. PBR may be able to improve the therapeutic ratio by both allowing dose escalation while minimizing toxicities from unintended dose to adjacent normal organs.
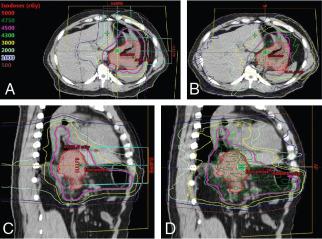
One early dosimetric study showed the potential benefit of PBR for the treatment of inoperable pancreatic cancer necessitating large fields. Zurlo et al. showed that for four patients for whom IMRT plans conferred a 5% higher risk of toxicity to the kidneys, liver, or bowel, proton plans were able to deliver the same target coverage without the excessive risk of morbidity. Other treatment planning studies have also shown the benefit of PBR for reducing low to moderate doses to normal tissue. One small study showed the feasibility of PBR to safely dose escalate to 59.4 GyE while reducing mean dose to the spinal cord, left kidney, right kidney, and liver by 78%, 73%, 42%, and 55%, respectively. Another treatment planning study performed at MD Anderson showed that PBR could allow safe dose escalation to 72 GyE with reduction in the V15 of the stomach and small bowel (48% vs. 5% and 61% vs. 9%, respectively). In the postoperative setting, treatment planning studies have also shown the ability to reduce dose to the small bowel, stomach, and kidneys with PBR. , However, there are some concerns regarding the robustness of PBR plans for the treatment of pancreatic cancer. One study showed that proton plans were highly susceptible to interfractional anatomic change, with coverage of the clinical target volume reduced by 8% as a result of the daily variability. A worst-case optimization strategy, which has been studied in carbon ion therapy for pancreatic cancer, may be useful to mitigate risks posed by interfractional anatomic changes. Fig. 10.2 shows IMRT and IMPT comparison plans for a patient receiving neoadjuvant SBRT for borderline-resectable pancreatic cancer.
![Fig. 10.2, Representative axial and sagittal slices for comparison intensity-modulated proton therapy (IMPT) (A and C) and intensity-modulated radiation therapy (B and D) plans for a patient with borderline resectable pancreatic adenocarcinoma treated with stereotactic radiation therapy to a total dose of 33 Gy (or Gy [relative biological effectiveness]) in 5 fractions. The maximum dose to the duodenum was similar, but the volume of bowel receiving lower doses (5, 10, and 15 Gy) was significantly lower with IMPT. Fig. 10.2, Representative axial and sagittal slices for comparison intensity-modulated proton therapy (IMPT) (A and C) and intensity-modulated radiation therapy (B and D) plans for a patient with borderline resectable pancreatic adenocarcinoma treated with stereotactic radiation therapy to a total dose of 33 Gy (or Gy [relative biological effectiveness]) in 5 fractions. The maximum dose to the duodenum was similar, but the volume of bowel receiving lower doses (5, 10, and 15 Gy) was significantly lower with IMPT.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Gastrointestinal/1_3s20B9780323733496000194.jpg)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here