Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Gene-therapy strategies involve replacing defective genes with functional variants, enhancing the expression of certain key genes, or suppressing genes that contribute to disease.
Methods of administering gene therapy include direct injection of DNA/RNA, use of specialized nonviral vectors such as nanoparticles, or administration of a viral vector.
More than 600 clinical trials have demonstrated the effectiveness of gene therapy in the treatment of a wide range of inherited and acquired diseases.
Gene therapy has shown significant potential within otolaryngology for the treatment of head and neck cancers to facilitate regrowth of hair cells within otology and for tissue engineering in reconstructive and plastic surgery.
Gene therapy continues to evolve with the introduction of new techniques such as genomic editing, which will revolutionize the treatment of disease in the twenty-first century.
Molecular biology is a relatively young scientific field that arose in the 1970s after the discovery of methods to study and manipulate DNA. The rapid development of these techniques enabled actual clinical application by the 1980s. Many diagnostic laboratory tests routinely used in clinical medicine, as well as methods used to develop and mass-produce pharmaceuticals, make use of molecular biology techniques.
The growing field of molecular biology has also led to the development of novel therapeutic strategies for disease. A new understanding of genes and the products of their expression has allowed the emergence of “gene therapy.” This rapidly expanding field is founded on the ability to introduce genetic material into the body to modulate the expression of genes and, thereby, treat disease.
This chapter reviews basic molecular terminology and introduces the concept of gene therapy. Emphasis is placed on the rationale, methods, and progress to date for clinical application of these exciting therapies.
The basic premise of molecular biology is to study cell function and regulation at the level of the genome. With this understanding comes insight into human disease because aberrances in cell function or regulation are the basis for most diseases. The following section reviews fundamental terminology and concepts on how information travels from the genetic code to the functional protein level. Included is a brief discussion on cell cycling, which is an important concept in tumor molecular biology.
The information for conducting all aspects of cellular function is contained within molecules of DNA located in the nucleus. The actual length of a human DNA strand is 1.8 m; however, it is coiled around nuclear proteins called histones to allow compression to the microscopic size of the cell's nucleus. Each strand of DNA contains thousands of genes, which are specific subunit sequences coding for the information required to synthesize a protein. Although every cell in the human body contains the same DNA, the expression of individual genes is not the same. Gene expression varies depending on the cell's function. These differences in gene expression result in the multiple cell and tissue phenotypes that constitute the human body as a whole.
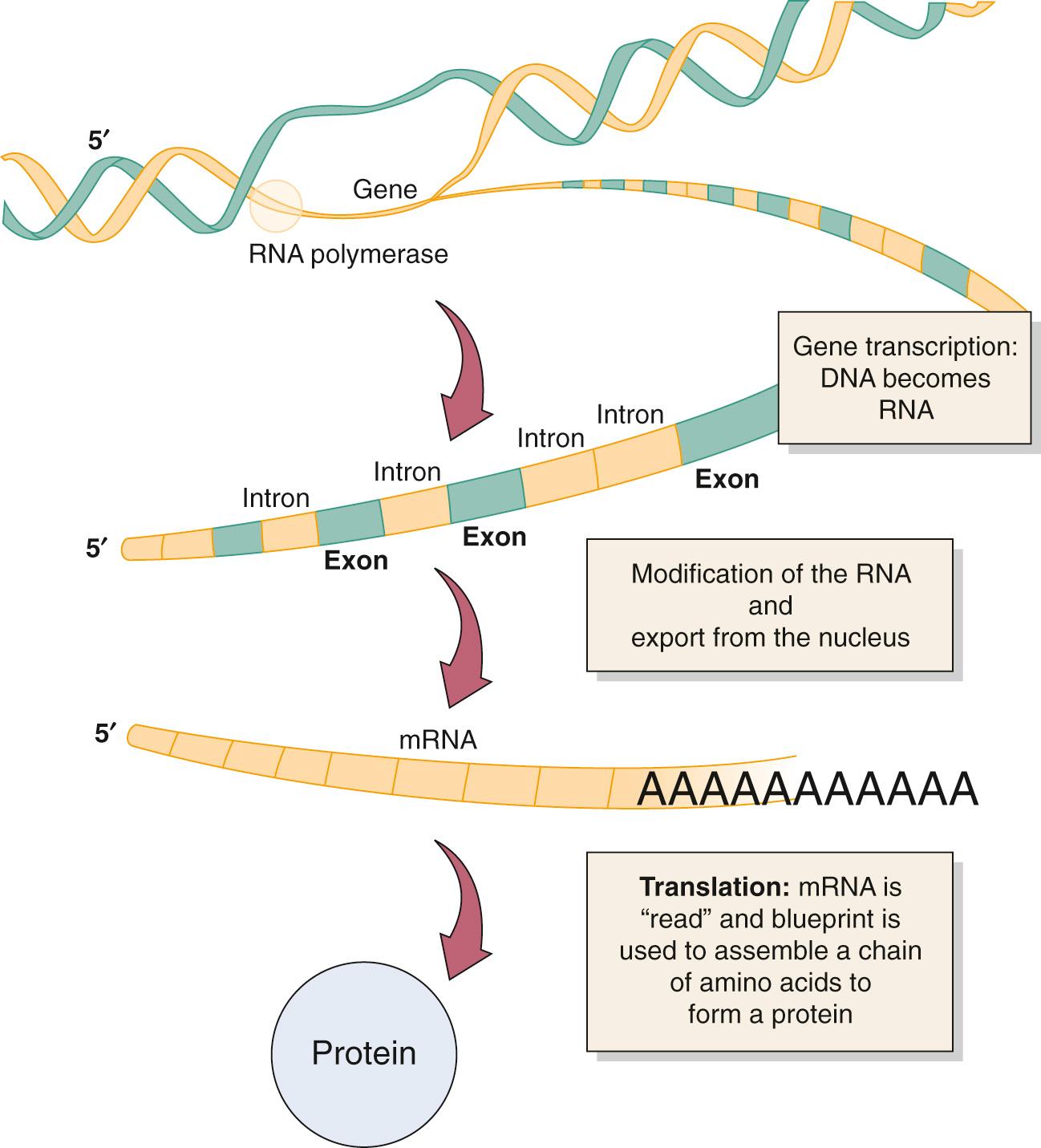
The process by which a gene codes for a specific protein begins with transcription, which is the formation of a single-stranded RNA molecule, which complements a single strand of the DNA subunit ( Fig. 72.1 ). This RNA molecule is subsequently modified to become the messenger that brings the genetic information from the nucleus to the cell's cytoplasm, where the actual synthesis of the functional protein occurs. After reaching the cytoplasm, the process of translation begins (see Fig. 72.1 ), during which the message from the RNA molecule directs the construction of a protein from its basic subunit, the amino acid. Once a protein is formed, it may require further modification to enable its designated function. These modifications include the addition of sugars, lipids, or phosphates to the protein backbone. These internal control steps are mediated by cytoplasmic enzymes, which, if defective, can also lead to defined diseases. Once a protein is formed within the cell cytoplasm, it can reside in the cell or be released to affect other tissues within the body. Depending on the original genetic program for the protein, it may serve its purpose and be rapidly degraded, or it may enter the circulation or reside in local or distant tissues for an extended period. Although stated simply here, both transcription and translation are complex processes that have many more modification and regulatory steps governed by regulatory genes and their protein products. When these delicate control mechanisms are lost, a disease state or a state of abnormal cell proliferation can ensue, as is exemplified in the development of cancer.
Disorders in cell function can lead either to cell death or proliferation. Abnormal stimulation of cell proliferation is the basis for the development of cancer. For a cell to divide, it must progress through the various phases of growth and reproduction that constitute what is called the cell cycle ( Fig. 72.2 ), the principal components of which are the replication of nuclear DNA and its distribution among the progeny (i.e., daughter) cells. The first phase of the cell cycle is called G1 , and it is in this phase that all the enzymes, nucleic acids, and other factors are generated that will enable DNA replication or synthesis , which occurs in the S phase . Once the DNA is replicated, a period of cell growth and duplication of cellular proteins and structures occurs in a period called G2 . After this cell growth, the actual distribution of the replicated DNA and the physical division of the parent cell into two daughter cells occur in the M phase . After cell division, the cell-cycle process can begin again, or the cell may enter a state of rest, called G0 .
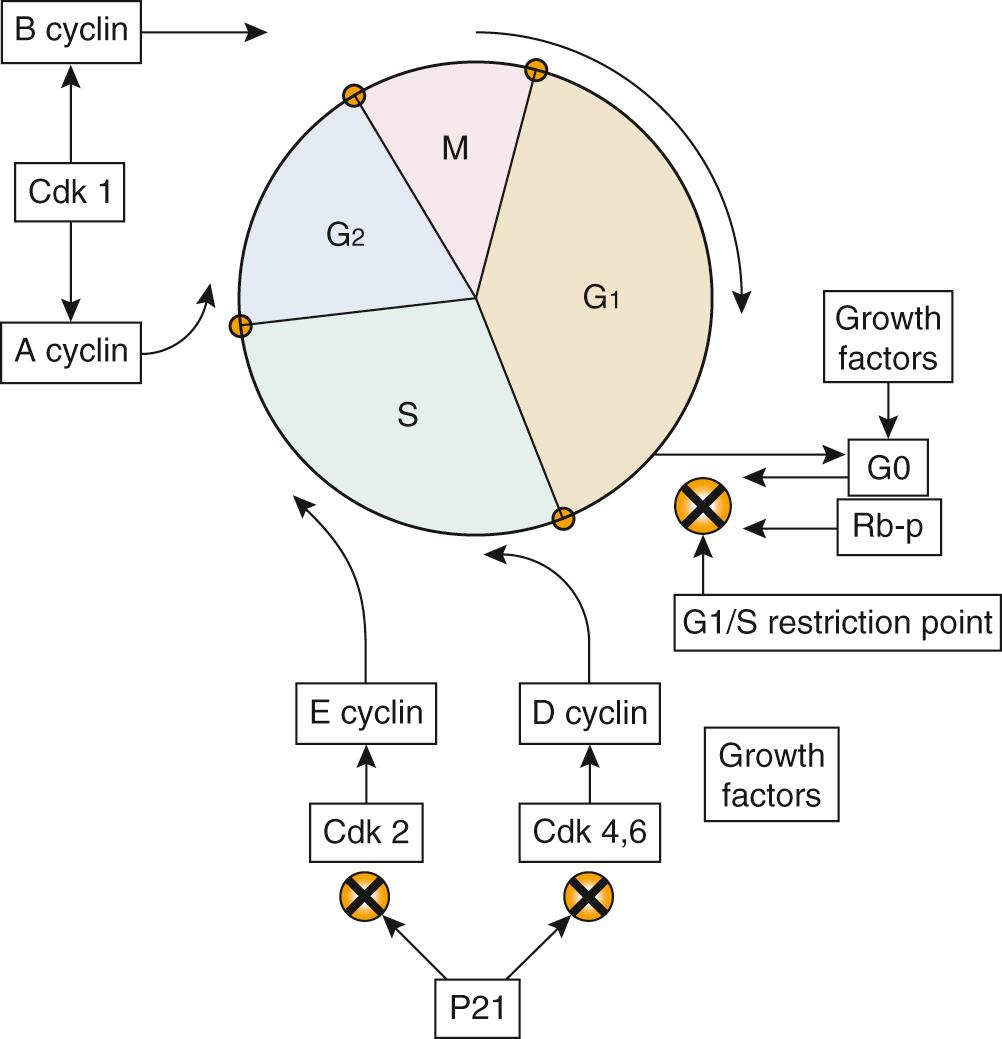
The signal to divide can come from either internal factors or exogenous growth factors, which bind to cell surface receptors and stimulate a cascade of events that lead to division. Important internal control mechanisms exist at the genetic level, which help regulate cell cycling. The “negative regulatory” genes that code for proteins that inhibit abnormal cell proliferation are called tumor suppressor genes . A mutation or loss of a tumor suppressor gene is a basic step in the development of many human cancers. Cell cycling can also be abnormally induced by cellular oncogenes, which naturally exist within cells but are typically kept dormant either by tumor suppressor genes or other regulatory mechanisms. Loss of negative regulation from tumor suppressor genes or amplification or mutation of the actual oncogene has also been associated with cancer formation.
Continued molecular research will provide further understanding of cell-cycle regulation and may lead to novel therapies for treating human cancer. A discussion of basic molecular biology techniques that have provided the means to understand gene expression, regulation, and cell cycling is found in Chapter 73 .
The basic principle of gene therapy is that the intrinsic expression of certain genes in body tissues can be modified to treat disease. This may involve replacing a defective gene with a functional version, enhancing the baseline expression level of a gene, or suppressing the expression of genes that may contribute to the pathologic process.
During the advent of gene therapy, the initial clinical targets were rare inherited diseases; these included adenosine deaminase (ADA) deficiency, enzymatic deficiencies that result in cystic fibrosis (CF) and liver disease, or coagulation pathway deficiencies that result in various types of hemophilia. Gene therapy could be used to introduce a normal gene into the body to compensate for the low or absent function of the patient's mutant gene. For example, a proportion of children born with severe combined immunodeficiency (SCID) have a deficiency in ADA as a result of a mutation in the gene that encodes this enzyme. Several clinical trials have introduced a functioning ADA gene into the bone marrow of children with SCID using a viral vector, reconstituting the patient's immune system. Clinical trials to assess the potential for the treatment of hemophilia B through the transfer of a functioning factor IX gene and treatment of CF via introduction of an intact CF transmembrane conductance regulator gene (CFTR) have also been completed. These studies strongly suggest future applicability for gene therapy in the treatment of inherited diseases.
In the past decade, it has become clear that gene therapy may have its most immediate and effective role in treating acquired diseases including various cancers, arthritis, and atherosclerosis. New approaches have been adopted that focus on increasing the expression of beneficial genes beyond normal levels. For example, in a clinical trial for the treatment of melanoma, tumor-infiltrating lymphocytes (TILs) were purified from a tumor at the time of surgical resection. The TIL cells were then grown in the laboratory, and the gene for tumor necrosis factor (TNF) was introduced into the cells. The genetically engineered cells were subsequently transfused into the patient and preferentially migrated to the site of residual tumor, delivering a therapeutic dose of TNF. As the molecular basis for disease is elucidated, interest in this form of gene therapy will likely increase.
As the molecular pathways that underpin disease—particularly cancers—have been unraveled, excitement has arisen about the possibility of suppressing key genes known to be involved in the disease process. For example, RET , a protooncogene implicated in the pathogenesis of medullary thyroid carcinoma, can be inactivated by inducing expression of a mutant form of the RET gene, resulting in tumor regression. This approach is dependent on the “dominant negative” effect, whereby the mutant gene encodes a protein that retains the ability to bind to other key proteins that normally interact with the wild-type gene. However, the mutant protein is nonfunctional, which creates a competitive inhibition of the wild-type functional variant. Dominant negative therapies are presently being translated to clinical trials, particularly within the field of oncology.
A new technique that may have immense potential for the future of gene therapy is RNA interference (RNAi), a method by which genes can be selectively “silenced.” RNAi is a ubiquitous mechanism used by the cell to effectively “turn off” specific genes, as described a decade ago in a landmark paper. The potential for RNAi in research and therapeutics was immediately recognized. In effect, any gene, including those involved in cancer, could be silenced, and the effect of this loss of function on phenotype could be elucidated.
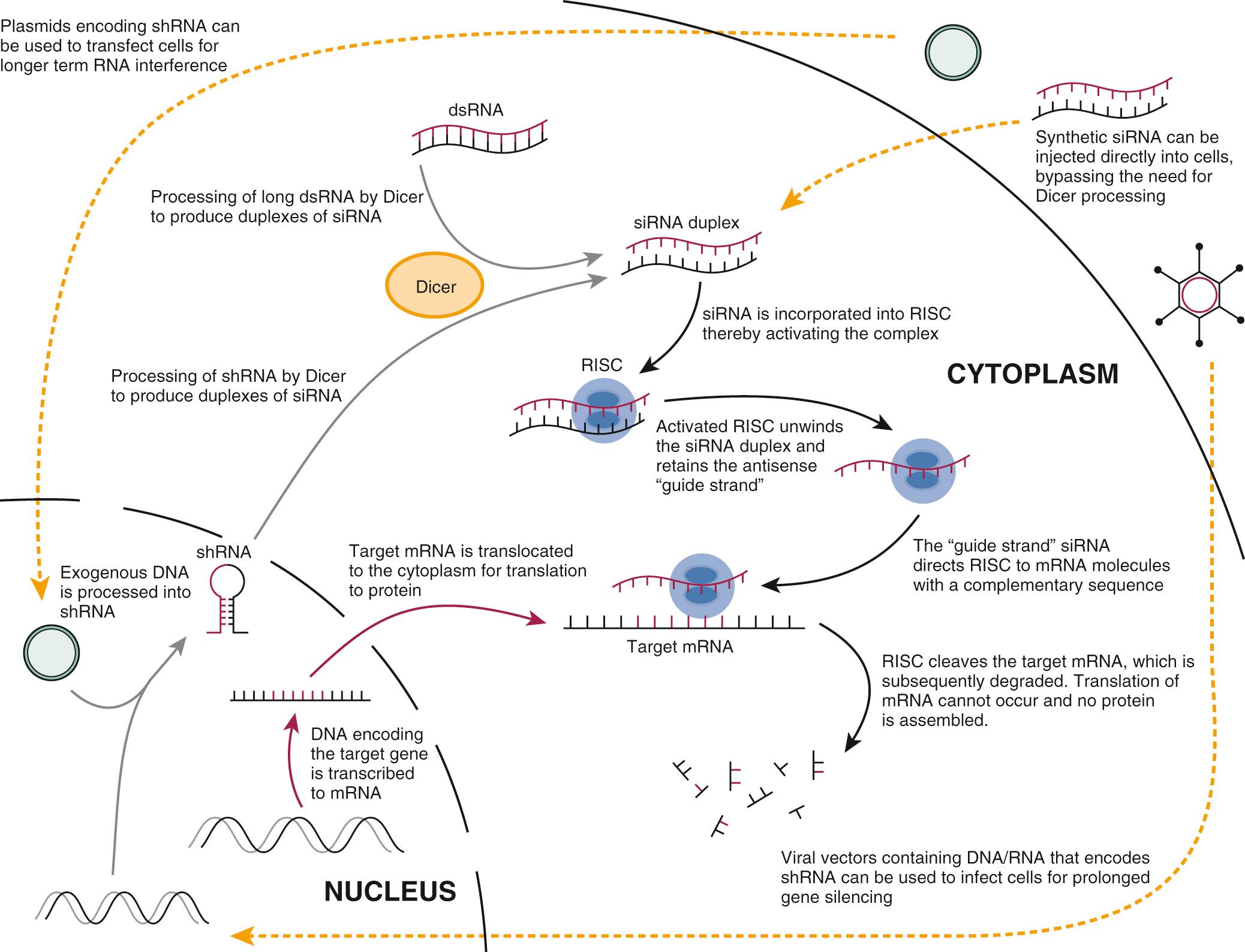
Subsequent studies have partially determined the mechanism for RNAi ( Fig. 72.3 ). In brief, an RNAase enzyme called Dicer cleaves dsRNA to form 21- to 23-nucleotide fragments of RNA. This short-interfering RNA (siRNA) is the actual effector molecule in RNAi. The siRNA is integrated into the RNA-induced silencing complex (RISC), after which the sense strand of the duplex is discarded. The antisense siRNA is retained and acts as the “guide strand” to direct RISC to messenger RNA (mRNA) molecules with a complementary sequence. RISC cleaves the targeted mRNA and thereby silences gene expression by blocking the translation of the mRNA sequence to protein.
Considerable progress has been made in applying RNAi to mammalian systems. The use of chemically synthesized 21-nucleotide siRNA molecules induces efficient, targeted gene silencing in mammalian cells without inducing a cytotoxic interferon response. These siRNAs can be used in subnanomolar concentrations to achieve a highly potent gene silencing by reducing mRNA levels by greater than 90%, while minimizing unsafe activation of the immune system. Unfortunately, a persisting problem associated with RNAi techniques are so-called off-target effects. This refers to unintended siRNA-mediated silencing of genes that have similar nucleic acid sequences. Even small amounts of sequence homology, as few as seven base pairs, are enough to produce off-target effects. Several computer algorithms have been designed to aid in the selection of siRNAs with minimal off-target effects. It is unclear what effect, if any, off-target effects will have in human trials, though studies using RNAi for the treatment of macular degeneration and neurodegenerative diseases have not revealed any significant side effects.
The last decade has seen the development of a new powerful technology that may revolutionize gene therapy. Genome editing allows for the addition, removal, or correction of genes. This process can be conducted ex vivo or, alternatively, the editing machinery can be delivered to target cells in vivo. In brief, an engineered nuclease acts as a “molecular scissor” and induces a double-strand break (DSB) at a specific site on the target DNA. This triggers DSB repair via nonhomologous end-joining (NHEJ) or homology-directed repair (HDR). NHEJ directly joins the broken ends of the DNA strand but is error prone, often inducing disruptive insertion/deletion mutations at the site of repair and, thereby, inactivating gene function. HDR uses a homologous sequence as a template to reconstitute DNA at the break point and is less prone to error than NHEJ. This process can be exploited by inserting a desired gene into a sequence that is homologous to the DNA flanking the DSB, which, after recombination, either corrects a mutation or inserts new desired gene sequences.
To induce the necessary DSB in a site-specific manner, customized DNA binding nuclease effector proteins are required. Transcription activator-like effector nucleases (TALENs) are synthesized by designing DNA binding domains that can recognize a specific DNA sequence, and fusing this domain to a nonspecific DNA cleaving domain. In effect, this allows these enzymes to cleave DNA at a specific site with extremely high accuracy. More recently, even more efficient methods of gene editing have been introduced. Doudna and Charpentier, in their landmark work, harnessed an antiviral defensive mechanism inherent to bacteria called clustered regularly interspaced short palindromic repeats (CRISPR). CRISPR-associated 9 (Cas9) nucleases process these sequences and cut matching viral DNA sequences. CRISPR-Cas9 can be targeted to a specific DNA site by using a short guide sequence of RNA (gRNA) that is complementary to the target site of interest. CRISPR-Cas9 has been successfully used to activate and deactivate genes, and even to correct single base mutations.
Though genomic editing remains in its infancy as far as clinical applications, this technology may overcome the drawbacks associated with other forms of gene therapy. The precision of TALENs and CRISPR-Cas9 platforms reduces the risk of off-target effects such as genotoxicity related to the activation of nearby proto-oncogenes, the deactivation of tumor suppressor genes, or disruption of normal promotors. However, the safety profile of genomic editing is an area of ongoing research. Continued work is attempting to clarify the extent of off-target mutations because of nuclease-induced NHEJ and HDR, and how to predict and detect on- versus off-target modifications to the genome. There are also hurdles related to the immunogenicity of nucleases when used in vivo and how to optimize delivery of the genomic editing machinery to the appropriate target tissue.
Despite these concerns, genomic editing is now being used clinically. TALENs have been used to delete specific T cell receptor genes on anti-CD19 T cells rendering them less likely to cause graft-versus-host disease. The modified, more tolerant T-cells were then administered to two patients with B cell acute leukemia with evidence of tumor response leading to Phase I clinical trials. Allogeneic T-cells from patients with acute myeloid leukemia have also been edited using TALENs to better target CD123, and this approach is also in clinical trials. Therapeutic gene insertion of the factor IX gene using genomic editing techniques is currently in clinical trials for the treatment of hemophilia B. Multiple clinical trials using CRISPR-Cas9 are underway in China, evaluating the potential benefit of knocking out the gene encoding the programmed cell death protein 1 (PD1). PD1 is a cell surface receptor expressed by T cells that promotes self-tolerance in T cells, thereby downregulating the immune system and preventing autoimmunity. This benefit is, however, double-edged in that immune tolerance may also compromise the ability of T cells to mount an anti-tumor response. By knocking out PD1 receptor expression on anti-tumor T cells, a more potent immune response against cancer cells could be generated.
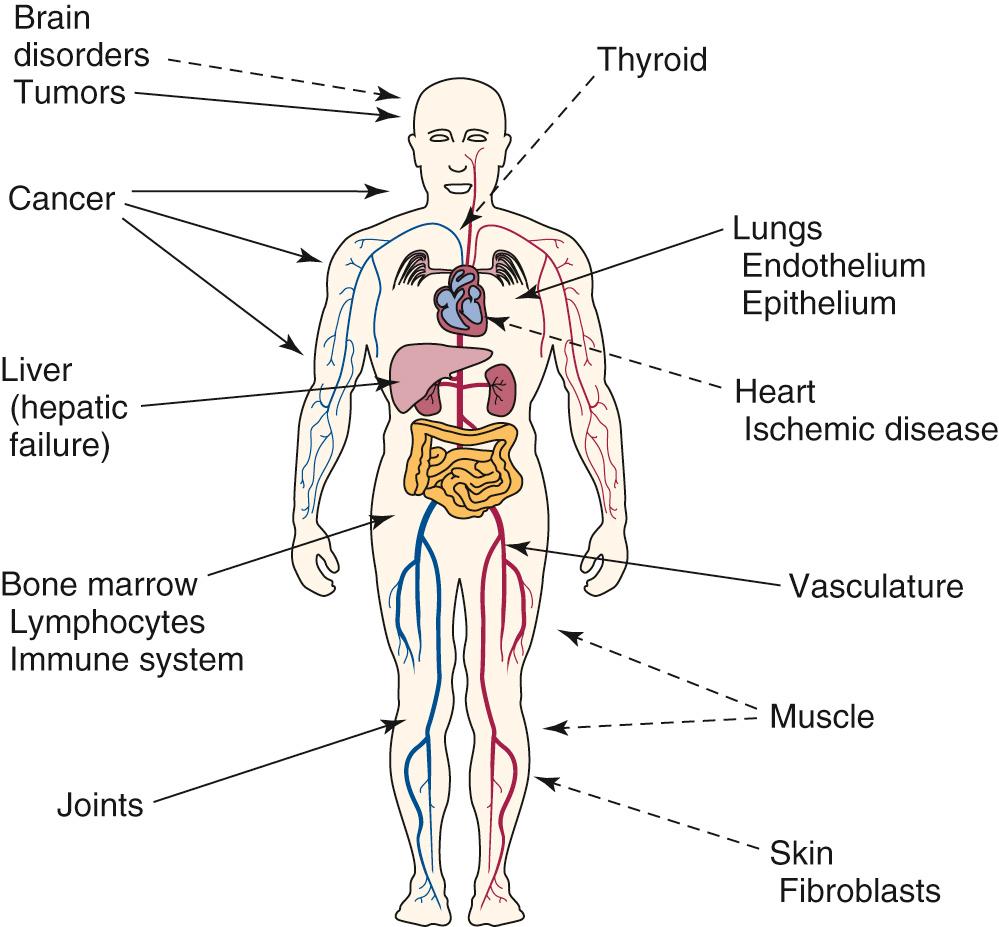
Gene therapy has two possible target cell types. Presently used targets are the somatic cells, or those cells that constitute the organs and postnatal tissues of the body. The second potential target is the germ cells, or those cells that produce the sperm or ovum and are passed on to a person's offspring. Many different organs and cell types are targets for somatic gene therapy, including bone marrow, liver, tumor cells, muscle, skin, endothelium, thyroid, and others ( Fig. 72.4 ). Genetic manipulation and therapy at these sites do not alter the inherited genetic material and raise few ethical issues. Genetic manipulation of the sperm or ovum, however, could prevent inherited diseases by altering the genetic constitution of offspring. Serious technical and safety issues, as well as profound ethical concerns, are inherent to this approach. One group, in China, used CRISPR-Cas9 to attempt to modify the hemoglobin gene in nonviable preimplantation human embryos, prompting renewed calls for the oversight of human genomic editing. Currently, in the United States, federal government funds cannot be used to research genetic manipulation of human germ cells.
A major focus in the field of gene therapy is the development of vehicles for introducing genetic material into selected target cells. Three general gene-therapy delivery methods can be distinguished: the first is direct administration of genetic material, which involves the injection of DNA or RNA into cells; the second is viral-mediated gene transfer, which involves packaging a therapeutic gene into a defective virus particle and using the process of viral infection to introduce the genetic material; and the third is the use of nanoparticles containing genetic material to bypass physiologic and immunologic barriers to delivery.
The gene that is delivered to a target cell is not itself therapeutic. Rather, it is the product encoded by the gene that is responsible for the resulting therapeutic effect. The gene product is typically a protein that has a specific function, such as a hormone or cytokine, but it could also be a bioactive RNA molecule, such as a gene-silencing siRNA that alters the regulation of pathologic processes. Thus it is the ability to achieve expression of the gene product at therapeutic levels that ultimately determines the effectiveness and efficacy of therapy.
The process of DNA-mediated gene transfer is called transfection , and the vehicle through which genetic material is transferred into a cell is called a vector . Functional DNA vectors are circular molecules of DNA called plasmids that contain various additional genetic elements that modulate gene expression, termed promoters and enhancers , that are required to achieve expression of the gene product at therapeutic levels ( Fig. 72.5 ). Plasmid vectors can be used to effectively deliver therapeutic genes to target cells, and have been used to prolong the transient, 3- to 7-day gene-silencing effect associated with the transfection of naked siRNAs.
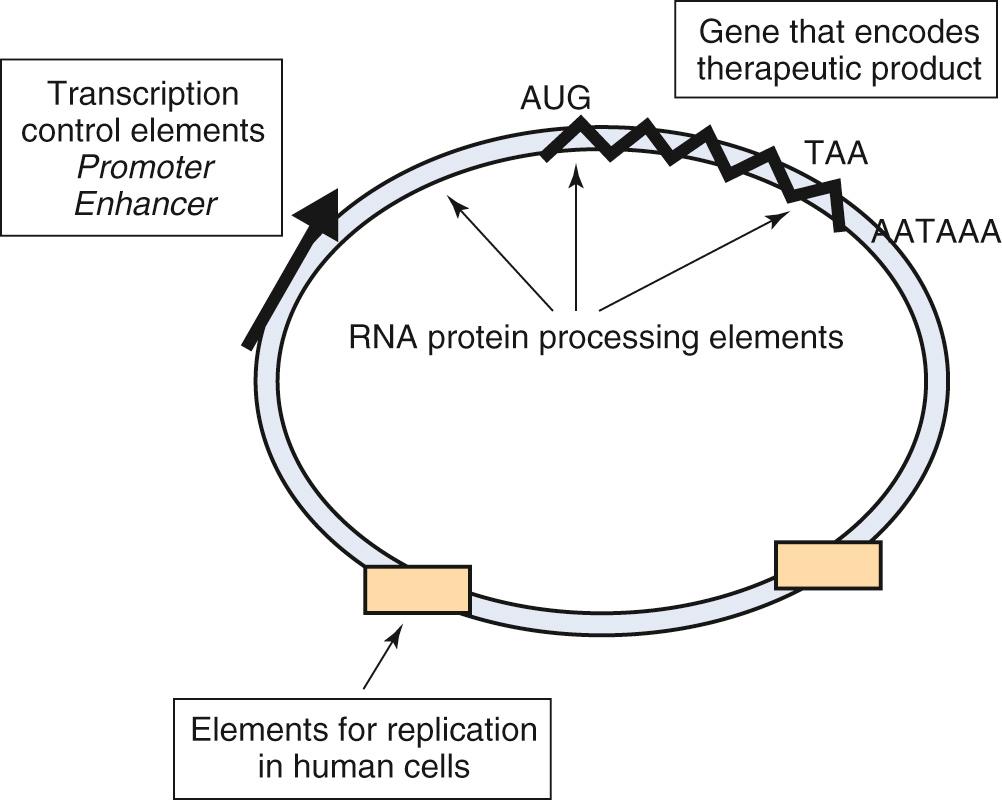
The delivery of DNA vectors into cells is possible via a variety of techniques. One classic method is to simply microinject plasmids directly into the cell nucleus. This technique is both time consuming and inefficient for achieving large numbers of transfected cells in living organisms. A more efficient process, which also is limited to in vitro application, is the process of electroporation, in which cultured cells are exposed to DNA in the presence of a strong electrical pulse. The electrical pulse creates pores in the cell membrane, which allow electrophoresis of plasmid DNA into the cell. The transfection efficiency of electroporation can be enhanced approximately fourfold by combining this technique with microbubble-assisted gene transfection, in which ultrasound is used to enhance permeability of the cell membrane.
It is possible to effectively introduce genes into muscle or thyroid simply by injecting DNA into these tissues in vivo, where the process of endocytosis enables cellular uptake. A common alternative method is the use of cationic lipids, which encase DNA vectors and fuse with the target cell membrane to enhance intracellular gene uptake. This process has been termed lipofection . Another method is to couple the DNA vector to proteins that bind to specific receptors on the target cell, leading to uptake of the DNA by receptor-mediated endocytosis. A novel approach to DNA vector delivery involves use of the “gene gun,” which uses electrical currents to project microscopic gold beads coated with plasmid DNA into target tissues and organs. This technique allows the simultaneous transfer of multiple genes, although the overall efficiency of transfection is low, and local inflammation may result. An important point to understand is that DNA-mediated gene transfer typically results in only transient residence of the therapeutic genes in the targeted cell due to degradation and elimination of the DNA vector. Different cell types eliminate the introduced genetic material at different rates. In muscle, for example, DNA vectors may persist in cells for many months and continue to express gene products. In contrast, DNA vectors injected into the thyroid have a shorter half-life, and the gene product is eliminated after 2 days. Vectors introduced into the liver are eliminated with a half-life of approximately 1 to 2 hours, and expression is significantly reduced after 6 to 24 hours.
DNA vectors are considered “safe” because they do not incorporate into the recipient cell's chromosome in vivo and thus should not alter the cellular genome. Moreover, DNA vectors have not induced any systemic immune response; thus they can be delivered repeatedly, which overcomes the potential limitation of transient therapeutic gene expression. The transient nature of gene expression does have an advantage in certain clinical scenarios, because the therapeutic gene can be administered by conventional oral, intramuscular, or IV routes to provide its beneficial effect over a predictable period. In contrast to conventional medications with short half-lives, DNA-mediated gene therapy could lead to prolonged expression of a gene product at continuous levels, thus eliminating the need for continuous infusions and enhancing compliance by minimizing the frequency of injections.
RNA can also be introduced into cells, opening the door to potential therapeutic implementation of gene silencing or genomic editing. TALENs or CRISPR-Cas9 nucleases, along with the targeting gRNA for the latter approach, can be transfected into cells as mRNA or RNA-protein complexes. The delivery of genomic editing enzymes into mammalian cells via RNA remains a subject of intense research. There have been significant advances in gene silencing via RNA-mediated delivery. In general, siRNA delivered locoregionally via the subcutaneous route, for example, can bypass first pass clearance by entering the microcirculation. However, delivery can be compromised due to phagocytosis by tissue macrophages. Oral administration is typically ineffective due to compromised passage across the intestinal epithelium and the stability of vectors in the gastrointestinal environment. Consequently, systemic administration is the most commonly used method of delivery via the IV route.
IV administration of siRNA targeting the vascular endothelial growth factor gene VEGF has been shown to significantly reduce tumor volume and intratumoral VEGF levels. Unfortunately, the gene-silencing effects of synthetic siRNAs last only minutes to a few days before they are degraded; however, chemical modification of the RNA can improve stability. The plasma half-life of an unmodified siRNA in a mouse model was 0.03 hours, the half-life of a chemically modified siRNA was 0.8 hours, and the half-life of a lipid-conjugated, modified duplex was 6.5 hours.
The most common method used to date for the delivery of siRNA is termed hydrodynamic delivery. This approach involves injecting a large volume of an RNA-containing solution as a rapid IV bolus. The hydrostatic pressure generated transiently disrupts the vascular endothelium and achieves siRNA delivery to tissues. Hydrodynamic delivery has been successfully used to deliver siRNA to skeletal muscle in rats, dogs, and rhesus macaques. Localized delivery can be achieved by application of a tourniquet to isolate a limb before IV injection. The potential for this approach in humans is under investigation and will likely transition to clinical trials.
The need for sustained gene silencing has led to the development of novel DNA plasmid expression vectors that direct the synthesis of short RNA over a prolonged period (see Fig. 72.3 ). The encoded short RNA sequence is transcribed and processed into the effector gene-silencing RNA molecule. One method involves genetically engineering a plasmid to encode a short-hairpin RNA (shRNA). An shRNA molecule consists of a special double-stranded RNA molecule that includes a sharp hairpin turn, and it is processed in the cell to yield a 21-nucleotide RNA construct capable of gene silencing. Use of DNA plasmid vectors can prolong in vivo mammalian gene silencing for up to several weeks. They are, however, limited by their inability to transfect nondividing cells. This limitation can be overcome, however, by using viral vectors to deliver siRNA, which is discussed in the next section.
Viral-mediated gene transfer involves the construction of synthetic virus particles that lack pathogenic functions, are incapable of replication, contain a therapeutic gene within the viral genome, and can deliver this gene to cells by the process of infection. By altering the type of viral vector used, gene expression can be designed to be transient in, for example, cancer therapy or permanent, as in the case of genetic diseases. However, concerns related to the potential for viral genome insertion into patient chromosomes, induction of a significant immune response, and costly production have limited the widespread use of viral vectors.
The original prototypes for viral-mediated gene transfer are retroviral vectors derived from the Moloney murine leukemia virus. Retroviral vectors were chosen as vehicles because of several useful properties. First, “defective” virus particles can be constructed that contain therapeutic genes and are capable of infecting cells but that contain no viral genes and express no pathogenic viral gene products. A general scheme for constructing a defective retroviral particle is illustrated in Fig. 72.6 . Second, retroviral vectors are capable of permanently integrating the therapeutic genes they carry into the chromosomes of the target cell. Because of this property, retroviral vectors are well suited for treating diseases that require permanent gene expression. Third, modifications can be made in retroviral vectors and in the cell lines that produce vectors that result in enhanced safety features.
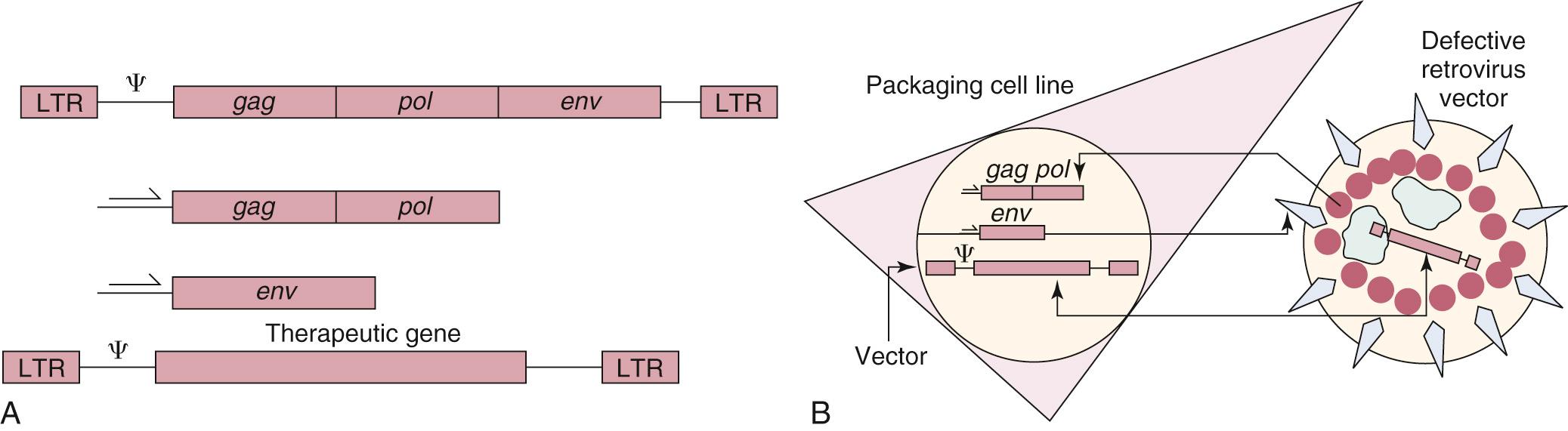
A major limitation for this strategy is that retroviruses will only integrate into actively dividing cells, and the efficiency of retroviral infection is relatively low. This shortcoming has been addressed to some extent with the development of lentiviral vectors, which are related to retroviruses but can insert into the genome of nondividing cells. Lentivirus vectors also preferentially integrate into the coding regions of genes, unlike retroviral vectors, which will integrate into the 5′ untranslated region of genes, potentially increasing the risk of oncogenic insertional mutagenesis. Perhaps the most serious problem, however, has been the difficulty in achieving stable, regulated gene expression from retroviral vectors in cells, despite the permanent genomic integration. Cells are able to shut off expression from retroviral vectors under certain conditions, which have not been clearly defined.
Previous experience in animal models and initial experiences in clinical trials suggest that these vectors are, generally, safe. However, serious complications have been reported in clinical trials that have used retroviral vectors to treat X-linked SCID, chronic granulomatous disease, and Wiskott-Aldrich syndrome. Although gene therapy produced excellent clinical outcomes in most children, several cases of acute myeloid and lymphoid leukemias were reported 2.5 to 5 years after therapy. This has been directly attributed to the activation of proto-oncogenes in proximity to the viral integration site, which occurs due to the tendency of retroviral vectors to insert near promoter regions. The powerful enhancers within the vectors that are used to drive expression of the therapeutic gene may also activate nearby oncogenes. Consequently, newer generation retroviral vectors, used in most modern clinical trials, are designed with removal of endogenous strong enhancer elements using a self-inactivating design to decrease oncogenicity. These newer vectors have been successfully used to treat X-SCID and Wiskott-Aldrich syndrome without induction of cancer.
In fact, retroviral vectors have been recently used to engineer T cells as a treatment for cancer. Chimeric antigen receptors (CARs) are custom receptors targeted toward a specific antigen that can effectively reprogram the specificity and function of T cells. Typically, CARs are transduced into T cells ex vivo and then administered systemically, triggering a rapid expansion in antigen-specific T cells. Given that the generation of CAR-T cells requires stable persistent gene expression, lentiviral vectors are often used to transduce T cells, though more recently, CRISPR-Cas9 has been used for this purpose. Therapy using CAR-T cells targeting CD19 has induced remission in refractory acute lymphoblastic leukemia and diffuse large B cell lymphoma. The responses were durable enough that the FDA approved two separate CAR-T based therapies in 2017. CAR-T treatment of solid tumors is under investigation, though the absence of tumor-specific cell surface antigens has complicated the targeting of these therapies. Furthermore, CAR-T therapy has triggered cytokine release syndrome and neurotoxicity, which has led to serious morbidity and even death in patients. The safety profile of CAR-T therapies will need to be established before widespread clinical use becomes mainstream.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here