Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter provides a snapshot of fetal and neonatal development of the diaphragm muscle (DIAm), the major inspiratory muscle in mammals, and partition between the thoracic and abdominal cavities. The DIAm appears rather late in evolution, being present only in mammals, while other vertebrates use different means of ventilation. The embryologic origin of the DIAm has been elucidated by studies using molecular markers of muscle development, reflecting a common origin to the DIAm in the pleuroperitoneal fold. The traditional view of DIAm development called for multiple sites of origin reflecting a complex derivation, consistent with its multiple anatomic origin and insertion sites. The mechanical actions of the DIAm reflect its functions during ventilatory (inspiration) and several nonventilatory motor behaviors. These nonventilatory behaviors include expulsive maneuvers such as coughing, defecation, emesis, micturition, parturition, and sneezing; postural activations while performing lifting activities; and emotional behaviors such as vocalization and musicianship.
Neural control of the DIAm is similar to other skeletal muscles and is based on recruitment and frequency coding of motor units. Motor units in the adult DIAm, each comprising a motor neuron and the muscle fibers it innervates, vary considerably in their mechanical, histochemical, and biochemical properties, and this heterogeneity provides the range of control of muscle force generation that occurs during different motor behaviors. It is the cumulative contractile and fatigue properties of the motor unit pool that determine the constraints under which the DIAm responds to the various mechanical demands placed upon it during different ventilatory and nonventilatory behaviors. Clearly, these motor demands can change during development, and the DIAm must adapt or remodel to accommodate these changing demands.
The DIAm becomes rhythmically active during late fetal development (fetal respiratory movements), and at birth, it must be ready to sustain ventilation. Postnatally, the DIAm is one of the most active skeletal muscles, with a duty cycle (time active versus relaxed) of ∼30% to 40%, compared to limb muscles (duty cycles ranging from 2% to 15%). It is not surprising, therefore, that the fetal and neonatal pathology of the DIAm often lead to ventilatory failure and premature death. Despite the vital importance of the DIAm, previous studies have provided mostly descriptive information about its development and growth. However, there is accumulating information about myogenesis and neural development that derives from in vitro model systems, which may be applicable to the mechanisms regulating DIAm development in vivo. Yet, the applicability of these in vitro results regarding the basis of myogenesis and neural development remains to be established, especially in the context of maturation of other systems, for example, the central nervous system (CNS), lung, and thoracic and abdominal walls. Unfortunately, such integrative information is lacking.
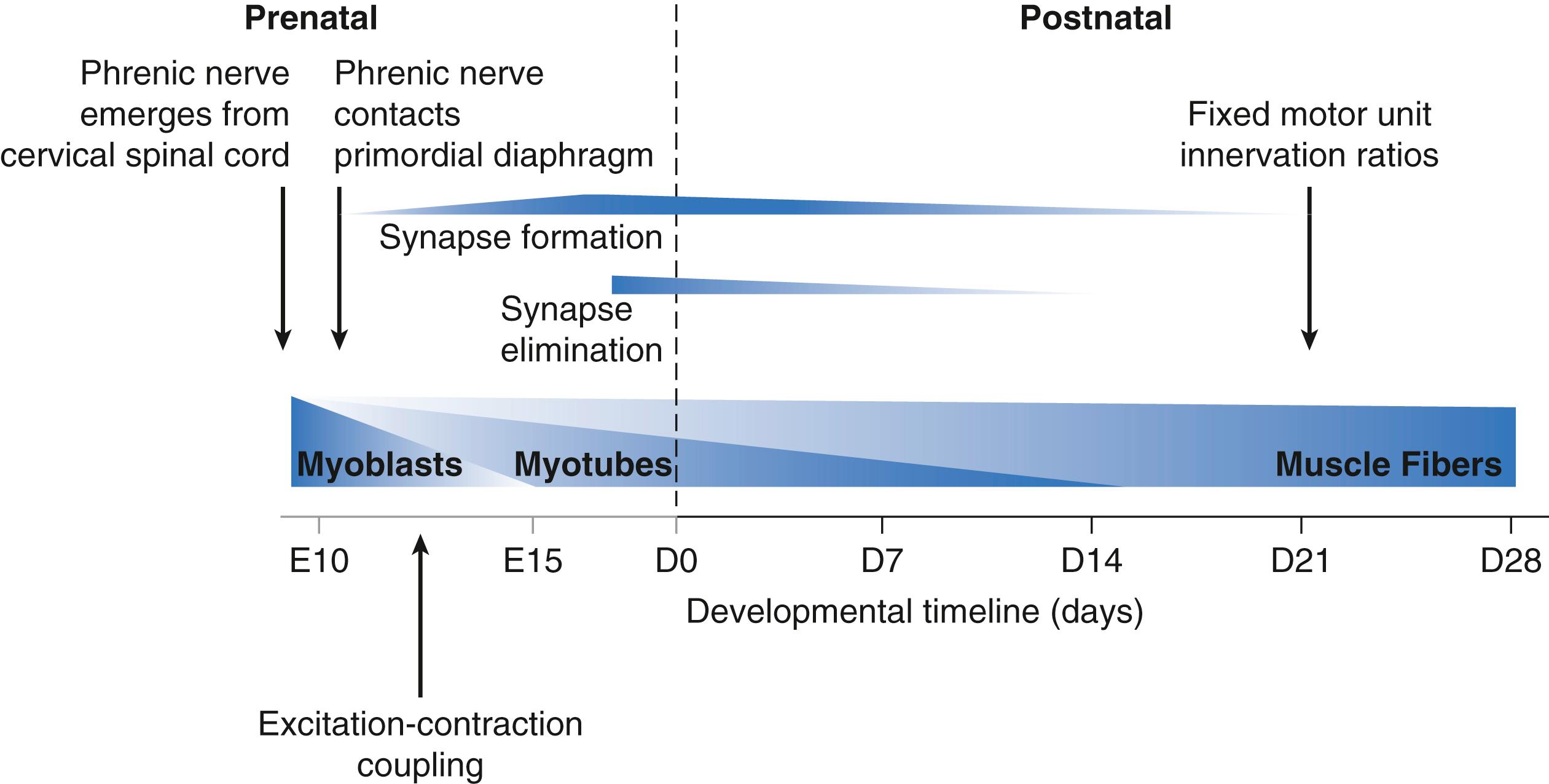
Rodent models are increasingly being used to explore the genetic basis for developmental plasticity in neuromotor control. Accordingly, in this chapter, we focus on recent results obtained in rats and mice. Benchmarks for the development of the mouse DIAm are summarized in Fig. 62.1 . The fetal and neonatal developmental benchmarks in rats are generally comparable to those in mice (offset by ∼2 days). In mice, developmental benchmarks for the DIAm begin at approximately embryonic day 11 or 12 (E11, E12) when the phrenic nerve makes initial contact with the primordial DIAm. In both rats and mice, the final pattern of adult motor units and muscle fiber types is not achieved until postnatal day 28 (D28). During this 5 to 6 week span, dramatic changes in DIAm innervation, contractile protein expression, and function occur. Obviously, the timeline for such developmental benchmarks in humans is far more protracted, but it is likely that similar convergence of neural and myogenic events also occurs. Therefore, much can be learned by studying the integrated aspects of myogenesis and neural development of the DIAm in rodent models.
Skeletal muscle fibers, including those in the DIAm, are formed in two stages: (1) commitment (also known as determination ), when mesodermal progenitor cells are transformed to myoblasts; and (2) terminal differentiation, when myoblasts fuse to form myotubes and myofibers ( Fig. 62.2 ). Myogenesis may proceed via an intrinsic genetic program, or it may require either the presence of positive extrinsic signals or the removal of inhibitory signals. None of these potential mechanisms can be presently excluded. However, there is converging evidence that myogenesis is induced in a variety of nonmuscle cells by the expression of proteins belonging to the basic helix-loop-helix (bHLH) superfamily. , Collectively, these are known as muscle regulatory factors (MRFs) and include MyoD, myogenin, Myf-5, and MRF4/Myf-6. In addition to providing a molecular mechanism for the regulation of myogenesis in fetal and neonatal muscle, MRFs are also involved in determining the expression of myosin heavy chain (MyHC) isoforms in adult muscle.
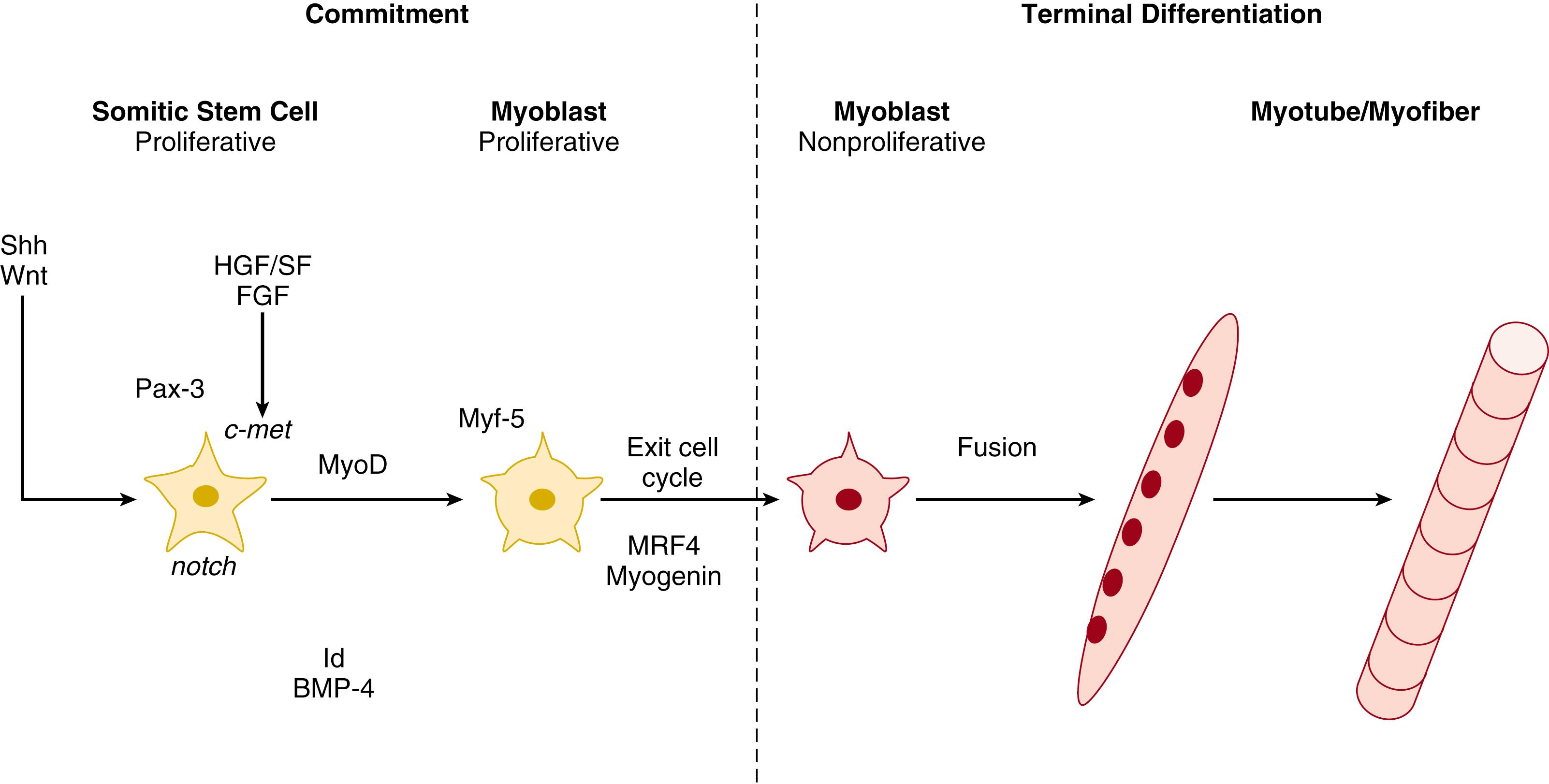
Genetic determinants of myoblast commitment have been most extensively studied in Drosophila , but this information is likely to be directly applicable to myoblast commitment in the mammalian DIAm as well. In Drosophila , it has been shown that neural-derived proteins (e.g., sonic hedgehog, Wnt 1, 3, and 4) promote commitment to myoblasts within a myotome. , Expression of sonic hedgehog and/or other signals commit mesodermal cells to form myoblasts possibly by releasing suppression of the expression of specific MRFs. The process of myoblast commitment is marked by the expression of the paired-box protein Pax-3 , which appears to positively regulate MRF expression, and in turn is regulated by Six1and Six4. For example, the ectopic expression of Pax-3 induces both MyoD and Myf-5 in embryonic tissue. The consequent increase in MRFs then initiates a cascade of signals that continues through differentiation, thus sustaining myogenesis. Indeed, transplantation of Pax-3 -expressing embryonic stem cells into dystrophic mice resulted in engraftment of stem cell precursors with adult myofibers and enhanced contractile function.
MRFs function as molecular switches, initiating myogenesis by inducing expression of muscle-specific genes through binding of the bHLH motif to cis -acting DNA control elements of muscle-specific genes, known as E-boxes . The E-box is a CANNTG sequence-containing motif present in the promoter regions of many skeletal muscle-specific genes. Transcriptional regulation mediated by MRFs may also involve interactions of MRFs with the family of MADS-box proteins termed myocyte enhancer factor 2 (MEF2A-D) , which may determine muscle-specificity for genes lacking MRF binding domains. Binding sites for MEF2 factors are located in the promoter regions of many muscle-specific genes, including myogenin. However, MEF2 binding alone is not sufficient to induce myogenesis.
It is also possible that myoblast commitment is determined by release from inhibition by signals that actively suppress MRF expression. For example, a class of HLH proteins termed Id factors (Id1–Id4) inhibit MRF activity by forming heterodimers with E2 products (ubiquitously expressed HLH-containing proteins encoded for by E2 genes). These products prevent dimerization with MRFs and thus block subsequent activation of skeletal muscle-specific genes. The Id proteins lack the basic DNA binding domain; therefore, the heterodimerization of bHLH proteins with Id proteins prevents the strong binding to DNA by either E-proteins or MRFs. Twist is another protein in mice that sequesters E-proteins that inhibit MRF activity by preventing the formation of MRF-E protein heterodimers. Yet another inhibitory factor is Mist1, which binds MyoD to form an inactive heterodimer. The balance between inhibitory and pro-myogenic signals likely determines the timing and location of muscle precursor development. Calcineurin was shown to activate MyoD indirectly by decreasing the expression of the Id inhibitory proteins. Others have shown that Nfix may also play a key role in the transition of embryonic to fetal skeletal muscle gene expression through binding with MEF2A. , A knockout mouse for Nfix (E16.5) shows disorganized sarcomeres, downregulation of β-enolase, and muscle creatine kinase (MCK) though myogenin expression remains intact. Myogenic inhibitory signals may be critical to prevent the ectopic formation of skeletal muscle because mesodermal somite cells can follow both myogenic and nonmyogenic programs.
In hypaxial muscles (e.g., limb muscles, chest, and abdominal wall muscles and DIAm) MyoD is the first MRF to be expressed. MyoD expression then initiates a cascade resulting in subsequent expression of Myf-5, MRF4, and myogenin. The sequence of MRF expression appears to be critical for the development of normal muscle. MyoD and Myf-5 are highly expressed in proliferating myoblasts, suggesting a primary role for these MRFs during this stage of myogenesis. In mutant mice lacking MyoD or MRF4, , there is no apparent effect on normal skeletal muscle development, but Myf-5 and myogenin are upregulated, respectively. Therefore redundant MRF expression may rescue the normal skeletal muscle phenotype in these animals. Similarly, Myf-5 deficient mice appear to have normal skeletal muscle, but they succumb to asphyxiation soon after birth due to rib cage abnormalities. In contrast, myogenin deficient mice have only myoblasts without the development of myotubes/myofibers, , suggesting that myogenin is indispensable for differentiation of myotubes/myofibers. In mice lacking both Myf-5 and MyoD, no myoblasts are present, suggesting that the expression of both of these MRFs is required for myoblast commitment. Mice lacking both Myf-5 and MRF4 resemble Myf-5 knockouts, suggesting that MRF4 regulates later aspects of myogenesis.
With terminal differentiation, committed mononucleated myoblasts are nonreversibly transformed into multinucleated myotubes/myofibers with the expression of contractile proteins (see Fig. 62.2 ). Thus terminal differentiation represents the irreversible exit of proliferating myoblasts from the cell cycle. Yet, not all myoblasts lose their ability to proliferate because a pool of myoblasts (satellite cells) persists into adulthood, and their proliferative capacity is important in processes of injury and repair as well as other conditions of muscle remodeling. Throughout life, proliferating myoblasts are susceptible to apoptosis, that is, programmed cell death, until they are terminally differentiated and exit the cell cycle. The balance between proliferation and apoptosis may play an important role in controlling the total number of muscle fibers of a given type. Terminal differentiation of myoblasts in hypaxial muscles does not occur in vivo until they have migrated from the myotome to their final location (e.g., thoracic or abdominal walls or to the limbs). As myoblasts migrate from the lateral dermomyotome, they express Myf-5 and c-met (a tyrosine kinase receptor for hepatocyte growth factor/scatter factor), which thus are used as markers for this process. In hypaxial muscle precursors (including DIAm), expression of Myf-5 and c-met begins at ∼E10 and continues through E12. The expression of MyoD is also important in the process of terminal differentiation and can be inhibited in vitro by bone morphogenic protein-4 or fibroblast growth factor-5, 17 both of which have been implicated in the maintenance of the myoblast proliferative capacity and inhibition of differentiation.
Following terminal differentiation, myoblasts fuse forming multinucleated myotubes/myofibers. Although the formation of myotubes/myofibers is coincident with the initial appearance of innervation in hypaxial muscles, converging evidence from in vitro and in vivo models indicates that terminal differentiation can be initiated in the absence of neural influence. Myotubes/myofibers can form in vitro in the absence of innervation, but at a much slower pace compared to that observed in vivo. This suggests that neural influence may facilitate the normal process of terminal differentiation and formation of myotubes/myofibers. This potential facilitating influence of innervation needs to be further explored. There is evidence, based on in vitro model systems, that terminal differentiation and myotube/myofiber formation depend on the expression of several non-neural proteins, including those present in the extracellular matrix (e.g., fibronectins and laminins), basal lamina (e.g., muscle cell adhesion molecule [M-CAM], neural cell adhesion molecule [N-CAM] and M-cadherin), cell membrane (e.g., β1-integrin), and cytoskeleton (e.g., actin and desmin). , Obviously, terminal differentiation and myotube/myofiber formation involve very complex interactions between differentiating cells and their surrounding environment. Current research is only starting to unravel these complex interactions . It should be noted that even though the process of myoblast fusion into myotubes/myofibers is called terminal differentiation , there is subsequent differentiation of these nascent muscle fibers into adult muscle fiber types (see below).
Other factors may also influence myogenesis. For example, in vitro passive mechanical strain results in both myotube hyperplasia and hypertrophy. , Thus, both myoblast proliferation and differentiation are affected by passive strain. Passive stretching can also prevent the atrophy of myotubes, which normally occurs in culture media lacking growth factors. The mechanisms responsible for the transduction of passive strain into a proliferative and/or trophic influence is unclear but may involve mediators such as prostaglandin (PG)F 2α , insulin-like growth factor-1 (IGF-1), or other cytokines. Although these in vitro studies employed passive strain, these results suggest that early mechanical activation of the fetal DIAm (e.g., via fetal respiratory movements) may be important in promoting further myogenesis.
Myosin is a hexameric protein (MW = 480 kDa) comprising two heavy chains and four light chains. At the rod-shaped COOH-terminus end of the myosin molecule, the two heavy chains (MW = 200 kDa) dimerize into a 200-nm α-helical tail. At the NH 3 -terminus, the heavy chains separate and form two distinct heads (S-1), which contain both actin and nucleotide-binding domains. The S-1 converts chemical energy into mechanical work through a process that involves stereo-specific docking of S-1 with actin, thereby reversing the intra-molecular conformational changes induced by ATP hydrolysis. , A number of MyHC isoforms exist, and all of which are encoded by a highly conserved multi-gene family located on chromosome 17 (human) or 11 (mouse). The rate of ATP hydrolysis at the S-1 varies among the different MyHC isoforms, thereby providing the molecular basis for fiber type differences in mechanical properties. , The essential (MLC 20 ) and regulatory myosin light chains (MLC 17 ) provide structural support and possibly modulate the mechanical performance of the MyHC. Different MLC isoforms may differentially modulate the kinetic properties of MyHC. There is also evidence that both Ca 2+ binding to MLC 17 and phosphorylation of MLC 17 modulate S-1 ATPase activity.
Adult skeletal muscle fibers are classified histochemically as type I, IIa, and IIb based on the pH lability of myofibrillar ATPase staining. Muscle fiber type classification in the adult corresponds with the expression of different MyHC isoforms; fibers classified as type I express MyHC Slow , type IIa fibers express MyHC 2A , and type IIb fibers express MyHC 2B and/or MyHC 2X . , Such histochemical classification of muscle fiber types is not possible during fetal and neonatal development. During this period, there are dramatic transitions in MyHC isoform expression, with a high incidence of co-expression of MyHC isoforms that precludes ready distinction of different muscle fiber types. In the fetal mouse and rat DIAm, an embryonic MyHC isoform (MyHC Emb ) is predominantly expressed together with MyHC Slow and MyHC 2A . At or close to birth, the predominant isoform expression switches from an embryonic MyHC isoform (MyHC Emb ) to a neonatal (MyHC Neo ) isoform, together with MyHC Slow and MyHC 2A . Thereafter,MyHC Neo expression gradually disappears and is totally absent in the mouse and rat DIAm by D28. Expression of MyHC 2X and MyHC 2B isoforms emerges only after D14 in the mouse and rat DIAm, and the proportion of fibers expressing these isoforms increases until about D28, when the adult pattern of MyHC isoform expression is fully established. This dramatic postnatal transition in MyHC isoform expression in the DIAm represents an important stage of muscle fiber differentiation, especially with respect to the development of mature contractile properties (see below). Beyond D28, the relative contribution of each MyHC isoform changes due to the disproportionate growth of DIAm fibers; for example, the growth of fibers expressing MyHC 2X and MyHC 2B is approximately two- to threefold greater than that of fibers expressing MyHC Slow and MyHC 2A isoforms. , Based on the temporal associations between innervation and the major developmental events in myogenesis (see the following section), it would seem reasonable that the nervous system can either directly (e.g., activity, nerve traffic) or indirectly (e.g., secretion of trophic factors) influence DIAm development. However, the precise mechanisms by which activation history and/or trophic factors influence DIAm development remain largely unknown.
The time course for developmental transitions in MyHC isoform expression in the DIAm is also influenced by the hormonal milieu (e.g., thyroid hormones, growth hormone, and IGF) surrounding the fibers. For example, MyHC expression is sensitive to thyroid hormone levels. , Nutritional status can also affect myogenesis and MyHC isoform expression.
The determination of skeletal muscle fiber type has received considerable attention. Expression of class II histone de-acetylases (HDACs) may determine muscle fiber type by inhibiting the transcription factor MEF2. MEF2 factors are required for the transcription of oxidative genes found in slow-twitch fibers. HDAC activity is also regulated posttranslationally via ubiquitination and proteasome degradation. Whether these mechanisms play a role in the developmental determination of muscle fiber type is still to be explored. We have examined MyHC mRNA expression during postnatal development of DIAm and found postnatal changes in mRNA and protein expression were not concordant for adult MyHC isoforms, suggesting that changes in MyHC expression in the developing rat DIAm are not driven solely by changes in mRNA expression. Furthermore, myonuclear domain size (reflecting the volume of myoplasm under transcriptional control by a single myonucleus) increased postnatally as fibers increased in cross-sectional area, indicating that changes in transcriptional activity (although present) do not exclusively determine the postnatal growth of the DIAm.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here