Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sudden unexplained death (SUD) is defined here as the death of an individual that remains unexplained after a thorough investigation, which often includes gross and microscopic examination by the medical examiners, detailed cardiac pathology and neuropathology examinations, laboratory tests (e.g., toxicology and microbiology), and a review of the available medical records. SUD occurs in all age groups from infants in their first year of life, to young and older adults alike.
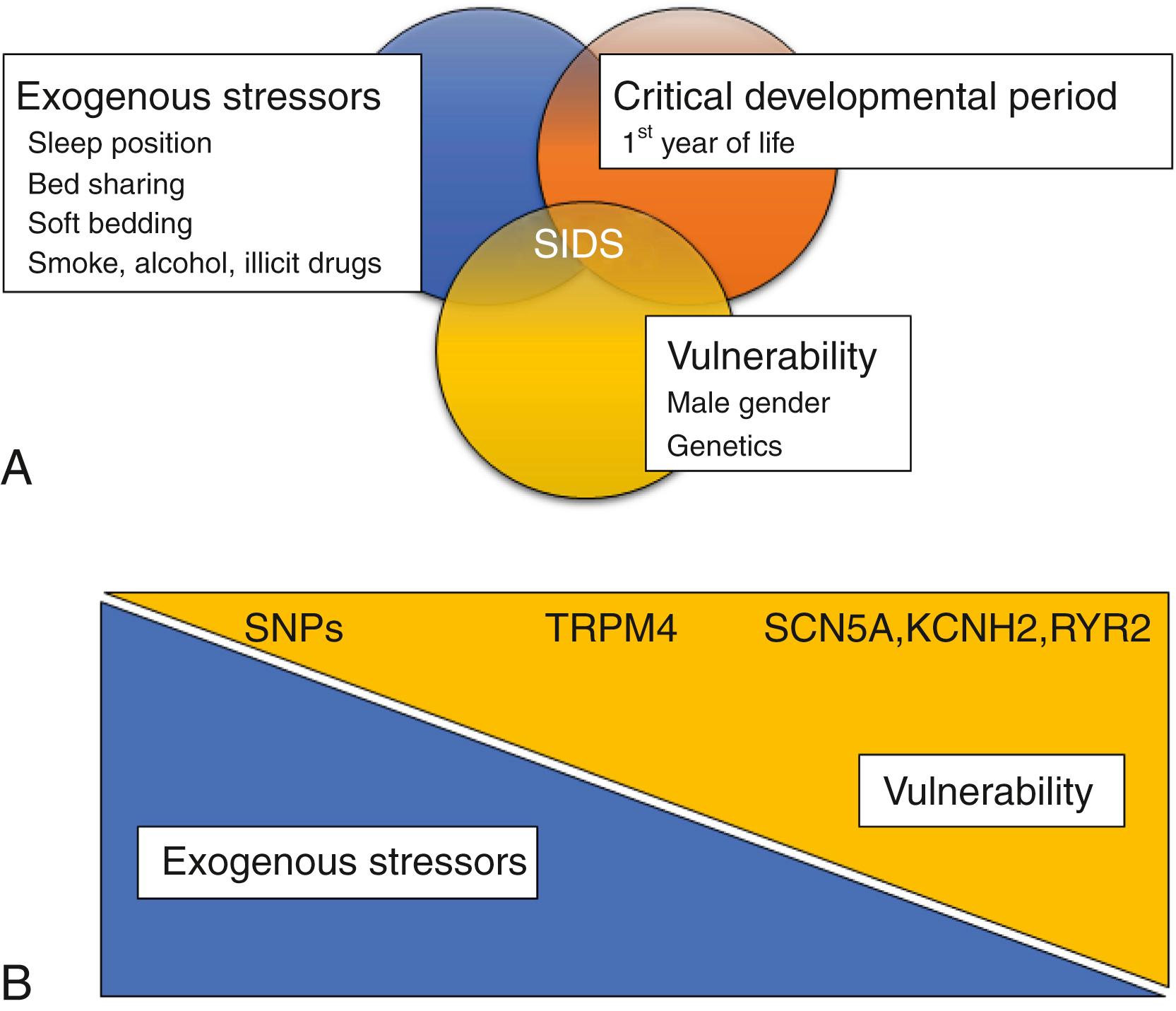
In apparently healthy infants, unexplained infant death is the leading cause of death before their first birthday, and it often occurs within the first 6 months of life. The infant mortality rate in the United States was approximately 45 deaths per 1000 live births in the 1960s. The American Academy of Pediatrics (AAP) recommended in 1992 that all babies should be placed on their backs to sleep. Although infant deaths from unexplained causes have subsequently declined, sleep-related deaths from other causes, including suffocation, entrapment, and asphyxia, have increased. New AAP guidelines, revised in 2016, include healthy sleep patterns; a firm sleep surface; avoiding co-sleeping in the same bed; breastfeeding; using a pacifier; and avoidance of overheating and exposure to tobacco smoke, alcohol, and illicit drugs. These guidelines have led to a further decline in sudden unexplained infant death (SUID). There remains, however, a persistent incidence, and a further reduction is a major challenge. Infants have about a 10 times higher risk of sudden cardiac death (SCD) compared with older children: 12.8 vs. 1.1 to 2.0 per 100,000, respectively, according the latest American Heart Association’s Heart and Stroke Statistics Update. Understanding the causes of SUID can potentially further curb this problem. The triple risk model for sudden infant death syndrome (SIDS) proposed in 1994 by Filiano and Kinney involves the intersection of three risks: (1) a critical developmental period in homeostatic control, (2) exogenous stressors, and (3) a vulnerable infant ( Fig. 13.1A ). Increasingly, studies are being performed to establish whether genetics may provide answers to at least one of these elements, namely to create a vulnerable infant.
Some forms of arrhythmias in children are related to congenital structural heart defects or manifest after corrective surgery. Many children, however, have congenital arrhythmias unrelated to structural heart defects. Data-driven studies of channelopathies in pediatrics are rare. An exception is long QT syndrome (LQTS), which was investigated prospectively in an Italian pediatric population. A neonatal electrocardiography (ECG) study that involved 44,596 infants of 3 to 4 weeks of age suggested the prevalence of LQTS to be as high as 1 in 2500 patients. Other forms of channelopathies, such as Brugada syndrome, short QT syndrome (SQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT), are recognized in children, but their prevalence has not been formally studied in large pediatric populations. Children also have other forms of arrhythmias. For example, congenital atrioventricular (AV) block is a rare disorder with an estimated prevalence of 1 per 20,000 live births. The vast majority of these cases are caused by maternal autoimmune disease, which leads to fibrosis of the fetal conduction system. In the remaining cases, variation in genes coding for ion channels subunits may be responsible for the defect.
Sudden death in adults is a well-reviewed topic. The majority of sudden deaths in adults are cardiac in origin; therefore it is often referred to as sudden cardiac death. SCD is a major health burden. The American Heart Association’s Heart and Stroke Statistics 2019 Update shows that out-of-hospital cardiac arrest (OHCA) in the United States is high and survival is low. Estimates are of more than 356,000 OHCAs annually in the United States, with nearly 90% of them being fatal. Although the overall incidence of OHCAs in children is lower, the mortality rate is high. Among adults, the risk of SCD increases exponentially with age, surpassing the risk for infants by the age of 40 years (20.3 per 100,000). A large proportion of OHCA in the general population results from coronary artery disease (CAD) and arrhythmias, which is most commonly associated with coronary heart disease, dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy (HCM). Most of the remaining SCDs are associated with underlying causes such as infiltrative, inflammatory, and valvular diseases. In a very small fraction of middle-aged and older adults (<1%), SCD is caused by disorders such as LQTS, Brugada syndrome, HCM, and right ventricular dysplasia, or to the developmental congenital disorders. This chapter is focused on SUD when, during autopsy, there is no evidence of overt or severe CAD, valvular diseases, cardiomyopathies, or congenital disorders. In these cases, the likelihood is strong that death may have resulted from spontaneous arrhythmias or some of the rarer cardiac disorders (e.g., LQTS or Brugada syndrome). In the adult, the triple risk hypothesis of SIDS clearly does not hold because of the absence of the critical developmental period. Nevertheless, it may be appropriate to consider adult SUD as a dual-risk disease model that involves (1) exogenous stressors and (2) a vulnerable individual, with genetic factors influencing the vulnerability element (see Fig. 13.1B ).
Medical examiners routinely assume jurisdiction over a probable natural death when other physicians are not available or willing to certify the death. Although many deaths coming to the attention of the medical examiner are due to natural causes, a definitive cause of death may not be identified in some cases, even after a complete and thorough autopsy, histology, and toxicology examination and other on- and off-site investigations. Such “negative autopsies” may prompt further investigations, including “molecular autopsies” or genetic screening. If applicable, or possible, family members may also undergo genetic screening. Genes often screened in cases of SUD and SIDS include those with a known involvement in metabolic diseases, cardiac disorders, neurotransmitter responses in the central and peripheral nervous systems, and the inflammatory response. The focus of this chapter is on genetic testing of ion channel genes (channelopathies) in SUD and SIDS.
Biologic variables that contribute to SUD may include age, sex, race, cardiac disease, arrhythmias, epilepsy, and HCM. The genetics of SUD is therefore expected to be quite complex. Nevertheless, a clear involvement of ion channel genes has emerged. In cardiac myocytes, the activities of several types of ion channels shape the exact shape of the action potential, determine the amount of Ca 2+ influx/efflux with each heartbeat, and determine rhythmicity and contractility. A small disruption in net ion current has the potential to upset the delicate balance between inward and outward ionic currents that is needed to maintain a normal action potential, which can precipitate life-threatening arrhythmias. Although dysfunction of any ion channel may be disruptive, some channels may have a more prominent role. For example, the late Na + current (I Na,L ) and the delayed rectifying voltage-gated K + current (I Kr ), respectively, coded by the SCN5A and KCNH2 genes, counterbalance each other to maintain the action potential duration. Other chapters in this text provide more information regarding specific ion channels, how they shape heart function, and how they can contribute to arrhythmias.
Dysfunction of ion channels caused by genetics, or channelopathies, has been associated with many human diseases, including cystic fibrosis, congenital hyperinsulinism, Bartter syndrome, Dravet syndrome, episodic ataxia, hyperkalemic/hypokalemic periodic paralysis, neuromyotonia, Timothy syndrome, and others. Not surprisingly, channelopathies also affect heart function and often are associated with the development of severe arrhythmias and sudden death. The best described conditions include Brugada syndrome, CPVT, LQTS and Romano-Ward syndrome, and SQTS ( Table 13.1 ).
| HGVSProtein | HGVSGenomic a | Frequency b (n/N, %) | Age | Sex | Ethnicity | Original Classification | Functional Effect | Possible Reclassification |
|---|---|---|---|---|---|---|---|---|
| TRPM4 | ||||||||
| p.Cys20Ser | g.19:49661482T>A | 1/246,216 (1/17,248 East Asian, 0.006%) | 43 y | F | Asian | VUS | LOF | VUS |
| p.Met49Val | g.19:49669350A>G | Novel | 2 y | M | Hispanic | VUS | LOF | VUS |
| p.Arg195Gln | g.19:49671652G>A | 6/246,184 (4/33,576 Latino, 0.01%; 2/111,666 European) | 22 y | M | Black | VUS | LOF | VUS |
| p.Ala380Val | g.19:49675354C>T | 3/277,140 (3/23,990 African,0.01%) | 13 y | M | Black | VUS | LOF | VUS |
| p.Glu497Gly | g.19:49686061A>G | 5/240952 (3/17188 East Asian, 0.02%; 1/30,758 South Asian; 1/107454 European) | 31 y | M | Asian | VUS | LOF | VUS |
| p.Gly534Arg | g.19:49686171G>C | 18/214356 (17/16384 East Asian, 0.1%; 1/94642 European, non-Finnish) | 4 m | M | Other | VUS | LOF | VUS |
| p.Val586Leu | g.19:49691910G>C | 29/277220 (17/126704 European (non-Finnish), 5/6466 other, 0.07%; 5/34420 Latino, 2/24036 African) | 33 y | F | Hispanic | VUS | LOF | VUS |
| p.Leu595Val | g.19:49691937C>G | 10/277,218 (10/24,028 African, 0.04%) | 1 y | F | Hispanic | VUS | LOF | VUS |
| p.Arg706Cys | g.19:49693561C>T | 11/277116 (5/24030 Africans, 0.02%; 5/30780 South Asians; 1/126634 European, non-Finnish) | 8 m | M | Black | Likely benign | LOF | Likely benign |
| p.Ser834Arg | g.19:49699988C>G | 20/185014 (20/13130 East Asians, 0.15%) | 17 m | F | Asian | VUS | LOF | VUS |
| p.Arg965His | g.19:49703983G>A | 20/277,020 (6/126,582 European; 2/24,020 African; 3/18,860 Asian; 5/30,778 South Asian; 4/6,464 other, 0.06%) | 7 w | F | Black | VUS | LOF | VUS |
| p.Ile1082Ser | g.19:49713579T>G | Novel | 1 m | F | Black | VUS | LOF | Likely pathogenic |
| p.Arg1086Gly | g.19:49713590C>G | 3/246182 (2/15296 African, 0.01%;1/33578 Latino) | 3 m | F | Black | VUS | LOF | VUS |
| SCN10A | ||||||||
| L388M | g.3:38798293G>T | Novel | 24 y | M | Black | VUS | LOF | Likely pathogenic |
| R756W | g.3:38781020G>A | 6/23,972 African; 9/126,154 European (non-Finnish); 15/150,126 total (0.01%) | 3 y | M | Black | VUS | None | VUS |
| L867F | g.3:38770074G>A | 7/34,322 Latino; 1/24,022 African 8/274,720; total (0.003%) | 49 d | F | Hispanic | VUS | LOF | VUS |
| P1102S | g.3:38764969G>A | 1/108,936 European (non-Finnish); 1/243,332; total (0.0004%) | 2 d | M | White | VUS | GOF | Likely pathogenic |
| V1518I | g.3:38743435C>T | 14/24,032 African; 1/18,864 East Asian; 1/34,410 Latino; 3/126,482 European (non-Finnish); 1/6,464 other; 20/276,950 total (0.007%) | 27 y | M | Black | VUS | LOF | VUS |
| V1518I | g.3:38743435C>T | See above | 2 m | F | White | VUS | LOF | VUS |
| ABCC9 | ||||||||
| p.His1305Tyr | g.12:21968807G>A | East Asian 6/19,944; African: 1/24,944 ; overall: 7/282,502 (0.002%) | 34 y | M | Asian | VUS | GOF | Likely pathogenic |
| p.Met941Val | g.12:22001129T>C | European: 1/21,640; overall: 1/251,384 (0.0004%) | 2 m | M | Hispanic | VUS | GOF | VUS |
| p.Lys1379Gln | g.12:21965059T>G | Novel | 2 m | F | White | VUS | GOF | Likely pathogenic |
| p.Ala355Ser | g.12:22063861C>A | Latino 2/34,492; European: 5/113,360; overall: 7/250,866 (0.003%) | 4 m | M | Black | VUS | GOF | Likely pathogenic |
| HCN4 | ||||||||
| p.E66Q | g.15:73660416C>G | Novel | 16 y | M | Asian | VUS | None | VUS |
| p.D546N | g.15:73617740C>T | 15/126,688 European: 0.01%; 15/277,194 total: 0.005% | 7 w | F | Black | VUS | GOF | Likely pathogenic |
| p.S935Y | g.15:73615630G>T | 7/17,022 African: 0.04%; 1/71,188 European: 0.001%; 1/4,522; other: 0.02% 9/173,412 total: 0.005% |
3 y | F | Black | VUS | None | VUS |
| p.R1044Q | g.15:73615303C>T | Novel | 23 y | M | Black | VUS | None | VUS |
| p. P1063T | g.15:73615247G>T | 32/15,464 African: 0.2%; 32/96,234 total: 0.03% | 8 w | F | Black | Likely benign | None | Likely benign |
| KCNH2 | ||||||||
| p.Gln1068Ter | g.7:150644093G>A | Novel | 18 y | F | Asian | Pathogenic | GOF | Pathogenic |
a Nucleotide positions are according to gnomAD.
b n, number of minor alleles; N, number of total alleles tested in gnomAD, by total and by ethnicity in parenthesis; %, minor allele frequency (shown are highest).
LQTS has been proposed as a possible cause of sudden infant death as early as 1976. , Between 1976 and 1994, a total of 34,442 infants were monitored by ECG shortly after birth with 33,034 followed up prospectively for 1 year. Half of the SIDS victims had prolonged QTc, whereas LQTS was not observed in non-SIDS; therefore this study concluded that QT interval prolongation of the ECG is a major risk factor in infant mortality. This finding was in keeping with another early study in which ECG testing was performed with 42 sets of parents who had at least one infant with SIDS. This study found that at least one member of 26% of the sets of parents (11 of 42) exhibited prolongation of the QT interval. However, no significant evidence was found of a prolonged QT interval in either the infants who later succumbed to sudden death or in their first-degree relatives. This topic has been hotly debated over the intervening years.
From the previously mentioned studies, it is clear that LQTS cannot be the sole factor responsible for SUD and SIDS and that other genetic mechanisms may well be involved. When classic forensic investigations to determine the cause of death have not yielded positive results (including autopsies, pathologic examinations, laboratory toxicology testing, and on-scene investigations), it has become more common to turn to genetic testing of genes previously implicated in cardiac death and arrhythmias. One of the early cases to strongly suggest a molecular link was a near-miss case of a 44-day infant with an OHCA. This infant, who was successfully resuscitated from ventricular fibrillation, was subsequently shown to carry a gain-of-function SCN5A S941N variant. Studies soon became larger. In a population-based molecular study to determine the cause of SIDS and undetermined infant death, postmortem molecular analysis between 1997 and 1999 of 93 such cases in Arkansas found two instances with rare gain-of-function SCN5A variants. This candidate gene approach was followed with subsequent individual gene studies, reporting that SIDS and SUD cases are associated with variants in a number of channelopathy genes, including SCN5A, KCNQ1, RYR2, KCNH2, CAV3, KCNJ8, and others. In a study of 91 unexplained intrauterine fetal death (stillbirth) cases, genetic testing was performed for LQTS-associated genes ( KCNQ1, KCNH2, and SCN5A ) and found variants in KCNQ1 and KCNH2 in approximately 3% of cases, consistent with the idea that arrhythmias may have contributed to premature fetal death. In 2011 a meta-analysis of such candidate gene approach studies estimated that 19.5% of SIDS victims carried a variant in one of the 16 cardiac ion channel–related genes studied, with evidence for channel malignancy in the majority of the cases.
Faster and cheaper sequencing technologies became available in the early to mid-2000s. It therefore became possible to screen a larger number of genes and to study larger cohorts. In a study in Germany conducted in 2006, sequencing was performed of five LQTS-associated channelopathy genes ( KCNQ1, HERG, SCN5A, KCNE1, and KCNE2 ) in 41 SIDS cases and reported only a single KCNQ1 variant. Patch-clamp experiments, however, demonstrated that the variant lacked adverse effects on channel function. In 2007 a genetic testing study performed in Norway with seven cardiac channelopathy genes found that rare or ultrarare gene variants occurred in 26 of 201 (12.9%) cases. Only 19 of these (9.5%), however, had a deleterious effect on channel function and may have been contributors to sudden death.
These two studies described earlier illustrate the need for functional testing of SIDS-associated or SUD-associated ion channel gene variants before inferences are made regarding their possible pathogenicity. In many subsequent genetic testing studies, however, functional testing was not performed mainly because (1) the pace of discovery with genetic testing far exceeds the capacity of traditional patch-clamp studies, and (2) patch-clamp facilities and expertise are often not available to those performing genetic testing of larger cohorts of SUD and SIDS cases. A New Zealand study in 2014, for example, performed genetic testing of 7 LQTS-associated genes in 102 sudden death cases of infants younger than 1 year and reported rare variants in eight cases. The genes in question were KCNQ1, KCNH2, and SCN5A, but it was not clear whether these variants led to altered ion channel function. Other large-cohort genetic testing had similar limitations. Postmortem genetic testing in 2014 of 123 autopsy-negative infant death cases in Southern China for Brugada syndrome–associated genes SCN5A, SCN1B, SCN2B, SCN3B, SCN4B, MOG1, and GPD1-L revealed seven unique (four novel) putative pathogenic mutations in SCN5A. There were additional variants of uncertain significance (VUS) in seven other cases. Genetic studies of a cohort consisting of 274 SUDs that occurred between 2008 and 2012 in New York City tested six major channelopathy genes ( KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, and RYR2 ). Interestingly, 51% of the cases (141 of 274) were infants younger than 1 year. Overall, a total of 22 previously classified cardiac channelopathy-associated variants were identified in this study, along with 24 novel putative channelopathy-associated variants in the infant group.
Modern and high-throughput gene sequencing has additionally led to the testing of an increasing number of genes. For example, a total of 59 “cardiac genes” were included in a study in Australia and New Zealand, which performed genetic testing in 113 cases of autopsy-negative sudden death in children and young adults between 2010 and 2012. This study reported “clinically relevant” cardiac gene mutations in 31 (27%) of the cases. Genes affected included ANK2, TPM1, TNNT2, RYR2, MYL2, MYH7, PKP2, LMNA, SCN5A, ACTN2, DES, CACNA1C, and others, but it was unclear whether these were rare variants (i.e., present at low allelic frequency in the “normal” population) or whether these variants affected gene function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here