Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Free-living amebae (FLAs) are aerobic, eukaryotic protists that comprise several genera. Clinically apparent infection of humans with FLAs is an infrequent but often fatal occurrence in both normal and immunocompromised individuals. Central nervous system (CNS) invasion by Naegleria fowleri, Acanthamoeba spp., and Balamuthia mandrillaris has been reported in hundreds of patients worldwide, with thousands of Acanthamoeba keratitis cases described. Other FLA species have each been reported as the cause of disease in single human cases and are discussed briefly below.
Distinct from other pathogenic protozoa by nature of their free-living existence, these organisms have no known insect vectors, no human carrier states of epidemiologic importance, and little relationship between poor sanitation and their transmission. Four distinct clinical syndromes are caused by the FLAs that infect humans: (1) primary amebic meningoencephalitis (PAM), (2) granulomatous amebic encephalitis (GAE), (3) disseminated granulomatous amebic disease (e.g., skin, pulmonary, and sinus infection), and (4) amebic keratitis.
PAM is caused almost exclusively by N. fowleri and occurs most commonly in healthy children and young adults with recent recreational freshwater exposure, usually warm lakes, rivers, or streams. Increasingly, however, it is being recognized that N. fowleri can be found in household water systems, with some infections occurring through tap water exposure. N. fowleri gains access to the CNS by direct invasion through the nasal mucosa and the cribriform plate and causes a rapidly fatal meningoencephalitis. GAE, caused by Acanthamoeba spp. and B. mandrillaris, is a subacute infection that likely spreads hematogenously from pulmonary or skin lesions to the CNS. The resultant focal neurologic deficits progress over days to months to a diffuse meningoencephalitis and death. Disseminated granulomatous amebic disease involving the skin, lungs, or sinuses (but without CNS infection) with Acanthamoeba and Balamuthia have also been reported. Acanthamoeba spp. also cause a subacute to chronic keratitis that is most often associated with contact lens use or corneal trauma, with rare reports of cases occurring after radial keratotomy and laser-assisted in situ keratomileusis (LASIK).
N. fowleri was named after Malcolm Fowler of Adelaide Children's Hospital of Australia, who (with R.F. Carter) described the initial cases of PAM. Of the approximately 30 species in the genus, N. fowleri is the only known pathogen of humans, although other species cause disease in mice (e.g., Naegleria australiensis and Naegleria italica ). Based on sequencing analysis, N. fowleri seems to have evolved from the nonpathogenic species Naegleria lovaniensis in North America. Globally, eight genotypes of N. fowleri have been identified by sequencing with types 1, 2, and 3 endemic to the United States. Type 1 is unique to the United States, and types 2 and 3 appear to be the most common worldwide. Although the pathogenicity among strains appears similar, genotyping isolates can help define connections between cases within an outbreak or cluster.
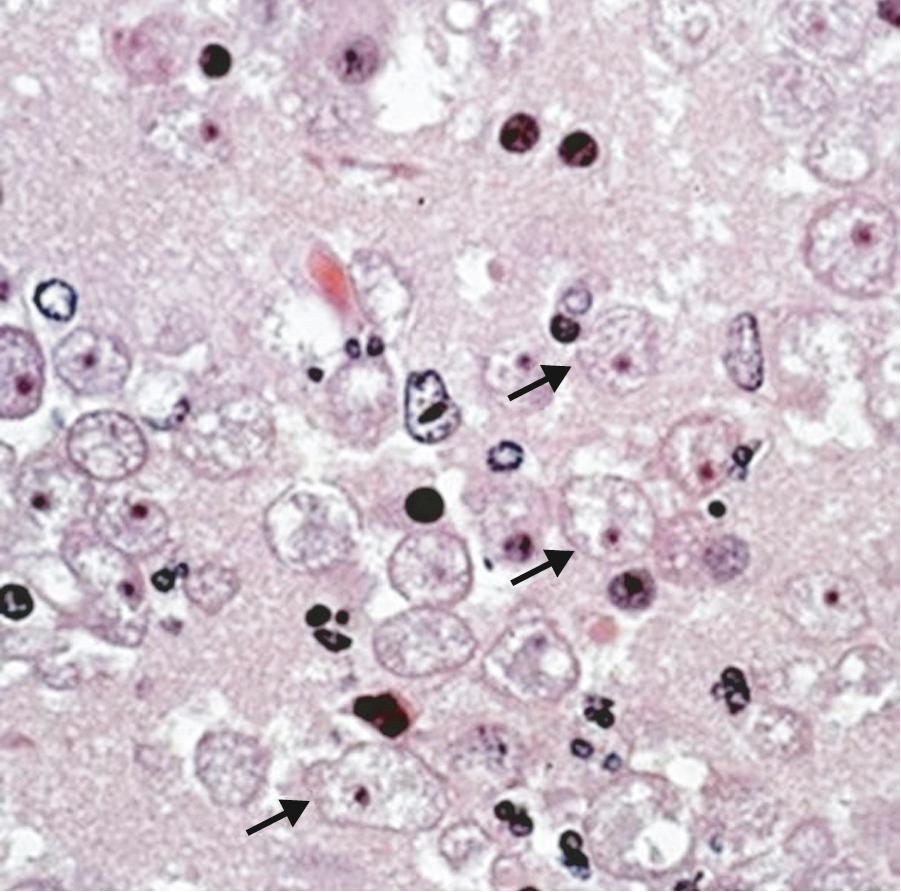
Naegleria spp. have three life-cycle stages: trophozoites, flagellates, and cysts ( Fig. 273.1 ). The trophozoites are the reproductive stage of the parasite and cause invasive human disease. Trophozoites feed predominantly on bacteria, are 10 to 25 µm in diameter, have pseudopodia, and have a clear nucleus with a prominent dense central nucleolus ( Fig. 273.2 ). The granular cytoplasm can contain ingested red blood cells and leukocytes along with cytoplasmic organelles. On transfer to distilled water or a nonnutrient medium, trophozoites can transform rapidly to a transitory flagellate form, which does not divide or feed. The flagellate form can spontaneously revert to the trophozoite. Among the FLAs, only Naegleria has a flagellated life-cycle stage. When trophozoites encyst, the cyst is resistant to environmental stresses and is approximately 9 µm in diameter with a central nucleus and a single-layered wall containing an average of two pores. N. fowleri is thermophilic, with trophozoites growing well at temperatures as high as 45°C. Organisms can be cultivated from clinical specimens and grow well in vitro on nonnutrient agar plates coated with bacteria. Like Acanthamoeba and Balamuthia, N. fowleri can be grown in a cell-free axenic medium, as well as in a chemically defined medium.
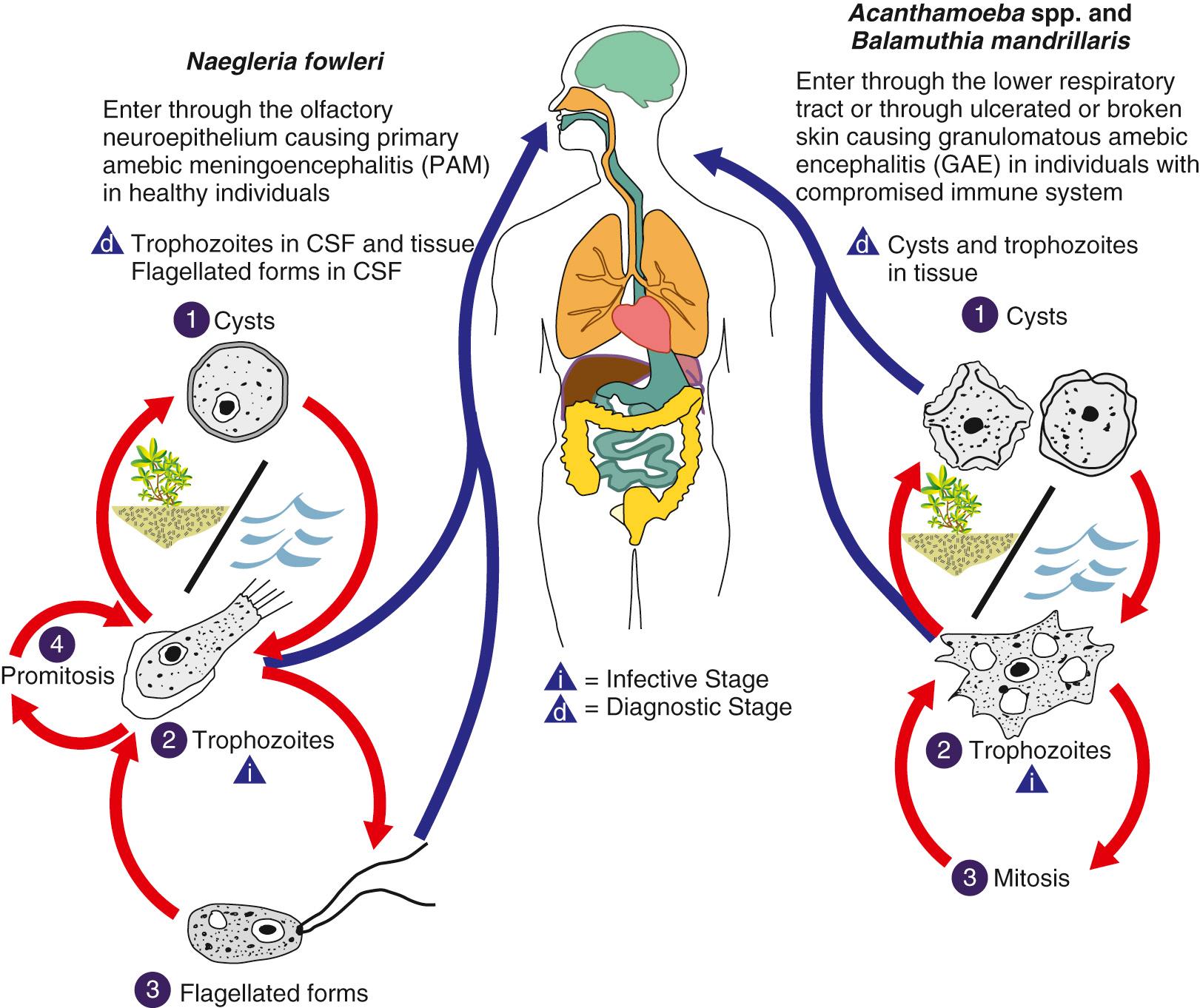
Previously, Acanthamoeba were grouped by morphology and cyst size into three groups (I, II, and III), but they are now grouped by genetic similarity via newer molecular mechanisms. The genotyping is generally consistent with the morphologic grouping. Seventeen genotypes (T1–17) have been described, and groups T1, T2a, T3–6, T10–12, and T15 have been associated with human disease. Genotype T4 (correlating to the Acanthamoeba castellanii complex) is the most commonly identified in the environment and in human disease. The life cycle of Acanthamoeba consists of trophozoite and cyst stages (see Fig. 273.1 ). Trophozoites are 15 to 50 µm in diameter, contain a single nucleus with a prominent central nucleolus, and have distinctive slender, spinelike projections of the plasma membrane ( Fig. 273.3 ). The cysts have a double-layered wall, are less than 18 to 30 µm in diameter, and like Naegleria may contain pores in the cyst wall ( Fig. 273.4 ). Trophozoites are the active form of Acanthamoeba, feeding on bacteria and environmental debris, whereas the cyst is the inactive but an environmentally resistant stage and may explain the resistance of the organism to antimicrobial therapy, especially in the setting of amebic keratitis. Acanthamoeba spp. can be easily cultivated in the laboratory; this is best accomplished by the use of tryptic soy agar with rabbit or horse blood, buffered charcoal yeast extract agar, and nonnutrient agar overlaid with live organisms, such as Escherichia coli or Enterobacter aerogenes . Like N. fowleri, most Acanthamoeba spp. can be grown in both cell-free axenic and chemically defined media.
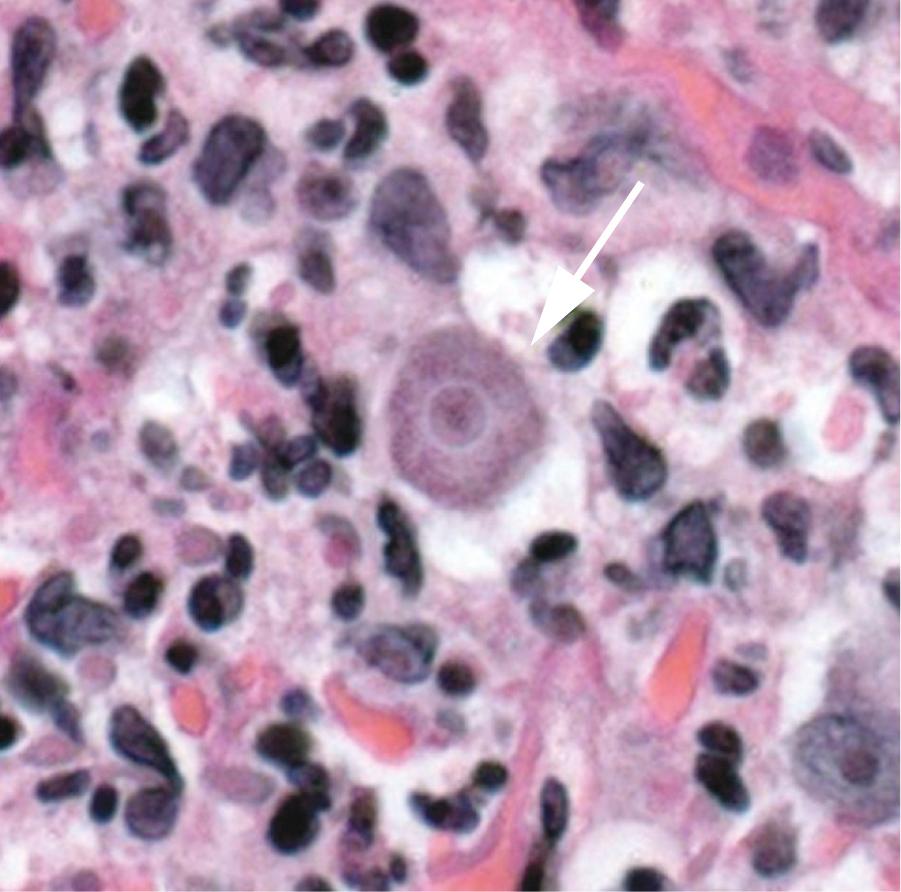
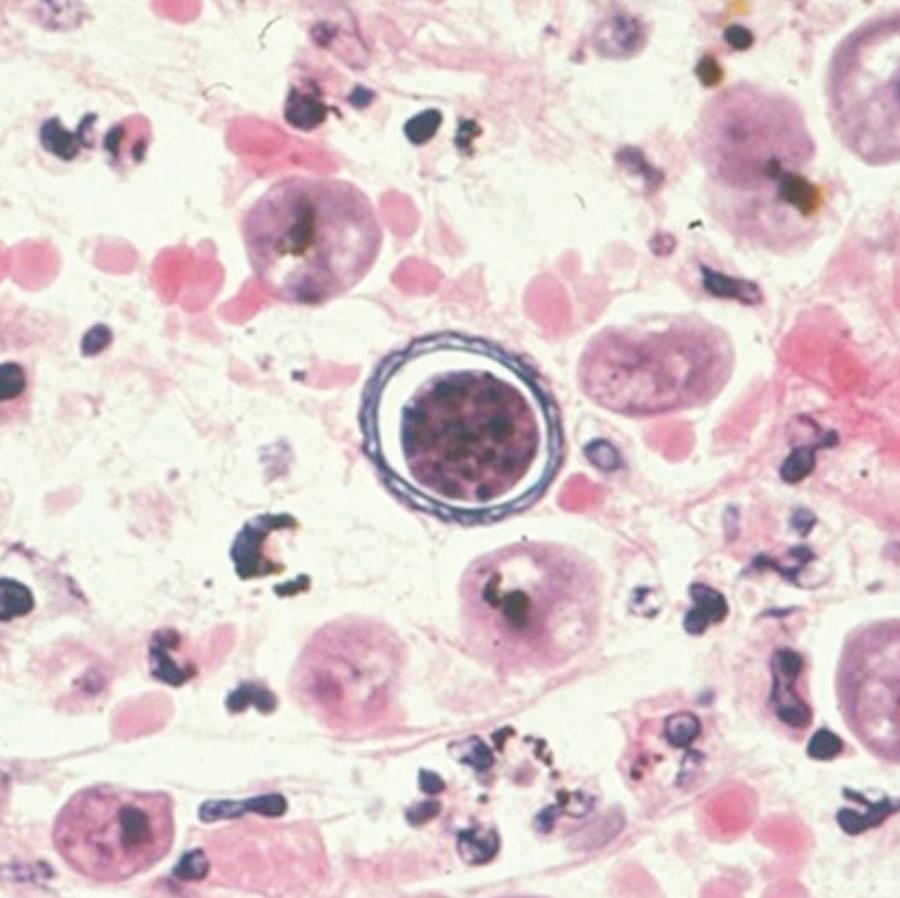
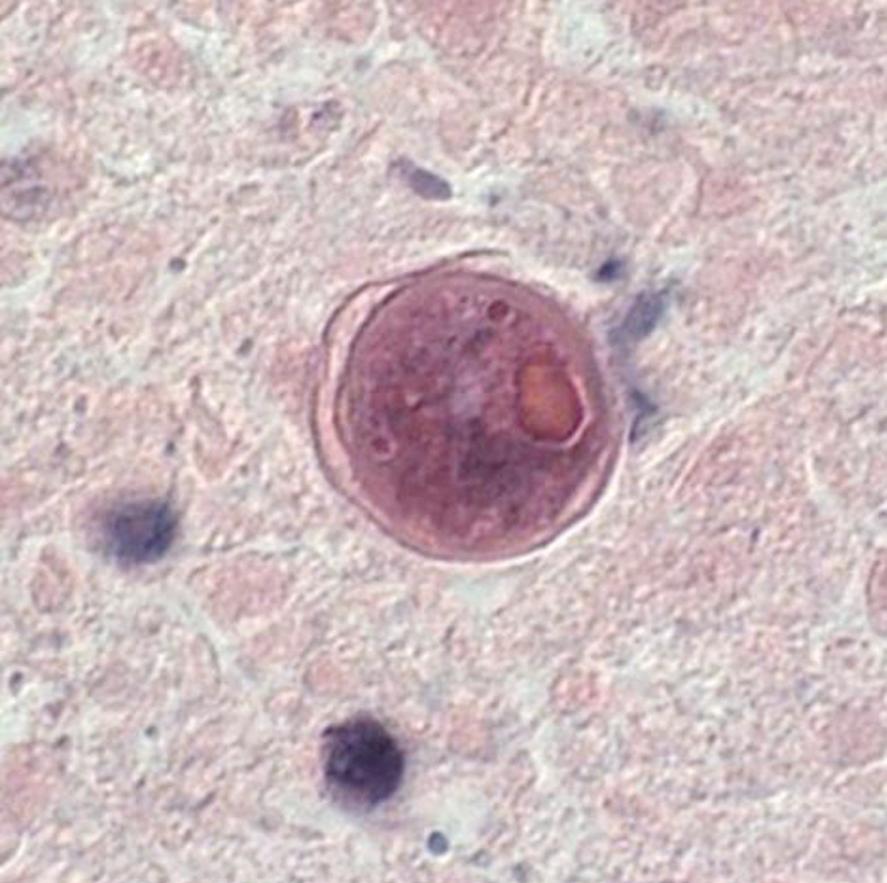
B. mandrillaris, formerly referred to as a leptomyxid ameba, is the only known species of this genus and was originally isolated from the brain of a mandrill baboon in 1986. The life cycle consists of the trophozoite and cyst stages (see Fig. 273.1 ). B. mandrillaris trophozoites have a mean diameter of 30 µm (range, 12 to 60 µm) and are uninucleate ( Fig. 273.5 ). Cysts have a mean diameter of 15 µm (range, 12–30 µm) with a wavy and irregular outer wall that is composed of three layers ( Fig. 273.6 ). On hematoxylin and eosin (H&E)-stained specimens, the organism cannot be reliably differentiated from Acanthamoeba . Laboratory growth of Balamuthia is more difficult than for Acanthamoeba or Naegleria because it does not grow on bacteria-coated agar plates and has a long doubling time. Currently, using a cell-free growth medium, axenization, and mammalian cell culture to grow Balamuthia in vitro, efforts are being made to better understand the components of the cyst and so offer new drug targets. In addition, whole-genome sequencing of several Balamuthia strains was recently done. Functional analyses of the genome will offer new insights into Balamuthia as an organism and identify potential drug targets.
Although Acanthamoeba spp. are the most common cause of amebic keratitis, Allovahlkampfia spp., Vahlkampfia spp., Hartmannella spp., and Paravahlkamfia spp. have each caused at least one human case of subacute keratitis, often in association with contact lens use or corneal trauma.
In 2009 a previously healthy man originally diagnosed with viral meningitis was subsequently determined to have PAM caused by Paravahlkamfia spp. A newly designed real-time polymerase chain reaction (PCR) assay contributed to this diagnosis and suggests that this is a new species of FLA, termed Paravahlkamfia francinae . Of note, he did not receive antimicrobial therapy active against FLAs but spontaneously recovered. This suggests the possibility that some previous survivors of Naegleria PAM, which is almost always fatal, may actually have been infected with Paravahlkamfia spp. instead. In addition, this case raises the possibility that some previously undiagnosed cases of “aseptic meningitis” may have been unrecognized PAM caused by a mildly virulent FLA.
In 2001 a report described Sappinia diploidea as the cause of GAE in a previously healthy 38-year-old man who survived the infection. The identification of this FLA as Sappinia diploidea was done by H&E staining and transmission electron microscopy of the patient's excised brain lesion. Two subsequent reports that used molecular diagnostics suggest that this Sappinia sp. was most likely either Sappinia pedata or a novel species of Sappinia that is molecularly closer to S. pedata than S. diploidea. This is the only case of Sappinia -associated human disease reported to date ( Fig. 273.7 ).
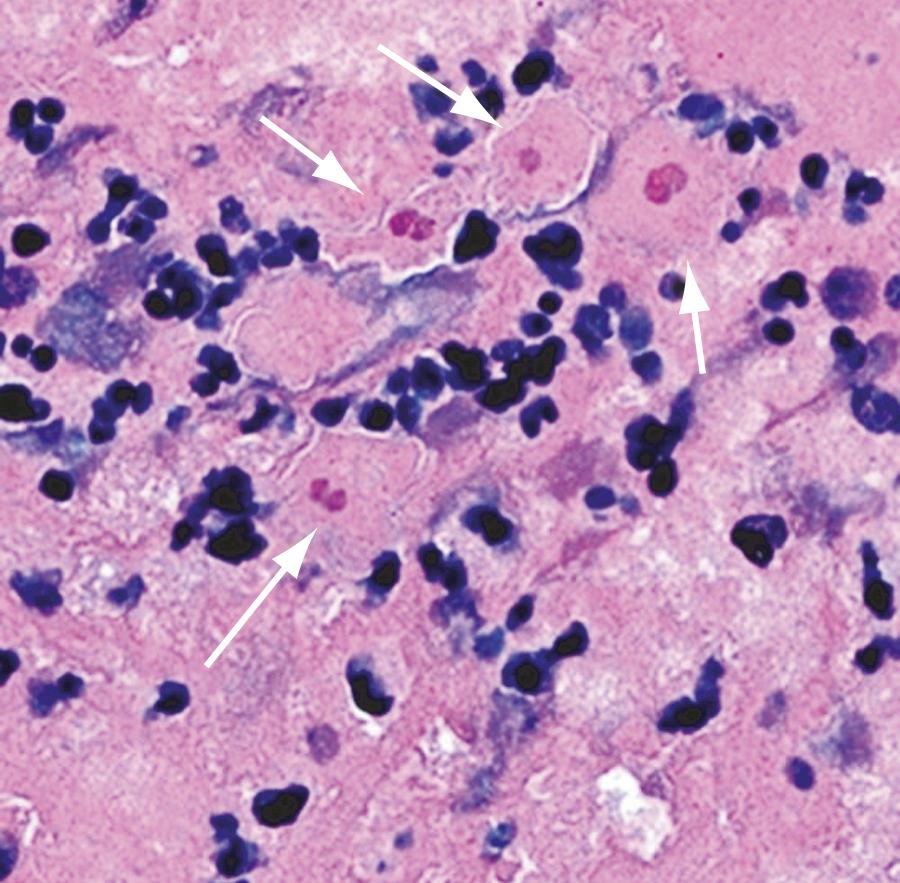
Overall, these single-case reports highlight the constant interaction between humans and FLAs and the need for a high index of suspicion for such infections to arrive at an accurate diagnosis.
N. fowleri has been found worldwide in river and lake water samples and in soil. Importantly, N. fowleri is not found in seawater. N. fowleri has also been found in thermal springs, thermal saline baths, and mud springs. Pathogenic N. fowleri are thermophilic and proliferate at temperatures up to 45°C. The presence of N. fowleri in fresh water is directly related to water temperature, and N. fowleri has been frequently isolated from thermally polluted waters in temperate climates, including as far north in the United States as Minnesota.
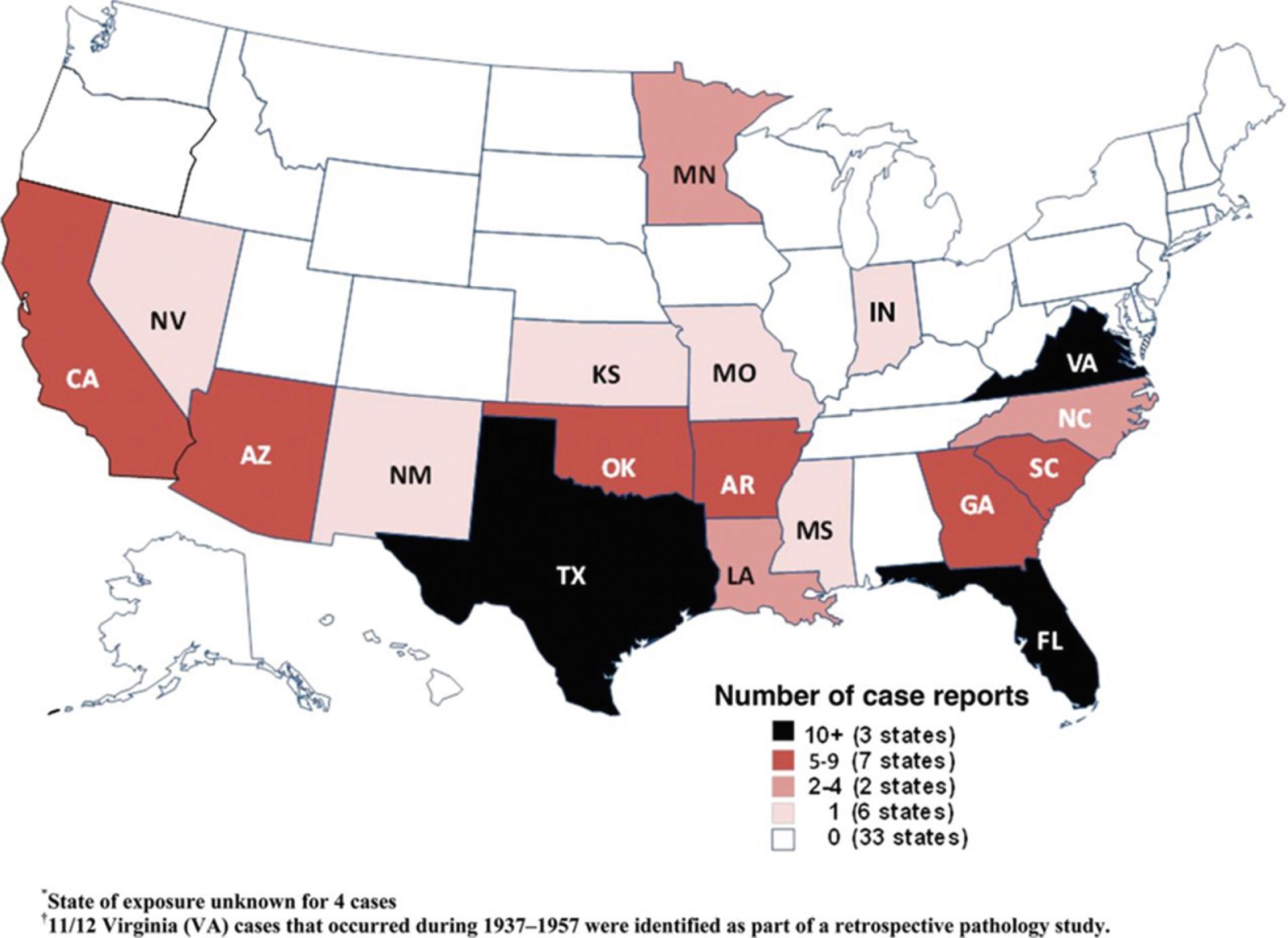
In semitropical locations such as Florida, it is not uncommon to isolate at least one N. fowleri organism per 25 mL of water. As water temperatures drop in winter, Naegleria is typically isolated from lake-bottom sediments; Naegleria cysts are stable for up to 8 months at 4°C. Although there have probably been billions of people exposed to Naegleria -contaminated fresh water, few develop PAM. The factors that protect most individuals from invasive Naegleria infection are not understood. In the southern United States the presence of serum-agglutinating activity against N. fowleri in many young adults (but not infants) indicates that subclinical infection or exposure to Naegleria is common. PAM has been reported throughout much of the world. Although the true incidence of this entity is unknown, hundreds of cases of PAM have been reported worldwide, with most having a recent history of recreational freshwater exposure. In the United States more than 100 cases of PAM were reported from 1962–2008. Annual reported case numbers ranged from 0 to 8 per year but do not appear to be increasing over time. The median age of patients was 12 years, 79% were male, and only one patient survived. Eighty-five percent of cases occurred in the summer months, and all were in southern-tier states, with more than half in Florida or Texas ( Fig. 273.8 ). Similar findings were noted in a review of 142 US cases from 1937–2013, including three well-documented survivors. In August 2010 a 7-year-old girl died of PAM due to Naegleria acquired in Minnesota, expanding the range of this organism over 500 miles north of the previously northernmost known human case in the United States. She had swum in two lakes in the area during the third-hottest summer on record in Minneapolis, possibly an unfortunate consequence of climate change. Several additional cases have been reported recently in non–Southern-tier states, including Kansas, Indiana, and another case in Minnesota.
New reports of PAM continue to appear in the literature, and the current incidence is undoubtedly higher, with some estimates placing the incidence at about 16 deaths annually in the United States. Clusters of PAM cases with common environmental exposures have occurred, including 16 deaths over a 3-year period that were retrospectively traced to a swimming pool in Czechoslovakia with a low chlorine concentration. A young woman died of PAM after swimming in a pool in California that was served by water that was piped in from a mountain hot spring. Recently, a young woman died of PAM after falling out of a raft and being submerged underwater at an artificial whitewater river facility in North Carolina; N. fowleri was detected in all of the water samples subsequently tested from the facility. In 2002 two previously healthy children from the same neighborhood in Arizona died of PAM due to N. fowleri within 1 day of each other, apparently acquired through exposure to household bathwater. Drinking water for the respective families came from the same untreated community well-water system, and PCR testing revealed N. fowleri in the water system. These cases were the first association of a drinking water system with N. fowleri infection in the United States. A subsequent study demonstrated N. fowleri (detected by PCR) in 16% of 185 wells sampled in Arizona, indicating that transmission in this manner does pose a potential risk. In 2011 two patients in Louisiana died of PAM due to Naegleria, both likely secondary to acquisition through sinus irrigation with contaminated tap water. N. fowleri was detected in water samples from both households; these were the first US cases reportedly due to exposure to contaminated treated municipal water. PAM deaths related to sinus irrigation or nasal ablution with contaminated tap water have been reported elsewhere, including recently in the US Virgin Islands. In 2013 a young boy died of PAM after likely acquiring the infection through exposure to tap water that was used on a backyard lawn water slide. This occurred at a house that was served by the same water system implicated in the 2011 Louisiana cases previously noted and represented the first time N. fowleri was cultured from a US municipal water system (not just the house at which the patients lived); the presence of N. fowleri in this water seems to have been related to undetectable chorine levels and high temperatures in the water. Two other recent clusters of PAM due to N. fowleri occurred in Pakistan, one a cluster of 13 cases likely due to ablution with contaminated tap water and another cluster of 19 cases exposed only to public water in Karachi. Naegleria has recently been found in other drinking water systems worldwide (including in Spain, Australia, Canada, the United Kingdom, and other parts of the United States). No known cases of N. fowleri transmission have occurred to date through organ transplantation; although it appears that N. fowleri can disseminate outside the CNS and that at least five organ donors had PAM as the cause of their death just before donation, none of the organ recipients in these cases have developed clinically apparent disease due to N. fowleri .
Acanthamoeba spp. have been isolated from soil, water, and air in diverse geographic locations. In contrast to Naegleria, Acanthamoeba growth is inhibited by temperatures above 35°C to 39°C, although recent evidence suggests that Acanthamoeba may be able to grow at least inefficiently at higher temperatures. Human exposure to Acanthamoeba spp. is likely a common event, as evidenced by the relatively frequent finding of the organisms in pharyngeal swab cultures of healthy individuals. In addition, serologic surveys have demonstrated serum anti- Acanthamoeba antibodies in 50% to 100% of some cohorts of healthy people. Persons of Hispanic descent may be less likely to develop antibodies to Acanthamoeba, especially Acanthamoeba polyphaga, than white persons; the clinical significance of this finding is currently unclear. Although exposure of the general population to Acanthamoeba appears to be common, GAE caused by Acanthamoeba spp. is mostly limited to debilitated or immunosuppressed individuals, although a number of recent cases in immunocompetent individuals have been reported. Underlying conditions reported in patients with GAE have included acquired immunodeficiency syndrome (AIDS), liver disease, diabetes mellitus, organ transplantation, steroid therapy, chemotherapy, and exposure to rituximab. Recently, chronic meningitis and meningoencephalitis secondary to Acanthamoeba spp. have been reported in several Indian children with no clear immunosuppression, other than malnourishment. Whether this represents a syndrome specific to this region or is due to better reporting is unclear at present. Disseminated Acanthamoeba infection without overt CNS manifestations has been increasingly recognized, most commonly in AIDS patients but also in transplant patients and those with long-term corticosteroid use. Most commonly, these patients have cutaneous disease, although involvement of the liver, lungs, and bones has been reported. Acanthamoeba infection associated with a total artificial heart has also been reported.
Unlike GAE or meningitis, which occurs most commonly in the immunosuppressed, amebic keratitis occurs predominantly in healthy people who wear contact lenses or have had corneal trauma. Worldwide the annual incidence ranges from 0.15 per million to 1.4 per million. In countries with high contact lens usage rates, greater than 80% of cases are likely related to this risk factor. The annual incidence among contact lens wearers is estimated at 1.65 per million to 19.50 per million contact lens wearers with significant associated health care expenditures. Amebic keratitis incidence increases during the warmer months, presumptively because of Acanthamoeba persistence in warmer temperatures and increased recreational water activity. Before 2005 poor contact lens hygiene practices, such as improper lens cleaning, swimming or showering with lenses, or rinsing the lens case with tap water, were associated with this disease, although it had also occurred in persons who reported none of these behaviors. In the United States, from 2005–07 a multistate outbreak involving at least 170 patients was associated with use of Advanced Medical Optics Complete Moisture Plus (AMOCMP), although no specific contamination of the solution was found. After the voluntary removal of AMOCMP the number of cases declined, but not to preoutbreak levels; this new increase in baseline amebic keratitis cases in the outbreak area is currently not understood. In addition, an increase of amebic keratitis in other parts of the world, not all of which can be accounted for by AMOCMP use, suggests that amebic keratitis will continue to become more common. These examples highlight the fact that commercially available disinfectant solutions are often ineffective against Acanthamoeba spp. and that high-risk behavior associated with contact lens use is highly prevalent. The cysts of Acanthamoeba spp. in particular may play a critical role in the establishment and persistence of amebic keratitis.
B. mandrillaris infects animals and humans worldwide. Until recently this organism had only rarely been isolated, mostly from soil and water. Now, new PCR methods and cell-free growth media/axenization have made identification and isolation of Balamuthia from the environment easier and more common. Studies using these methodologies suggest that Balamuthia is found worldwide (with regional variability) and that this ameba is more commonly associated with soil than water. Like Naegleria and Acanthamoeba, serologic studies of healthy populations in the United States, Africa, and Australia suggest that many people are exposed to Balamuthia, but few develop clinical disease. The first human cases of infection with this organism were reported in 1990. The isolation of Balamuthia from potting soil in the home of a fatally infected 3-year-old child that matched the clinical isolate from the patient confirmed the free-living and pathogenic status of B. mandrillaris . Worldwide there have been more than 200 cases of human disease reported to date, with a very high mortality rate. Across the world only a small number of patients (12) have survived Balamuthia encephalitis in response to a variety of antimicrobial treatments. Analysis of US cases suggests that risk factors for balamuthiasis include male sex, Hispanic race, and residence in the most southern states of the United States; infections are more commonly seen in immunocompetent patients than in the immunocompromised. Soil exposure appears to be a common risk factor (85%), though water exposure was also common (66%). Whether this epidemiologic pattern is a result of common environmental exposures or a genetic predisposition is currently unknown.
There have now been three documented clusters of transmission of Balamuthia through organ transplantation. All three donors were young, immunocompetent males. Although one donor was reported to have died of a stroke and the other of acute disseminated encephalomyelitis, the latter donor was subsequently determined to have died of Balamuthia GAE. The third donor died from trauma. His postmortem brain and lung examination, done after the liver recipient died of GAE, showed no evidence of Balamuthia , although the donor was found to be seropositive for Balamuthia . This third case suggests that Balamuthia can be transmitted from donors who are asymptomatic. Although 13 patients received organs from these three donors, only 5 (2 liver, 1 kidney-pancreas, and 2 kidney recipients) became symptomatic and were confirmed to have acquired Balamuthia from the transplant. In 4 of these patients, illness developed 17 to 24 days posttransplant. Four of these 5 symptomatic patients died despite treatment; the lone survivor had a poor neurologic outcome. Of the remaining 8 patients, 6 were Balamuthia seropositive (titer 1:64 or greater). All 8 were empirically treated with a variety of regimens, with the seropositive patients showing declines in antibody titer after treatment. Given the very low rate of GAE among the seropositive patients, it is unclear if any of them would have developed GAE had they not received preemptive therapy.
PAM produces a diffuse meningoencephalitis, which affects the cortical gray matter most severely. Animal models and autopsy studies indicate that CNS invasion by N. fowleri occurs after nasal inoculation with amebae by disruption of the olfactory mucosa. The amebae penetrate the respiratory epithelium, as well as the submucosal nervous plexus and the cribriform plate, and gain access to the CNS. In the CNS cortical hemorrhage and edema are seen, and the olfactory bulbs are hemorrhagic and necrotic. Naegleria trophozoites are found in the olfactory nerves and the adventitia and perivascular spaces of small to midsize arteries and arterioles. They can be identified in wet mounts of cerebrospinal fluid (CSF) in patients with acute meningoencephalitis. No cysts are seen in the brain. A prominent fibrinopurulent leptomeningeal inflammatory infiltrate is usually seen. In addition, in a series of 16 autopsies of patients with PAM, myocarditis was present in 7, although no congestive heart failures or arrhythmias were noted. The inflammatory infiltrate was predominantly neutrophilic, and no amebae were seen in the myocardium. The tissue necrosis elicited by Naegleria is likely mediated in part by secreted cysteine proteases and direct phagocytosis by feeding cups found on the trophozoites. The protein Nfa1, which mediates contact between N. fowleri pseudopods and target cells, is an important virulence factor for this organism, and mice immunized against this protein exhibit increased survival in experimentally induced PAM.
Other potential virulence determinants include nitric oxide production, pore-forming proteins, low-molecular-mass thiol compounds, and calcium-mediated complement resistance. Virulence appears to be mediated by an interaction between the environment and N. fowleri, resulting in alterations of the expression of virulence factors by the organism.
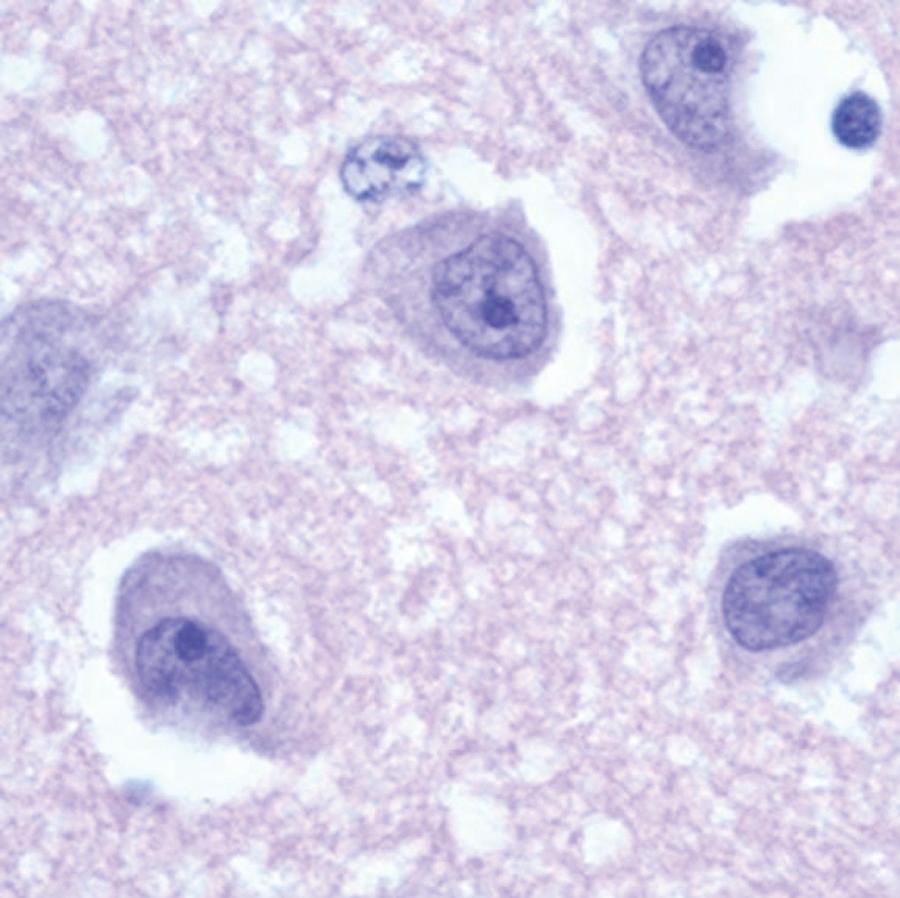
Both Acanthamoeba spp. and B. madrillaris likely gain entry to the body via the skin or the respiratory tract. Although initially contained at the site of entry by the immune system in immunocompromised/debilitated individuals, the amebae can enter the circulation and disseminate to the brain and other organs. The histologic appearance of GAE from Acanthamoeba spp. is of parenchymal necrosis and granulomas, although granulomatous tissue reaction may not be present in immunocompromised individuals. When the leptomeninges are involved, two patterns are seen: infiltrates containing equal numbers of polymorphonuclear cells, lymphocytes, and macrophages or infiltrates that are predominantly of lymphocytes and macrophages. Moderate-to-severe cerebral edema occurs, and thus bilateral uncal or cerebellar tonsillar herniations can occur. Necrotizing granulomatous lesions containing perivascular trophozoites and cysts are most frequently located in the cerebellum, midbrain, and brainstem. Multinucleated giant cells are occasionally present within the granulomas. The perivascular location of amebic trophozoites and cysts suggests a hematogenous dissemination of Acanthamoeba to the CNS; this is also supported by identification of Acanthamoeba in the skin, lung, adrenal glands, and lymph nodes. Amebic skin lesions, sinusitis, and pneumonitis may be sites of primary human infection that lead to hematogenous dissemination.
The histologic appearance of amebic keratitis is similar to that of Acanthamoeba infections of other organs. Both amebic cysts and trophozoites are found within the cornea. There is an acute or mixed inflammatory infiltrate that may contain epithelial and giant cells. However, amebae have also been found in tissue in the absence of an inflammatory infiltrate. Corneal neovascularization occurs to a variable extent. Involvement of the posterior chamber of the eye is a rare complication of amebic keratitis. Sterile inflammation of the posterior segment occurs without isolation or visualization of amebic cysts or trophozoites. The corneal ringlike infiltrate caused by Acanthamoeba appears to be caused by the neutrophil chemoattractant effect of antigen-antibody complexes in the cornea. Amebae adhere to corneal epithelial cells and secrete proteinases that facilitate invasion of the cornea and stroma. Trophozoites and cysts are seen between the lamellae of the cornea, and inflammatory infiltrates in the superficial and middle layers of the corneal stroma are common. Infiltration of nerves causes radial keratoneuritis, and later a characteristic stromal ring infiltrate develops. Anterior uveitis is common. In late stages amebic keratitis is characterized by necrosis, ulceration, descemetocele formation, and perforation of the cornea.
Significant progress has been made toward understanding the molecular basis of pathogenesis in Acanthamoeba infections, especially keratitis. The pathology induced by Acanthamoeba can be classified into contact-dependent mechanisms, which require the ameba to physically contact the host cell, and contact-independent mechanisms. Keratitis begins with the contact-dependent action of trophozoites using a mannose-binding protein to adhere to mannose glycoproteins on the corneal epithelium. The mannose glycoproteins also stimulate the release of cytopathic factors from the parasites, leading to the killing of corneal cells and destruction of the extracellular matrix. In addition, amebic ecto–adenosine triphosphatases (ATPases), neuraminidases, elastases, phospholipases, glycosidases, and proteases, some of which act in a contact-independent manner, potentially play a role in corneal destruction. Acanthamoeba also evade the immune response by degrading human immunoglobulin A (IgA) antibodies with secreted proteases, switching from trophozoites to cysts, and infecting the cornea, a site that lacks resident antigen-presenting cells, thus avoiding a delayed-type hypersensitivity response or a serum IgG response. Finally, a recent study has shown that Acanthamoeba express a pore-forming protein, which can kill both bacteria and mammalian host cells, although the role of this acanthaporin in vivo is unknown.
An ongoing development in the understanding of Acanthamoeba pathogenesis at the cellular level is the observation that many bacteria (including Legionella spp., some Mycobacteria, Shigella spp., Chlamydia spp., Pseudomonas aeruginosa, Vibrio cholerae, and Burkholderia spp.), viruses, and even yeast and protozoa survive within the ameba. This endosymbiosis has several potential sequelae. First, it may render the intracellular microbe more pathogenic for the human host; second, it may facilitate gene transfer between the ameba and bacteria ; third, it may allow the microbe to survive an otherwise inhospitable environment; and fourth, in coinfections, Acanthamoeba may shield the intracellular microbe from the immune response and antibiotics, thus leading to more fulminant infections with these bacterial species. Although the endosymbiotic relationship of intracellular microbes and ameba continues to be explored, the actual impact of this relationship on human disease remains unclear.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here