Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Free-functioning muscle transfer involves the transfer of a muscle from a distant location to replace a major functional deficit.
It is a complex procedure that requires high patient motivation and compliance.
The technique involves microvascular anastomoses and neural coaptation.
Meticulous attention must be paid to proper positioning and tensioning of the transferred muscle. Postoperatively, a structured long-term rehabilitation program is required.
In the appropriate patient with free joints, good functional results can be achieved.
Access video content for this chapter online at Elsevier eBooks+ ![]()
Free-functioning muscle transfer is a complex procedure for use when no other alternative is available.
This technique is applicable to restoration of finger, thumb, and wrist flexion and extension, thumb opposition, elbow flexion and extension, and shoulder flexion.
Muscle placement and restoration of its resting length are critical to achieving full range of motion.
Selecting a viable and adequately sized donor nerve is essential for sufficient muscle function.
Meticulous microsurgical technique and diligent postoperative monitoring are required; vascular compromise may lead to muscle loss.
A free-functioning muscle transfer describes the micro-neurovascular transfer of a functioning muscle to a recipient site in the upper limb from elsewhere in the patient’s body to restore a major functional deficit. The functional deficit is often the result of traumatic or iatrogenic muscle loss (e.g., following sarcoma resection, Volkmann's ischemic contracture, or longstanding brachial plexus injury). Accordingly, free-functioning muscle transfers to the upper limb have been used to reconstruct functional deficits in finger flexion and extension, thumb abduction, thumb opposition, elbow flexion and extension, and shoulder abduction.
The viability of the transferred muscle is maintained by microvascular anastomosis of the muscle's artery and vein(s) to corresponding blood vessels at the recipient site. To reanimate the muscle, motor nerve fibers are rerouted to the transferred muscle by coapting a donor nerve at the recipient site to the nerve of the muscle. Several muscles may serve as suitable donors for free-functioning muscle transfers. For upper extremity reanimation, the gracilis muscle is most commonly used based on appropriate size, muscle excursion and strength as well as its moderate donor site morbidity, surgical accessibility, and suitability of the neurovascular pedicle.
Free-functioning muscle transfers are complex surgical procedures that require long-term rehabilitation. Satisfactory functional recovery depends on muscle viability, sufficient neural regeneration as well as correct muscle flap positioning, and the patient’s adherence to the rehabilitative program. In the appropriate patient, free-functional muscle transfers can reliably achieve good functional results following otherwise devastating injuries, however, patients must be counseled to be realistic about their expectations for this type of surgery.
After numerous failed attempts to transfer and maintain non-vascularized free muscle grafts, the first functioning muscle transfer was reported in 1970. Tamai et al . successfully transplanted the rectus femoris muscle in dogs using microsurgical vascular anastomosis and nerve coaptation and demonstrated electrophysiological and histological evidence of muscle reanimation. In 1973, at the Sixth People's Hospital in Shanghai, microsurgeons successfully transplanted the lateral portion of pectoralis major into the forearm of a patient with Volkmann's ischemic contracture. This was the first clinical application of free functional muscle transplantation in the upper limb. A good range of finger motion and substantial grip force were reported. In 1976, Ikuta et al . demonstrated the efficacy of free functional muscle transfer and almost normal muscle histology experimentally. In the same year, Harii et al . reported free functioning muscle transfers for facial reanimation Since then, multiple authors have pursued functional muscle transfers throughout the human body to restore lost function. Functional muscle transfers have been reported in the upper and lower limb and are frequently used for facial reanimation.
Free-functioning muscle transfer is applicable to patients with permanent loss of a critical upper extremity function where no local option for functional reconstruction is available. Treatment options that should be preferably considered include reinnervation of local muscle via nerve reconstruction or local nerve transfers, or rerouting of local muscles via tendon transfers. However, following severe extremity trauma, Volkmann's ischemic contracture, extensive tumor resection, and in longstanding brachial plexus lesions, a free-functioning muscle transfer may be the only viable option to restore critical function. Other indications may include electrical burns, post gas gangrene and post-replant patients ( Algorithm 27.1 ).
Although free-functioning muscle transfers are frequently used to restore a specific function such as finger flexion, it is possible to reconstruct function in multiple joints, such as elbow flexion and wrist extension, with a single free-functioning muscle transfer. However, better functional outcomes are usually achieved when a single muscle is used to restore only one function. Further, in patients with global loss of upper extremity function, a double free functional muscle transfer may be used to reconstruct the most critical functions such as elbow flexion, finger extension, and finger flexion. However, a free-functioning muscle transfer is a highly involved surgical procedure that requires a motivated patient who is fully equipped to adhere to the oftentimes intensive pre- and postoperative rehabilitation program that is required for a successful outcome. The patient must be aware that the maximal outcome of the procedure will not be realized until 1–2 years postoperatively.
Preoperative planning is a key component of successful free-functioning muscle transfer. Prior to surgery, a complete medical history and detailed physical examination are essential to identify comorbidities, particularly hypercoagulopathies, and concomitant injuries, as well as to map the remaining muscle function and sensation in the recipient extremity. Sensation in the hand should be near normal to achieve optimal results, although this is not an absolute requirement. In patients with brachial plexus injuries, upper limb sensation may be limited. Possible intraplexal and extraplexal donor nerves should be assessed. Electromyograms (EMG) and nerve conduction studies may help to define the level and severity of brachial plexus injury and to identify suitable donor nerves. Sometimes the intercostal nerves are the only remaining option. The patient must have stable skeletal structure and functional joints with normal or near normal range of motion in the hand, wrist, and elbow. There must be adequate soft-tissue cover over the planned tendon-gliding site. If there is insufficient or scarred soft-tissue cover, preliminary procedures such as tissue expansion may be necessary prior to muscle transfer. To enable tension-free wound closure, the muscle can be harvested with a skin paddle.
Suitable recipient artery and vein(s) must be available to supply the transferred muscle ideally without the use of interposition vein grafts. Preoperative Duplex examination and/or angiography can be very helpful to map the vascular status of the extremity and identify suitable donor vessels. To reanimate the muscle flap, a suitable donor nerve must be available and there should be adequate antagonist function in the arm to counterbalance the transferred muscle ( Table 27.1 ). When a donor muscle is selected, the donor site should be examined for any indications of previous trauma (e.g., scars) and confirmation of intact donor muscle function.
| Available, undamaged motor nerve, artery, and vein at the site of muscle transplantation |
| Adequate skin coverage for the distal half of the muscle |
| Supple joints and gliding tendons |
| Good hand sensibility and intrinsic function |
| Adequate antagonist muscle function |
| Good patient motivation |
| No simpler solution for the patient's problem |
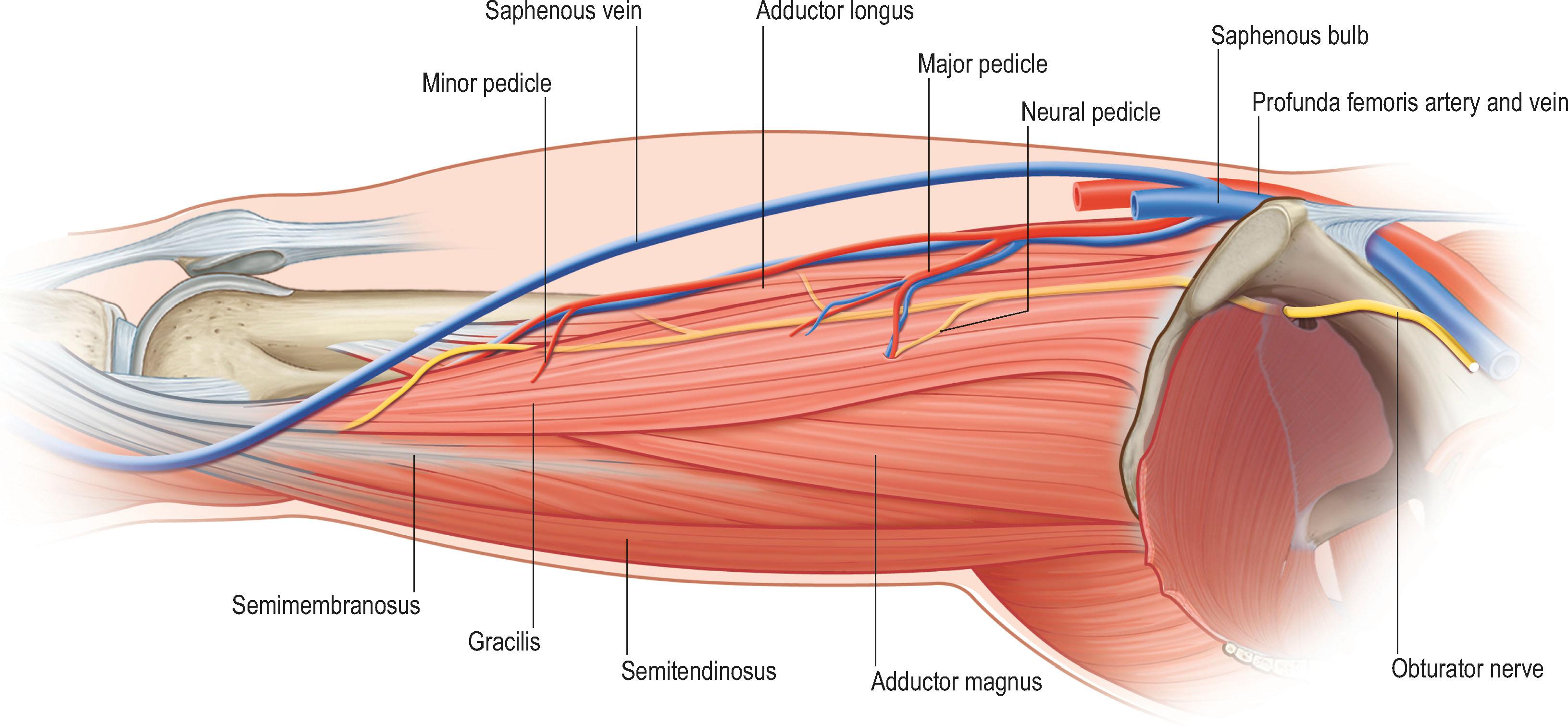
Multiple different muscles have been used for free-functioning transfer in the upper limb, including latissimus dorsi, rectus femoris, serratus anterior, gastrocnemius, hemisoleus, flexor carpi ulnaris, pectoralis major, and tensor fasciae latae. Presently, the gracilis muscle ( Fig. 27.1 ; see Fig. 27.7 ) is most widely used for restoration of upper extremity function as it fulfills many of the preferred selection criteria for free-functioning muscle transfers. The donor site morbidity should be non-critical, and other muscles should be able to compensate the sacrificed donor muscle function. Of note, depending on occupation and activity level, the impact of a given functional deficit on activities of daily living may vary among different patients. Detailed preoperative discussion of the expected deficit and alternative options are mandatory. Ideally, the transferred muscle should have a single dominant vascular pedicle (Mathes and Nahai type 1, 2 or 5) of sufficient length and diameter to allow easy anastomosis after transfer without the need for vein grafts. The muscle should be powered by a single motor nerve of sufficient length and in the correct orientation to allow for tension-free coaptation to the donor motor nerve, without interposition nerve grafts. Further, the muscle ideally should have adequate fascia and tendon to allow secure attachments at the recipient site.
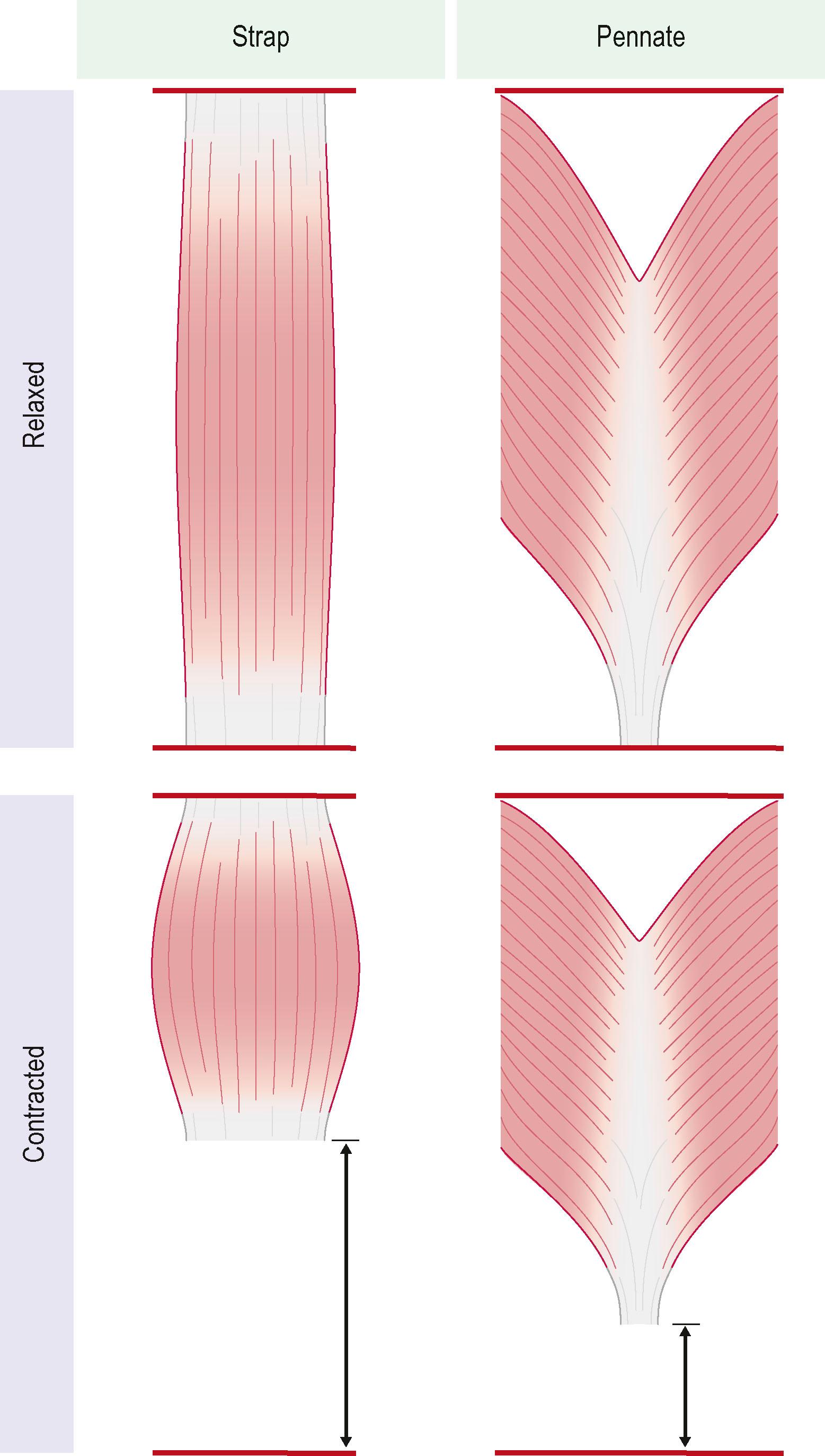
To restore the full range of motion, the transferred muscle should generate adequate excursion during muscle contraction and provide sufficient latitude for unobstructed antagonist muscle function. Finger flexors normally have an excursion of approximately 7 cm and finger extensors around 5 cm. As a rule of thumb, muscle fibers can be stretched to approximately 150% of their resting length and shorten to around 40% of their resting length when maximally contracted. In muscles where the muscle fibers are aligned along the longitudinal muscle axis (strap muscles e.g., gracilis muscle, rectus abdominis muscle) those metrics approximately translate to the expected excursion of the muscle flap. In pennate muscles however, shorter fibers are oriented at an angle to the axis of force generation (e.g., rectus femoris, gastrocnemius muscle) and consequently provide less muscle excursion but can generate more force ( Fig. 27.2 ). As a result, strap muscles may be of advantage for restoring motor functions with a wide range of motion such as finger flexion.
The functional amplitude of the gracilis muscle can be up to 12–16 cm (around 40% of the muscle resting length). The donor site functional deficit is not critical, and the resulting scar on the inner thigh may widen over time but is placed in a relatively inconspicuous location for most patients. The neural pedicle can be harvested with up to 10 cm in length and contains around 800 myelinated axons usually grouped in 2–3 motor fascicles supplying discrete portions of the muscle. As such, it is possible to use the muscle split into two components for dual functionality with separate motor innervation ( Fig. 27.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here