Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
ELECTROLYTE DISTURBANCES ARE COMMON in children because of their small size, large ratio of surface area to volume, and immature homeostatic mechanisms. As a result, fluid management can be challenging. On the ward, in the operating room, or in the intensive care unit (ICU), additional difficulties may result when fluid management is not tailored to the individual or when therapeutic decisions are based on extrapolations from adult data. To better understand the former and to limit the latter, this chapter reviews the basic mechanisms underlying fluid and electrolyte regulation, the developmental anatomy and physiology of fluid compartments, and the management of selected pediatric disease states relevant to anesthesia and critical care.
Water is in thermodynamic equilibrium across cell membranes, and it moves only in response to the movement of solutes ( E-Fig. 9.1 ). Movement of water is described by the Starling equation:
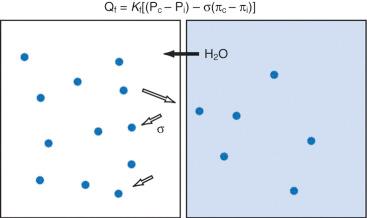
where Q f is fluid flow; K f is the membrane fluid filtration coefficient (a proportionality constant); subscripts c and i refer to capillary and interstitial; P c and P i are hydrostatic pressures and π c and π i are osmotic pressures on either side of the membrane; and σ is the reflection coefficient for the solute and membrane of interest. The reflection coefficient gives a measure of a solute's permeability and, consequently, its contribution to osmotic force after equilibration. Across the blood-brain barrier, for example, the σ for sodium approaches 1.0, whereas in muscle and other cell membranes, σ is on the order of 0.15 to 0.3. Therefore, when isotonic sodium-containing solutions are given intravenously, usually only 15% to 30% of administered salt and water remains in the intravascular space, whereas the remainder migrates to the interstitium. In contrast, hypertonic solutions permit greater expansion of circulating blood volume with smaller fluid loads and less fluid in the interstitium (e.g., as edema).
Both the amount and the concentration of solute are tightly regulated to maintain the volumes of intravascular and intracellular compartments. Because sodium is the primary extracellular solute, this ion is the focus of homeostatic mechanisms concerned with maintenance of intravascular volume. When osmolality is held constant, water movement follows sodium movement. As a result, total body sodium (although not necessarily serum Na + ) and total body water (TBW) generally parallel one another. Because sodium “leak” across membranes limits its contribution to the support of intravascular volume, this compartment also critically depends on large, impermeable molecules such as proteins. In contrast to sodium, albumin molecules, for example, follow the Starling equilibrium with a reflection coefficient in excess of 0.8. Soluble proteins create the so-called colloid oncotic pressure, approximately 80% of which is composed of albumin.
Although the presence of albumin supports intravascular volume, protein leak into the interstitium (and consequent water movement) may limit its effectiveness. It has been observed, for example, that the reflection coefficient for albumin decreases by as much as one-third after mechanical trauma. Furthermore, because of ongoing leakage, a slow continuous infusion of albumin is superior to bolus administration for increasing the serum albumin concentration in critically ill individuals.
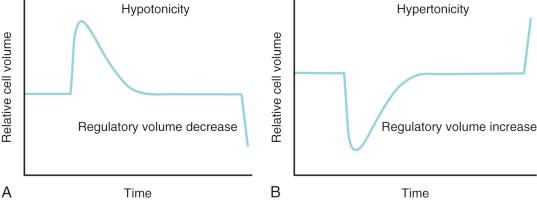
Potassium is the primary intracellular solute, with approximately one-third of cellular energy metabolism devoted to Na + /K + exchange. Sodium continuously leaks into cells along its concentration gradient, yet it is rapidly extruded in exchange for potassium. As the cell is exposed to varying osmolarity, water movement occurs, causing cell swelling or shrinkage. Because stable cell volume is critical for survival, complex regulatory mechanisms have evolved to ensure that stability is maintained. The processes by which swollen cells return to normal size are collectively termed regulatory volume decrease processes, and those returning a shrunken cell to normal are termed regulatory volume increase processes ( Fig. 9.1 ). With sudden, brief changes in osmolality, regulatory volume increase or decrease processes are activated after small (1%–2%) changes in cell volume, returning cell volume to normal primarily through transport of electrolytes. If anisosmotic conditions persist, chronic compensation occurs through the accumulation or loss of small organic molecules termed osmolytes or idiogenic osmoles. These osmogenic agents can also be cytoprotective under stress and include polyols, sorbitol, myoinositol, amino acids and their derivatives (e.g., taurine, alanine, proline), and methylamines (e.g., betaine, glycerylphosphorylcholine).
Like intracellular volume, circulating blood (intravascular) volume is also tightly controlled. Increases in intravascular volume result from increases in sodium and water retention, whereas decreases in intravascular volume result from increases in excretion of sodium and water. As noted earlier, serum osmolality must be maintained within a very narrow range if serum sodium is to be an effective focus of intravascular volume control. Serum osmolality is usually maintained between 280 and 300 mOsm/L; changes in osmolality as small as 1% trigger compensatory mechanisms.
Serum osmolality is primarily regulated by arginine vasopressin (AVP), thirst, and renal concentrating ability. Because the indirect aim of osmolar control is actually volume control, these same osmoregulatory mechanisms are also influenced by factors such as blood pressure (BP), cardiac output, and vascular capacitance. In pathologic conditions such as ascites or hemorrhage, intravascular volume preservation takes precedence over osmolality and osmoregulatory mechanisms operate to restore intravascular volume, even at the expense of disrupting solute balance.
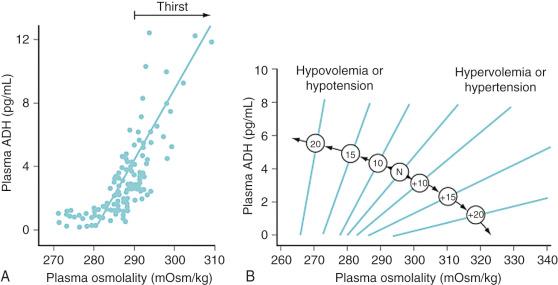
AVP is released from neurons within the supraoptic and paraventricular nuclei of the hypothalamus. Microelectrode recordings suggest that different subpopulations of neurons are responsive to osmotic input, baroreceptor-mediated input, or both. Osmoresponsive cells react to osmolar fluctuations in cell size, so solutes that readily permeate cell membranes (such as urea) increase the serum osmolality without triggering the release of antidiuretic hormone (ADH). Infusion of solutes with large actual or effective cell membrane reflection coefficients (σ) (e.g., sodium, mannitol) elicit a robust AVP release. Typically, AVP release begins when the serum osmolality reaches a threshold of approximately 280 mOsm/L. In keeping with our understanding of cell volume regulation, rapid increases in osmolality lead to greater release of AVP rather than slow increases. Meanwhile, baroreceptors on the arterial side of the circulation (left ventricle, carotid sinus, aortic arch, and juxtaglomerular apparatus) provide tonic inhibition of nonosmotic AVP release. Hypovolemia and hypotension diminish this inhibition, release AVP stores, and increase the overall “gain” of the system ( E-Fig. 9.2 ). Thus, in a volume-depleted or hypotensive child, brisk AVP release occurs even in the presence of plasma osmolalities as low as 260 to 270 mOsm/L. On balance, baroreceptor signals always override osmotic signals so that water is retained as needed to maintain circulatory homeostasis.
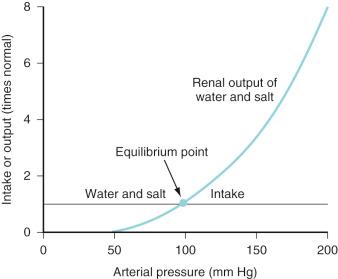
Intravascular fluid volume, salt and water intake, electrolyte balance, and cardiovascular status are interrelated at several levels. For example, as the veins and arteries distend with fluid and the systemic BP increases, AVP release decreases, and both pressure diuresis and natriuresis begin. The resulting relationship between urine output and arterial pressure is termed the renal function curve and its intersection with salt and water intake determines the equilibrium point at which arterial BP ultimately stabilizes ( Fig. 9.2 ). Equilibrium (chronic) BP is influenced only by shifts of the renal function or fluid intake curves. Transient changes in arterial pressure secondary to peripheral vascular resistance changes are always resolved by opposing shifts in total body salt and water.
In response to a decreasing arterial pressure, the renin-angiotensin system is also activated. With decreased renal perfusion, juxtaglomerular cells release renin, which in turn converts renin substrate (angiotensinogen) to angiotensin I. Angiotensin I is then rapidly converted to angiotensin II by angiotensin-converting enzyme present in lung endothelium. Angiotensin II supports arterial pressure in three ways: (1) direct vasoconstriction, (2) increased salt and water retention (via renal vasoconstriction and decreased glomerular filtration), and (3) stimulation of aldosterone secretion ( Fig. 9.3 ).
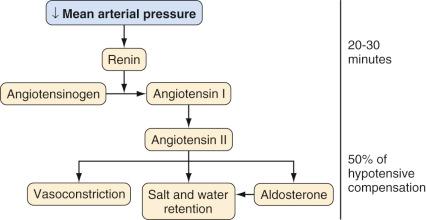
AVP, pressure diuresis, and the renin-angiotensin system permit wide ranges in salt and water intake while maintaining the BP and volume status within narrow ranges; all serve to support the systemic circulation when threatened and to complement the more immediate activity of the sympathetic nervous system. In addition to high-pressure sensors such as aortic arch and carotid sinus baroreceptors, intravascular volume information is provided by low-pressure thoracic sensors. For this reason, effective increases or decreases in intrathoracic blood volume may mimic changes in whole-body volume status and produce natriuresis, diuresis, or fluid retention. Intravascular volume may also be sensed by atrial muscle fibers; as the fibers stretch, atrial natriuretic peptide (ANP) is released. Although its complete physiologic role is uncertain, ANP may serve to “fine-tune” the volume status by vasodilating modestly, gently increasing the glomerular filtration rate (GFR), and decreasing reabsorption of sodium. The combination of complex autoregulatory mechanisms with complementary actions operating on varying time scales, all responding to different, yet interrelated, effector stimuli, yields an elegant system by which the mature individual may maintain circulation amid a variety of challenges. In this context, it is interesting to observe that successful heart transplant recipients, despite general cardiovascular stability, typically manifest fundamental derangements in body fluid homeostasis.
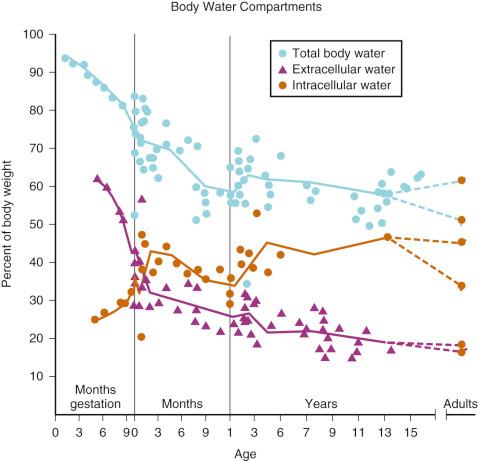
Much of our understanding of the development of body water compartments is derived from deuterium oxide dilution studies performed in the 1950s. In a series of 21 neonates, TBW was found to be approximately 78 ± 5% of body weight. Subsequent measurements in fewer subjects showed that TBW decreased to approximately 60% in the second 6 months of life with most of the loss being extracellular. A smaller decrease (to about 57%) is observed late in childhood ( Fig. 9.4 ).
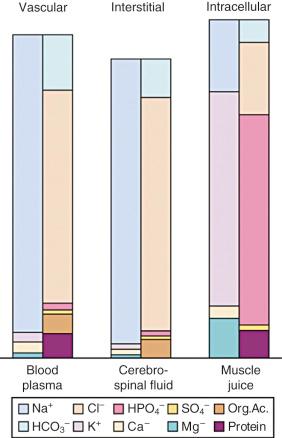
The importance of the extracellular compartment, its relationship to the intracellular space, and much of the chemical anatomy of both were first described by Gamble in educational monographs issued during the first part of the 20th century ( E-Fig. 9.3 ). The chemical compositions of mature body fluid compartments are provided in Table 9.1 .
| Extracellular Fluid | Intracellular Fluid | |
|---|---|---|
| Osmolality (mOsm) | 290–310 | 290–310 |
| Cations (mEq/L) | 155 | 155 |
| Na + | 138–142 | 10 |
| K + | 4.0–4.5 | 110 |
| Ca 2+ | 4.5–5.0 | — |
| Mg 2+ | 3 | 40 |
| Anions (mEq/L) | 155 | 155 |
| Cl − | 103 | — |
| HCO 3 − | 27 | — |
| HPO 4 2− | — | 10 |
| SO 4 2− | — | 110 |
| PO 4 2− | 3 | — |
| Organic acids | 6 | — |
| Protein | 16 | 40 |
The blood volume in neonates was determined to be 82 ± 9 mL/kg using an iodine 121–labeled human serum albumin technique, although substantial variability may result from the degree of placental-fetal transfusion. In low–birth-weight (LBW), preterm, or critically ill infants, values as high as 100 mL/kg have been measured. Blood volume increases slightly during the first few months of life, reaching its zenith at 2 months of age (approximately 86 mL/kg), then returns to near 80 mL/kg and finally stabilizes at 70 mL/kg by the end of the first year of life. In general, the ratio of blood volume to weight decreases with growth. The most accurate basis for prediction of blood volume is lean body mass, the consideration of which removes any male/female variation even into adulthood. An estimate of the circulating blood volume is presented in Table 9.2 .
| Age | Estimated Blood Volume (mL/kg) |
|---|---|
| Preterm infant | 100 |
| Full-term neonate | 90 |
| Infant | 80 |
| School age (5 years) | 70 |
| Adults | 70 |
Renal development begins at approximately 5 weeks of gestation and continues in a centrifugal pattern until the full complement of nephrons is in place by about the 38th week. In the outermost regions of the renal cortex, postnatal nephron differentiation may continue for several weeks to months. In the early stages of gestation, renal blood flow is approximately one-fifth of normal. Initially this is related to structural immaturity, and later it is caused by increased renovascular resistance. By 38 weeks of gestation, renal blood flow is approximately one-third of normal. High renovascular resistance protects the developing nephron from both pressure and volume overload. The resulting renal contribution to metabolic homeostasis in utero is limited.
As with the pulmonary bed, vascular resistance in the kidney decreases after birth, leading to abrupt increases in renal blood flow and GFR. In utero, despite a low GFR, urine output is brisk, owing to poor reabsorption of salt and water. Plasma renin activity is increased in utero, decreases immediately after birth, and then increases again as excess extracellular water is mobilized and excreted. Aldosterone levels are increased in cord blood and are maintained at this level for the first 3 days of life. The increased aldosterone may be necessary for sodium retention during periods of increased anabolism early in life.
Intrarenal gradients of NaCl and urea are less steep in the immature kidney, and full nephron length has yet to be achieved. Consequently, urine-concentrating ability is limited in neonates, with maximum urine osmolality being about half that of the adult (700–800 mEq/L vs. 1300–1400 mEq/L). In part, this also relates to low circulating ADH levels and decreased renal responsiveness to ADH. Although overall ADH production is not impaired, excessive secretion may occur in some disease states. Limited urine-concentrating ability necessitates large urine volumes for elimination of large solute loads.
Renal plasma flow and GFR (based on body surface area or allometry) are 30% of adult values in neonates. Both increase during the first year, reaching 50% of adult values by 6 months age (350 and 70 mL/minute per meter squared, respectively, and 90% by approximately 1 year see Figs. 7.11 and 7.12 ). At birth, the serum creatinine reflects the maternal concentration, which may be increased in term and preterm infants, but normalizes in the second month after birth, reflecting creatinine production. Fractional excretion of sodium (FE Na ) is markedly increased in preterm infants, decreases somewhat by term, and stabilizes at adult rates by the second month of life. Although the adult kidney may easily achieve FE Na values as small as 0.5%, the 34-week-gestation infant is limited to no less than 2%.
These maturational features limit the ability of the preterm or young infant to handle large fluctuations in fluid and solute loads. Both sodium conservation and regulation of extracellular fluid volume are impaired in comparison with the older child and adult. Limited GFR makes excretion of a fluid challenge difficult. Excessive urinary sodium loss leads to increased maintenance requirements; hyponatremia is common. Conversely, diminished concentrating ability increases free water losses during excretion of a solute load, whereas the high ratio of surface area to volume increases evaporative water loss. Consequently, fluid requirements are relatively high, and dehydration is common. Any errors in fluid management are poorly tolerated. As a rule, the most severe impairment exists in preterm infants, and the majority of homeostatic mechanisms are fully developed after the first year of life.
Holliday summarized the evolution of contemporary hydration therapy. In 1831, Latta first reported the use of intravenous (IV) fluids in the resuscitation of patients dehydrated by cholera. In 1918, growing information on the subject permitted Blackfan and Maxcy to successfully treat nine infants by intraperitoneal injection. In 1923, Gamble and associates detailed the anatomy of fluid and electrolyte compartments, introducing the use of milliequivalents to clinical practice. This paved the way for the development of the “deficit therapy” regimen of Darrow.
In subsequent decades, various recipes to replace the extracellular and intracellular fluid losses were suggested. For the most part, these failed because of excessive potassium and insufficient sodium content. Hyponatremia was common. When the focus of the treatment shifted to replacing the extracellular fluid deficit, rapid restoration of extracellular fluid volume using solutions with sodium concentrations similar to those in blood became commonplace. This, along with oral rehydration, is the preferred method of treatment today.
The concept of “maintenance fluids” is a complex subject. Although water and salt are required to sustain life, it is fair to say that for an individual child at any particular time, the precise amounts necessary are unknown (and perhaps unknowable). Instead, fluids and electrolytes, like anesthetics, are titrated to effect with general guidelines provided by clinical assessment, basic physiologic principles, and limited published data. The term maintenance fluids is often more limiting than helpful, and in all cases it is less precise than other terms familiar to anesthesiologists, such as minimum alveolar concentration (MAC) or median effective dose (ED 50 ).
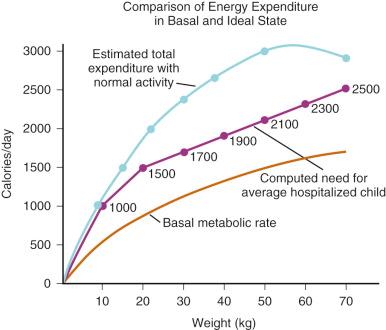
Holliday and Segar provided calculations for a first approximation of “the maintenance need for water in parenteral fluid therapy” in 1957. Integrating the relevant known physiology at that time, these authors observed that “ insensible loss of water and urinary water loss roughly parallel energy metabolism and do not parallel weight .” However, because water utilization parallels energy metabolism, energy metabolism follows surface area, and surface area follows weight, it should be possible to estimate water requirement from weight alone. The authors then proceeded under a series of assumptions to extrapolate from limited data to a “relationship between weight and energy expenditure that might easily be remembered.”
Assuming energy requirements of “ hospitalized patients ” to be “ roughly midway between basal and normal levels ,” they constructed a curve of caloric requirement versus weight. This curve could be seen as consisting of three linear sections: 0 to 10 kg, 10 to 20 kg, and 20 to 70 kg ( Fig. 9.5 ). Viewing the curve in this manner, the authors reasoned that “fortuitously, the average need for water, expressed in milliliters, equals energy expenditure in calories”: 100 mL/kg per day for weights to 10 kg, an additional 50 mL/kg per day for each kilogram from 11 to 20 kg, and 20 mL/kg per day more for each kilogram beyond 20 kg. In anesthetic practice, this formula has been further simplified, with the hourly requirement referred to as the “4-2-1 rule” (4 mL/kg per hour for the first 10 kg of weight, 2 mL/kg per hour for the next 10 kg, and 1 mL/kg per hour for each kilogram thereafter; Table 9.3 ).
| Weight (kg) | MAINTENANCE FLUID REQUIREMENTS | |
|---|---|---|
| Hour | Day | |
| <10 | 4 mL/kg | 100 mL/kg |
| 10–20 | 40 mL + 2 mL/kg for every kg >10 kg | 1000 mL + 50 mL/kg for every kg >10 kg |
| >20 | 60 mL + 1 mL/kg for every kg >20 kg | 1500 mL + 20 mL/kg for every kg >20 kg |
For decades, the simplicity and elegance of the Holliday and Segar formula has made it the starting point for fluid management in healthy children. Until recently, the majority of consultant anesthetists in the United Kingdom administered hyponatremic glucose-containing solutions intraoperatively and postoperatively to children undergoing elective surgery. However, the uncritical use of these solutions was never intended, and their blind application in the operating room, or in any clinical situation, resulted in instances of hyponatremia, aspiration, and death. Further, as Holliday has since pointed out, their original approach involved hyponatremic glucose-containing solutions rather than near-isotonic solutions such as 0.9% saline or lactated Ringer's solution (LR). In addition, these requirements were assessed at a basal metabolic state and not when the child was acutely ill or under physiologic stress during which increased levels of ADH were present. As the authors cautioned, “understanding of the limitations and of exceptions to the system [is] required. Even more essential is the clinical judgment to modify the system as circumstances dictate. ” General water losses for infants and children are summarized in Table 9.4 .
| Cause of Loss | Volume of Loss (mL/100 kcal) |
|---|---|
| Output | |
|
70 |
|
|
| Skin | 30 |
| Respiratory tract | 15 |
| Hidden intake (from burning 100 calories) | 15 |
| Total | 100 |
More recently, Holliday and colleagues revised the approach to fluid therapy in children that he and Segar enshrined with several caveats. In a related commentary, he pointed out several problems with applying the original 4-2-1 rule to acutely ill children. Namely, dysregulation of ADH is a hallmark of critical illness because ADH secretion is affected by a variety of nonosmotic factors such as pain, stress, mechanical ventilation, and medications ( Table 9.5 ). As a result, the choice of IV fluid and the rapidity of deficit replacement must be approached with care. The authors recommended a relatively simple strategy for healthy children undergoing elective surgery (including outpatients) to turn off ADH secretion and prevent perioperative water retention and subsequent hyponatremia. When a child (who is without significant heart or kidney disease) presents with marginal to moderate hypovolemia (e.g., after fasting for surgery), 20 to 40 mL/kg of isotonic fluids should be given during the surgery and postanesthesia care unit stay (as rapidly as 10–20 mL/kg per hour). Clinical judgment must always allow for modification of these recommendations if indicated for an individual child. If hypovolemia is more severe (e.g., after an extensive bowel preparation), 40 to 80 mL/kg may be necessary during the perioperative period. Some have expressed concern that such volumes of fluid could cause volume overload even in healthy children. However, evidence suggests that children handle crystalloid volumes much more efficiently than adults.
| Nonosmotic |
|---|
| Pain |
| Inflammation |
| Stress, catecholamines |
| Surgery; laparoscopic surgery |
| Vomiting |
| Hypoxia |
| Hypercapnia |
| Medications (e.g., opioids, amiodarone, vincristine) |
| Respiratory diseases (e.g., asthma, pneumonia, atelectasis) |
| Central nervous system disorders (e.g., head injury, tumors) |
| Osmotic |
| Fasting |
| Hypovolemia |
| Hypertonicity |
| Hypotension |
| Renal insufficiency |
| Hepatic insufficiency |
Postoperative IV fluid therapy should consist of an isotonic solution infused to replace ongoing fluid losses plus about half the rate described in the original 4-2-1 fluid regimen (i.e., 2 mL/kg for the first 10 kg, 1 mL/kg for the next 10 kg, and 0.5 mL/kg for each additional kilogram thereafter). Historically, when a child is unable to tolerate oral intake postoperatively, routine maintenance fluid therapy has been resumed with hypotonic saline solution (e.g., 0.45% saline). Although this regimen was believed to limit the ADH response and reduce the risk of postoperative hyponatremia and hypernatremia, pain and surgical stress can maintain increased ADH levels and risk the development of hyponatremia with seizures and encephalopathy. In the postoperative setting therefore, an isotonic salt solution is a better choice for maintenance at a rate of 2 : 1 : 0.5 rule for the first 12 hours then returning to the 4 : 2 : 1 rule thereafter, until the child tolerates oral fluids. Regardless of the fluids administered, it remains prudent to serially monitor plasma electrolyte concentrations until the child is drinking normally and homeostasis is restored.
In the first few postnatal days, isotonic losses of salt and water cause the healthy neonate to lose 5% to 15% of body weight. Although GFR increases rapidly, urine output is initially minimal, and renal losses are modest. Day 1 fluid requirements of the wrapped neonate, therefore, are relatively small. Over the next few postnatal days, losses and fluid requirements increase. In the poorly feeding infant, progression to hypernatremia and dehydration are common. When intake is appropriate, the term infant will regain body weight during the first week.
Three distinct phases of fluid and electrolyte homeostasis have been described in LBW and very low–birth-weight (VLBW) infants. In the first postnatal day, there is minimal urine output, and body weight is stable despite limited fluid intake. In the second phase, days 2 and 3 of life, diuresis occurs irrespective of the amount of fluid administered. By the fourth and fifth days, urine output begins to vary with changes in fluid intake and state of health.
Prematurity increases neonatal fluid requirements substantively. Fluid requirements are therefore estimated and then titrated to the infant's changing weight, urine output, and serum sodium concentration.
No less important is glucose homeostasis. In the ninth month of gestation, the fetus begins to form glycogen stores at a rate of more than 100 kcal/day. In the unstressed, term infant, hepatic glycogen stores are 5% of body weight. Immediately after birth, glycogenolysis depletes most of these stores within the first 24 to 48 hours. Gluconeogenesis must then proceed to yield glucose at a rate of approximately 4 mg/kg per minute.
At birth, the serum glucose concentration in the fetus is 60% to 70% of the maternal value. This may decrease within the first postnatal hours before recovering but should exceed 45 mg/dL to avoid neurologic injury. Symptoms of hypoglycemia may include jitteriness, lethargy, temperature instability, and convulsions. Ten percent dextrose in water (D 10 W) may be given as a bolus of 2 to 4 mL/kg followed by a continuous infusion (using a pump) at 4 to 6 mg/kg per minute. The serum glucose concentration is then analyzed frequently and the infusion adjusted as necessary to prevent hypoglycemia and hyperglycemia. Alternately, neonatologists are switching to a 40% dextrose gel that is applied to the buccal mucosa for rapid absorption and resolution of hyponatremia. This obviates the need for IV access if one has not been established by this time. It is important that the amount of glucose being provided be calculated in milligrams per kilogram per minute to avoid errors during fluid changes and to facilitate the diagnosis of persistent hypoglycemia.
Typical day 1 infant fluid orders recommend 70 to 80 mL/kg of D 10 W. Because D 10 W contains 10 g of glucose per deciliter, this regimen provides
On day 2, fluids are routinely increased to at least 100 mL/kg per day, and sodium is added at 2 to 3 mEq/dL. After urine output is established, potassium is added at 1 to 2 mEq/dL. The final solution, containing 30 mEq Na + and 10 to 20 mEq K + per liter, approximates the 0.2% saline “maintenance” solution commonly used previously in older children.
In the neonatal ICU, fluid management focuses on provision of adequate nutrition, maintenance of electrolyte balance, and limitation of fluid overload. The last factor is of particular concern because plasma oncotic pressure is reduced in preterm infants and the whole-body protein reflection coefficient is less than that in adults. VLBW infants are at particular risk for fluid and electrolyte imbalances. Even modest fluid overload may exacerbate pulmonary edema, prolong ductal patency, and more readily produce congestive heart failure. This perspective typically accompanies the infant to the operating room, where the primary considerations are routinely quite the opposite: restoration of circulating blood volume after third-space accumulation, maintenance of intravascular volume amid ongoing blood loss, replacement of potentially massive evaporative losses, and maintenance of BP despite anesthetic-induced vasodilatation and increased venous capacitance. During surgery, these concerns must take precedence, yet unnecessary administration of fluid is best avoided.
In infants and children, the first step toward intraoperative fluid management is often the most challenging: that is, establishing IV access. In general, simple procedures in healthy children are successfully approached using a single peripheral IV line. Although preferences vary among anesthesiologists, establishing IV access is most easily accomplished after induction of anesthesia. In young children, anesthesia is often induced by inhalation, and a catheter is inserted by an assistant into a hand or foot vein. In older children, or when IV access is desirable before anesthesia is induced, IV access may be facilitated by the use of topical anesthesia (e.g., EMLA [eutectic mixture of local anesthetics] cream, amethocaine, lidocaine infiltration) or sedation or both.
Complex surgeries in sicker children usually require at least two large-bore catheters. In pediatrics, however, “large-bore” is a relative term, with 22-gauge catheters typically providing sufficient access in infants. Preferred sites for larger catheters include the antecubital and saphenous veins (see Fig. 49.1 ). In cases in which access to the central circulation is required (as for pressure monitoring, infusion of vasoactive medications, or prolonged access), longer catheters may be placed via the femoral, subclavian, or internal jugular vein (the latter usually via a high, anterior approach; see Fig. 49.2, Fig. 49.3, Fig. 49.4, Fig. 49.5 ). Although secure access may also be obtained via the external jugular vein, it is often difficult to negotiate the J -wire or catheter tip into the central circulation. Peripherally inserted central catheter (PICC) lines have become common among hospitalized children. Although they represent a long-term means of delivering IV fluids and medications to those who need them, their intraoperative utility is very limited for several reasons. First, modern patient safety practices prohibit repeated access to central lines and practitioners must maintain strict sterile technique whenever entering them. Second, flow resistance is great within long, small-diameter catheters, precluding their use for large-volume resuscitation. Finally, PICCs are often placed for delivery of hyperalimentation or other solutions, which may be incompatible with anesthetic needs. For these reasons and others, separate IV access is often required and secure larger-bore shorter IV catheters with much lower resistance must always be placed if large fluid shifts or significant blood loss is anticipated.
In selecting the appropriate IV catheter, it is useful to consider the relative effects of catheter length and diameter on solution flow rates. Longer catheters offer more resistance to flow than shorter ones and are therefore less desirable when rapid infusion of a large volume of fluids is necessary (see E-Figs. 52.1 , 52.2 , and Fig. 51.1 ). In vitro, catheters designed for peripheral venous access had 18% to 164% greater flow rates compared with the same-gauge catheters designed for central venous use. Under pressure, as might be used during emergent volume resuscitation, rates differed up to 17-fold. Although this seems to suggest that short peripheral catheters should be preferred, in vivo data are more complex. In animal models, overall catheter flow rates are less than in vitro rates, and central access presents somewhat less resistance to flow than peripheral access. Finally, when the risks and benefits of central versus peripheral access are compared, central administration of resuscitation medications may provide little practical advantage compared with peripheral administration.
Intraosseous devices are now commonly used in the initial resuscitation of critically ill or injured children (see E-Figs. 49.1 and 49.6 and 49.7). Flow rates via these devices depend less on needle diameter than on resistance in the marrow compartment. In the operating room, the intraosseous route has been used for both induction and maintenance of anesthesia. However, onset of drug effect is less predictable, and the device is more easily dislodged than an IV catheter. Potential complications include compartment syndrome and, very rarely, damage to the growth plate. Such devices are probably best considered an emergency or last-resort option.
To prevent accidental volume overload, the amount of IV fluid available to administer to a child at any one time should not exceed the child's calculated hourly requirement. Particularly in infants, a volumetric chamber should be used to limit the amount of fluid available for infusion. Similarly, a microdrip infusion set limits the rate of fluid administration and permits much greater control. Although a fluid infusion pump provides the most precise mode of regulating the rate of fluid administration (and is therefore very useful in providing supplemental fluids or medications), such devices are impractical on primary access lines because they hinder the ability to administer drugs and fluids rapidly. In addition, the clinician should be mindful that pumps may continue to infuse through dislodged catheters, giving misleading reassurance that adequate IV access is present and fluids are being administered. Administering large volumes of fluid and drugs interstitially will not deliver the anticipated results. Moreover, if an identification or allergy bracelet is on a limb proximal to the IV insertion site, it may act as a tourniquet if the IV is interstitial, possibly causing ischemia to digits. Therefore access to the IV site is important in children, as well as removing all bracelets proximal to ipsilateral IV insertion sites.
In neonates and small infants, when rapid infusion of resuscitation solutions or blood products is anticipated, many practitioners insert a stopcock manifold into the IV infusion. Additional fluids may be prepared in syringes and warmed separately; during periods of sudden blood loss, stored syringes may then be inserted into the manifold and a fluid volume rapidly infused.
Finally, in prolonged surgeries or when volume replacement is great, all IV infusions may be warmed to maintain thermal homeostasis. Also, in younger infants and children in whom communication exists between the right and left sides of the circulation (e.g., patency of the foramen ovale), an in-line “bubble” filter is desirable.
In the early 1960s, simultaneous measurements of plasma and extracellular fluid volumes demonstrated that, during surgery, plasma volume is supported at the expense of the extravascular space. At the same time, it was classically observed that isotonic resuscitation fluids temporarily redistribute from the intravascular spaces to what was originally believed to be a third, nonfunctional space. However, when the endothelial glycocalyx is perturbed, as can occur with surgical trauma, fluid may shift from the intravascular to interstitial space. Therefore the historical “third space” may simply represent the reversible expansion of the interstitium. Because of the differences in fluid distribution and renal function in infants compared with older children, it was at first unclear that these findings could be extended to infancy. Thus fluid restriction remained the standard of care until careful studies specifically demonstrated that fluid and electrolyte requirements are often extremely large in neonates who are undergoing major surgical procedures.
Historically, hypotonic fluids were used as the maintenance solutions throughout the hospital, but this is no longer the practice. Isotonic solutions are preferred intraoperatively for several reasons. First, most ongoing volume losses are isotonic, consisting of shed blood and interstitial fluids. Second, large volumes of hypotonic solutions may rapidly diminish serum osmolality, producing very low concentrations of electrolytes (in particular, sodium) and undesirable fluid shifts. Indeed, even large volumes of “isotonic” fluids significantly decrease the serum osmolality in adult volunteers. Third, as discussed earlier, the plasma volume expansion that is necessary in response to diminished vascular tone under anesthesia is difficult to achieve even with isotonic fluids. Finally, increases in ADH levels and other elements of intraoperative physiology retain free water in excess of sodium if inadequate amounts of the latter are provided.
The compositions of commonly used IV solutions are presented in Table 9.6 . Assuming normal plasma osmolality is 275 to 290 mOsm/L, 0.9% NaCl (normal saline, NS) is theoretically hypertonic to plasma but is effectively isotonic when the in vivo activities of its constituents are considered. For dextrose-containing solutions, the osmolality decreases rapidly as sugar is metabolized, resulting in increased volumes of free water. Therefore, administration of 5% dextrose in water is ultimately equivalent to administering free water.
| mOsm/L | CATIONS (mEq/L) | ANIONS (mEq/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na + | K + | Ca 2+ | Mg 2+ | NH 4 + | Cl − | HCO 3 − | HPO 4 − | ||
| Extracellular fluid | 280–300 | 142 | 4 | 5 | 3 | 0.3 | 103 | 27 | 3 |
| Lactated Ringer's (LR) solution | 273 | 130 | 4 | 3 | 109 | 28 | |||
| 0.45% NaCl | 154 | 77 | 77 | ||||||
| 0.9% NaCl (normal saline) | 308 | 154 | 154 | ||||||
| PlasmaLyte A a | 294 | 140 | 5 | 3 | 1.6 | 98 | 98 | ||
| 3% NaCl | 1024 | 513 | 513 | ||||||
a PlasmaLyte is a trademark of Baxter International Inc., its subsidiaries or affiliates. (Plasmalyte also contains acetate 27 mEq/L and gluconate 23 mEq/L.)
Controversy regarding the perioperative use of colloid versus crystalloid fluid replacement remains unresolved. Colloid solutions carry the theoretical benefit of more effective expansion and retention of intravascular volume. Crystalloid solutions are much less expensive, easier to store, and carry few side effects. Although an initial meta-analysis of adult studies suggested worse outcomes after albumin resuscitation, this was not confirmed in a subsequent randomized controlled trial. However, subgroup analyses from the latter trial suggested that some patients, such as those with head injury, may be harmed by albumin, whereas others, such as those with septic shock, may realize some benefit. Thus the choice of solution may depend on the underlying medical conditions and remains a matter of clinical judgment.
It is worth noting that aside from 5% albumin, synthetic colloids are gaining popularity among pediatric practitioners. One reason for this is the development of newer synthetic colloids with a more favorable side effect profile. Hydroxyethyl starches (HES) are synthetic colloids that are simply modified polysaccharides. Circulating amylases quickly degrade natural polysaccharides, but HES solutions are not quickly degraded because the solutes contain hydroxyethyl groups in place of hydroxyl groups at carbon positions C-2, C-3, and C-6, rendering the molecules resistant to hydrolysis. These compounds are characterized by three attributes: average mean molecular weight (MW), molar substitution (MS), and the C-2/C-6 ratio, which relates to the relative positions of hydroxyethyl groups on the polysaccharide molecule.
HES solutions with a greater MW/MS ratio remain in the intravascular space for longer periods than those with smaller ratios. However, they also are prone to more adverse effects including hypocoagulability. Newer, low MW/low MS solutions have much less effect on hemostatic mechanisms than older, higher MW/higher MS solutions. The precise mechanism by which HES compounds affect coagulation remains unclear, although it has been attributed to interference with von Willebrand factor, factor VIII, and platelet function. A greater C-2/C-6 ratio is responsible for a slower degradation of the starch by amylase with fewer adverse effects. When renal function is normal, newer HES solutions (e.g., HES 130/0.42/6 : 1) are safe for children undergoing elective surgery, since they maintain hemodynamic stability and produce only mild to moderate changes in acid-base status. Synthetic colloids such as these can therefore be considered in surgical patients who demonstrate the need for aggressive intraoperative fluid resuscitation (see also Chapter 12 ). Use of these solutions in cardiac surgery remains controversial given the effects on coagulation factors and platelet function induced by the cardiopulmonary bypass circuit.
The routine intraoperative use of glucose-containing solutions has also been a subject of debate. As a rule, operative stress evokes physiologic responses that increase serum glucose. In practice, therefore, hypoglycemia is seldom a problem in healthy, fasted children when glucose is omitted from perioperative IV fluids. Indeed, the risk should be particularly small if the period of fasting is limited to less than 10 hours. At the same time, rapid administration of dextrose solutions may certainly produce acute hyperglycemia and hyperosmolality. Therefore, glucose-containing electrolyte solutions should not be used to replace fluid deficits, third-space losses, or blood losses, but they may be used as a background electrolyte maintenance solution. Some populations, such as debilitated infants, children who are malnourished, neonates and infants younger than 6 months of age, and those undergoing cardiac surgery, are at risk for intraoperative hypoglycemia. The use of glucose-containing solutions (1%–2.5% dextrose), along with intraoperative glucose monitoring, may be beneficial in these children.
It is now common practice that critically ill children arrive in the operating room with hyperalimentation solutions infusing. Common contents of hyperalimentation solutions are shown in Table 9.7 . In general, children require 0.5 to 3.0 mg/kg per day of protein, 6 to 9 mg/kg per minute of glucose, and 0.5 to 3 g/kg per day of fat. Children receiving parenteral nutrition preoperatively should continue to receive those infusions separately, and a corresponding volume should be deducted from isotonic operative fluids. Hyperalimentation typically consists of two infusions: fat (e.g., Intralipid, Fresenius Kabi AB, Uppsala, Sweden) and a concentrated glucose/protein solution. It is prudent to discontinue the Intralipid solution during surgery, but if that is not possible, then every effort should be made to avoid accessing any ports in the line to reduce the risk of contaminating the Intralipid. Conversely, the concentrated glucose/protein solution should be continued at the same rate (because circulating insulin concentrations have acclimated accordingly). Because of hyperglycemic responses to the stress of surgery and reduced metabolism related to anesthesia and hypothermia, some practitioners routinely decrease hyperalimentation infusion rates by one-third to one-half. If the latter practice is followed, clinicians should consider checking serum glucose concentrations at regular intervals to monitor for hypoglycemia. Under no circumstances should concentrated glucose solutions (such as D 10 or D 20 ) be abruptly discontinued, because high concentrations of circulating insulin may cause a precipitous and profound decrease in the serum glucose concentration.
| Carbohydrates |
| 10%, 12.5%, 20%, 25%, 30% Dextrose |
| Limited to D 10 or D 12.5 if through a peripheral catheter |
| Protein |
| In the form of amino acids |
| 0.5, 1.0, 1.5, 2.0, 2.5, or 3.0 g/kg per day |
| Lipids |
| 10%, 20% Lipids |
| Standard Additives |
| Sodium: 30 mEq/L |
| Potassium: 20 mEq/L |
| Calcium: 15 mEq/L |
| Magnesium: 10 mEq/L |
| Phosphorus: 10 mmol/L |
| Heparin |
a Common contents of parenteral nutrition solutions containing dextrose, protein, lipids, and standard additives such as electrolytes. These values represent standard starting points that may be modified based on individual patient needs.
Concerns regarding the routine intraoperative use of dextrose-containing solutions increased with recognition that hyperglycemia may exacerbate neurologic injury after an ischemic or hypoxic event. As a result, many clinicians now elect to avoid dextrose-containing solutions during routine surgery. When dextrose-containing solutions are used, appropriate monitoring is advised to avoid serum glucose extremes. Many practitioners administer glucose-containing solutions as a separate piggyback infusion using an infusion pump or other rate- or volume-limiting device to avoid accidental bolus administration. Alternatively, evidence indicates that isotonic solutions that contain reduced glucose concentrations (e.g., 1% or 2.5% vs. 5%) are safe alternative solutions for intraoperative use. In the United States, several Food and Drug Administration (FDA)-approved solutions containing 2.5% dextrose are available but none with concentrations lower than 2.5%. In Europe, 1% dextrose electrolyte solutions are available. Because intraoperative administration of solutions containing 5% dextrose (D 5 LR) frequently causes hyperglycemia, prudent anesthesiologists should selectively administer dextrose-containing solutions to those who are at particular risk for intraoperative hypoglycemia (i.e., neonates, chronic malnourished children, and cachectic children). In these instances, it may be sensible to administer solutions with a reduced dextrose concentration.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here