Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Tendons transmit forces generated by muscles to move joints or to create action power. Flexor tendon injuries are common, but recovery of satisfactory function, particularly after injuries within the digital sheath, is sometimes difficult. Lacerated flexor tendons should be treated by primary surgical repair whenever possible.
The current trend of end-to-end surgical tendon repairs is to use multistrand core sutures (four-strand repairs such as cruciate, double Tsuge, Strickland, modified Savage, or six-strand repairs such as modified Savage, Tang, or triple Kessler).
In tendon repairs in the digital sheath area, a number of surgeons advocate that the A2 pulley can be released up to two-thirds of its length, and the A4 pulley can be entirely released when necessary and tendon repair is in the proximity of the pulley, given the integrity of the other pulleys. The release may reduce the resistance to tendon motion and the chance of repair ruptures.
Postoperatively, early tendon mobilization should always be employed, except in children or in some rare instances; motion protocols vary greatly among different treatment centers.
Repair rupture, adhesion formation, and joint stiffness are major complications of primary surgery.
Combined use of multistrand core repairs, release of constricting pulley parts, and well-designed postoperative combined passive and active motion protocols – that do not overload, but sufficiently move the tendon – can help minimize adhesions, avoid repair ruptures, and restore optimal function. Full extension and flexion of the operated digit should be tested right after repair, ideally in a wide-awake setting, to ascertain no gapping of the repair and smooth tendon gliding.
Secondary surgeries include tenolysis, free tendon grafting, and staged tendon reconstruction. Tenolysis is indicated when restricting adhesions hamper tendon gliding and soft tissues and joint conditions of the hand are favorable. Free tendon grafting is a salvage operation for failed primary repairs, delayed treatment (>1 month) of an acute cut, or lengthy tendon defects. Staged reconstruction is indicated in case of extensive scar formation or multiple failed surgeries. Preservation or reconstruction of major annular pulleys is vital to restoring function of the digits during these secondary surgeries.
Closed rupture of flexor tendons usually requires surgical repair.
Success of flexor tendon surgery is very expertise-dependent. A thorough mastery of anatomy and meticulous surgical technique are requirements for satisfactory restoration of function.
Access video content for this chapter online at Elsevier eBooks+ ![]()
Tendons are composed of dense connective tissues that transmit forces generated by muscles to move the joints or to create action power. Functionally, the hand is dependent upon the integrity and ample gliding of the tendons. Among all the tendons in the body, those in the hand are most frequently injured, due to their length and the varied nature of the activities of the hand. The pursuit of ideal repair techniques has drawn the attention of surgeons ever since hand surgery became a subspecialty. For over a century, flexor tendon repairs have presented challenges to hand surgeons.
Difficulties in restoration of function of digital flexor tendons relate chiefly to the intricate anatomy of flexor tendon systems: the coexistence of superficialis and profundus tendons within a tight fibro-osseous tunnel. Peritendinous adhesions jeopardize tendon gliding. Tendons within the synovial sheath (intrasynovial tendons) were once considered to lack self-reparative capacity; therefore, ingrowth of adhesions from peritendinous tissues was believed to be a prerequisite in the tendon healing process. As the concepts regarding tendon healing biology evolved, tendon cells have been proven capable of proliferating and of producing collagen to heal tendons. However, the tendon is innately low in cell density and growth factor activity, limiting its early healing strength.
In the early and middle twentieth century, secondary tendon grafting dominated the repair of digital flexor tendons. During this period, tendon implants were developed for staged tendon reconstruction. However, as the practice of primary repair prevailed in recent decades, the number of cases indicated for secondary tendon grafting or staged reconstruction decreased drastically. Primary repair of injured digital flexor tendons was pioneered by Verdan and Kleinert et al . in the 1960s and is the essential approach underlying current practice. Current primary repair and early tendon motion are based on the recognition of intrinsic healing capacity of the tendon in the 1970s and 1980s by Lundborg, Manske et al ., and Gelberman et al .
Nevertheless, despite the widespread use of primary repairs, surgical outcomes remained unpredictable, and sometimes even disappointing. In the last two to three decades, major efforts were thus devoted to tackling this problem, with the goal of achieving consistently optimal outcomes and minimizing repair ruptures and adhesions. In this regard, a number of multistrand core surgical repairs – such as Savage, Strickland, cruciate, Lim–Tsai, or Tang techniques – have been developed to replace weaker, conventional two-strand repairs. Subdivisions of zones 1 and 2 of digital flexor tendon systems were proposed by Moiemen and Elliot and Tang, which offer precise nomenclature in recording the locations of tendon cuts, discussing treatments, and comparing outcomes. Surgical procedures to release critical parts of the pulleys have been advocated by Tang and Kwai Ben and Elliot to decompress the tendon and “free” tendon motion. In the past few years, we have witnessed reports in which repair ruptures have been minimized, with recovery of excellent or good function in the majority of cases. These recent reports represent some remarkable steps towards satisfactory flexor tendon repairs and highlight the promise of predictable tendon repairs ( Box 9.1 ).
Repairing flexor tendons is based on a thorough mastery of anatomy and biomechanics. The surgeon should know the anatomy in detail, including the lengths of major pulleys, characteristic changes in the diameter of the sheath, and tendon gliding amplitude.
Primary repairs should be performed by experienced surgeons whenever possible.
Mastery of atraumatic techniques is essential for the operator. The outcomes of the repairs are very expertise-dependent; repair of tendons by an inexperienced surgeon is a frequent cause of tendon adhesions and poor function, and thus should be avoided.
Conventional two-strand repairs are weak; stronger surgical repairs are preferable.
Complete closure of the tendon sheath is not necessary. Venting of a part of sheath (<2.0 cm), including a critical portion of the pulleys, provides easy access to the injured tendons, and may decrease resistance to tendon gliding after surgery; this procedure does not lead to loss of digital function when other sheath parts are intact.
Surgeons should emphasize strong suture techniques and decreasing compression to the tendons, which will aid in achieving improved outcomes with hand therapy.
There are 12 flexor tendons in the hand and forearm region. They include finger and thumb flexors and wrist flexors. Finger flexor tendons are the flexor digitorum superficialis (FDS) and the flexor digitorum profundus (FDP), and the tendon in the thumb is the flexor pollicis longus (FPL). They originate from muscles at about the mid forearm. The tendons of the FDP, except the index finger, come from a common muscle belly. The tendons of the FDS originate from the separate muscle bellies, which allow more independent finger flexion. The FPL tendon arises from the volar aspect of the midportion of the radial shaft and from its adjacent interosseous membrane. Three wrist flexors are flexor carpi radialis (FCR) and ulnaris (FCU), and palmaris longus. Palmaris longus is absent in about 15–20% of the normal population. Wrist flexion power is not affected by absence of this muscle.
Within the carpal tunnel, nine tendons exist – four FDS, four FDP, and one FPL. The relationship of these tendons within the carpal tunnel is fairly constant. The FDS tendons to the ring and middle fingers lie superficially, deeper are the FDS tendons to the index and small fingers, and deeper still are the FDP tendons. The FPL tendon is located deep and radially adjacent to the scaphoid and the trapezium. After emerging from the carpal tunnel, the tendons enter the palm. At about the level of the superficial palmar artery arch, the lumbrical tendons originate from the FDP tendons.
The most intricate portions of the flexor tendons are within the fingers, where the tendons glide within a closed fibro-osseous sheath with segmental, semirigid, constrictive dense connective tissue bands present. The digital sheath forms a closed synovial compartment extending from the distal palm to the middle of the distal phalanx. Proximally, the synovial sheath ends just proximal to the neck of the metacarpus, forming the proximal reflection of the digital flexor sheath. The FDS tendons lie superficial to the FDP tendons up to the bifurcation of the FDS tendon at the midportion of the proximal phalanx. Then, FDS tendons become two slips coursing laterally and then deeper to the FDP tendons. This FDS bifurcation is in the A2 pulley area. This part of the FDS tendon also serves to constrain the FDP tendon; the FDS segment at bifurcation may be viewed as a structure functioning similarly to a pulley. Deep to the FDP tendon, the FDS slips rejoin to form Camper's chiasm (a fibrous interweaved connection between two FDS slips), and distally insert on the proximal and middle parts of the middle phalanx as two separate slips. The FDP tendon inserts into the volar aspect of the distal phalanx. The FPL tendon is the only tendon inside the flexor sheath of the thumb and inserts at the distal phalanx.
The digital flexor sheath consists of synovial sheath and interwoven condensed fibrous bands (“pulleys”). Synovial sheath is a thin layer of continuous smooth paratenon covering the inner surface of the fibrous sheath, providing a smooth surface for tendon gliding and nutrition to the tendons. The pulley system of the digital flexor tendon is unique; it consists of annular pulleys (condensed, rigid, and heavier annular bands) and cruciate pulleys (filmy cruciform bands) ( Fig. 9.1 ). There are five annular pulleys (A1–A5), three cruciate pulleys (C1–C3), and one palmar aponeurosis pulley. The A1, A3, and A5 pulleys originate from the palmar plates of the metacarpophalangeal (MCP), proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints, and the A2 and A4 pulleys originate from the middle portion of the proximal and middle phalanges, respectively. The broadest annular pulley is the A2 pulley, which covers the proximal two-thirds of the proximal phalanx and encompasses the bifurcation of the FDS tendon at its middle part. The A4 pulley is located at the middle third of the middle phalanx. The A2 and A4 pulleys are the largest among five annular pulleys and have the most important function. The annular pulleys maintain the anatomical paths of tendons close to bones and phalangeal joints, thus optimizing the mechanical efficiency of digital flexion. The more compressible cruciate pulleys allow for digital flexion to occur with condensation of the fibro-osseous sheath at the inner part of flexed fingers. This is called the “concertina effect”.
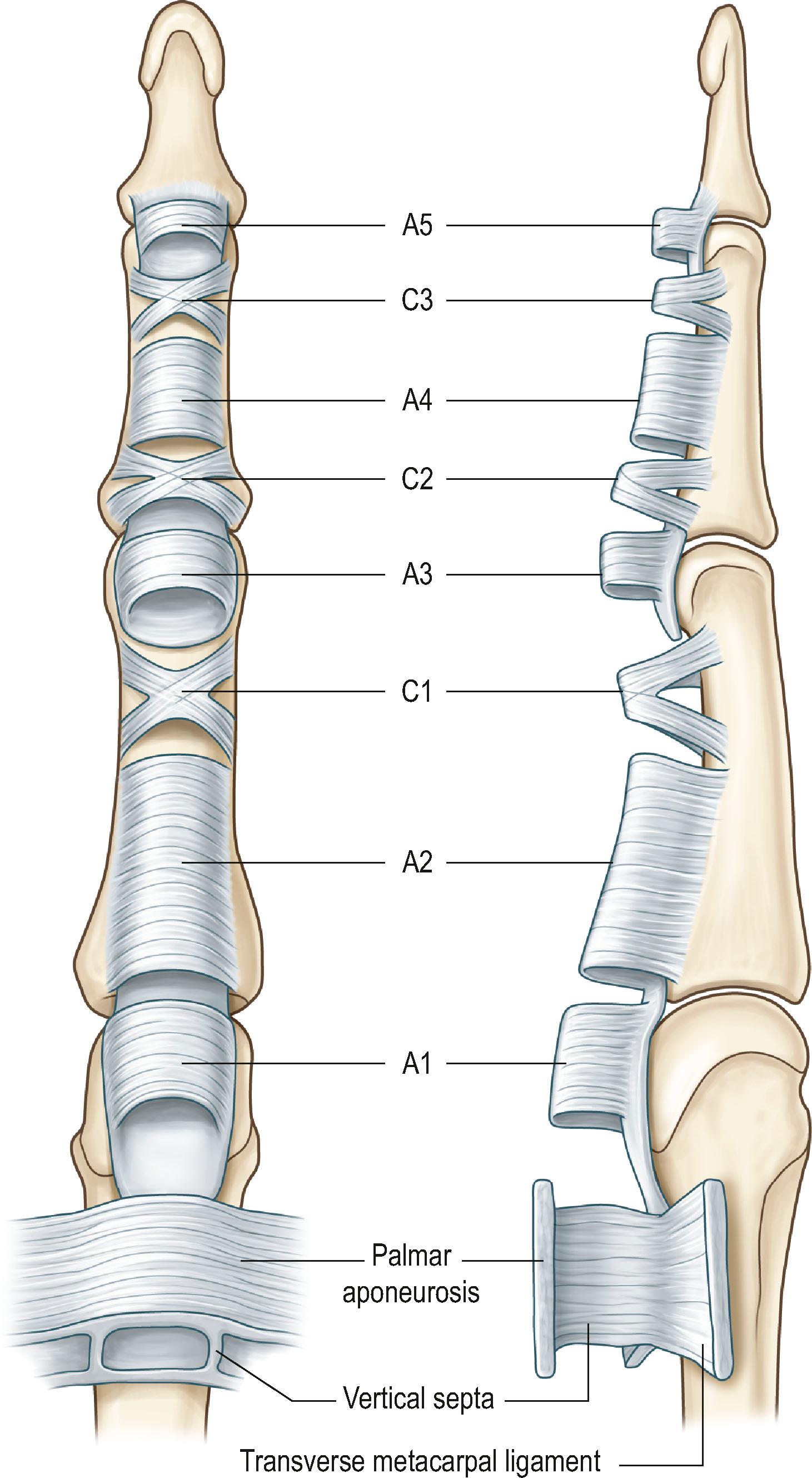
The length of the A2 pulley is about 1.5–1.7 cm in the middle finger of an average adult, and the length of the A4 pulley is about 0.5–0.7 cm. The diameter of the flexor sheath is at its narrowest at the level of the A4 pulley and in the middle and distal parts of the A2 pulley. The A2 and A4 pulleys are easily recognizable, because both are remarkably denser and more rigid than their adjacent flexor sheath. The A1 pulley, with a length of about 1.0 cm, is located proximal to the A2 pulley; in some instances, both A1 and A2 pulleys merge to form an especially lengthy pulley complex. The A3 pulley is located palmar to the PIP joint, but it is very short (0.3 cm) and may be difficult to distinguish from the synovial sheath.
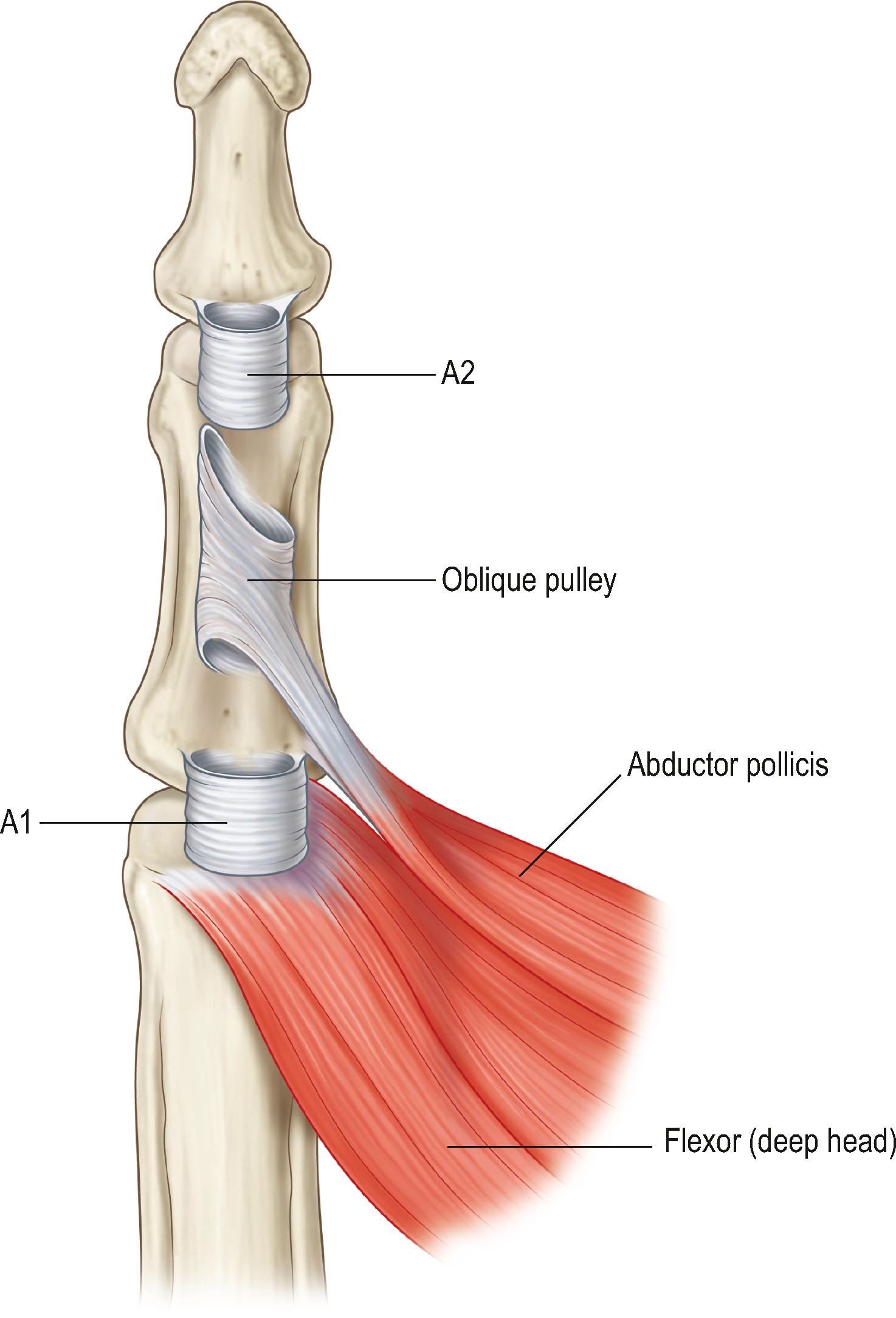
In the thumb, there are three pulleys (A1, oblique, and A2) with no cruciate pulleys ( Fig. 9.2 ). The A1 and oblique pulleys are functionally important. The A1 pulley, 0.7–0.9 cm long, is located palmar to the MCP joint. The oblique pulley, 0.9–1.1 cm long, spans the middle and distal parts of the proximal phalanx. The A2 pulley is near the site of insertion of the FPL tendon, which is thin and 0.8–1.0 cm long.
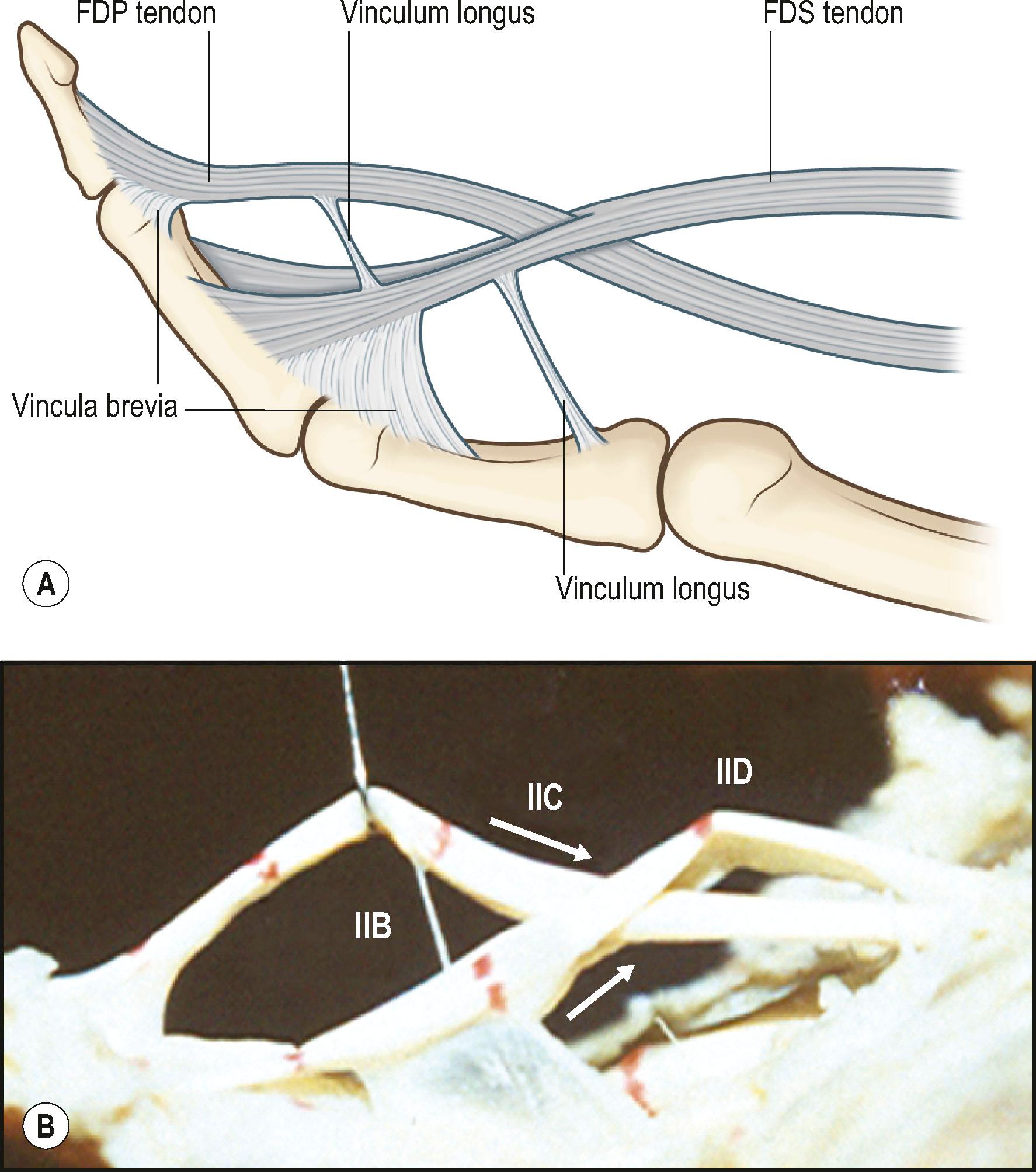
The FDP tendon has two vincula: a fan-like short vinculum and a cord-like long vinculum. The short vinculum is located at the insertion of the FDP tendon ( Fig. 9.3 ). The long vinculum connects the FDP tendon through the short vinculum of the FDS tendon to the floor of the palmar surface of the phalanges. The FDS tendon also has two vincula; one connecting to the proximal phalanx, and another at the insertion of the FDS tendon. Vincula carry blood vessels to the dorsum of these tendons, providing limited nutrition. Tendon insertion sites to bones also carry vessels into tendons over a very short distance.
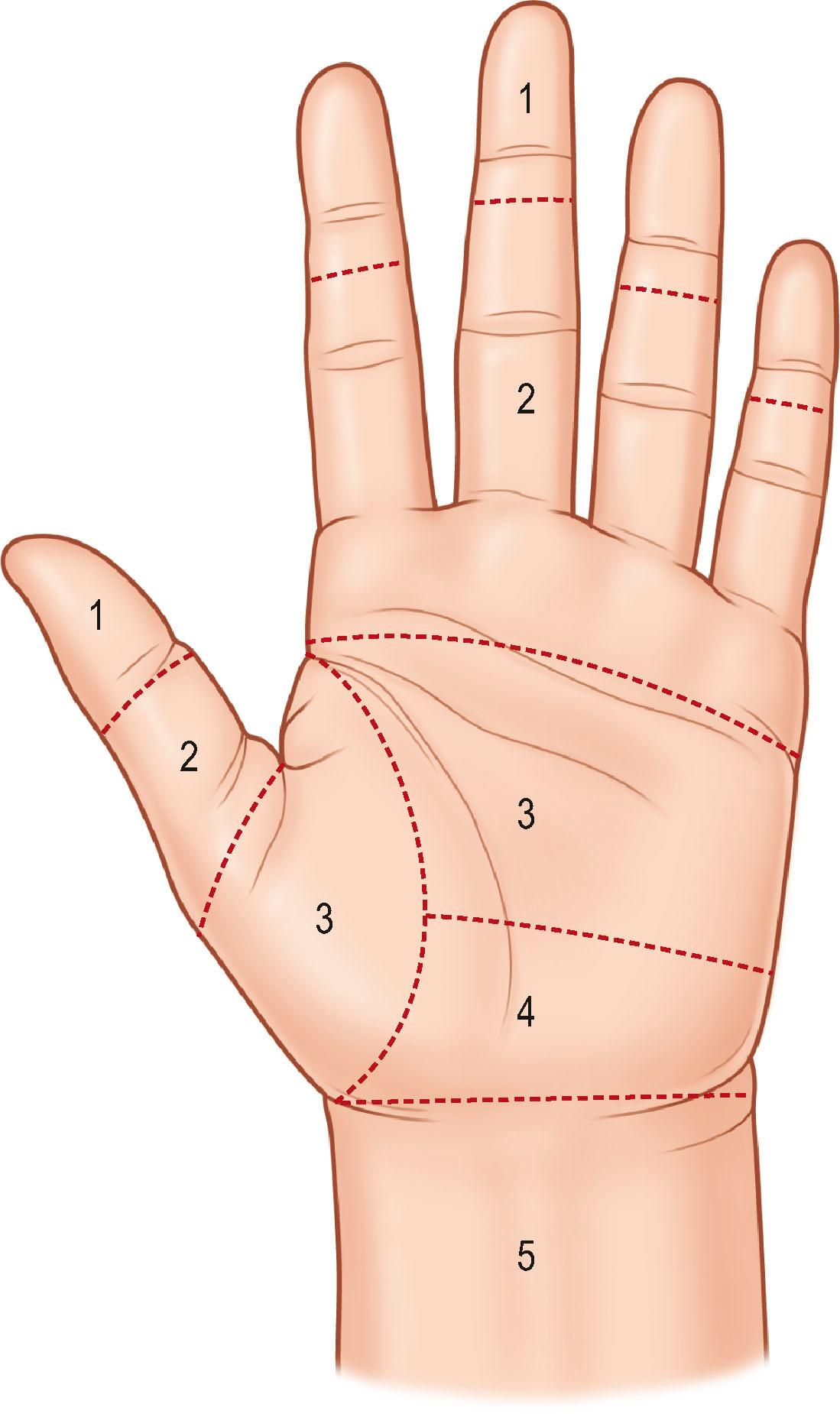
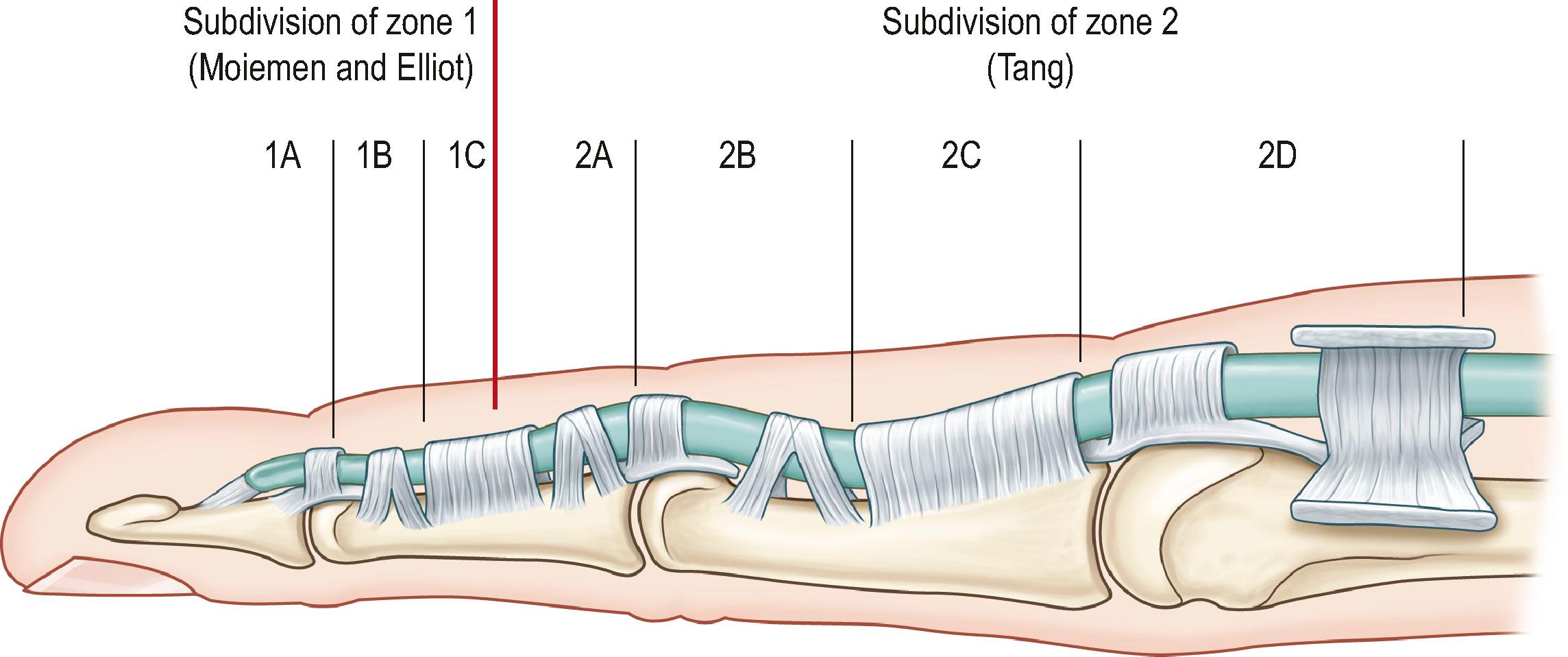
According to anatomical features, the flexor tendons in the hand and forearm are divided into five zones, which offers the fundamental nomenclature for flexor tendon anatomy and surgical repairs. In the 1990s, the most complex areas – flexor tendons in the digital sheath – were subdivided by Moiemen and Elliot, and by Tang. The zoning is described below, and its relation to the locations of pulleys is shown in Figs. 9.4 and 9.5 :
Zone 1: from the insertion of the FDS tendon to the terminal insertion of the FDP tendon
Zone 2: from the proximal reflection of the digital synovial sheath to the FDS insertion
Zone 3: from the distal margin of the transverse carpal ligament to the digital synovial sheath
Zone 4: area covered by the transverse carpal ligament
Zone 5: proximal to the transverse carpal ligament
In the thumb, zone 1 is distal to the interphalangeal (IP) joint, zone 2 is from the IP joint to the A1 pulley, and zone 3 is the area of the thenar eminence.
The subdivisions of zone 1 by Moiemen and Elliot are:
1A: the very distal FDP tendon (usually <1 cm)
1B: from zone 1 A to distal margin of the A4 pulley
1C: the FDP tendon within the A4 pulley
The subdivisions of zone 2 by Tang are:
2A: the area of the FDS tendon insertion
2B: from the proximal margin of the FDS insertion to the distal margin of the A2 pulley
2C: the area covered by the A2 pulley
2D: from the proximal margin of the A2 pulley to the proximal reflection of the digital sheath
Documentation and treatment of tendon injuries dates back to antiquity. Hippocrates and other ancient physicians observed a white slender tubular structure entering skeletal muscle, and considered it as a nerve. Galen, who lived in the Roman Empire in the second century, advised physicians not to repair tendons because it was believed to result in pain, twitching, and convulsions of the limb. Over the following 15 centuries, surgeons were largely influenced by the teaching of Galen. The seminal challenge to Galen's assertion did not occur until the mid-eighteenth century when Von Haller showed that the placement of a suture in canine Achilles tendons did not have detrimental effects.
The first experimental investigation of the tendon healing process was performed in 1767 by John Hunter, who recorded that the canine Achilles tendon healed by the formation of callus, similar to that seen in healing bone. However, specific studies of the flexor tendon healing within digital sheath were not started until about 150 years later, in the early twentieth century. Around 1920, Bier and Saloman observed poor tendon healing of sutured canine flexor tendons. Saloman thus advocated to leave a defect in the tendon sheath which allowed contact between the repaired tendon and the subcutaneous tissue. Bunnell and Garlock observed frequent occurrence of restrictive adhesions at the flexor tendon laceration site. Bunnell coined the term “no man's land” to describe the region where the flexor tendons passed through the digital sheath and advised surgeons to be cautious when repairing tendons within this region. In 1940, Mason advised some specific conditions for repairing lacerated digital flexor tendons, which included never repairing both tendons, wide excision of the overlying sheath, and adequately eliminating contaminates from the wound. Because of the generally unsatisfactory results of primary flexor tendon repairs, many surgeons preferred tendon grafting, despite the fact that a few surgeons had demonstrated that primary surgeries were practicable at that time.
In the early half of the twentieth century, repairing the lacerated tendons within the digital sheath was predominantly by means of secondary tendon grafting. The first series of flexor tendon grafts in the hand was reported by Lexer in 1912. He used grafts to repair flexor tendons after rupture, old lacerations, infections and cases with ischemic contracture. In 1916, Mayer published articles that have served as the basis of the present day concepts of free tendon grafting. He emphasized the need for existing surgical techniques, direct juncture of the tendon to bone, the use of adequate muscle as a motor, and preserving peritenon around a graft to lessen adhesions. In 1918, Bunnell published a classic article on tendon grafting in which atraumatic technique, a bloodless field, perfect asepsis, and preservation of pulleys were emphasized. The surgical techniques of tendon grafting were subsequently modified by various leaders in the field, including Pulvertaft, Graham, Littler, Boyes and Stark.
In an effort to combat serious scarring in the tendon gliding bed in which conventional one-stage free tendon graft likely failed to restore tendon function, various materials have been implanted into the fingers to stimulate formation of a smoother tendon gliding bed. In 1936, Mayer inserted a celloidin tube into seriously scarred tendon gliding bed, and after 4–6 weeks of implantation he then introduced a tendon graft. Mayer brought forth the pseudosheath concept. In the 1950s, Bassett and Carroll began working with flexible silicone rubber rods to build pseudosheaths in badly scarred fingers, adding passive motion into this procedure. This method was later refined into a two-stage tendon reconstruction by Hunter et al . in the 1960s. Hunter et al . also developed the active tendon implant. These implants and two-stage reconstruction procedures are still used currently, effective for those patients with badly scarred digits and serving as a salvage procedure after other surgery attempts failed.
It was not until the 1960s that reports of surgical techniques and outcomes of primary repair from Verdan, and Kleinert et al . became the turning point in establishing the practice of primary repair of digital flexor tendons. The emerging popularity of primary tendon repair stimulated experimental studies to elucidate mechanisms of the healing process. In the 1970s and 1980s, Manske et al . demonstrated diffusion of nutrients through synovial fluid to be an effective source of nutrition of intrasynovial tendon, thus obviating the need of vascularization in the healing process. Matthews and Richards observed healing of lacerated rabbit flexor tendons within the intact digital sheath in the absence of adhesions. Lindsay et al . noted that both epitenon and endotenon cells proliferate and migrate to the laceration site, subsequently bridging it. Lundborg et al . demonstrated healing of lacerated flexor tendon segments when placed within the synovial environment of the knee joint or in a synthetic membrane pouch placed in a subcutaneous pocket of the back of the rabbit. In the 1980s, Manske, Lesker, Gelberman, and Mass et al . demonstrated healing of lacerated flexor tendon segments of different animals when placed in tissue culture in the complete absence of extrinsic cells.
In the last three decades, major advancements in the field of flexor tendon repairs were made with respect to the development of novel surgical repair techniques, accumulation of biomechanical information regarding tendon repairs or gliding mechanisms, modifications of postsurgical rehabilitation methods, and an emerging trend of exploring biological approaches to enhance tendon healing. In 1985, Savage published an influential experimental study that later stimulated a widespread interest in developing multistrand suture techniques to repair the tendon. The cruciate repair, a simple yet strong four-strand repair developed by McLarney et al . in the late 1990s, is used in many hand centers. In the same period, multistrand repair using Tsuge's looped suture lines, as developed by Lim and Tsai, Tang (and its modification, M-Tang method), were used by surgeons in hand centers in Asia, Europe, and North America. In 1995 and 1998, Tang and Elliot presented an anatomical study and clinical results of the release of a major flexor pulley (A2) to accommodate gliding of the repaired tendons, respectively. Elliot and Tang also proposed to vent the A4 pulley when the tendon repairs are in the areas.
The flexor tendons were divided into five zones by Verdan in the 1960s. In the 1990s, subdivisions of zone 1 by Moiemen and Elliot and zone 2 by Tang were added into the existing zoning system, in order to describe the location of injuries and repairs more specifically. These zoning and sub-zoning systems provide hand surgeons with the nomenclature to document the tendon injuries and outcomes, and discuss the principles of treatment.
In the past 30 years, biomechanics of the flexor tendon system has become a subject of intense interest for both surgeons and basic scientists. A large volume of information regarding tendon gliding and repair biomechanics has been obtained, including surgical repair techniques (mainly from labs of Gelberman, Manske, Mass, McGrouther, Tang, Trumble, and Wolfe), gliding resistance of tendons within the sheath or against annular pulleys (Amadio and Tang), mechanics of the pulleys (Amadio, Cao, Tang, and Wu), postsurgical rehabilitation (Amadio and Boyer), and edema formation (Tang). In recent years, as advocated by Lalonde, flexor tendon repair and tenolysis are popularly performed in some centers around the globe under wide-awake settings (without tourniquet) with local anesthesia plus epinephrine. This approach allows the tendon to move actively during surgery to ascertain good quality of tendon repair or tenolysis.
In the past three decades, investigations exploring molecular events in the tendon healing process, tendon tissue engineering, and biological approaches to enhance the healing came into the center stage of basic science investigations. Chang et al . reported a series of investigations exploring function of growth factors in tendon healing, molecular methods to prevent adhesions, and tendon tissue engineering. Tang and colleagues performed a series of studies of molecular events in tendon healing and gene therapy approaches to enhance the strength or to limit adhesions.
Comprehensive history and development of flexor tendon repairs were covered in more detail in the reviews written by Verdan (1972), Manske (1989), Strickland (1995), Elliot (2003), Manske (2005), and Tang (2007) and Tang (2018).
Flexor tendons derive nutrition from both synovial and vascular sources. Flexor tendons outside the synovial sheath are supplied with a segmental vascular network through the paratenon, and the vascular supply plays an important role in nutrition of these tendons. However, the tendons within the synovial sheath are mostly deprived of a vascular network. Only limited dorsal regions around vincular insertions are vascularized. A series of experiments by Manske et al . showed that intrasynovial flexor tendons are nourished by synovial fluid and that nutrition through vascular supplies is insignificant. While the general healing process of the tendon has long been recognized as having early inflammatory, middle collagen production, and late remodeling phases, the healing potential of the intrasynovial flexor tendon was a subject of intense investigations and debate over several decades in late twentieth century. The work in that period of time led to conclusions that cells in the intrasynovial tendon can proliferate and participate in the healing process, making the tendon itself capable of healing without forming adhesions. This became the scientific basis of early postoperative tendon mobilization.
It is now agreed that intrasynovial flexor tendons can heal through two mechanisms – intrinsic and extrinsic. Intrinsic healing takes place through the proliferation of tenocytes and production of extracellular matrix by intrinsic cells. Growth of tissues or cell seeding from outside the tendon is extrinsic healing. The tendon's intrinsic healing capacity is innately weak; extrinsic healing becomes dominant when intrinsic healing capacity is disabled (such as in the case of severe trauma to the tendon or peritendinous tissues). Tendon healing exclusively through the intrinsic healing mechanism occurs only under a few experimental conditions. Clinically, the lacerated tendon heals through a combination of both intrinsic and extrinsic mechanisms, whose balance depends upon the condition of the tendon and surrounding tissues. Extrinsic healing may act on the tendon healing process through either forming adhesions or seeding of the extrinsic cells without adhesions to laceration site. On the other hand, adhesions do not necessarily consist of extrinsic cells. Tenocytes may migrate out of the laceration site for a very limited distance to become part of adhesions. Conceptually, extrinsic healing does not equal adhesion formation. However, it is extrinsic healing in the form of restrictive adhesions that hampers tendon function.
The following five variants (grades) of adhesions are seen clinically: (1) no adhesions; (2) filmy adhesions: formation of visible, filmy, and membranous tissue from tendon to outside tissues; (3) loose adhesions: loose and largely movable; (4) moderately dense adhesions: of limited mobility; and (5) dense adhesions: dense, almost immovable, and invading deep into the tendon.
The first two grades do not affect tendon motion; the third affects motion mildly. Because the fourth and fifth affect motion dramatically, those are the adhesions that surgeons seek to prevent. The density of adhesions relates to the tissues from which they arise. The adhesions arising from bones, periosteum, or major annular pulleys are dense. The density of adhesions can be altered to some extent by tendon motion. Some adhesion fibers can be disrupted as well. Surgeons should do their utmost to preclude or minimize the formation of adhesions that will restrict tendon gliding.
Many strategies have been attempted or suggested to prevent adhesion formations, including medications, use of artificial or biological barriers, and chemical or molecular approaches, with varied results. However, few medications or barriers have become clinically routine. So far, the most effective methods to prevent adhesions in clinic are meticulous surgery and early postoperative motion; the prime cause of adhesions is tendon repair by inexperienced surgeons.
Forces generated during normal hand action range from 1 to 35 N, except tip pinch, according to in vivo measurements. Therefore, a surgically repaired tendon should be able to withstand a tension of at least 40 N during motion, with power sufficient to resist gap formation. The repair should be able to withstand cyclic loads under both linear and curvilinear load conditions. Laboratory tests have shown that conventional two-strand core repairs plus running peripheral sutures yield a maximal strength from 20 to 30 N ; this is lower than forces generated during normal hand actions and explains why some repairs are disrupted during postoperative motion exercise. Studies showed that failure forces of four-strand repairs are around or beyond 40 N ; six-strand repairs fail with loads over 50–60 N.
Many factors affect the strength of a surgical repair ( Fig. 9.6 ): (1) the number of suture strands across the repair sites – strength is roughly proportional to the number of core sutures ; (2) the tension of repairs – most relevant to gap formation and stiffness of repairs ; (3) the core suture purchase ; (4) the types of tendon–suture junction – locking or grasping ; (5) the diameter of suture locks in the tendons – a small-diameter lock diminishes anchor power ; (6) the suture calibers (diameter) ; (7) the material properties of suture materials ; (8) the peripheral sutures ; (9) the curvature of tendon gliding paths – the repair strength decreases as tendon curvature increases ; and (10) above all, the holding capacity of a tendon, affected by varying degrees of trauma and post-traumatic tissue softening, plays a vital role in repair strength.
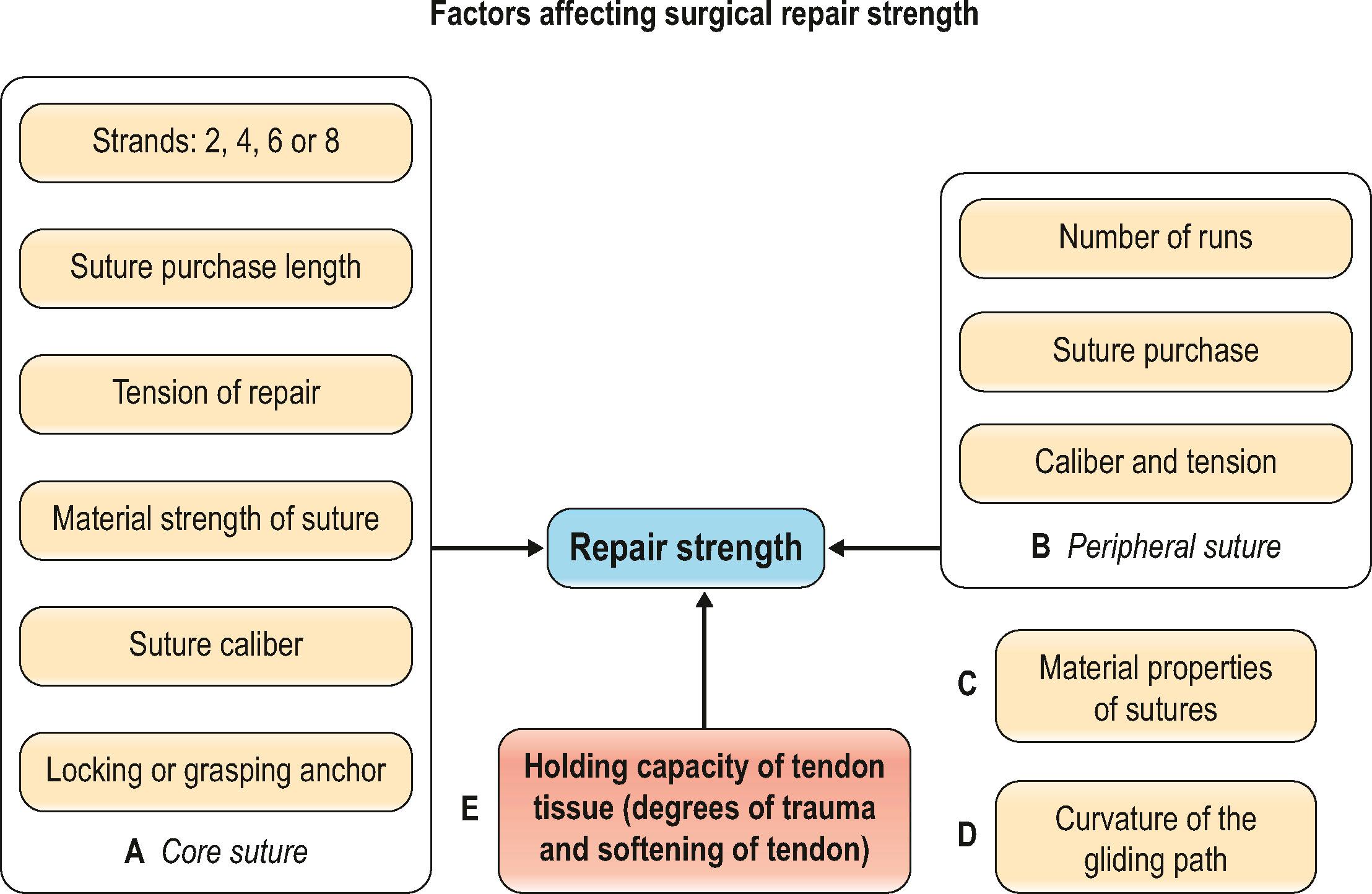
To achieve an optimal surgical repair, the above-outlined factors must be considered and incorporated into repair design. A core suture purchase of at least 0.7 to 1.0 cm is necessary to generate maximal holding power, as recommended by Tang et al . and Cao et al . Slight tension in the core sutures is necessary to resist gapping at the repair site. A locking tendon–suture junction is generally better than a grasping junction in terms of holding power. Diameter of the suture locks must reach or exceed 2 mm, according to Xie et al . Tan and Tang recommend a greater core suture purchase (>1.2 cm) and locking repairs for an obliquely cut tendon. Barrie et al . and Taras et al . greatly improved repair strength by increasing suture calibers. Clinically, the caliber of suture used in adults is either 3–0 or 4-0; sutures of 2–0 or greater are too large and rigid in the hand.
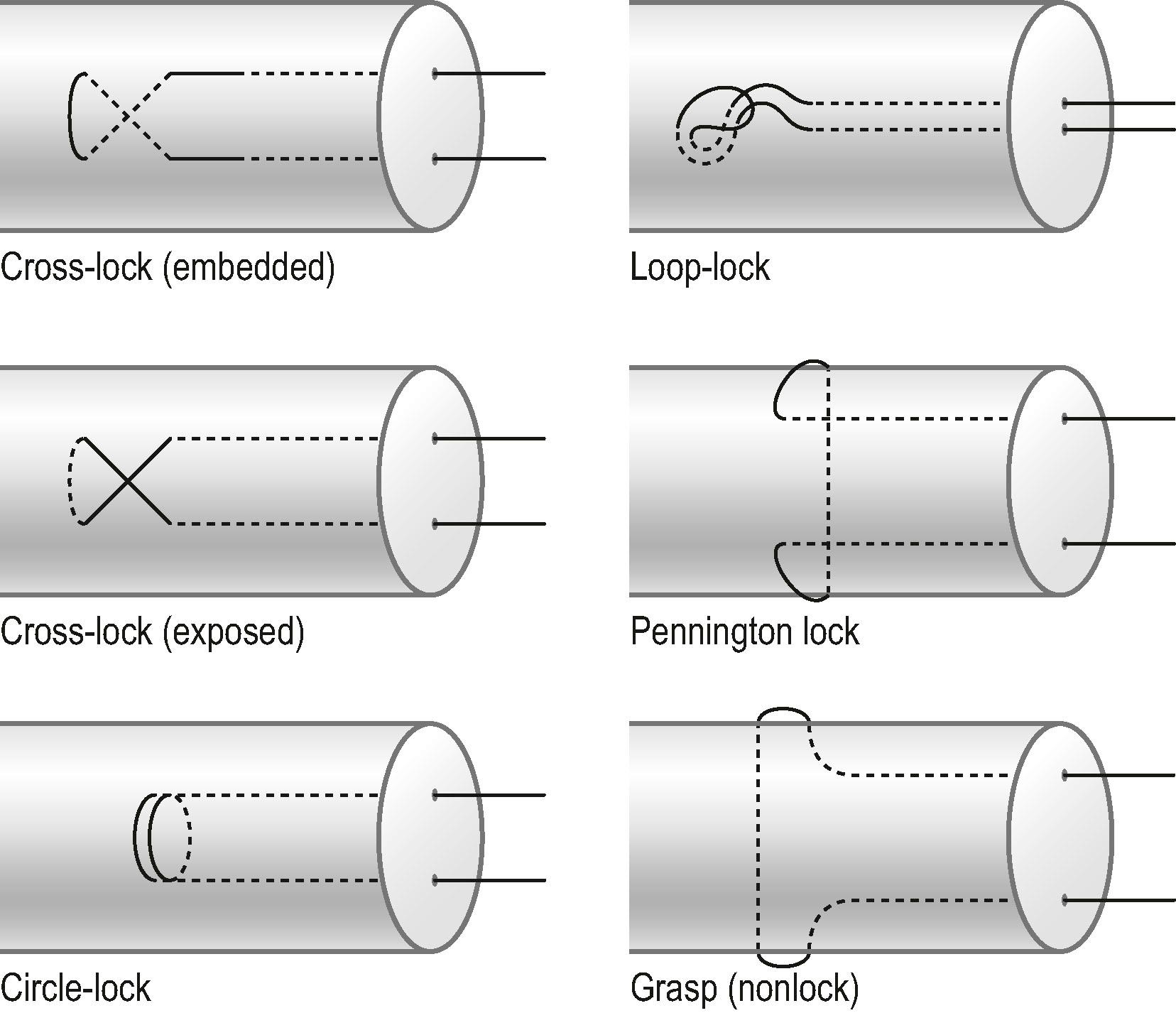
Tendon–suture junctions in surgical repairs are either grasping or locking; locking junctions vary greatly ( Fig. 9.7 ). Grasping repairs are generally weaker than locking repairs. Among locking junctions, cross-locks provide identical strength to circle-locks. Exposed and embedded cross-locks create the same strength. With an identical number of suture strands across the tendon, different locking junctions result in minor differences in the strength. Nevertheless, repairs with cross- or circle-locks appear slightly stronger than Kessler-type repairs with Pennington locks. Pennington locks provide a looser junction than cross- or circle-locks.
Peripheral sutures serve to “tidy up” the approximated tendon stumps; they may add strength to repairs as well. Deep-bite peripheral sutures increase repair strength. Increases in suture purchase or complex peripheral sutures, as typified by the Silfverskiöld method, increase overall strength. However, most surgeons choose to insert only simple peripheral stitches. Some surgeons even do not supplement peripheral stitches when multistrand core sutures have been used. In the presence of a strong multistrand core repair, peripheral sutures contribute little in terms of strength. In fact, to simplify repair maneuvers, multistrand core sutures (with or without a few peripheral sutures) may be sufficient.
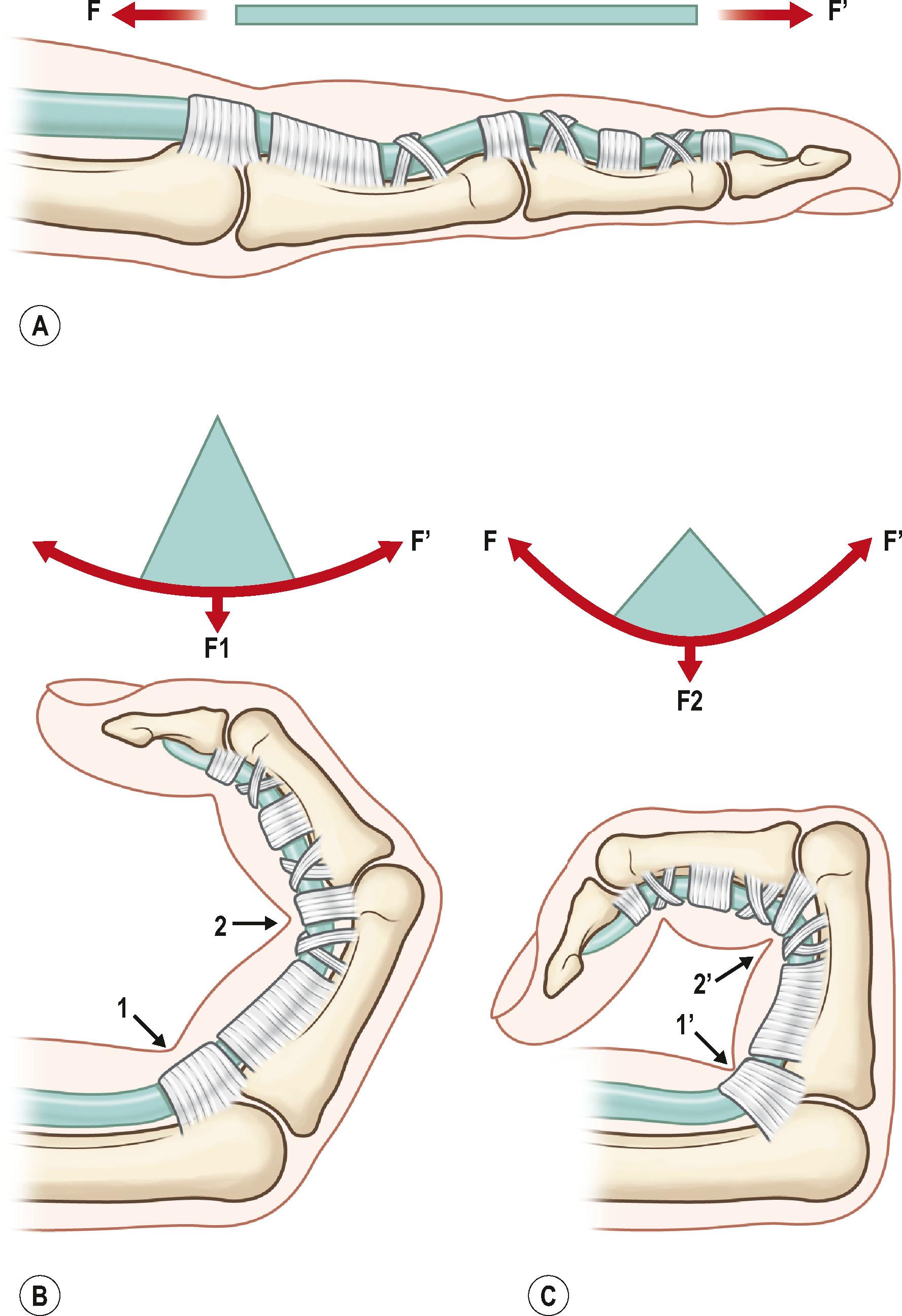
In addition to surgical factors, tendon curvature affects strength as well. Surgical repair in a tendon under a curvilinear load is weaker than that under a linear load; the repair strength decreases as the curvature increases. Mechanically, a tendon under linear tension is pulled without being bent, whereas a tendon under curvilinear tension is subjected to both linear pulling and bending forces. Therefore, the repair fails more easily in the flexed finger under curvilinear loads. When the finger moves to approach full flexion, a strongly bent tendon is particularly prone to fail ( Fig. 9.8 ).
Annular pulleys are critical to the function of the digital flexor tendons. The pulleys keep the tendons' course close to the phalanges for optimal mechanical efficiency of tendon excursion. Lengthy loss of the sheath and pulleys causes anterior displacement – bowstringing – of the flexor tendon during finger flexion. In fingers, the A2 and A4 pulleys are most critically located and functionally important. Preservation or reconstruction of the two pulleys is necessary in the absence of other pulleys or sheath. Nevertheless, given the presence of other pulleys and sheath, loss of any individual pulleys – including the A2 or A4 pulley – appears to result in few detrimental consequences. Both Tang and Tomiano et al . showed that incision of the A2 pulley up to one-half or two-thirds of its length or of the entire A4 pulley results in no tendon bowstringing and little loss of digital flexion. In in vivo settings, incision of the A2 pulley decreases resistance to tendon motion and lessens the chance of repair failure. Loss of the A3 pulley alone has few consequences as well, but a lengthy sheath cut adjacent to the A3 pulley, containing the C1 or C2, causes tendon bowstringing. Therefore, significant loss of sheath should be avoided to maintain tendon function; however, loss of a small portion (<2 cm in length) of the sheath–pulley, even including a part of the most critically located A2 pulley, has no substantial mechanical consequence.
Flexor tendons in the digits glide in a fairly resistance-free synovial environment. Resistance to tendon motion is increased when the tendons are injured and repaired. The following create resistance to tendon gliding: (1) rough tendon gliding surface; (2) biological reactions of the wound, e.g., subcutaneous and tendon edema; (3) friction caused by exposure of suture materials; (4) increases in tendon bulkiness due to placement of sutures; (5) tight closure of sheath or pulleys that narrows the tendon gliding tunnel; (6) tendon-catching at pulley or sheath edges; (7) postsurgical extensor tethering and joint stiffness that burden the movement of the flexor tendon; and (8) adhesions that restrict tendon gliding.
Following trauma and surgery, tendons undergo inflammation, healing, and edema. The volume of the tendons is increased, which increases the resistance within the narrow sheath tunnel. Subcutaneous edema outside the sheath also impedes tendon motion. These factors affecting the resistance to tendon gliding should be considered in deciding how rigorous the postoperative motion program should be. The safety margin can be enhanced by a strong surgical tendon repair or appropriately decompressing the tendon to avoid repair ruptures.
Biological healing strength is a central issue underlying all tendon repairs. Urbaniak et al ., Aoki et al ., Kubota et al . and Boyer et al . have characterized the strengths of the healing flexor tendon using animal models. They found that strength either remained consistent or actually decreased somewhat over the initial few weeks after surgery. Decreases in strength, typically those in the second postsurgical week, are thought to be caused by softening of the tendon stumps, which lower the suture's holding power. Our investigations using a chicken model indicated that the strength of a healing tendon is steady during the initial 4 weeks, followed by a substantial increase (greater than threefold) in the fifth and sixth weeks; thereafter, the tendon heals strongly and is difficult to disrupt. The fifth and sixth weeks after surgery appear very crucial to regaining strength. Accelerating the healing, aiming to move this critical “strength gain” period to earlier weeks, is a current focus of research into molecular modulation of tendon healing.
Flexor tendon injuries are open in most cases, resulting from a sharp cut or a crush, but they can also present as closed injuries. Open injuries due to extensive trauma are frequently associated with neurovascular deficits. Closed injuries often relate to forced extension during active flexion of the finger. Flexor tendon rupture can also occur as a result of chronic attrition in rheumatoid disease, Kienböck disease, scaphoid non-union, a hamate or a distal radius fracture.
Careful attention to the patient's history and the mechanism of injury can alert the surgeon to the extent of the tendon trauma and associated injuries. The natural resting posture of the wounded digits is important for evaluation. Complete lacerations of both FDP and FDS tendons are easily diagnosed when the affected fingers are seen in a relatively extended position with loss of active finger flexion at PIP and DIP joints. If the patient can actively flex the DIP joint while the motion of the PIP joint is blocked, no injuries or only partial injuries to the FDP tendon can be diagnosed ( Fig. 9.9 ). To assess the continuity of the FDS tendon, the adjacent fingers are held in full extension by the examiner. If the patient cannot actively flex the PIP joint, the FDS tendon is severed completely ( Fig. 9.10 ). Variations of the FDS tendons in the little finger are frequent. The FDS in 30–35% of the little fingers has a connection with the FDS in the ring or middle fingers. Some little fingers (10–15%) are missing an FDS tendon. These patients have limited or no PIP flexion of the little finger during testing. Weakness during resisted finger flexion indicates a possible partial tendon cut. To test the FPL tendon, the thumb MCP joint is stabilized in neutral position. The patient is asked to flex the IP joint. Loss of active flexion at the joint indicates complete severance of the FPL tendon.
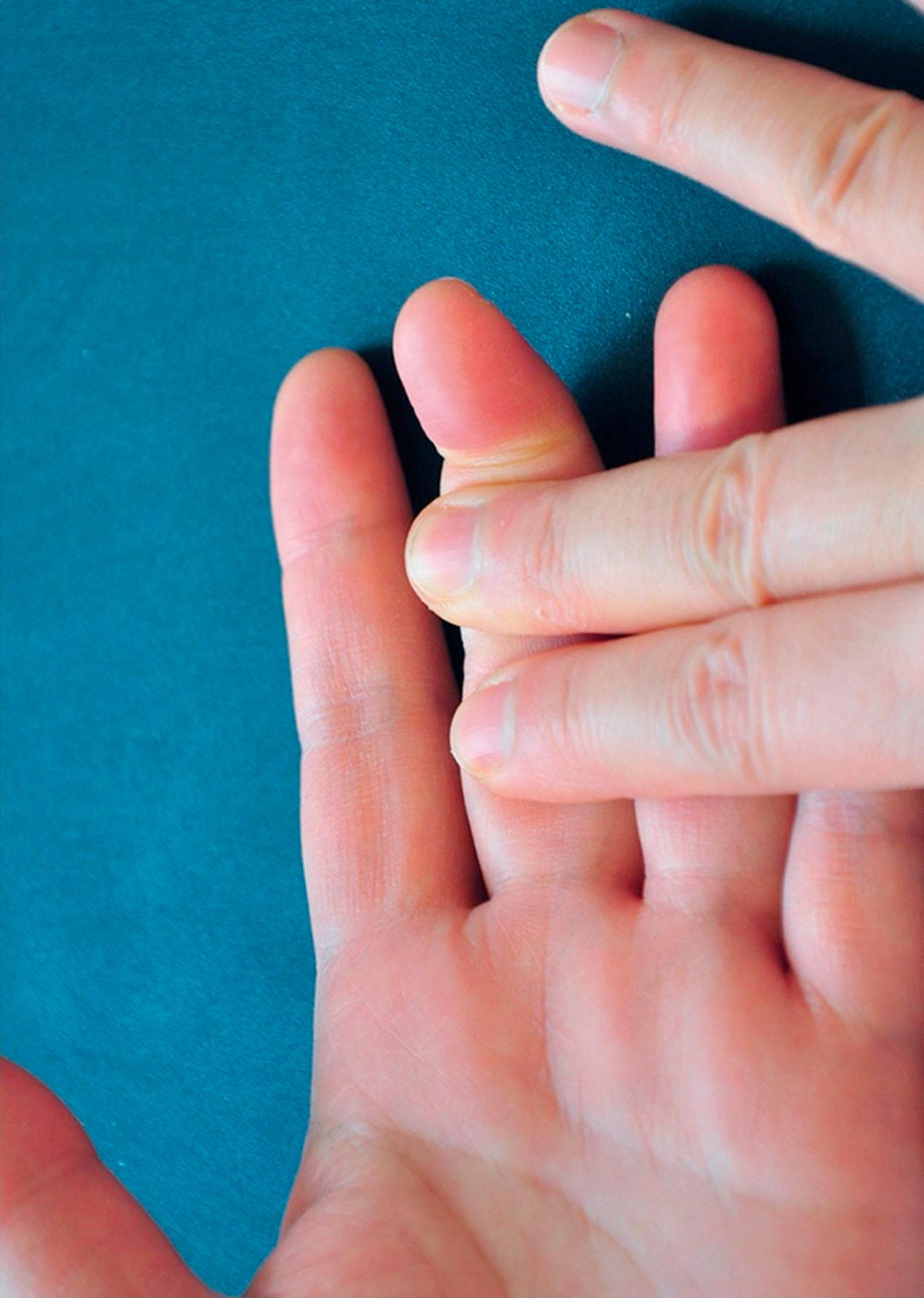

Nerve and vascular function should be assessed routinely, because accompanying injuries in the neurovascular bundles in one or both sides of the fingers or median and ulnar nerves at carpal tunnel or distal forearm are common. Loss of sensation in the finger pulps or loss of function of some intrinsic muscles in the hand are indicative of such accompanying injuries; treatment of neurovascular injuries must be included when planning surgical strategies. If fingers or hands are found to be hypovascular or avascular due to vascular lacerations, vascular anastomosis should be a surgical emergency. Otherwise, after wound debridement, either the lacerated flexor tendons can be repaired (when experienced surgeons are readily available) or the skin can be closed to allow for delayed primary repairs within days by experienced surgeons.
Radiographs should always be taken. Associated fractures are not infrequent and require treatment. Computed tomography (CT) scan or magnetic resonance imaging (MRI) are usually not necessary to diagnose open tendon injuries. However, for diagnosis of closed tendon ruptures or suspected ruptures of the primary end-to-end surgical repair, these tools are of particular value. MRI should be utilized if there is a suspicion of closed tendon rupture. Ultrasonographic examination is an important tool to assess repaired tendon and may reveal rupture of the repaired tendons.
Whenever possible, acutely lacerated flexor tendons in the hand and forearm should be treated primarily or at the delayed primary stage. Primary tendon repair is the end-to-end repair performed immediately after wound cleaning and debridement, usually within 24 hours after trauma. Delayed primary repair is defined as the repair performed within 3 or even 4 weeks after tendon lacerations. No clinical investigations actually validated the best time for primary repair. The ideal situation is that of a patient with digital flexor tendon lacerations brought into the clinic soon after injury; surgery begins within a few hours, and an experienced surgeon is readily available. The tendon injured in critical areas (such as zone 2) should not be repaired by an inexperienced surgeon. Rather, the tendon repair can be delayed until an experienced surgeon is available. My preferred period of deliberate delay is 4–7 days, when the risk of infection can be properly addressed and edema has reduced. The direct repair is feasible 3–4 weeks after trauma, but delay beyond that may cause myostatic shortening of the muscle–tendon unit; for these late cases, lengthening the tendon within the muscles in the forearm can ease the tension ( Algorithm 9.1 ).
Rupture of the repaired flexor tendons after surgery can be re-repaired if the rupture occurs within a few weeks up to a month after surgery; secondary tendon grafts may be the only choice for ruptured cases in the presence of obvious retraction of the tendon end or extensive scarring in the intact FDS tendon when the FDP tendon ruptures.
Primary or delayed primary end-to-end tendon repairs are indicated mainly in clean-cut tendon injuries with limited damage to peritendinous tissues. Neurovascular injury is not a contraindication for primary repairs. Loss of soft-tissue coverage over the tendon and the presence of fractures are borderline indications. Local defects in skin and subcutaneous tissues can be covered by flap transfer. A simple fracture limited to the phalangeal or metacarpal shaft can be securely fixed with screws, wires, or intramedullarly placed headless screws, and then tendons can be repaired. However, serious crush injuries, severe wound contamination, loss of extensive soft tissues, or extensive destruction of pulleys and tendon structures are contraindications for primary tendon repairs. Severe injuries to multiple joint components and fractures involving bones at different levels or not yielding stable internal fixation are contraindications for primary tendon repairs ( Box 9.2 ).
Clean-cut tendon injuries
Tendon cut with limited peritendinous damage, no defects in soft-tissue coverage
Regional loss of soft-tissue coverage or fractures of phalangeal shafts are borderline indications
Within several days or 3 or 4 weeks after tendon laceration
Severe wound contamination
Bony injuries involving multiple joint components or extensive soft-tissue loss
Destruction of a series of annular pulleys and lengthy tendon defects
Experienced surgeons are not available
Brachial plexus block is usually sufficient; general anesthesia can also be used when associated injuries are severe. A tourniquet is placed on the upper arm. Nevertheless, local anesthesia without tourniquet with the patient being wide awake during entire surgery can be more beneficial, called “wide-awake” flexor tendon repair as advocated by Lalonde. This approach allows the patient to actively flex and extend the operated digits, enabling the surgeons to confirm that the motion is smooth, does not lead to gapping in the repair site, and the repair strength is sufficient for early active motion on the operating table. The anesthetic mixture of 1% lidocaine with 1:100,000 epinephrine is given in the surgical waiting room 20–30 minutes before making surgical incisions ( Box 9.3 ). Epinephrine acts to stop bleeding from capillaries and produces maximal capillary constriction about 25 minutes after infiltration to the surgical field.
Primary or delayed primary repair of the flexor tendons can be performed under local anesthesia, without tourniquet, while the patient is wide awake during entire operation.
For the repair in zones 1–3, the method of local anesthesia is to inject 1% lidocaine premixed with 1 : 100,000 epinephrine. The surgeon should prepare 10 mL of such an anesthetic for each finger with tendon repair. The local anesthetic is injected 20–30 minutes before beginning the surgical repair.
For zone 1 and 2 injuries, the point of first injection is at the midpalm level, just proximal to the MCP joint; a total of 5 mL of anesthetic is injected. The needle should be perpendicular to the skin to minimize pain, and injection is into the subcutaneous tissue.
After waiting 5–10 minutes, the second injection (2 mL) is given to the middle part of the proximal phalanx at the volar midline. Immediately after that, the third and fourth injections (2 mL and 1 mL, respectively) are administrated to the middle parts of the middle and distal phalanx along the palmar midline.
15 minutes after completing the above four injections, surgery can begin.
For repair in the forearm (zone 5), 20–50 mL of 1% lidocaine with 1 : 200,000 epinephrine is injected to the multiple sites where surgical incisions will be made 15 minutes before surgery.
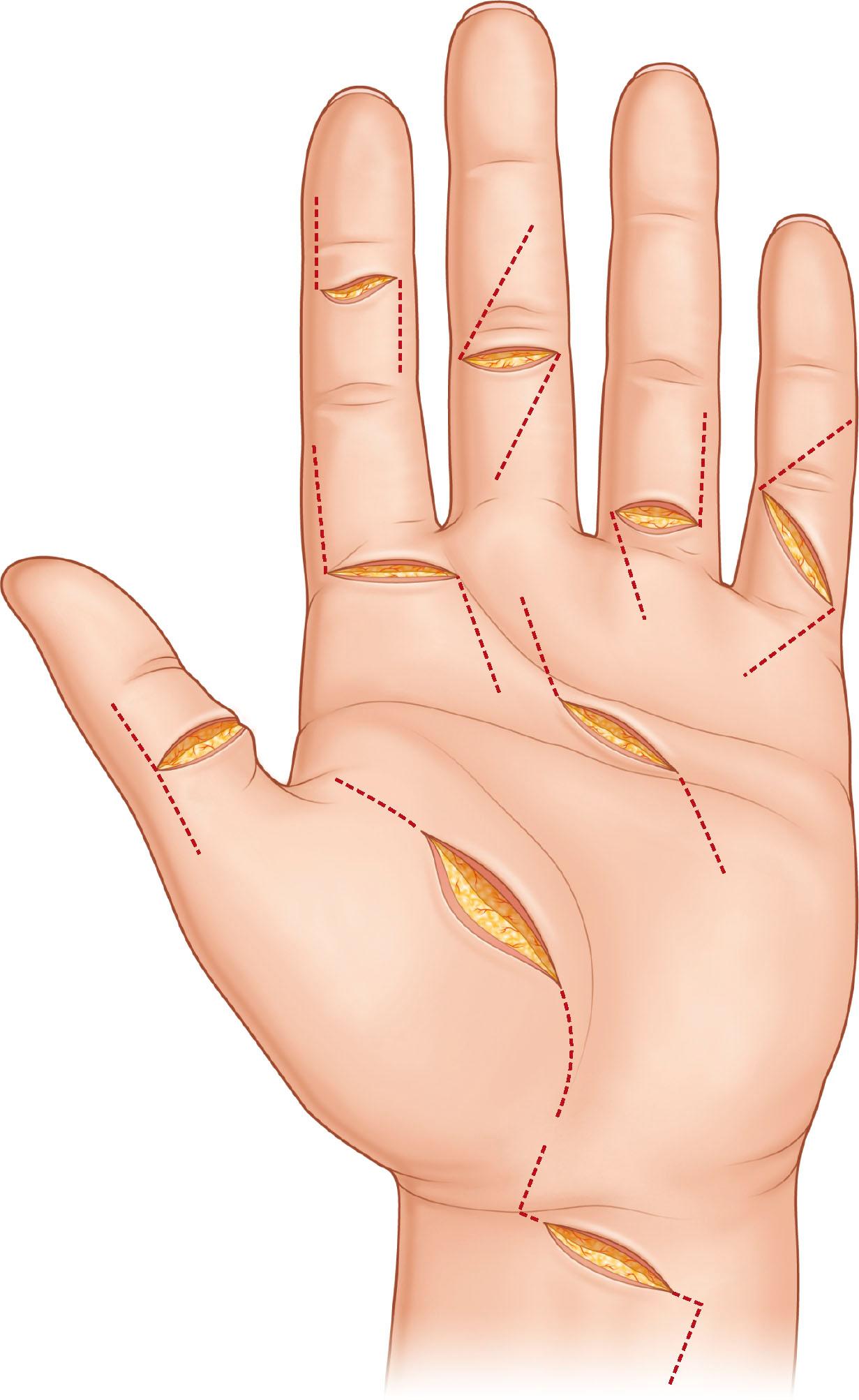
The wounds should be thoroughly debrided, devitalized tissues excised, and the wounds washed with antibiotic solution. Position of the fingers or hand is determined by levels of cuts in the tendons in relation to their superficial tissues. The hand is usually held by an assistant, so that it can be adjusted during surgery. Loupe magnification is advised for surgery. The tendons are exposed through zigzag skin incisions on the volar side of the fingers, e.g., Bruner's incision, or a lateral incision. When the wounds are in the palm or forearm, incision by extending the opening of wounds is often necessary ( Fig. 9.11 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here