Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Flaccid paralysis of the elbow severely limits a patient's ability to position and stabilize the hand for function. Paralysis of elbow flexors (biceps, brachialis, and brachioradialis) makes reaching the face impossible. Most other extremity functions require frequently changing levels for lifting and carrying, also impossible with elbow flexor dysfunction. Elbow extension is necessary to work with the extremity above the horizontal position. Any overhead task is impossible as are tasks such as stabilizing an object on a surface and supporting body weight during transfers, using crutches, or standing from a seated position. Forearm supination is necessary for many lifting and carrying tasks. Control of both pronation and supination is important for positioning the hand for many activities such as writing, keyboarding, or driving.
Many older surgical procedures to restore elbow flexion were developed for poliomyelitis patients. Today, most adult cases are traumatic peripheral nerve or brachial plexus injuries resulting from motor vehicle accidents. Birth-related trauma may also result in flaccid elbow paralysis in infants. Most of these injuries are the result of traction force affecting the supraclavicular plexus (C5 and C6 nerve roots, or upper trunk). Injury to these structures alone results in a typical pattern of injury commonly described by its eponym, Erb's palsy. This includes flaccid paralysis of all elbow flexors as well as deltoid, supraspinatus, and infraspinatus muscles. More distal nerve injury must affect this C5–C6 pathway to both the musculocutaneous-innervated biceps and brachialis muscles as well as the radial-innervated brachioradialis muscle in order to see loss of all elbow flexion. Extensive direct muscle injury occasionally necessitates tendon and muscle transfer to replace lost function.
Restoration of elbow extension is commonly performed in patients with tetraplegia. Elbow extension allows improved positioning of the hand in space and allows use of the extremity to shift sitting position for pressure relief, better propel a wheelchair, and even perform transfers independently. In brachial plexus injury, elbow extension has lower priority than elbow flexion or shoulder stability. It is often required, however, as a part of reconstruction of grasp to control elbow positioning. When shoulder function is sufficient to fully extend and abduct the humerus, triceps paralysis will prevent needed elbow extension positioning against gravity and must be reconstructed.
Reconstructive procedures available for restoration of elbow function include nerve grafts or transfers, tendon transfers, and free functioning muscle transfers. Combinations of nerve transfers and novel uses of free functioning muscle transfers have offered more function in patients with devastating injuries. Each of the basic types of reconstructive procedures, the timing, principles, anatomy, outcome, and our overall recommendations will be detailed in this chapter.
Time is an important factor in planning appropriate intervention following any neurologic injury resulting in elbow paralysis. Nerve graft or transfer for peripheral nerve injury is most appropriately performed within 6 months of nerve injury. Direct repair or graft of the musculocutaneous nerve itself will provide superior results and minimal donor morbidity when possible. In more proximal nerve injury, nerve transfer may also permit biceps or brachialis reanimation. In an upper trunk (Erb's palsy pattern) of brachial plexus injury, for example, intraplexal nerve transfers using two to three fascicles of the ulnar nerve, median nerve, or both may be useful. When the entire plexus is avulsed, use of direct nerve transfers from the spinal accessory nerve or intercostal motor nerves are often successful when performed within this limited time range. Results diminish with elapsed time, making other options preferable after 6 months and absolutely necessary after 12 months. Tendon or free muscle transfers, however, have no such time limit. In spinal cord injury, transfers should not be performed until neurologic recovery has stopped and initial rehabilitation is complete. Surgery is delayed in very young children to allow adequate assessment and cooperation.
Paralysis of the elbow cannot be reconstructed successfully without a functional arc of passive motion. Nerve reconstruction is time-dependent and will often need to be performed without delay, before joint mobilization is assured. Any other surgical plan should be delayed until passive flexion of at least 120 degrees is achieved, either with physiotherapy, static splints (see Chapter 16 ), or surgical release.
A transferred muscle must provide both adequate power and amplitude to move the elbow. The muscle to be transferred should contract against gravity resistance (grade 4, “M4”) if significant function is to be anticipated ( Table 117.1 ). Power is directly proportional to its cross-sectional area (3.65 kg/cm 2 ), while excursion or amplitude is a function of average fiber length. When multiplied together, these two parameters define its work capacity. The biceps brachii contribution to elbow flexion is 4.8 kg/m and to forearm supination 1.1 kg/m. Motors used to replace biceps should ideally provide similar values.
| Grade | Definition | Explanation |
|---|---|---|
| 0 | Absent | No function |
| 1 | Trace | Slight contraction, no motion |
| 2 | Poor | Complete motion, gravity eliminated |
| 3 | Fair | Complete motion against gravity |
| 4 | Good | Complete motion against gravity and some resistance |
| 5 | Normal | Apparently normal strength |
Muscles used for transfer should ideally be expendable—that is, not cause significant functional loss when used. Triceps-to-biceps transfer must be carefully considered before use, for example. Muscles contracting in synchronicity with the desired function rehabilitate easily and are stronger when only moving one joint (one joint–one function).
Nerve repair or transfer is the treatment of choice whenever possible. In cases of flaccid elbow paralysis resulting from a brachial plexus injury, prompt treatment will allow restoration of biceps and/or brachialis function even when the limb is flail and all nerve roots avulsed. Reinnervation may be provided by extraplexal or intraplexal direct nerve transfer or by direct repair/graft of an isolated musculocutaneous nerve injury. Extraplexal transfers reported commonly include intercostal, spinal accessory, or even the contralateral C7 nerves. Intraplexal transfers use fascicles of the ulnar or median nerve. This method is preferred when strong wrist flexion is present, such as in cases of upper trunk or Erb-Duchenne palsy.
A functioning free muscle flap provides an additional reconstructive option in brachial plexus injury when treatment delay or failure prevents biceps reinnervation and tendon transfers are not available.
Several tendon transfers are available for elbow flexion. These include use of the flexor/pronator muscle group (Steindler flexorplasty) a
a References .
triceps, pectoralis major, pectoralis minor alone, sternocleidomastoid, flexor carpi ulnaris, and latissimus dorsi muscles. b
b References .
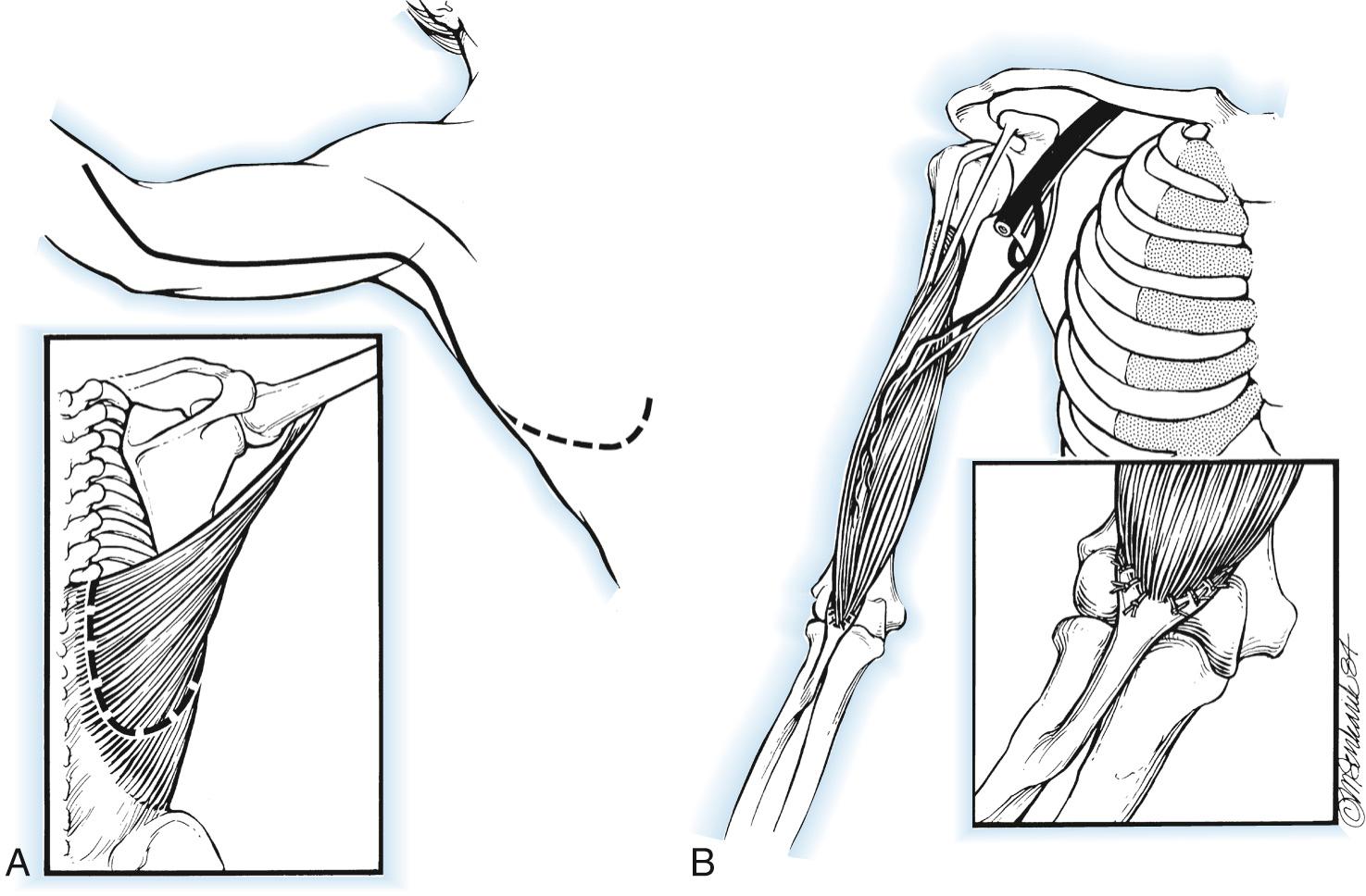
Injury to the lower motor neurons originating from the C5 and C6 nerve roots will result in loss of elbow flexion as well as shoulder abduction and external rotation. Restoration of elbow flexion may generally be accomplished with either nerve grafts or nerve transfers when intervention is not delayed beyond 6 months. Thereafter, recovery rates diminish and are minimal at 12 months. The pattern of motor and sensory injury on exam and electrodiagnostic testing will generally reveal the location of the injury, most commonly occurring at or proximal to the point of formation of the upper trunk and proximal to the origin of the suprascapular nerve. Most such injuries are stretch or traction injuries related to birth trauma or collisions and result in extensive longitudinal injury. Brachial plexus reconstruction methods selected will depend upon a number of factors, including more extensive plexus injury, integrity of the C5 and C6 roots, patient age, and elapsed time from injury. Axon regrowth through grafts from C5 or C6 to the musculocutaneous nerve require approximately 1 year to reach the biceps. Transfer of nerves from extraplexal or intraplexal sources permit recovery of elbow flexion in cases of root avulsion and often result in improved results due to generally shorter reinnervation times. Early reports of spinal accessory and intercostal nerve transfers prolonged with nerve grafts suggested the potential of this method but required improvements in microsurgical technique before acceptable rates of recovery were reported. Most important was the description of direct intercostal nerve transfer without use of grafts. Intercostal nerve transfer is most commonly performed in global plexus injury when no intraplexal nerve transfers are possible ( Fig. 117.1 ).
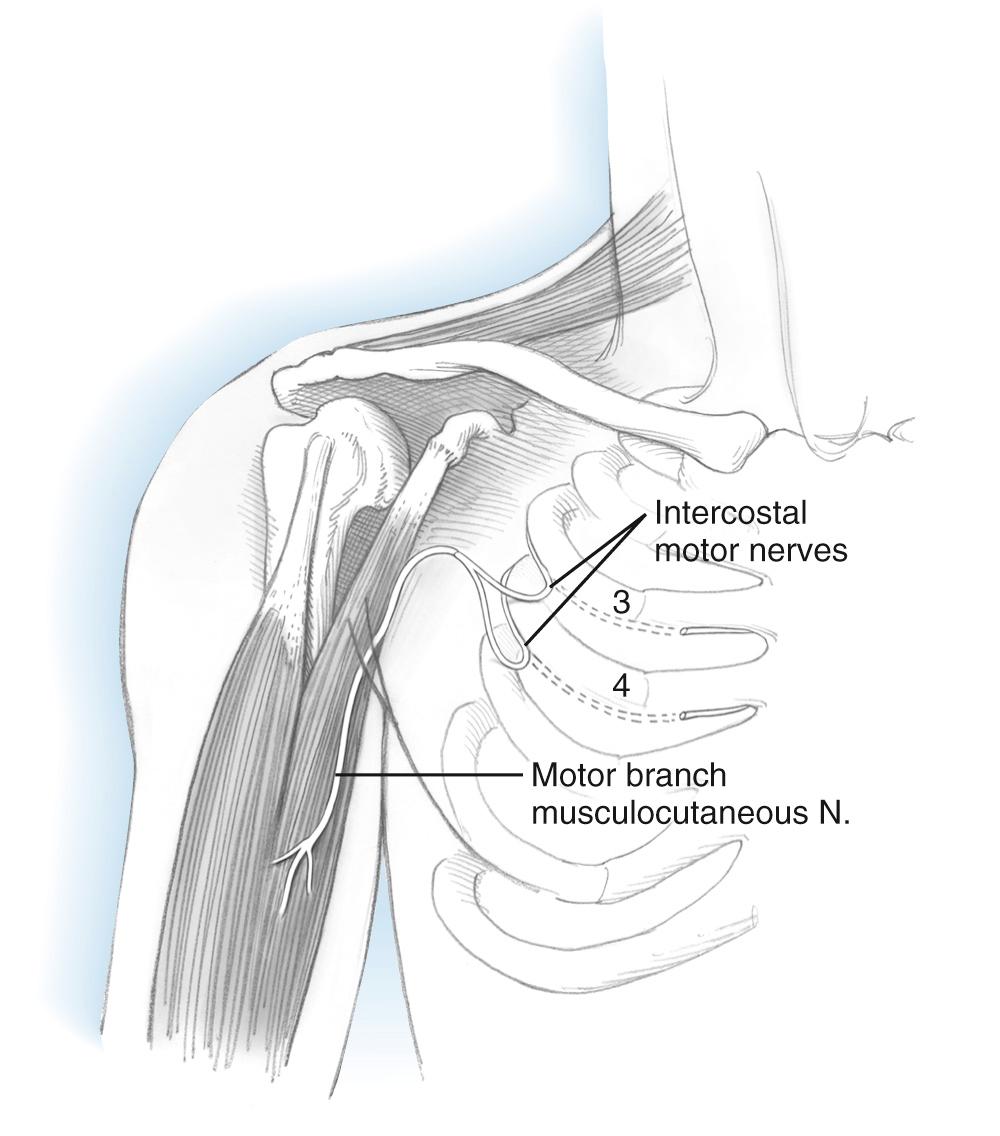
When the lower plexus is uninjured, direct transfer of ulnar nerve flexor carpi ulnaris fascicles to the biceps motor branch, with or without flexor carpi radialis median nerve fascicles to the brachialis motor branch of the musculocutaneous nerve, has proven to be the best option in most such circumstances ( Fig. 117.2 ). Use of many other nerve transfers, both intraplexal and extraplexal in origin, have been described but are much less commonly used. Examples include thoracodorsal, medial pectoral, phrenic, and hypoglossal nerves and high cervical nerve roots. The spinal accessory nerve has been shown to reach distal plexus targets when an extended harvest is employed, as shown by an anatomical study.
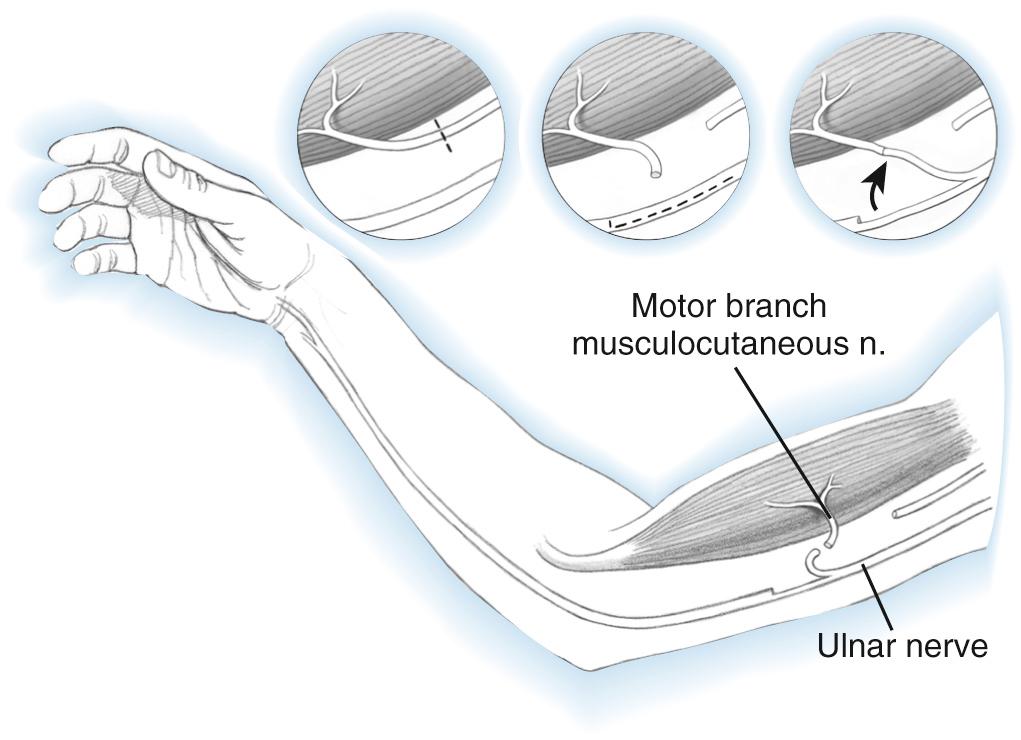
Results of nerve transfers, when performed with less than 6 to 9 months' delay, will result in recovery of at least M3 strength in most patients and are generally at least as successful as nerve grafting from cervical roots. A metaanalysis of the English literature (26 studies, 965 nerve transfers) demonstrated recovery of at least M3 biceps strength in 72% of direct intercostal transfers and 77% of spinal accessory transfers prolonged with nerve grafts.
In cases of C5–C6 palsy, transfer of ulnar nerve fascicles to the biceps motor branch results in much shorter reinnervation times and recovery of M3 or greater strength in more than 90% of patients. The double fascicular transfer (ulnar and median nerves to biceps and brachialis branches) provides similar results.
Steindler described the proximal shift of the flexor-pronator muscle origin to increase its elbow flexion moment arm in 1918. Steindler recommended the procedure only if the wrist flexors were near normal.
Adequate strength is demonstrable by ability to fully flex the elbow with the arm abducted 90 degrees. Technical modifications to improve flexor strength and decrease pronation tendency have been described, including extension of the common tendon of origin with a fascia lata graft and inclusion of medial epicondylar bone, fixed 5 to 7.5 cm proximal to the joint. Similar use of the extensor/supinator group from the lateral epicondyle has not proven reliable.
An anteromedial incision is made 7 cm proximal to the elbow, passing just posterior to the medial epicondyle and then curving anteriorly for 10 cm onto the forearm. The ulnar nerve is isolated and protected, identifying flexor carpi ulnaris motor branches. The lacertus fibrosus is divided, and the median nerve visualized to protect its flexor-pronator motor branches. The common flexor-pronator muscle origin is then detached with a flake of epicondyle, freeing the muscles from the anterior surface of the joint and coronoid process. The ulnar head of the flexor carpi ulnaris origin is detached from the ulna. When the elbow is flexed 120 degrees, traction on the bone flake determines its maximal proximal position, usually 5 to 7.5 cm proximal to the joint line. Anterior transposition of the ulnar nerve will reduce potential tension. The brachialis is split longitudinally to expose the anterior humerus, and a small cortical opening is made with a burr on the lateral aspect. The flexor-pronator origin is secured at this position to the humerus. A posterior splint maintains the elbow in 120-degree flexion and the forearm in full supination. Active flexion and extension exercises are begun at 4 weeks, with orthoplast splinting between exercises for an additional 4 weeks. A dynamic extension splint is often useful if the patient has no triceps function ( Fig. 117.4 ).
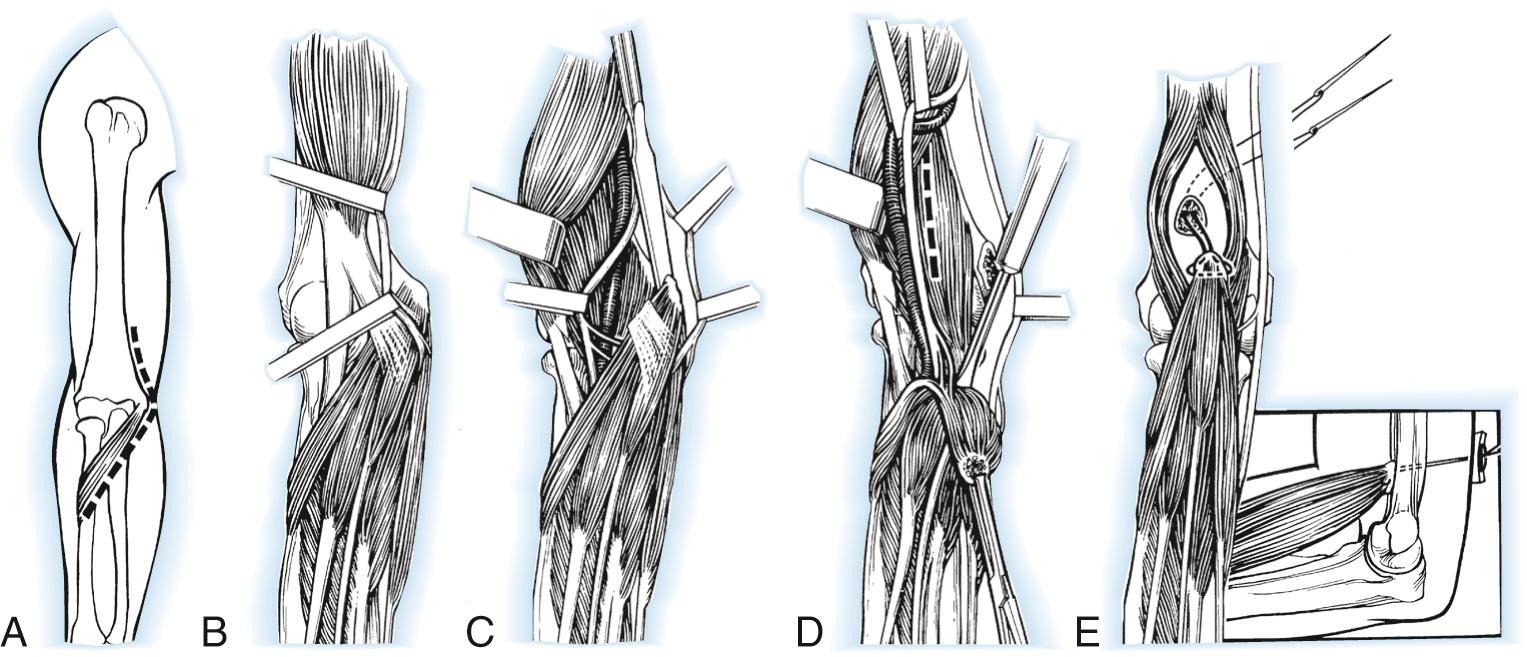
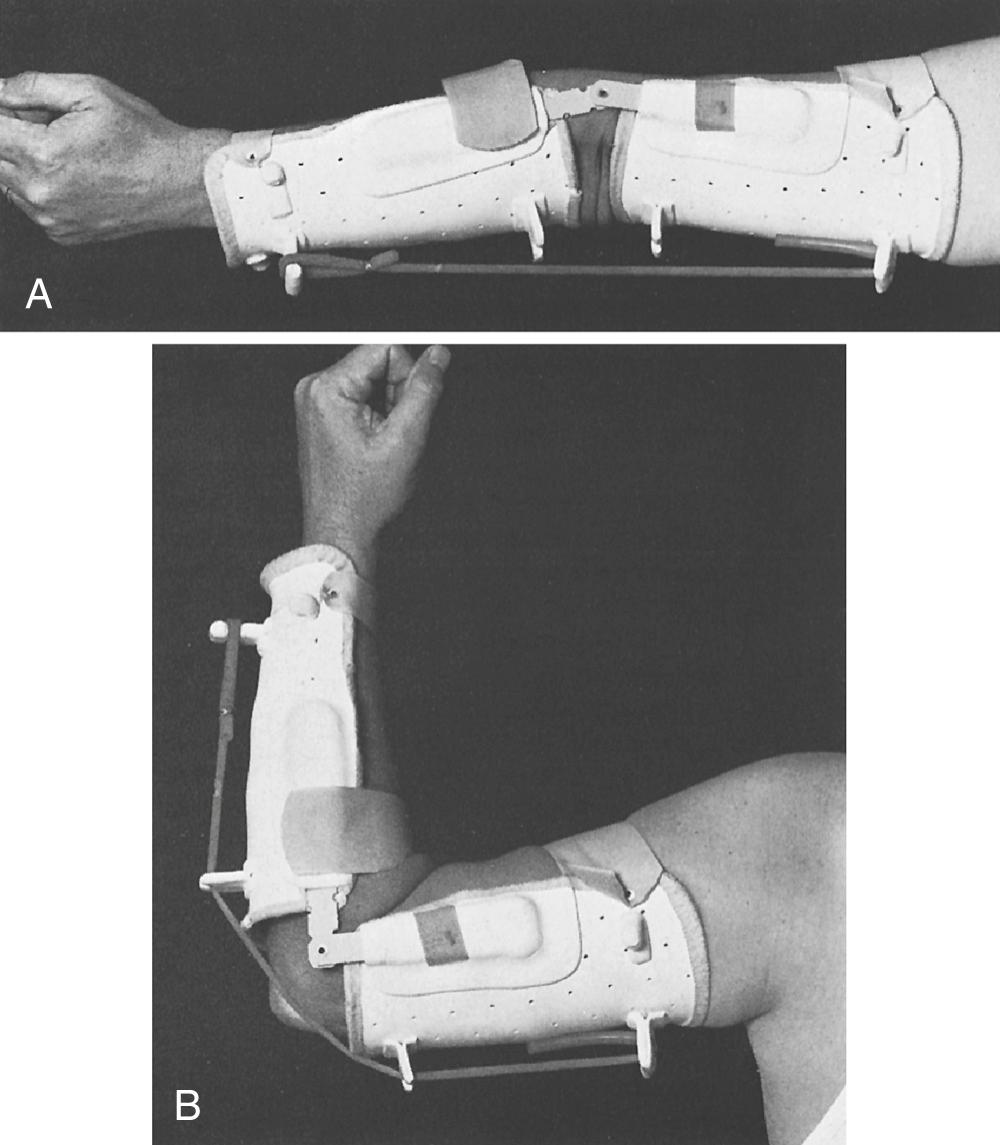
Outcome of the Steindler flexorplasty is dependent upon a variety of factors. Proper patient selection is critical. Flexor-pronator group strength must be at least grade 4. The procedure works best when some elbow flexor function is present preoperatively. Proper technique is important, including correct tensioning and optimal placement of the epitrochlear mass. Using strict criteria for success, Mayer and Green recorded 11 excellent, five good, four fair, and two poor results among 22 flexorplasties ( Fig. 117.5 ), results similar to other reports in the literature. Carry-lift strength in patients with good results is generally weak (between 1 and 6 lb in one study). Prior use of one or more flexor-pronator tendons for transfers does not negatively affect outcome. Results are enhanced with arthrodesis of the shoulder, permitting abduction at the scapulothoracic joint that decreases the gravitational forces of elbow flexion.
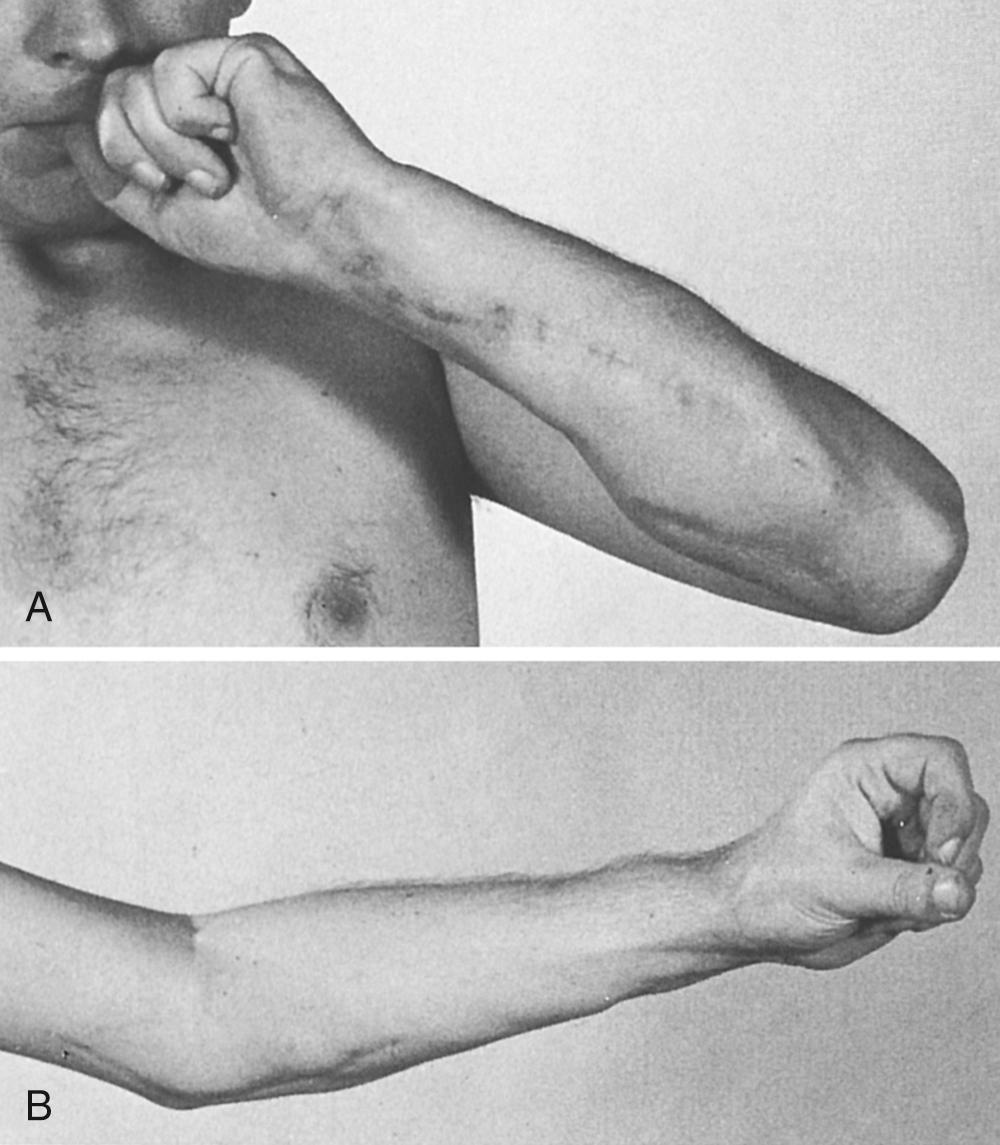
Pronation positioning occurring with elbow flexion, the so-called Steindler effect, occurs in as many as 50% of patients after the procedure. The problem is due to preoperative absence of active supination and enhanced effectiveness of the pronator teres resulting from its proximal shift. Inserting the transfer more anteriorly and laterally on the humerus diminishes pronation tendency.
Modification of the transfer to include only the flexor carpi ulnaris, flexor carpi radialis, and palmaris longus, when placed anteriorly on the humerus has been reported to largely prevent the problem. Transfer of the flexor carpi ulnaris to the flexor carpi radialis or extensor carpi radialis brevis either primarily or secondarily may improve resting position.
Flexion contracture of the elbow generally occurs, and tends to be most problematic in patients with weak flexor-pronator muscles, prolonged immobilization, and poor triceps strength. Some flexion posture is deemed acceptable, and even desirable when strength rather than cosmesis is the major operative goal. The permissible degree of flexion contracture in the literature ranges from as little as 15 degrees to as much as 60 degrees. Turnbuckle splints may be used postoperatively to minimize the flexion deformity.
Dutton and Dawson reported transient ulnar nerve paresthesias in two patients. Lindholm and Einola reported on two patients with postoperative ulnar nerve paresthesias, one of whom was still having symptoms at follow-up. Detachment of the transferred muscle occasionally occurs at times, requiring reinsertion.
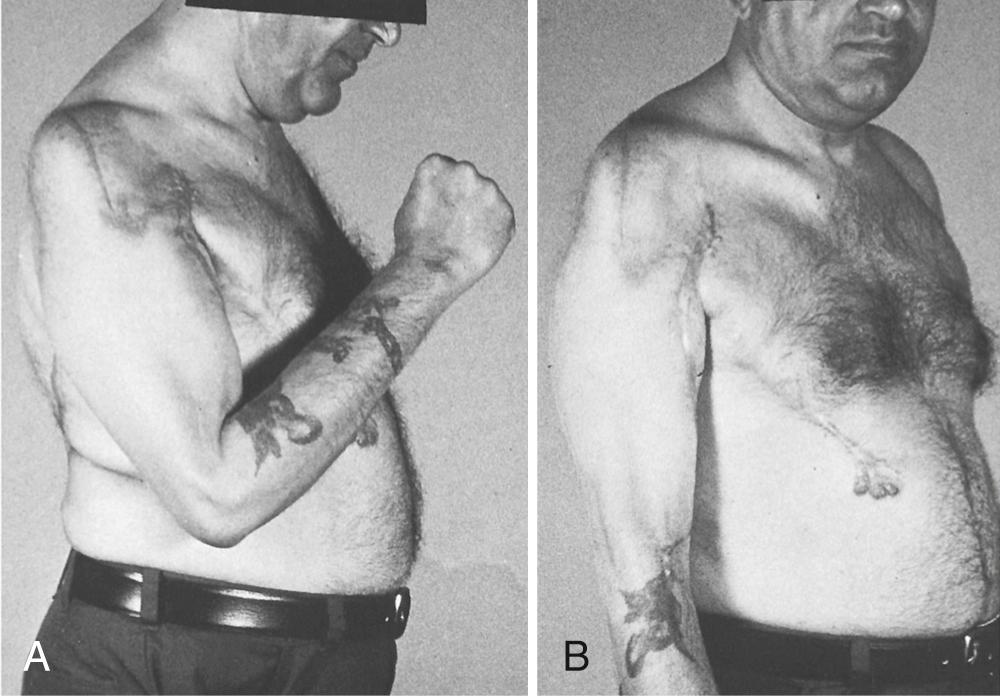
The latissimus muscle flap is widely used as a pedicled muscle flap for restoration of elbow flexion. Its cross-sectional area, fiber length, and excursion compare favorably to the biceps and brachialis muscles, and its function is generally expendable. As originally described, the entire muscle origin is mobilized from the chest wall and its humeral tendon of insertion released. This allows the muscle to be rotated on its thoracodorsal neurovascular pedicle. The entire muscle is thus positioned anteriorly in the arm and fixed by reattaching its insertion to the coracoid and its origin to the distal biceps muscle and tendon. This bipolar transfer method restores both contour and function.
Alternatively, the origin alone may be mobilized and moved to the biceps insertion—a unipolar transfer described by Hovnanian. Bipolar transplantation is mechanically more efficient for elbow flexion, due to the anterior placement of the origin into the coracoid process, with a vector parallel to the biceps and brachialis muscles. Once transposed, tensioning of the muscle is easier than the unipolar method. Both methods require care to avoid torsion or tension-related impairment of the thoracodorsal pedicle. Selection of the optimal method may be assessed at the time of surgery. Following mobilization of the origin, its line of pull must be directed in the plane of elbow motion without tension on the neurovascular pedicle if the procedure is to be completed as a unipolar transfer. When either is unsatisfactory, conversion to a bipolar transfer is advised.
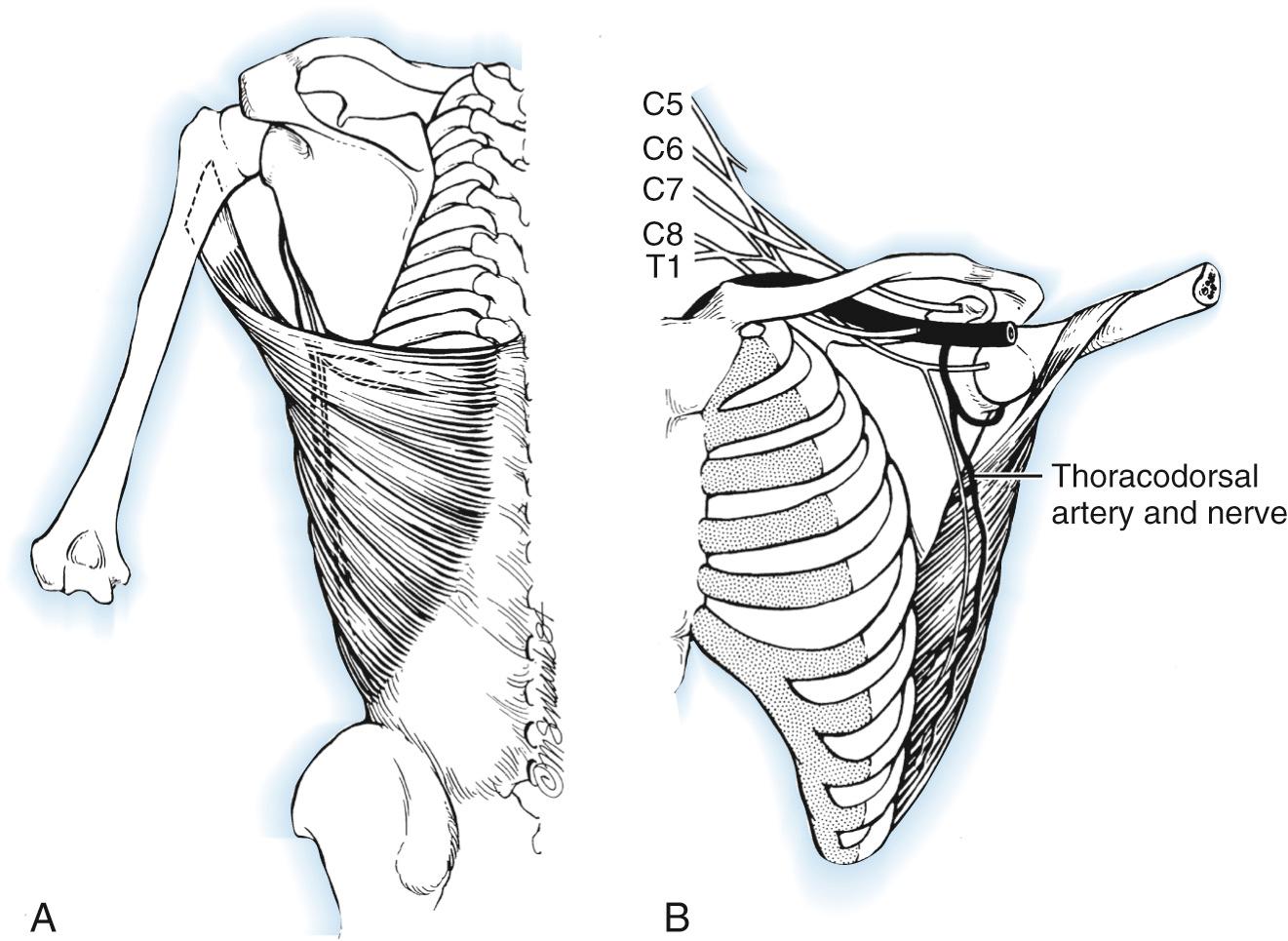
The latissimus dorsi is a broad, thin muscle ( Fig. 117.6 ) with a wide origin from the lower six thoracic vertebrae, spinous processes of the lumbar and sacral vertebrae, and an aponeurosis from the iliac crest. It inserts into the intertubercular groove of the humerus. Its major vascular pedicle is the thoracodorsal artery, a terminal branch of the subscapular artery with additional minor vascular pedicles. It is innervated by the thoracodorsal nerve, a branch of the posterior cord. It enters the muscle with the thoracodorsal vessels on its deep surface 10 cm from its insertion. One to three vascular branches supplying the serratus muscle must be ligated to allow sufficient mobilization for a bipolar transfer. The vascular pedicle to the latissimus dorsi has an average length of 11 cm and the thoracodorsal nerve, 12.3 cm.
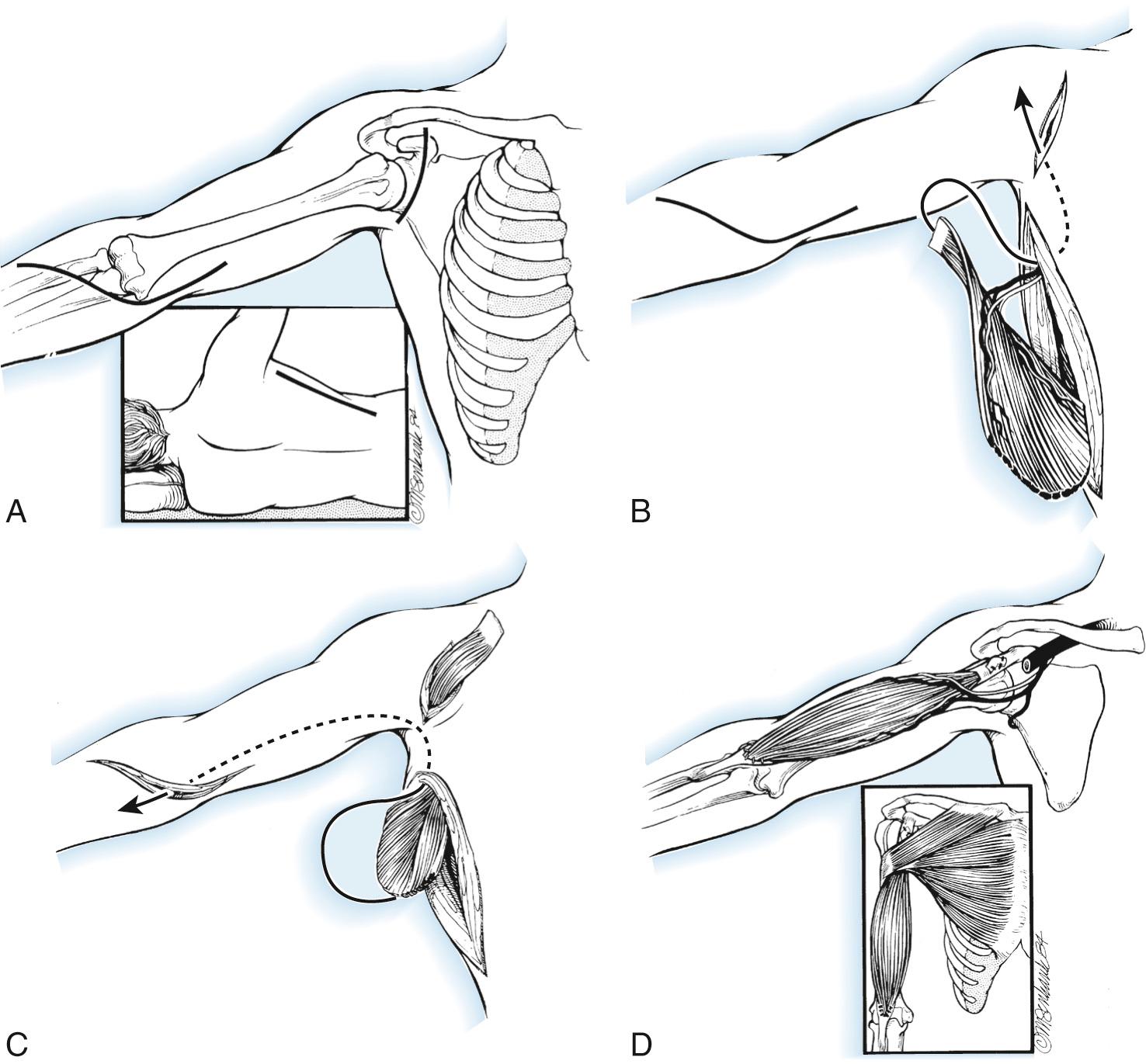
The arm and hemithorax are draped free with the patient in the lateral position. A longitudinal incision is made parallel to the anterior border of the latissimus dorsi, extending from the posterior axilla to the iliac crest. Identify the anterior latissimus border, and then create a 4- to 6-cm-wide elliptically shaped skin island extending longitudinally the length of the normal biceps muscle. Elevate skin flaps from the underlying muscle. To best judge tension later, place several sutures at 5-cm intervals with the arm forward flexed and abducted fully. Elevate the muscle origin from the chest wall distally followed by its vertebral border, including some fascia. Lastly, separate the muscle from the teres major and minor muscles at the inferior angle of the scapula. The thoracodorsal neurovascular pedicle is then visualized on its deep surface. The pedicle must be sufficiently mobilized to avoid kinking, ligating multiple more proximal branches, and releasing any fascial constraints. The tendon of insertion is released from the humerus, and two Krakow 2-0 nonabsorbable sutures are placed.
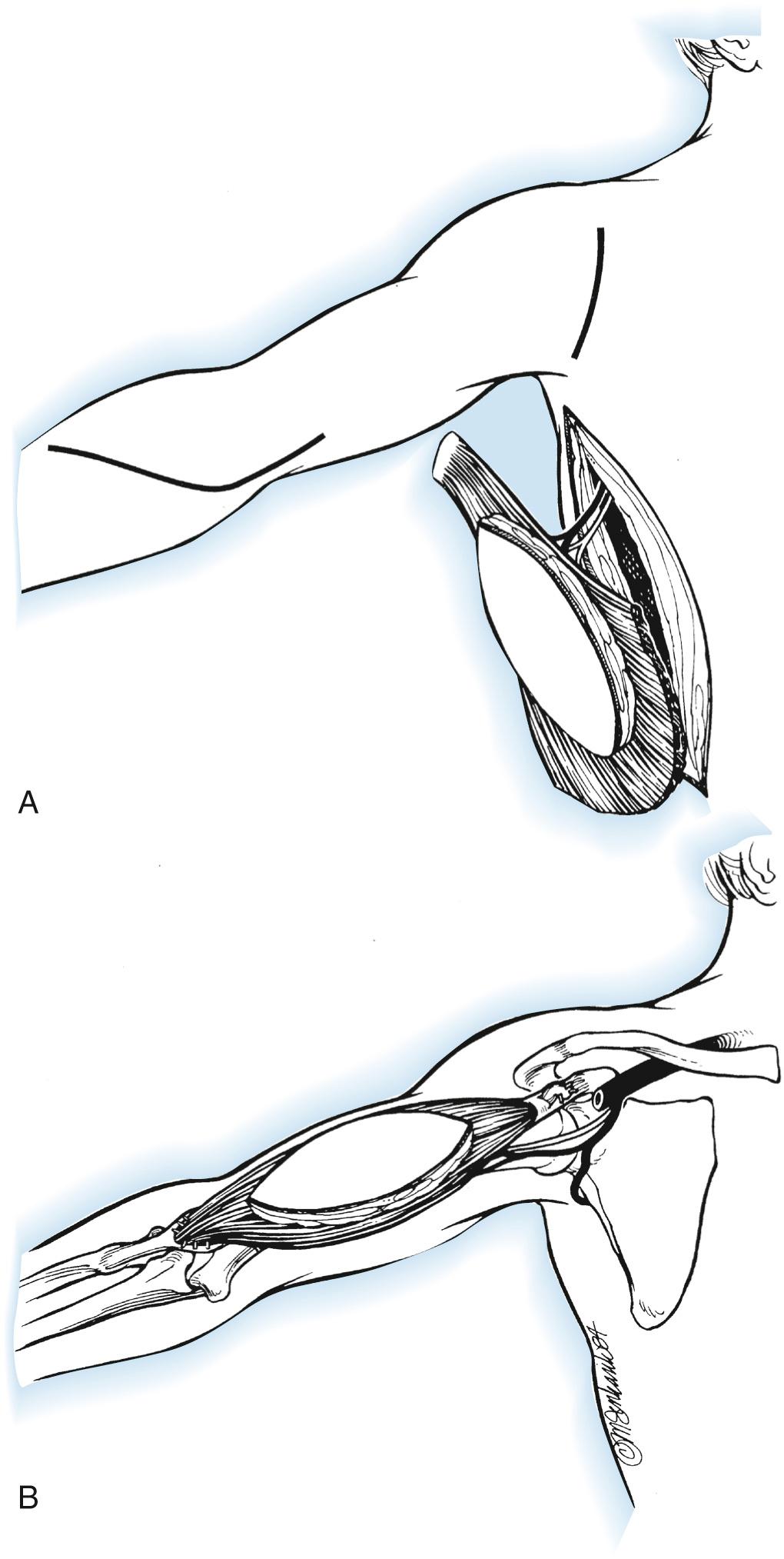
Anteriorly, make a transverse incision at the coracoid and an additional longitudinal deltopectoral incision overlying the biceps muscle to the level of the antecubital fossa. Place four mattress sutures in the biceps tendon in preparation for securing the latissimus dorsi.
A subcutaneous pocket is created in the axilla by elevating the medial flap of the deltopectoral incision superficial to the fascia, wide enough to admit the surgeon's hand and connecting to the posterior incision. The muscle origin is tubularized by bringing the vertebral border to the lateral border, secured with fascial sutures placed every 2 to 3 cm. The muscle is carefully passed anteriorly into the deltopectoral incision while protecting the thoracodorsal pedicle from kinking or tension, beginning with the distal (humeral) tendon of insertion. Through the anterior incision, lift the inferior border of the pectoralis muscle proximally to find the coracoid process and advance the humeral tendon to overlie the coracoid. The entire remaining muscle is then positioned anterior to the biceps. With the elbow extended, tension the muscle to restore 5-cm spacing between the resting-length sutures. Secure the distal latissimus to the biceps tendon, wrapping it circumferentially around the tendon with the previously placed sutures. The proximal tendon is then anchored to the coracoid process with suture anchors, positioning the elbow in 100 degrees of flexion with full supination. The skin island is used to close the deltopectoral incision without tension. Inspect the pedicle for evidence of venous compression or torsion and verify normal color and capillary refill in the skin island. Dark blood in the vein indicates impaired outflow and requires inspection and correction. Two deep drains are placed posteriorly to prevent a seroma.
Isometric contraction of the latissimus is begun at 4 weeks, but prevent any motion using an abduction shoulder immobilizer for 8 to 9 weeks. Thereafter, allow progressive extension at a rate of 10 to 20 degrees per week, and begin reeducation of the latissimus. Biofeedback is useful for this purpose. Later, exercise against resistance will produce powerful flexion of the elbow.
The patient is positioned as for the bipolar transfer. The incision begins on the chest wall, extending along the lateral margin of the latissimus to the posterior axillary fold, and continues across the axilla and downward along the medial arm to the elbow. The muscle elevation and pedicle mobilization are as described above, without detaching the humeral insertion. The muscle is then transferred to the anterior arm, where it is attached to the biceps tendon and periosteal tissues of the bicipital tuberosity. The arm is bandaged to the thorax for 3 to 4 weeks, at which time active and passive exercises are started.
Patients are able to hold weights between 8 and 15 kg and obtain excellent elbow flexion in most cases ( Fig. 117.11 ). Clinical evidence of a functional deficit after transfer or transplantation of the latissimus dorsi is unusual.

Clark reported rotation of the sternocostal origin of the muscle with its separate nerve (medial pectoral) and blood supply, passed subcutaneously into the arm and attached to the biceps tendon. Seddon modified Clark's operation by elevating a segment of rectus abdominis sheath in continuity with the distal end of the transplant to act as a tendon. Brooks and Seddon described a unipolar transfer of the entire pectoralis major muscle, employing the devascularized long head of the biceps as its tendon of insertion. They recommended this operation instead of a Clark transfer when either the lower part of the pectoralis major was paralyzed but the clavicular head strong or the whole muscle was weak.
In 1955, Schottstaedt and others first reported bipolar pectoralis major transplantation, using only the sternocostal origin (lower two-thirds) of the pectoralis major, but with simultaneous detachment of the humeral insertion, to be attached to the coracoid process. Carroll and Kleinman modified this technique, using the entire pectoralis major muscle and both pectoral nerves. The muscle origin was prolonged with the anterior rectus abdominis sheath secured to the biceps tendon. The tendon of insertion was transferred to the acromion.
The addition of the pectoralis minor to the above procedure may improve strength and further protects both pectoral nerves during dissection. Prerequisites to this technique are that the sternocostal part of the pectoralis major muscle is of normal strength, and the elbow has full passive range of motion (ROM).
The unipolar transfer approach is based on that of Clark and Holtmann et al. The patient is supine with a sandbag behind the shoulder. The arm is draped free and supported on a hand table or Mayo stand. An incision is made parallel to the lateral border of the pectoralis major muscle from the axilla to the seventh rib. The origin of the lower third of the muscle is detached from the sternum and fifth and sixth costal cartilages in continuity with a 6-cm segment of anterior rectus abdominis sheath. The sternocostal segment is carefully freed from the rest of the pectoralis major muscle to protect its nerve (medial pectoral) and vascular (lateral thoracic branches) supplies.
The biceps tendon is exposed through an oblique incision over the anterior aspect of the distal arm and elbow. Large forceps are then thrust upward from this distal incision to create a subcutaneous tunnel continuous with the upper end of the other incision. The muscle is pulled through the tunnel, and its rectus sheath segment is woven through the biceps tendon with the elbow in 125 degrees of flexion and the forearm fully supinated. With the shoulder in adduction and internally rotated, the elbow and forearm are immobilized in acute flexion and supination for 6 weeks. Isometric contractions are begun at 3 weeks, followed by active elbow flexion from an initial position of 90 degrees of elbow flexion.
This procedure is based on that of Carroll and Kleinman ( Fig. 117.12 ). With the patient supine and the upper extremity draped free, a curvilinear incision is made from the seventh sternocostal joint, extending proximally to two fingerbreadths inferior to the clavicle. The incision continues laterally to the coracoid process and then distally along the anteromedial aspect of the arm to the level of the axilla.
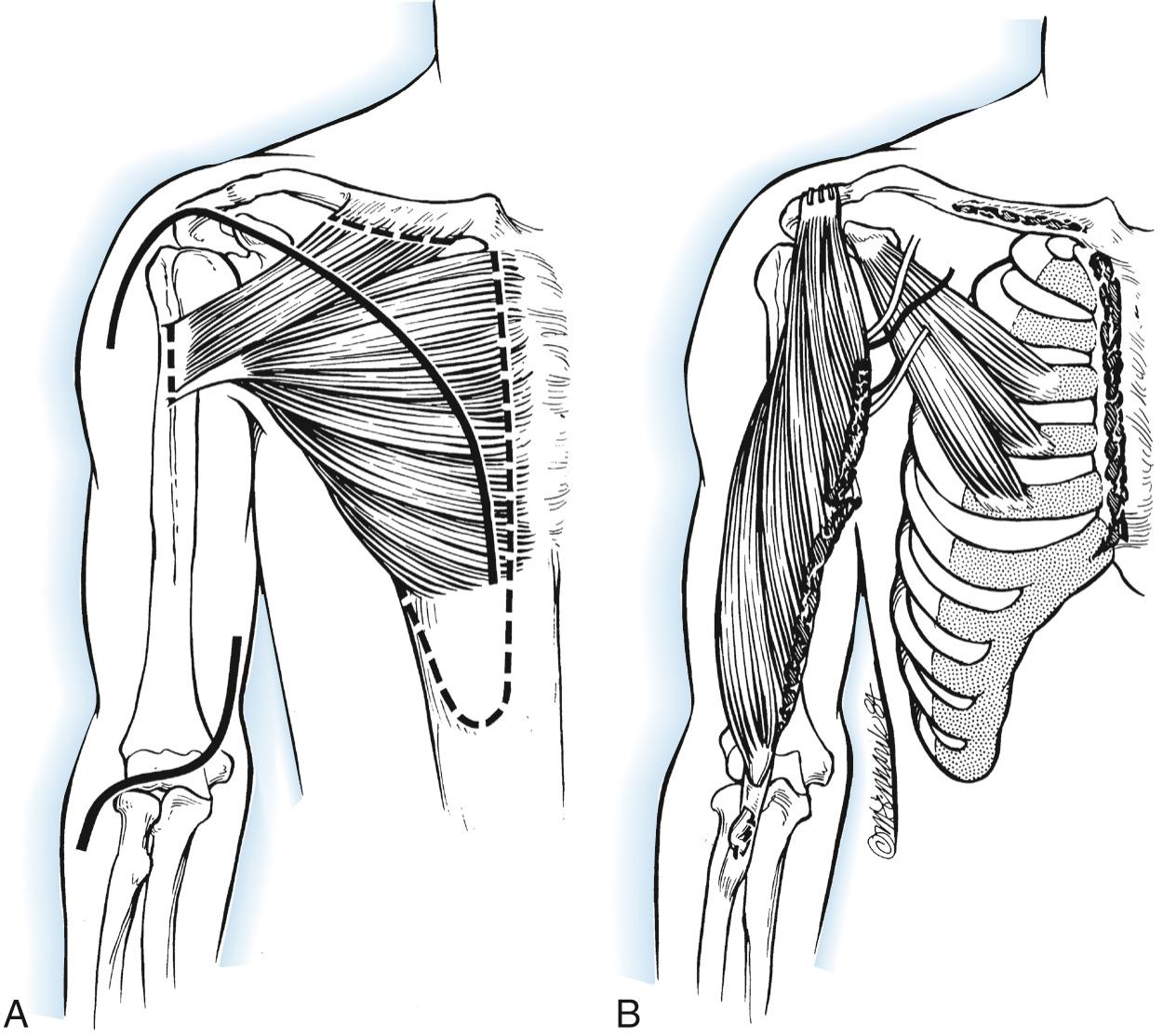
With the acromion and the entire pectoralis major muscle thus exposed, a second antecubital incision is made with a transverse limb across the fossa and a longitudinal limb extending medially and distally 6 cm. The entire pectoralis major origin is then detached, including both the clavicular head and the sternocostal portion. Care must be given to protect the pectoral nerves, avoiding dissection between the pectoralis major and minor muscles over the central portion of the muscle. A 10- by 4-cm strip of anterior rectus abdominis fascia is included.
The entire muscle mass is then rotated 90 degrees. The clavicular and sternocostal origins are rolled into a tube and directed through a subcutaneous tunnel to the antecubital incision. With the elbow flexed 135 degrees, the fascial tube is secured to the biceps tendon under maximal tension. The tendon of insertion is then detached, directed proximally, and anchored securely to the anterior acromion. The elbow is immobilized for 6 weeks, with the joint flexed 135 degrees.
The results of pectoralis flexorplasty will depend in part on the extent of the injury. Brachial plexus trauma with elbow paralysis will generally result in loss of the lateral pectoral nerve as well, leaving only the pectoralis minor and sternocostal portion of the muscle innervated. In such cases, bipolar transfer including the pectoralis minor may be advisable. Tsai and others used modified bipolar transplantation of the pectoralis major (leaving the lateral half of the clavicular origin intact), supplemented with a unipolar transfer of the pectoralis minor, in four patients. Three of the four achieved excellent results (full extension with at least 60 degrees of flexion), and the other patient required a secondary Steindler's flexorplasty.
Seddon noted excellent results (powerful flexion against gravity and resistance) in only 7 of 16 of Clark's patients. In the remainder, the elbow could be flexed against gravity and slight resistance. Clark also included the pectoralis minor when the pectoralis major was weak. Unipolar transfer as reported by Brooks and Seddon achieved three excellent results of 10 patients. Two were failures, and two others required a later triceps to biceps transfer due to problems with co-contraction of the transfer with the triceps.
In addition to problems with weakness and co-contraction, others have reported need for subsequent shoulder fusion, cosmetic disfigurement in female patients, and occasional need for a supplemental Steindler flexorplasty.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here