Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
On completion of this chapter , you should be able to:
Describe in detail the embryology of the fetal skeleton
Describe the variety of musculoskeletal anomalies that can occur in the fetus
Differentiate sonographically among the most common skeletal dysplasias
List limb abnormalities and the anomalies that are associated with and unique to specific defects
The majority of the musculoskeletal system forms from the primitive mesoderm arising from mesenchymal cells that are the embryonic connective tissue. These cells arise from different regions of the body. The vertebral column and ribs arise from the somites, and the limbs arise from the lateral plate mesoderm. The formation of the head is more complex in that the cranial bones that form the roof and base of the skull arise from mesenchymal cells of the primitive mesoderm, but the facial bones actually arise from mesenchymal cells arising from the neural crest, which is ectodermal in origin. The skeleton initially appears as cartilaginous structures that later undergo ossification.
Limb development begins the 26th or 27th day after conception with the appearance of upper limb buds. Lower extremity development begins 2 days later. Although the stages of development for the upper and lower extremities are the same, lower extremity development continues to lag behind that of the upper extremities. Initially the limbs have a paddle shape with a ridge of thickened ectoderm, known as the apical ectodermal ridge, at the apex of each bud. Digital rays begin to differentiate from the apical ectodermal ridge around day 41 through a process of cell death of the ridge between the digits. The fingers are distinctly evident by day 49, although they are still webbed, and by the eighth week of development the fingers are longer. The development of the feet and toes is essentially complete by the ninth week, although the soles of the feet are still turned inward at this time.
Anomalies of the skeletal system often result from genetic factors, although the cause may be unknown or be the result of environmental factors, including drug or mechanical effects.
Skeletal dysplasia is the term used to describe abnormal growth and density of cartilage and bone, and the incidence is 3 in 10,000 births, with a significantly greater incidence in stillbirths. This grouping of disorders is characterized by abnormal shape, growth, and integrity of the skeletal system. Although the prenatal diagnosis can be challenging, routine ultrasound in conjunction for familial history and other assessments of risk can prove beneficial to diagnosing these conditions. There are more than 450 types of skeletal anomalies, and not all of them are amenable to sonographic detection. The perinatal team may be able to isolate a skeletal dysplasia when abnormal skeletal structures are observed, such as bone shortening or hypomineralization. Dwarfism is the condition of a disproportionately short stature; it occurs secondary to a skeletal dysplasia.
Skeletal dysplasias are considered rare, and many are considered incompatible with life. What determines whether these conditions are potentially lethal is the assessment for the presence of pulmonary hypoplasia as a result of a skeletal dysplasia limiting the ability to intubate or provide adequate ventilation once the neonate is born. The lethal forms characteristically are extremely severe in their prenatal appearance, as with severe micromelia. Nonlethal skeletal dysplasias tend to manifest in a milder form. The sonographer should become familiar with the sonographic characteristics of the more common skeletal dysplasias that can be diagnosed in utero.
There are multiple anomalies of the musculoskeletal system that may be identified with ultrasound. Many of these osteochondrodysplasias have similar features, although often there are distinguishing features that can lead to a diagnosis. The primary focus should be on identifying those features suggestive of lethality. A list of short-limb skeletal dysplasias, ultrasound characteristics, and their distinguishing features are listed in Table 65.1 .
| Anomaly | Sonographic Findings | Distinguishing Characteristics |
|---|---|---|
| Thanatophoric dysplasia |
|
Cloverleaf skull |
| Achondrogenesis |
|
|
| Achondroplasia |
|
|
| Camptomelic dysplasia |
|
|
| Osteogenesis imperfecta (type II) |
|
|
| Short-rib polydactyly syndrome |
|
|
| Hypophosphatasia |
|
|
The patient whose fetus is at risk for a skeletal dysplasia is commonly referred to a maternal-fetal center for genetic counseling and a targeted ultrasound. Although many skeletal dysplasias are inherited, sporadic occurrences and new mutations do occur, so it is important to screen for skeletal dysplasias as part of every obstetric ultrasound examination. Most prenatally diagnosed skeletal dysplasias occur in association with polyhydramnios or other fetal anomalies or when there is a risk for recurrence.
When a skeletal dysplasia is suspected, the protocol of the obstetric ultrasound examination should be adjusted to include the following criteria:
Assess limb shortening. All long bones should be measured. A skeletal dysplasia is suspected when limb lengths are more than 2 standard deviations below the mean ( Tables 65.2 and 65.3 ).
| Week | Tibia Percentile | Fibula Percentile | ||||
|---|---|---|---|---|---|---|
| 5th | 50th | 95th | 5th | 50th | 95th | |
| 12 | — | 7 | — | — | 6 | — |
| 13 | — | 10 | — | — | 9 | — |
| 14 | 7 | 12 | 17 | 6 | 12 | 19 |
| 15 | 9 | 15 | 20 | 9 | 15 | 21 |
| 16 | 12 | 17 | 22 | 13 | 18 | 23 |
| 17 | 15 | 20 | 25 | 13 | 21 | 28 |
| 18 | 17 | 22 | 27 | 15 | 23 | 31 |
| 19 | 20 | 25 | 30 | 19 | 26 | 33 |
| 20 | 22 | 27 | 33 | 21 | 28 | 36 |
| 21 | 25 | 30 | 35 | 24 | 31 | 37 |
| 22 | 27 | 32 | 38 | 27 | 33 | 39 |
| 23 | 30 | 35 | 40 | 28 | 35 | 42 |
| 24 | 32 | 37 | 42 | 29 | 37 | 45 |
| 25 | 34 | 40 | 45 | 34 | 40 | 45 |
| 26 | 37 | 42 | 47 | 36 | 42 | 47 |
| 27 | 39 | 44 | 49 | 37 | 44 | 50 |
| 28 | 41 | 46 | 51 | 38 | 45 | 53 |
| 29 | 43 | 48 | 53 | 41 | 47 | 54 |
| 30 | 45 | 50 | 55 | 43 | 49 | 56 |
| 31 | 47 | 52 | 57 | 42 | 51 | 59 |
| 32 | 48 | 54 | 59 | 42 | 52 | 63 |
| 33 | 50 | 55 | 60 | 46 | 54 | 62 |
| 34 | 52 | 57 | 62 | 46 | 55 | 65 |
| 35 | 53 | 58 | 64 | 51 | 57 | 62 |
| 36 | 55 | 60 | 65 | 54 | 58 | 63 |
| 37 | 56 | 61 | 67 | 54 | 59 | 65 |
| 38 | 58 | 63 | 68 | 56 | 61 | 65 |
| 39 | 59 | 64 | 69 | 56 | 62 | 67 |
| 40 | 61 | 66 | 71 | 59 | 63 | 67 |
| Week | Ulna Percentile | Radius Percentile | ||||
|---|---|---|---|---|---|---|
| 5th | 50th | 95th | 5th | 50th | 95th | |
| 12 | — | 7 | — | — | 7 | — |
| 13 | 5 | 10 | 15 | 6 | 10 | 14 |
| 14 | 8 | 13 | 18 | 8 | 13 | 17 |
| 15 | 11 | 16 | 21 | 11 | 15 | 20 |
| 16 | 13 | 18 | 23 | 13 | 18 | 22 |
| 17 | 16 | 21 | 26 | 14 | 20 | 26 |
| 18 | 19 | 24 | 29 | 15 | 22 | 29 |
| 19 | 21 | 26 | 31 | 20 | 24 | 29 |
| 20 | 24 | 29 | 34 | 22 | 27 | 32 |
| 21 | 26 | 31 | 36 | 24 | 29 | 33 |
| 22 | 28 | 33 | 38 | 27 | 31 | 34 |
| 23 | 31 | 36 | 41 | 26 | 32 | 39 |
| 24 | 33 | 38 | 43 | 26 | 34 | 42 |
| 25 | 35 | 40 | 45 | 31 | 36 | 41 |
| 26 | 37 | 42 | 47 | 32 | 37 | 43 |
| 27 | 39 | 44 | 49 | 33 | 39 | 45 |
| 28 | 41 | 46 | 51 | 33 | 40 | 48 |
| 29 | 43 | 48 | 53 | 36 | 42 | 47 |
| 30 | 44 | 49 | 54 | 36 | 43 | 49 |
| 31 | 46 | 51 | 56 | 38 | 44 | 50 |
| 32 | 48 | 53 | 58 | 37 | 45 | 53 |
| 33 | 49 | 54 | 59 | 41 | 46 | 51 |
| 34 | 51 | 56 | 61 | 40 | 47 | 53 |
| 35 | 52 | 57 | 62 | 41 | 48 | 54 |
| 36 | 53 | 58 | 63 | 39 | 48 | 57 |
| 37 | 55 | 60 | 65 | 45 | 49 | 53 |
| 38 | 56 | 61 | 66 | 45 | 49 | 54 |
| 39 | 57 | 62 | 67 | 45 | 50 | 54 |
| 40 | 58 | 63 | 68 | 46 | 50 | 55 |
Assess bone contour. Thickness, assessment for abnormal bowing or curvature, fractures, and a ribbon-like appearance should be performed.
Estimate degree of ossification. Decreased attenuation of the bones with decreased shadowing suggests hypomineralization. Special attention should be focused toward this assessment of the cranium, spine, ribs, and long bones.
Evaluate the thoracic circumference and shape. A long, narrow chest or a bell-shaped chest may be indicative of specific dysplasias.
Survey for coexistent hand and foot anomalies, such as talipes and polydactyly.
Evaluate the face and profile for facial clefts, frontal bossing, micrognathia, hypertelorism, and other facial anomalies that may be associated with skeletal dysplasias.
Survey for other associated anomalies, such as hydrocephaly, heart defects, and nonimmune hydrops.
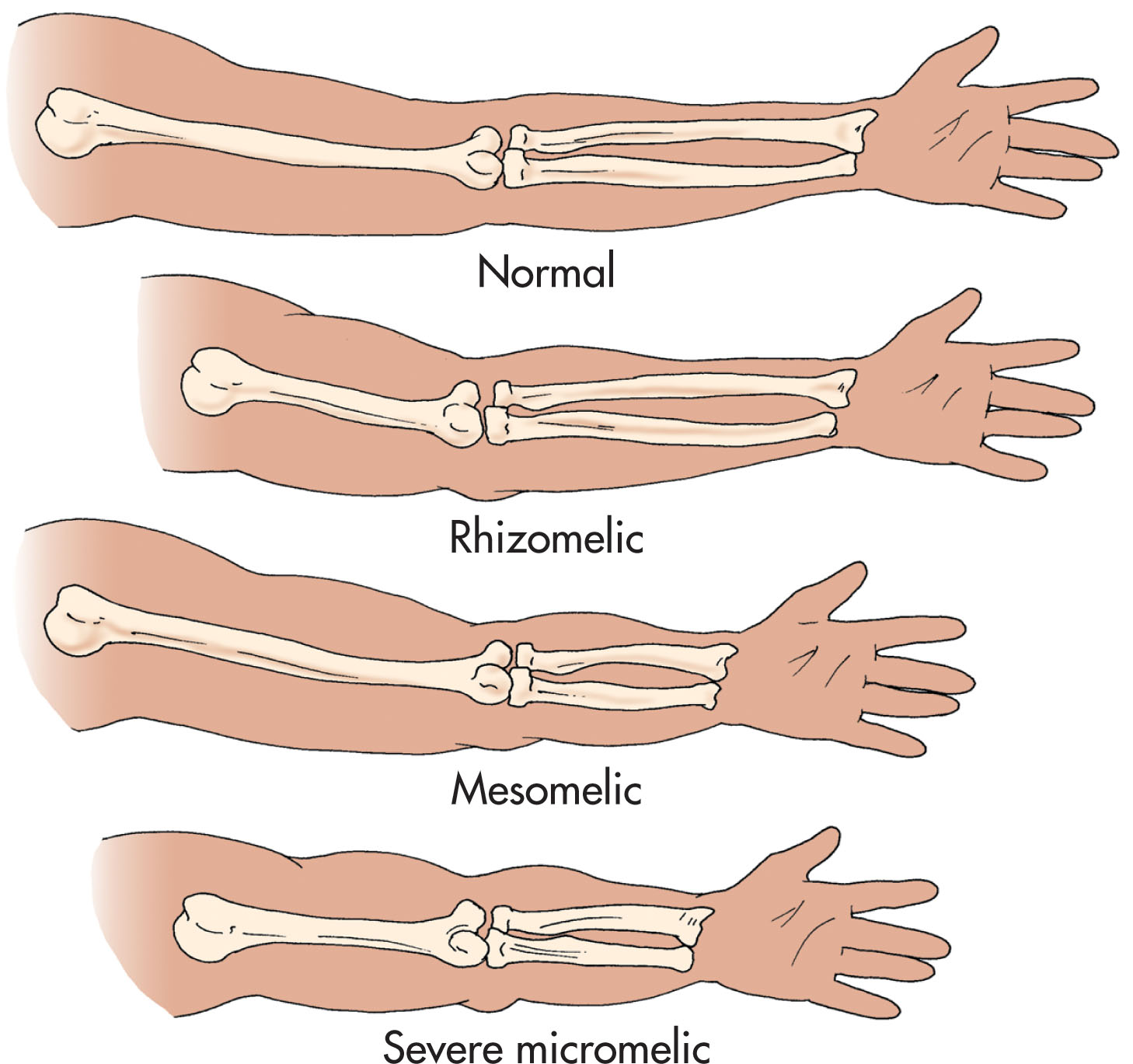
The manifestation of skeletal dysplasias varies based on the specific dysplasia, and three-dimensional ultrasound may aid in the diagnosis. First trimester assessment may present with an increased nuchal translucency, which increases the risk for lethality, and chorionic villus sampling may provide important diagnostic information regarding the type of skeletal dysplasia. In addition, long bones are affected in different patterns ( Fig. 65.1 ) according to the dysplasia. Rhizomelia is shortening of the proximal bone segment (humerus and femur). Mesomelia refers to shortening of the middle segments (radius/ulna and tibia/fibula). Micromelia describes the shortening of the entire extremity. Sonographic examination of the long bones should include an assessment to define whether there is segmental shortening or micromelia, because this will aid in the diagnosis. Three-dimensional evaluation may provide further definition of the abnormality.
Thanatophoric dysplasia is the most common lethal skeletal dysplasia and occurs in 1 in 20,000 to 40,000 live births ( Fig. 65.2 ). The term thanatophoric comes from the Greek word thanatos , which means death personified . The two main subdivisions of thanatophoric dysplasia are types I and II. Type I is characterized by short, curved femurs and flat vertebral bodies. Type II is characterized by straight, short femurs, flat vertebral bodies, and a cloverleaf skull. Most cases of thanatophoric dysplasia are sporadic occurrences and the result of mutations in the fibroblast growth factor receptor 3 (FGFR3) gene. Prenatal molecular analysis can aid in making the definitive diagnosis.
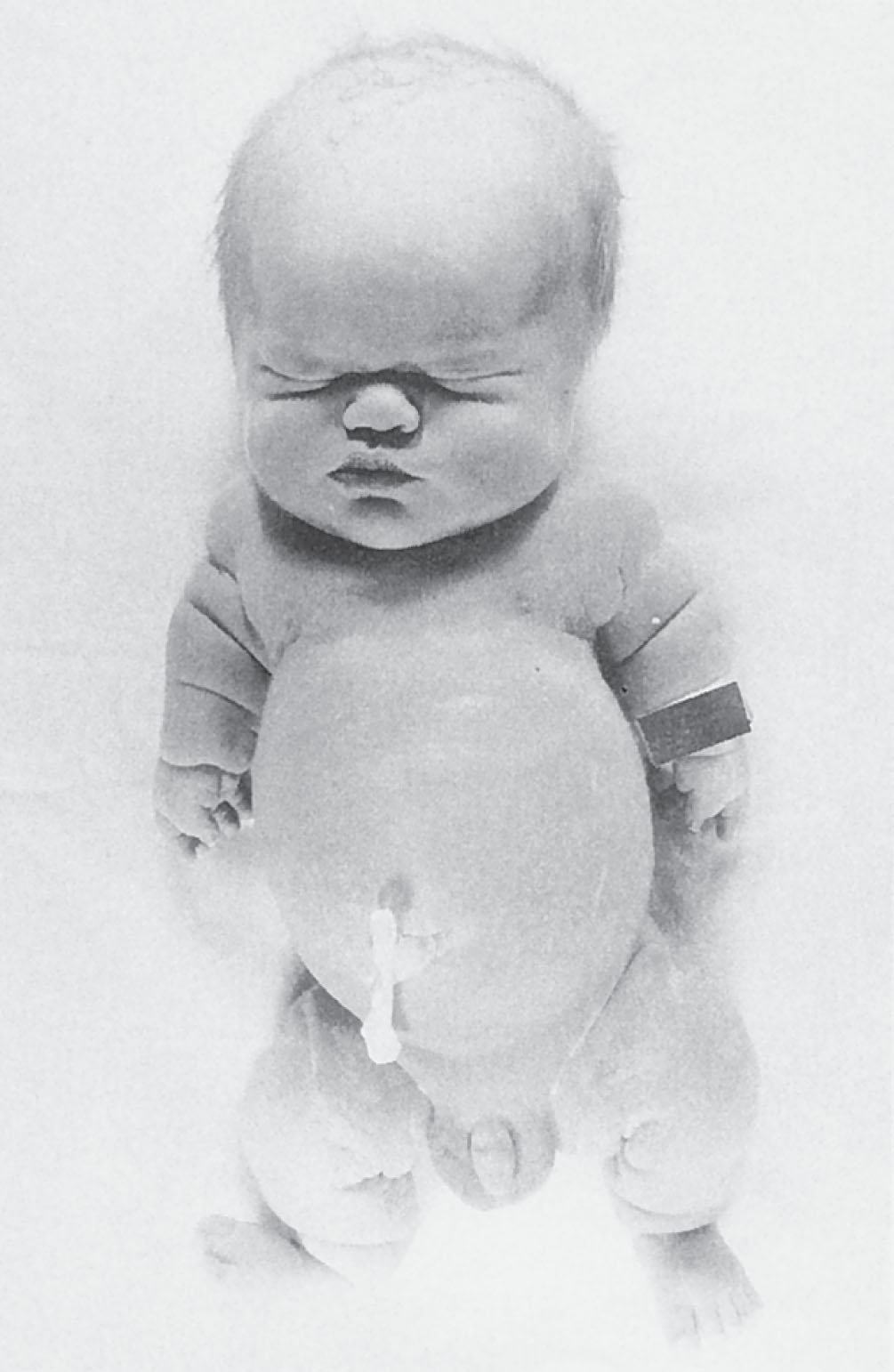
The prognosis for thanatophoric dysplasia is extremely grim. It is considered a lethal anomaly, with most infants dying shortly after birth as a result of pulmonary hypoplasia, which results from the narrow thorax.
The sonographic features of thanatophoric dysplasia include the following:
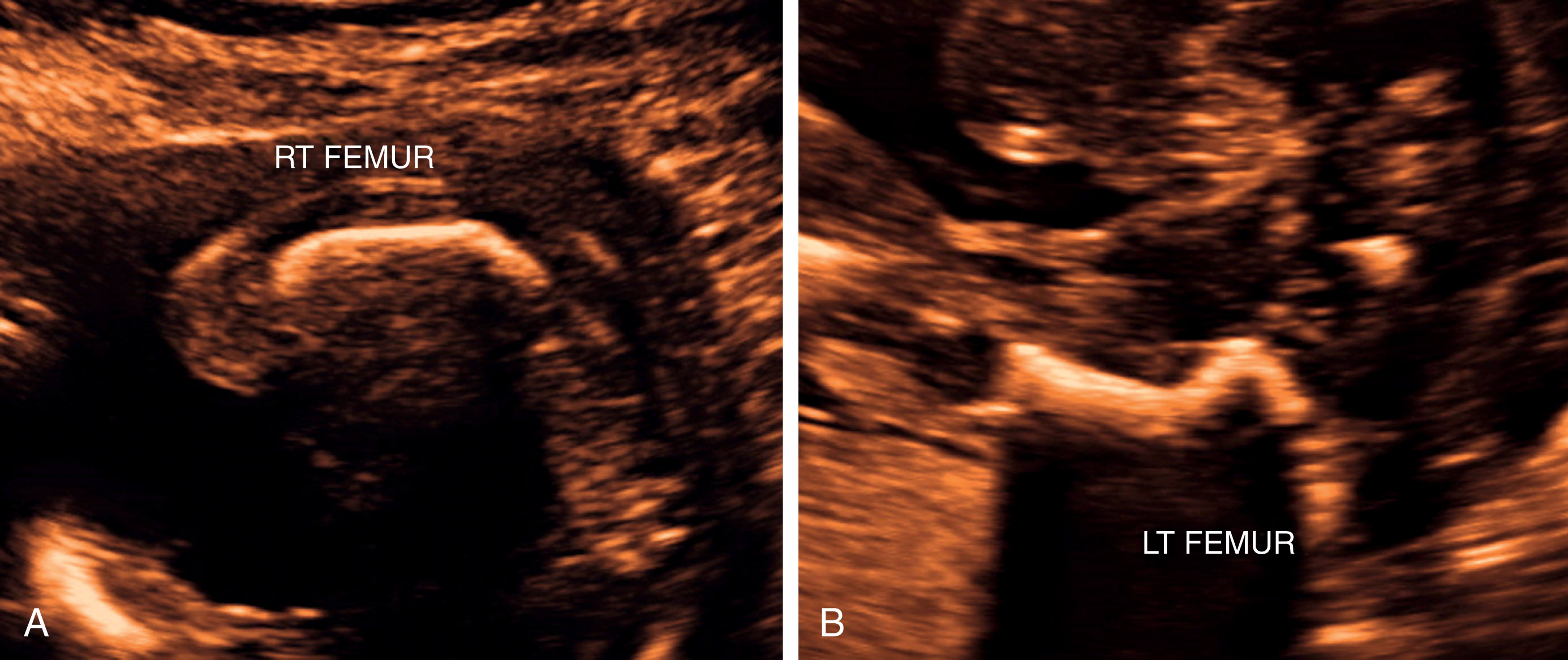
Severe micromelia ( Fig. 65.3A–B ), especially of the proximal bones (rhizomelia)
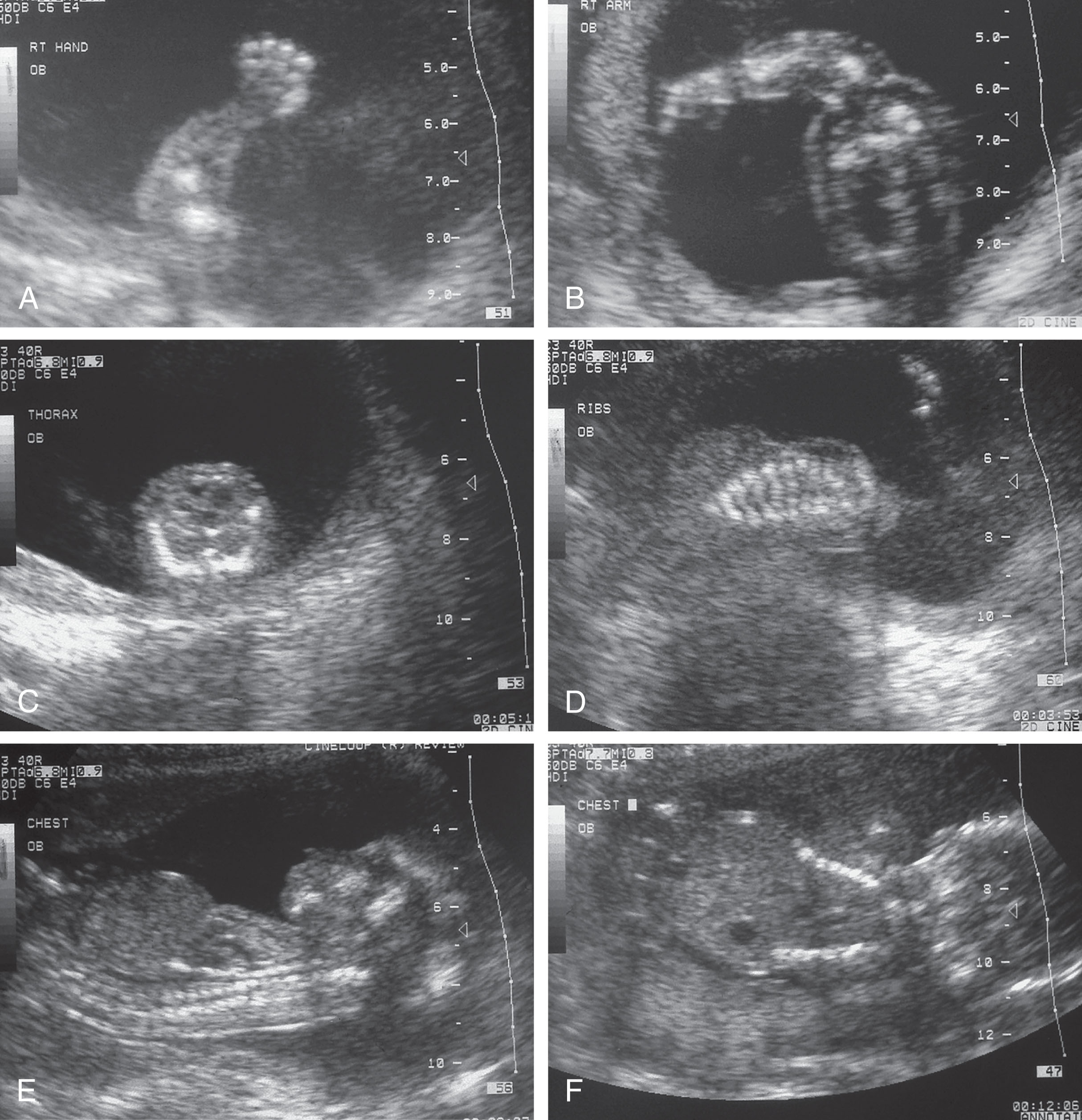
Cloverleaf deformity (Kleeblattschädel skull), which occurs as a result of premature craniosynostosis and may be associated with agenesis of the corpus callosum
Narrow thorax with shortened ribs (see Fig. 65.3C–D )
Protuberant abdomen
Frontal bossing (bulging forehead)
Hypertelorism (widely spaced eyes)
Flat vertebral bodies (platyspondyly)
Other sonographic findings that may be associated with thanatophoric dysplasia include severe polyhydramnios, hydrocephalus, and nonimmune hydrops.
Achondroplasia is the most common nonlethal skeletal dysplasia and occurs in approximately 1 in 10,000 to 30,000 live births. It results from decreased endochondral bone formation, which produces short, squat bones. It is most commonly the result of a spontaneous mutation but can also be transmitted in an autosomal fashion. Advanced paternal age increases the risk for this dysplasia.
The prognosis for achondroplasia depends on the form. Heterozygous achondroplasia , inherited from one parent, has a good survival rate with normal intelligence and a normal life span. Health problems may include neurologic complications that may require orthopedic or neurologic surgical intervention. Homozygous achondroplasia , inherited from two parents, is considered lethal, with most infants dying shortly after birth from respiratory complications. With this form, sonographic findings are more severe and include a narrow thorax.
The sonographic features of achondroplasia may not be evident until after 22 weeks of gestation, when biometry becomes abnormal. Ultrasound-guided chorionic villus sampling may aid in the diagnosis in the first trimester for parents with this genetic disorder, and amniocentesis may also be used. Sonographic findings include the following:
Rhizomelia
Macrocephaly
Trident hands (short proximal and middle phalanges)
A depressed nasal bridge
Frontal bossing
Mild ventriculomegaly may be identified
Achondrogenesis is a rare, lethal skeletal dysplasia occurring in 1 in 40,000 births and is caused by cartilage abnormalities that result in abnormal bone formation and hypomineralization. The two types of achondrogenesis are types I (Parenti-Fraccaro) and II (Langer-Saldino). Type I is considered more severe and is transmitted in an autosomal recessive mode, whereas type II is less severe, is more common, and is the result of a spontaneous mutation.
The prognosis for achondrogenesis is grim. It is a lethal abnormality with infants either being stillborn or dying shortly after birth from pulmonary hypoplasia.
The sonographic features ( Figs. 65.4–65.6 ) of achondrogenesis include the following:
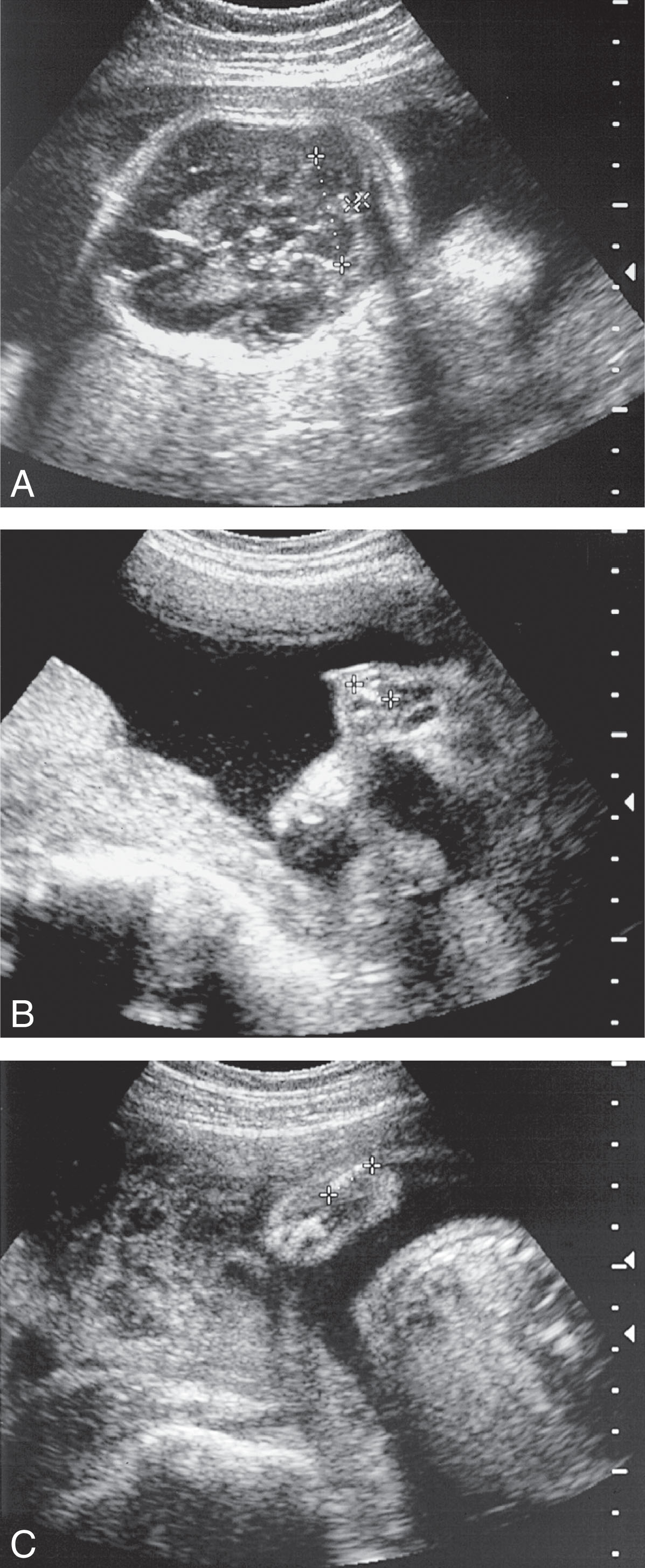
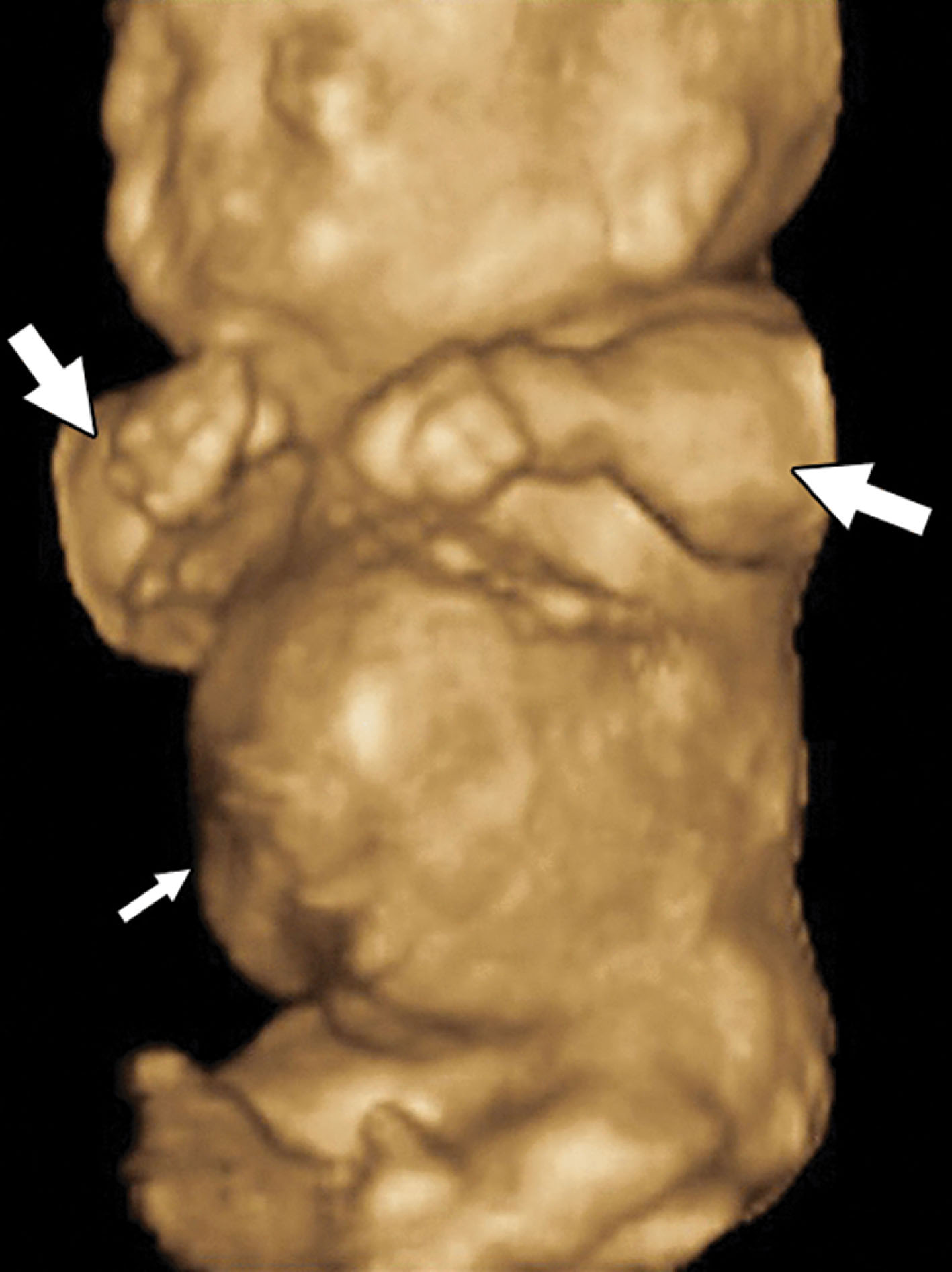
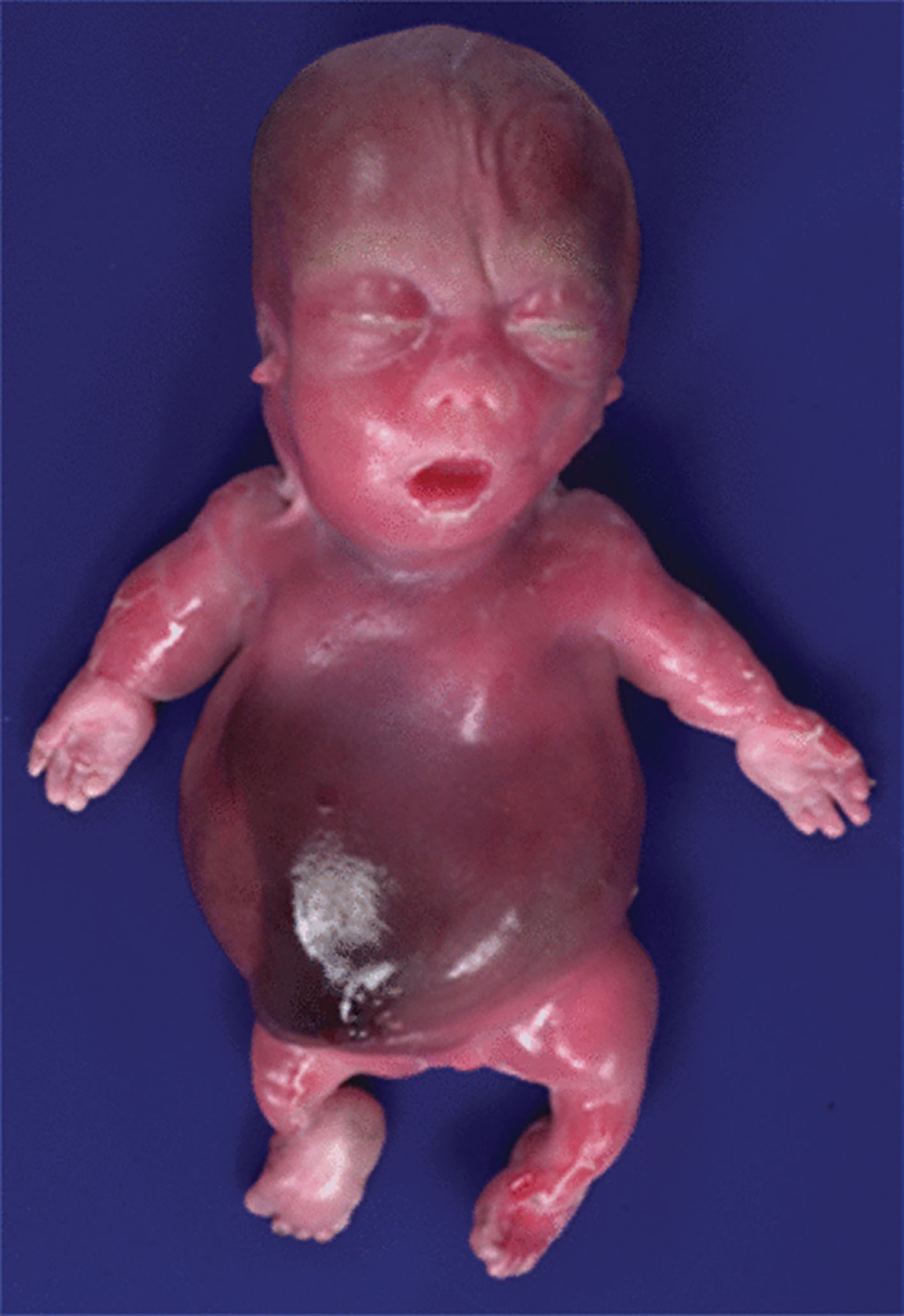
• Severe micromelia
• Decreased or absent ossification of the spine
• Macrocephaly
• Short trunk
• Short thorax and short ribs
• Micrognathia
• Polyhydramnios
• Hydrops possibly identified
Osteogenesis imperfecta is a rare disorder of collagen production leading to brittle bones, blue sclera, and manifestations in the teeth, skin, and ligaments. It has been linked to mutations in the COL1A1 and COL1A2 genes. There are four classifications, types I to IV. Types I and IV are the mildest forms, and it would be unlikely that a diagnosis would be made in utero. Types I and IV are transmitted in an autosomal dominant fashion. Type III is a severe form that may be transmitted in an autosomal dominant or autosomal recessive manner. Type II ( Fig. 65.7 ) is considered the most severe form of osteogenesis imperfecta, having a lethal outcome. It is inherited in an autosomal dominant or autosomal recessive fashion or may result from a spontaneous mutation.
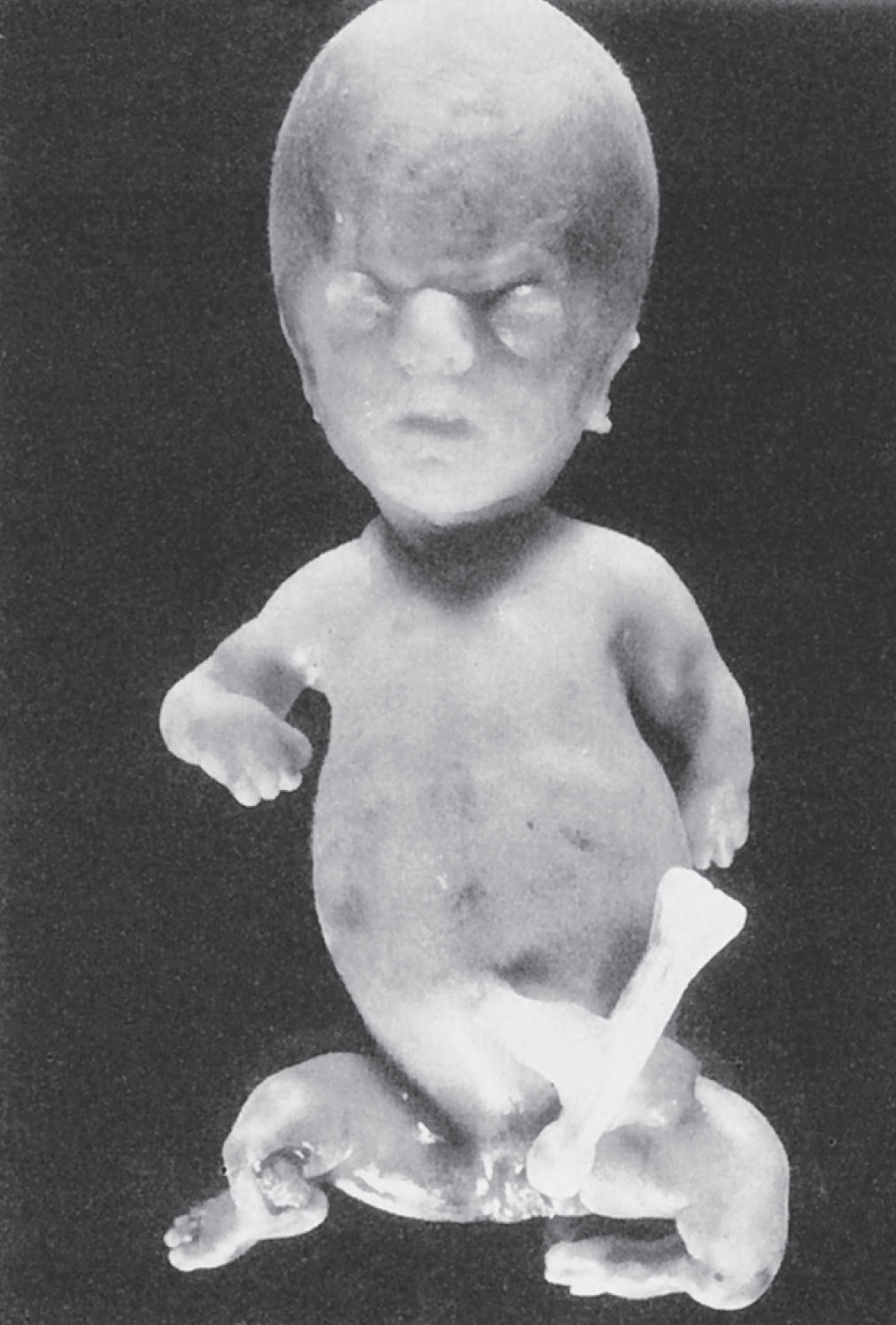
The prognosis for osteogenesis imperfecta depends on the type. Children with types I and IV may have multiple fractures during childhood and may be short. Type I children may also have kyphoscoliosis and deafness. Because of the severity of the brittle bones and multiple fractures, osteogenesis imperfecta type III may produce significant disabilities with progressive deformities of the long bones and spine. Infants with type II usually die shortly after birth because of respiratory complications.
Osteogenesis imperfecta has presented with an increased nuchal translucency in the first trimester of pregnancy. Other more specific sonographic features of osteogenesis imperfecta type II include the following:
Generalized hypomineralization of the bones, especially the calvarium ( Fig. 65.8 )
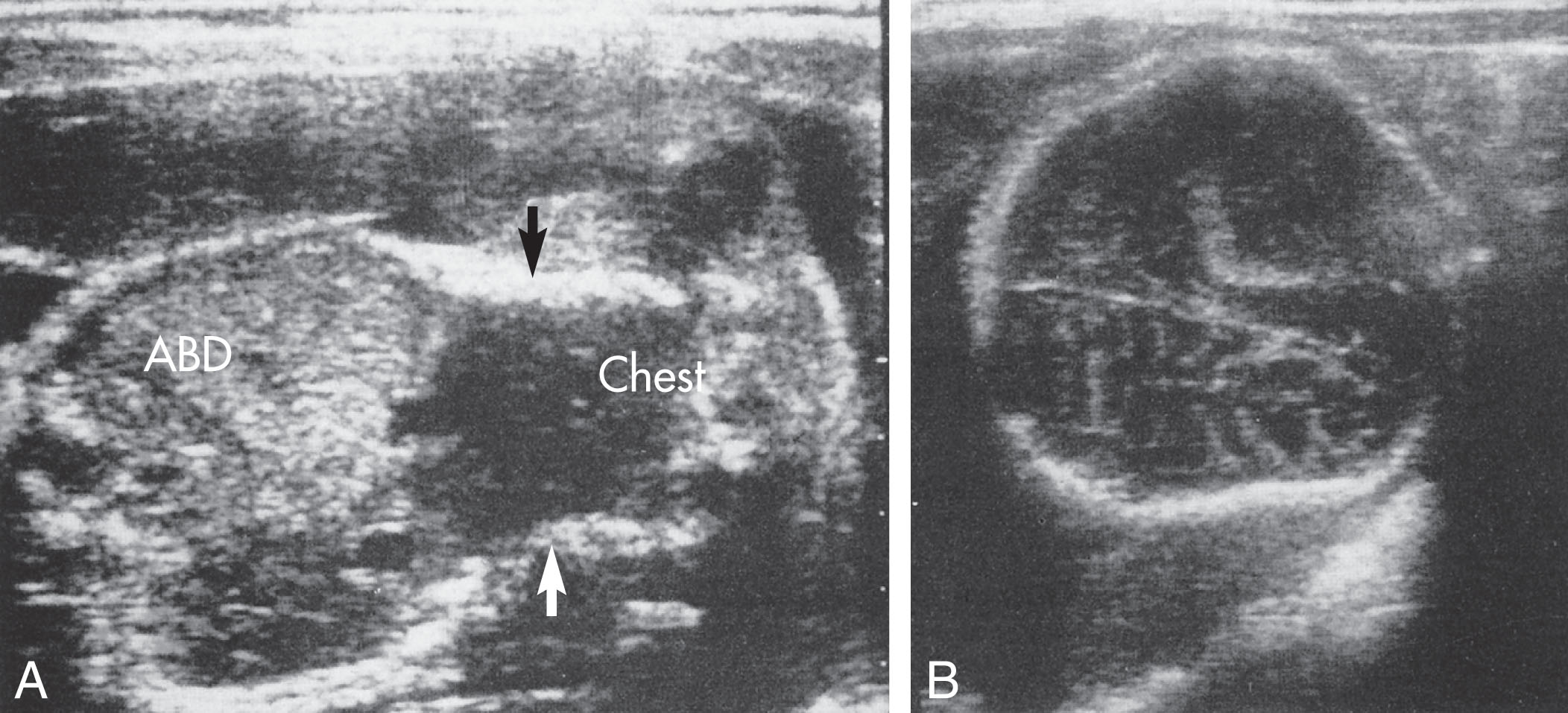
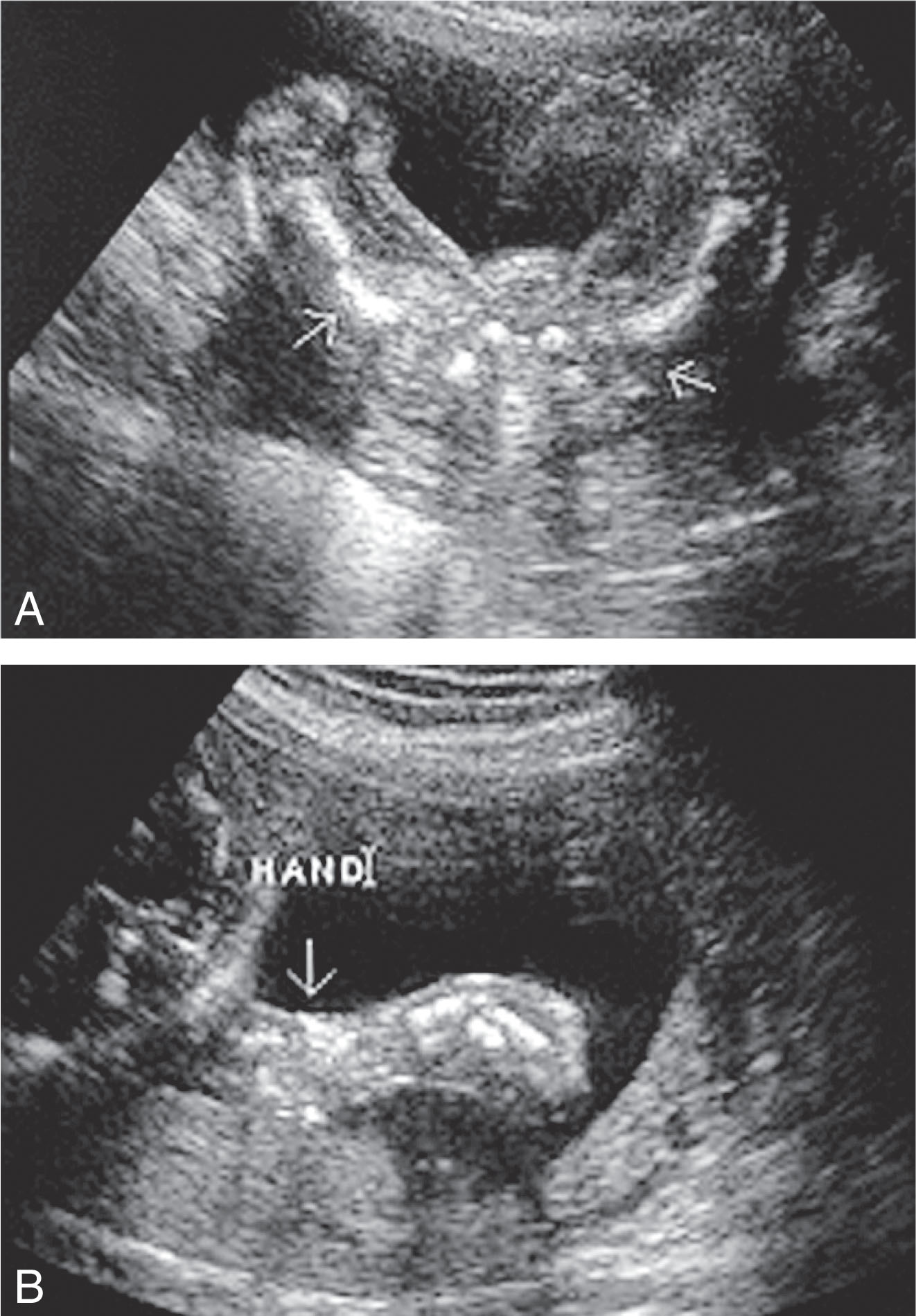
Multiple fractures of the long bones, ribs, and spine ( Figs. 65.9 and 65.10 )
Narrow thorax (see Fig. 65.8A )
Micromelia
In addition to these findings, brain structures are clearly visualized because of the hypomineralization of the calvarium. The calvarium will also be compressible. The multiple fractures that have occurred during the course of pregnancy may leave the bones bowed, thickened, and sharply angulated. Polyhydramnios may also be evident.
The sonographic features of osteogenesis imperfecta type III are similar to those of type II, although it is less severe.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here